Abstract
Background
The use of animals to augment traditional medical therapies was reported as early as the 9th century but to our knowledge has not been studied in an orthopaedic patient population. The purpose of this study was to evaluate the role of animal-assisted therapy using therapy dogs in the postoperative recovery of patients after THA and TKA.
Questions/purposes
We asked: (1) Do therapy dogs have an effect on patients’ perception of pain after total joint arthroplasty as measured by the VAS? (3) Do therapy dogs have an effect on patients’ satisfaction with their hospital stay after total joint arthroplasty as measured by the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS)?
Methods
A randomized controlled trial of 72 patients undergoing primary unilateral THA or TKA was conducted. Patients were randomized to a 15-minute visitation with a therapy dog before physical therapy or standard postoperative physical therapy regimens. Both groups had similar demographic characteristics. Reduction in pain was assessed using the VAS after each physical therapy session, beginning on postoperative Day 1 and continuing for three consecutive sessions. To ascertain patient satisfaction, the proportion of patients selecting top-category ratings in each subsection of the HCAHPS was compared.
Results
Patients in the treatment group had lower VAS scores after each physical therapy session with a final VAS score difference of 2.4 units (animal-assisted therapy VAS, 1.7; SD, 0.97 [95% CI, 1.4–2.0] versus control VAS, 4.1; SD, 0.97 [95% CI, 3.8–4.4], p < 0.001) after the third physical therapy session. Patients in the treatment group had a higher proportion of top-box HCAHPS scores in the following fields: nursing communication (33 of 36, 92% [95% CI, 78%–98%] versus 69%, 25 of 36 [95% CI, 52%–84%], p = 0.035; risk ratio, 1.3 [95% CI of risk ratio, 1.0–1.7]; risk difference, 23% [95% CI of risk difference, 5%–40%]), pain management (34 of 36, 94% [95% CI, 81%–99%], versus 26 of 36, 72% [95% CI, 55%–86%], p = 0.024; risk ratio, 1.3 [95% CI of risk ratio, 1.1–1.6]; risk difference, 18% [95% CI of risk difference, 5%–39%]). The overall hospital rating also was greater in the treatment group (0–10 scale) (9.6; SD, 0.7 [95% CI, 9.3–9.8] versus 8.6, SD, 0.9 [95% CI, 8.3–8.9], p < 0.001).
Conclusions
The use of therapy dogs has a positive effect on patients’ pain level and satisfaction with hospital stay after total joint replacement. Surgeons are encouraged to inquire about the status of volunteer-based animal-assisted therapy programs in their hospital as this may provide a means to improve the immediate postoperative recovery for a select group of patients having total joint arthroplasty.
Level of Evidence
Level II, randomized controlled study. See Instructions for Authors for a complete description of levels of evidence.
Introduction
Animal-assisted therapy is defined by the American Veterinary Medical Association as “a goal-directed intervention in which an animal, meeting specific criteria, is an integral part of the treatment process” [4]. One study focused on the use of dogs in a therapeutic capacity [9]. This is attributable in large part to the fact that dogs seem particularly well suited for this role and because dog and handler teams are inherently more accessible than other species [9, 11, 19]. Therapy dogs have been shown to be efficacious in the treatment for a wide range of patients from children recovering from surgical procedures to adults with congestive heart failure [1, 7, 8, 14, 16, 23, 25, 31, 35, 37]. As the popularity of animal-assisted therapy has increased, practice-based guidelines have been developed to assist hospitals and nursing homes with implementation of safe and effective programs [29, 47]. Although there was initial concern regarding the risk of zoonotic infections with hospitalized patients, two longitudinal studies with thousands of patients have not shown a zoonotic infection or adverse event [26, 30].
Despite its widespread use, animal-assisted therapy, to our knowledge, has not been studied in an orthopaedic population. The population undergoing total joint arthroplasties most closely resembles other populations shown to derive benefit from animal-assisted therapy owing to its high volume and focus on postoperative physical therapy [16]. The effect of animal-assisted therapy is mediated by a patient’s impression of the interaction and thus is best evaluated using validated measures of pain (VAS) and hospital satisfaction (Hospital Consumer Assessment of Healthcare Providers and Systems [HCAHPS]) which focus on these subjective aspects of a patient’s hospitalization.
The purpose of this study was to evaluate the role of animal-assisted therapy, using therapy dogs, during the immediate postoperative period after THA and TKA. We hypothesized that patients receiving animal-assisted therapy would perceive lower pain levels after physical therapy and greater satisfaction with their hospital stay than matched control subjects.
This study attempts to answer the following questions: (1) Does animal-assisted therapy using dogs decrease patients’ pain after THA and TKA as measured by VAS? (3) Is use of animal-assisted therapy associated with a greater proportion of highest ratings in HCAHPS hospital satisfaction scores after total joint arthroplasty?
Patients and Methods
Study Design and Setting
Approval was obtained from our institutional review board for a randomized controlled trial. We performed a prospective randomized controlled study from August 2013 to September 2013 at a tertiary care hospital in an urban environment.
Participants/Study Subjects
The operative schedule for all involved surgeons was screened by the principal investigator (CMH). All patients meeting inclusion criteria were invited to participate through a letter and followup telephone call. Confirmation of enrollment was made after obtaining informed consent on postoperative Day 1.
Inclusion/Exclusion Criteria
The inclusion criteria were (1) age older than 18 years; (2) unilateral primary THA or TKA; (3) ability to read, write, and speak English; and (4) ability to provide informed consent. Exclusion criteria were (1) fear of dogs; (2) allergy to dogs or dog dander; (3) active immunosuppression or a preoperative white blood cell count less than 4500 cells/μL; (4) patients undergoing chemotherapy; (5) patients with a positive test for methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, or Clostridium difficile; (5) patients in shared rooms whose roommates objected to having a dog in the room; (6) patients with symptoms of delirium or those not oriented to person, place, and time; (7) patients scheduled to be “fast tracked” or discharged on postoperative Day 1; and (8) patients randomized to the treatment arm whose roommate was serving as a control subject.
Basic demographic information including age, sex, comorbidities, prior total joint arthroplasty, and current dog ownership was collected from patients and their medical records. Comorbidities were estimated with the Charlson Comorbidity Index, which provides a weighted score to predict short- and long-term outcomes taking into account the number and severity of predefined comorbid conditions [13].
Randomization
Patients were randomized to visitation with a therapy dog or no visitation with the use of a computer-driven randomizing equation. Patients were enrolled and randomized sequentially.
Description of Experiment, Treatment, or Surgery
From August 2013 to September 2013, 95 patients were assessed for eligibility, and 84 of them were found to be eligible for the study. We enrolled 72 (85%) patients (35 THAs and 37 TKAs). Thirty-four of the patients who had THAs and 36 of the patients who had TKAs were placed in double rooms while one patient who had a THA and one who had a TKA were placed in single rooms. Of the patients who elected not to enroll, six did not like dogs, four had family members who were allergic and elected not to participate, and two stated that their family did not like dogs. There were no dropouts, crossover, or loss to followup in either the control or the treatment arm (Fig. 1).
Fig. 1.
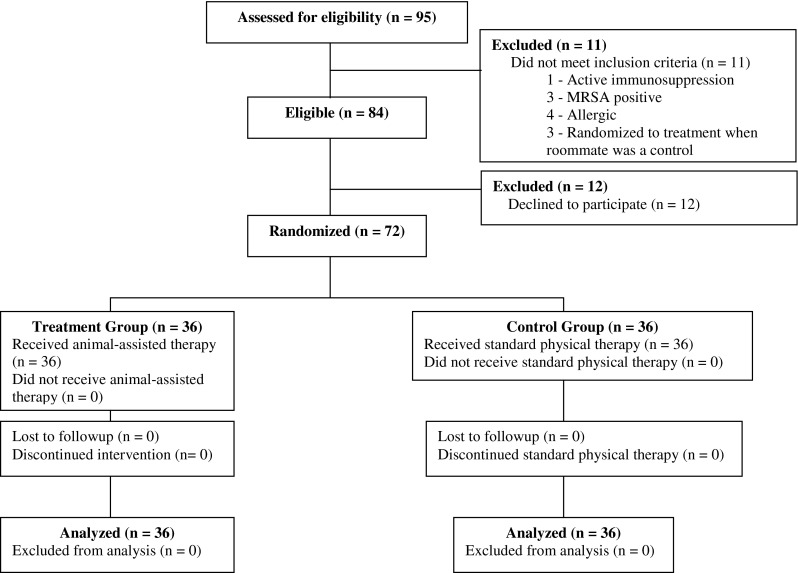
Eighty-five percent of eligible patients elected to enroll in the study. There was no crossover or loss to followup. MRSA = methicillin-resistant Staphylococcus aureus.
Treatment
Our institution has a standardized physical therapy protocol for patients undergoing THA and TKA. Patients undergo physical therapy at the bedside twice per day on postoperative Day 1. On postoperative Day 2, patients undergoing TKA receive two physical therapy sessions, whereas patients undergoing THA receive an occupational therapy session in the morning and a physical therapy session in the afternoon. Both patient populations typically are discharged home or to a rehabilitation facility at the end of postoperative Day 3. Thirty minutes before undergoing a physical therapy session, patients were visited by the therapy dog and handler (CMH) team. The dog was a 5-year-old curly-coated retriever named Holden (Fig. 2), and the handler was an orthopaedic surgery resident. Both dog and handler were certified through Therapy Dogs International. The same dog and handler were used for all interactions. The experimental group received the animal-assisted therapy, which consisted of a 15-minute visit following a standard animal-assisted therapy protocol [16, 27]. Before starting the visit, the patient and the handler washed their hands. The handler introduced himself and sat in a chair or stood approximately 1.2 m (4 feet) from the patient’s head letting the patient know that the visit would last for 15 minutes if the patient was amenable to it. After hands were cleansed, visitations followed a standard series of events: (1) the dog sat by the patient’s bed or chair with its head within reach; and (2) patients were permitted to pet the dog and talk to the dog and the volunteer. Conversations between participants and the dog’s handler generally focused on the dog (breed, age, training) and dog-related topics. Postoperative management and medical discussions specific to the patient were discouraged. Then, (3) the patient and handler washed their hands after the visit. No patient requested to end any earlier than 15 minutes. After the visitation, patients underwent physical therapy per hospital protocol. This same protocol was followed for each visitation. All patients received three visitations.
Fig. 2.

The therapy dog in our study, a 5-year-old curly-coated retriever named Holden, is shown.
Patients assigned to the control group underwent physical therapy per hospital protocol without any changes to the normal hospital routine.
Variables, Outcome Measures, Data Sources, and Bias
Outcome Assessment
Immediately after the first, second, and third physical therapy sessions (on postoperative Days 1 and 2) and before administering analgesic medication, nursing staff administered a standard VAS form for pain to the patients in which a mark was made indicating the patients’ current level of pain [17]. At the time of discharge, patients were given the Hospital Consumer Assessment of Healthcare Providers and Systems survey (HCAHPS) [12]. HCAHPS was selected because, as of October 2012, the Center for Medicare & Medicaid Services is distributing funds to hospitals based on several quality measures, of which 30% is based on patients’ rating of their hospital experience through HCAHPS, although questions still remain regarding the validity of HCAHPS [46, 48]. Results were recorded based on the percentage of “top box” scores (the highest possible score in each category).
Statistical Analysis, Study Size
Sample Size Estimations
Given an expected difference between the two treatment groups on the VAS equaling 1 SD, power analysis indicated that a sample size of 46 patients (23 per group) would provide 90% power using a two-sample t-test and a two-tailed α (Type I error) level of 0.05. Standard deviation was estimated at 2 and minimum clinically important difference was estimated at 1.3 (increased to 2 to improve specificity) based on prior studies [22, 45]. To ensure sufficient power, we increased the required number by 10% to account for 10% possible dropout or loss to followup and therefore planned to enroll a minimum of 51 patients in this study (46/0.9 = 51). We designated this study at 90% power based on our rationale of wanting to capture with high probability a clinically important improvement as measured on the VAS (difference greater than or equal to 2) [45].
Statistical Analysis
Student’s t-test and Fisher’s exact test were used to compare the baseline characteristics between patients in the treated group and the control group. We also used Student’s t-test and Fisher’s exact test to assess the associations between HCAHPS scores and therapy dog treatment.
We used a multivariate longitudinal regression model to examine the effect of the therapy dog intervention on postoperative VAS pain levels measured at three physical therapy sessions after the surgery. In this mixed-effect regression model, we accounted for correlated repeated measures within subjects. The primary outcome is VAS pain level measured on postoperative Days 1 and 2. The independent variables include treatment status (therapy dog treatment versus control), time (ordinal variable for the times of the physical therapy sessions), and the interaction of treatment status by time (which represents the additional change of VAS level with each additional unit of time among treated subjects relative to those in the control group).
Demographics, Description of Study Population
A total of 72 patients participated in the study (36 treatment and 36 control). All patients underwent three visitations with the therapy dog for a total of 108 visits. All visitations lasted 15 minutes. There were no demographic differences between groups regarding age, sex, laterality, hip or knee replaced, operating surgeon, history of prior joint arthroplasty, Charlson Comorbidity Index, dog owner status, length of stay, and proportion of patients who refused occupational or physical therapy (three owing to nausea and two owing to hypotension) (Table 1).
Table 1.
Patient demographics
| Variable | Treatment n = 36 | Control n = 36 | p value |
|---|---|---|---|
| Age (years)* | 67 ± 10 | 66 ± 11 | 0.64 |
| Gender | |||
| Male | 16 (44%) | 14 (39%) | 0.81 |
| Female | 20 (56%) | 22 (61%) | |
| Laterality | |||
| Right | 20 (56%) | 22 (61%) | 0.81 |
| Left | 16 (47%) | 14 (39%) | |
| Joint | |||
| Hip | 19 (53%) | 16 (44%) | 0.64 |
| Knee | 17 (47%) | 20 (56%) | |
| Surgeon | |||
| TST | 9 (25%) | 7 (19.5%) | |
| JW | 12 (33.3%) | 12 (33.3%) | 0.95 |
| JR | 8 (22.2%) | 9 (25%) | |
| GWB | 7 (19.5%) | 8 (22.2%) | |
| Prior joint replacement | 8 (22%) | 9 (25%) | 1.0 |
| Charlson Comorbidity Index* | 3.9 ± 1.9 | 3.5 ± 1.8 | 0.35 |
| Current dog owner | 10 (28%) | 10 (28%) | 1.0 |
| Length of stay (days)* | 2.3 ± 0.5 | 2.5 ± 0.5 | 0.62 |
| Physical therapy refusal† | 2/108 (1.8%) | 3/108 (2.7%) | 1.0 |
* Mean ± SD; †sum of all refusals for every patient in group.
Results
Do Therapy Dogs Have an Effect on Patients’ Perception of Pain After Surgery?
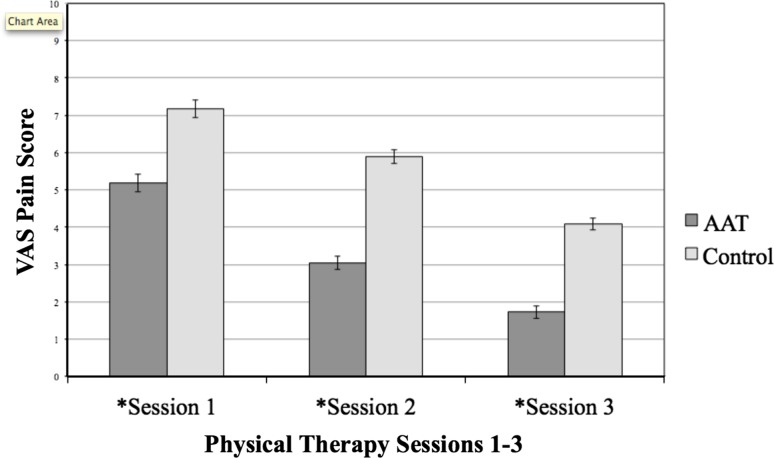
Patients undergoing animal-assisted therapy had lower VAS scores for each session compared with control subjects. After the first session the treatment group (VAS, 5.2; SD, 1.4; 95% CI, 4.71–5.64) had an average VAS pain score two units lower than that of the control group (VAS, 7.2; SD, 1.4; [95% CI, 6.71–7.3], p < 0.001). This difference was maintained during the three physical therapy sessions with a final VAS score difference of 2.4 units (animal-assisted therapy, 1.7; SD, 0.97 [95% CI, 1.4–2.04] versus control, 4.1; SD, 0.97; [95% CI, 3.77–4.41], p < 0.001) after the third physical therapy session (Fig. 3). Additionally, patients undergoing animal-assisted therapy had a more rapid decrease in VAS between the first and second physical therapy sessions (5.2; SD, 1.4 [95% CI, 4.2–5.64] to 3.1; SD, 1.08 [95% CI, 2.7–3.4] versus 7.2; SD, 1.4 [95% CI 6.71–7.63] to 5.9; SD, 1.08 [95% CI, 5.54–6.24]) compared with control subjects (p = 0.003 for the interaction between treatment and the interval between first and second sessions). Specifically, between the first two physical therapy sessions, the treated group had 0.85 units (95% CI, 0.34–1.36) more decrease in VAS pain than the control group. From Session 1 to Session 3, however, the average decrease in VAS pain was 3.1 units in the control group (7.2 to 4.1) and 3.5 units in the treatment group (5.2–1.7), which was not significant (p = 0.10).
Fig. 3.
The VAS for the animal-assisted therapy group after physical therapy was significantly lower than that of the control group at three times *Session 1: 5.2 ± 1.5 versus 7.1 ± 1.3, p < 0.001; *Session 2: 3.05 ± 1.3 versus 5.8 ± 0.74, p < 0.001; *Session 3: 1.71 ± 0.88 versus 4.07 ± 1.05, p < 0.001.The bars indicate standard error. AAT = animal-assisted therapy.
We performed a separate subgroup analysis within treatment groups and found no difference between VAS scores between patients undergoing THA or TKA at any of the three physical therapy sessions (physical therapy Session 1 animal-assisted therapy: hip, 5.1, SD, 1.4; versus knee, 5.2, SD, 1.7; p = 0.72. Control hip, 7.3, SD, 0.81; versus knee, 7.0, SD, 1.5; p = 0.29).
Do Therapy Dogs Have an Effect of Patients’ Satisfaction With The Hospital Stay?
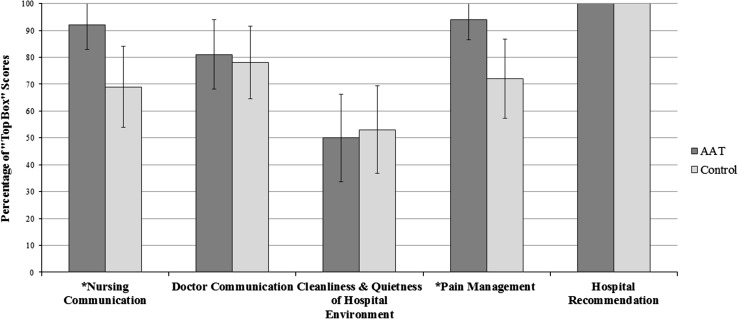
The treatment group had a higher proportion of top box scores in the categories of nursing communication (33 of 36, 92% [95% CI, 78%–98%] versus 25 of 36, 69% [95% CI, 52%–84%], p = 0.035; risk difference, 23% [95% CI, 5%–40%); and pain management (34 of 36, 94% [95% CI, 81%–99%] versus 26 of 36, 72% [95% CI, 55%–86%], p = 0.024; risk difference, 18% [95% CI, 5%–39%) compared with the control group. Overall hospital rating was found to be significantly higher in the treatment group compared with the control group (9.6, SD, 0.7 [95% CI, 9.3–9.8] versus 8.6, SD, 0.9 [95% CI, 8.3–8.9], p < 0.001) compared with the control group. The categories of doctor communication (29 of 36, 81% [95% CI, 64%–92%] versus 28 of 36, 78% [95% CI, 61%–90%], p = 1.0), cleanliness and quietness of the hospital environment (18 of 36, 50% [95% CI, 34%–66%] versus 19 of 36, 53% [95% CI, 37%–69%], p = 1.0), and hospital recommendation (36 of 36 [100%] versus 36 of 36 [100%], p = 1.0) showed no difference between the groups regarding the percentage of top box scores (Fig. 4).
Fig. 4.
The Hospital Consumer Assessment of Healthcare Providers and Systems survey (HCAHPS) showed the treatment group had a greater proportion of top box scores for *Nursing Communication (33 of 36 [92%] versus 25 of 36 [69%], p = 0.03) and *Pain Management (34 of 36 [94%] versus 26 of 36 [72%], p = 0.02) compared with the control group. The bars indicated 95% CI. AAT = animal-assisted therapy.
No adverse events or surgical site infections occurred as of 12 months postoperatively.
Discussion
Daily interaction with dogs has been shown to have measurable health benefits [2, 3, 5, 21]. Therefore, the use of dogs in a clinical setting was a logical step and has been shown to be beneficial for a wide range of patients [1, 14, 23, 25, 35, 37]. To our knowledge, our study is the first to evaluate the role of therapy dogs in the acute postoperative period after total joint arthroplasty. We found that the inclusion of animal-assisted therapy during the immediate postoperative period resulted in substantial improvement in VAS pain scores and HCAHPS scores compared with standard postoperative protocols which served as controls.
This study has several limitations. Although our study is randomized, it was not possible to blind patients to treatment because of its interactive nature. The greatest limitation to our study was that one dog and handler (CMH) team was used for our treatment group. While reducing treatment heterogeneity this limits the studies’ external validity and thus its generalizability to a broader population (particularly because the handler also was an orthopaedic resident). Owing to the unique characteristics of each dog and handler team we would anticipate variability in patient response to different teams. We did not assess the effect that visitation with a handler alone had on patients. This decision was based on previous work, which showed that response to a dog and handler team was greater than that of the response to a handler alone [16]. Finally, we did not control for the amount or timing of analgesic administration but attempted to mitigate the effect of any variation by administering VAS scores before analgesic delivery. Although unlikely to serve as a confounding factor as a result of the randomization of patients and the strong effect seen in the treatment arm, this variable could have an effect on outcomes.
Patients receiving animal-assisted therapy before physical therapy showed lower VAS pain scores after each session compared with matched control subjects. The effect was cumulative with pain scores declining with subsequent sessions at a greater rate than for control subjects. Using animal-assisted therapy as an adjunct for pain control has been studied in pediatric and adult populations [1, 8, 23, 24]. Instituting a therapy dog program has been shown to decrease the amount of narcotics required by pediatric patients experiencing sickle cell crisis and the overall amount of narcotic drugs consumed by geriatric patients in a long-term rehabilitation setting [32, 43]. Marcus et al. [34] reported that 23% of adult patients with chronic pain achieved “clinically meaningful” pain relief when interacting with a therapy dog before their outpatient clinic appointment. A theory proposed to explain this phenomenon focuses on the neuroendocrine effect associated with animal-assisted therapy [38]. Patients interacting with a therapy dog during a 10-minute period were shown to have statistically significant increases in β-endorphin, oxytocin, prolactin, β-phenylethylamine, and dopamine with corresponding decreases in cortisol compared with control subjects [38]. These hormones have been linked with the subjective emotions of comfort and relaxation with β-endorphins having analgesic effects [10]. The neuroendocrine response in the dogs interacting with patients is similar, lending scientific weight to the anthropomorphic observation that therapy dogs “enjoy” their work [38]. As a result of the proven analgesic effect of animal-assisted therapy, some centers have formalized the role of animal-assisted therapy in inpatient and outpatient settings where therapy dogs are seen as an integral part of the pain management regimen [33, 39].
In addition to lower pain scores, we observed increased patient satisfaction in several portions of the HCAHPS among the treatment group; specifically nursing communication, pain control, and overall hospital rating. Total joint arthroplasty is a known physical and emotional stressor. Patients with poor coping skills, psychopathologic disorders, or higher levels of anxiety have a greater incidence of postoperative pain and lower satisfaction with surgical outcomes despite similar functional results [18, 20, 41]. Research supports improving patients’ self-efficacy and decreasing anxiety as an important method in addressing dissatisfaction after total joint arthroplasty [36]. Numerous studies have shown animal-assisted therapy to be an effective tool for reducing depression and anxiety in patients [21, 24, 28, 42, 44]. By providing patients with such an experience after total joint arthroplasty, we suspect that animal-assisted therapy has an anxiolytic effect on patients that may improve the perception of their hospital course. Interestingly, patients’ perceptions of nursing care and communication also were greater. Although this has yet to be fully evaluated, it is known that healthcare professionals need only 5 minutes of interaction with a therapy dog to show an improvement in mood [6]. In a study evaluating nursing staff perception of animal-assisted therapy, 100% of the staff found that patient visitation with a therapy dog was beneficial, and 100% of the staff themselves wanted to visit with the dog [34]. It is possible that when animal-assisted therapy is taking place, patients perceive an improvement in nursing staff mood, which correlates with greater satisfaction [8, 15].
Animal-assisted therapy is an effective adjunctive modality. We found that the use of animal-assisted therapy in the form of a therapy dog has a positive effect on patients’ pain level and satisfaction with their hospital stay. The ability to improve hospital satisfaction is becoming an increasingly important priority to hospital administrations owing to changes in government reimbursement [40]. More work is needed to investigate the applicability of animal-assisted therapy to patients receiving treatment for orthopaedic disorders. We recommend that surgeons inquire regarding the status of pet therapy programs at their hospital and not hesitate to use animal-assisted therapy and therapy dogs in particular as an adjunctive modality in the recovery of their patients.
Acknowledgments
We acknowledge and thank the orthopaedic physical therapy and orthopaedic nursing teams for their assistance in coordinating visits and administering surveys. We also thank Beth D. Harper MD (Department of Medicine, Boston Children’s Hospital, Harvard Medical School), for training the therapy dog and guidance and support during the design and implementation of this study.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Abate SV, Zucconi M, Boxer BA. Impact of canine-assisted ambulation on hospitalized chronic heart failure patients’ ambulation outcomes and satisfaction: a pilot study. J Cardiovasc Nurs. 2011;26:224–230. doi: 10.1097/JCN.0b013e3182010bd6. [DOI] [PubMed] [Google Scholar]
- 2.Allen K, Shykoff BE, Izzo JL., Jr Pet ownership, but not ace inhibitor therapy, blunts home blood pressure responses to mental stress. Hypertension. 2001;38:815–820. [PubMed] [Google Scholar]
- 3.Allen KM, Blascovich J, Tomaka J, Kelsey RM. Presence of human friends and pet dogs as moderators of autonomic responses to stress in women. J Pers Soc Psychol. 1991;61:582–589. doi: 10.1037/0022-3514.61.4.582. [DOI] [PubMed] [Google Scholar]
- 4.American Veterinary Medical Association. Guidelines for animal assisted activity, animal-assisted therapy and resident animal programs. Available at: https://www.avma.org/KB/Policies/Pages/Guidelines-for-Animal-Assisted-Activity-Animal-Assisted-Therapy-and-Resident-Animal-Programs.aspx. Accessed July 1, 2013.
- 5.Anderson WP, Reid CM, Jennings GL. Pet ownership and risk factors for cardiovascular disease. Med J Aust. 1992;157:298–301. [PubMed] [Google Scholar]
- 6.Barker SB, Knisely JS, McCain NL, Best AM. Measuring stress and immune response in healthcare professionals following interaction with a therapy dog: a pilot study. Psychol Rep. 2005;96:713–729. doi: 10.2466/pr0.96.3.713-729. [DOI] [PubMed] [Google Scholar]
- 7.Beck AM, Katcher AH. Pets as Therapists. Between Pets and People: The Importance of Animal Companionship. West Lafayette, IN: Purdue University Press; 1996:125–164.
- 8.Beck CE, Gonzales F Jr, Sells CH, Jones C, Reer T, Zhu YY. The effects of animal-assisted therapy on wounded warriors in an Occupational Therapy Life Skills program. US Army Med Dep J. 2012;Apr-Jun:38–45. [PubMed]
- 9.Brodie S, Biley F, Shewring M. An exploration of the potential risks associated with using pet therapy in healthcare settings. J Clin Nurs. 2002;11:444–456. doi: 10.1046/j.1365-2702.2002.00628.x. [DOI] [PubMed] [Google Scholar]
- 10.Bruehl S, Burns JW, Chung OY, Chont M. What do plasma beta-endorphin levels reveal about endogenous opioid analgesic function? Eur J Pain. 2012;16:370–380. doi: 10.1002/j.1532-2149.2011.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burch MR. A History of Animal-Assisted Therapy. Volunteering with Your Pet: How to Get Involved in Animal-Assisted Therapy with Any Kind of Pet. Hoboken, NJ: Howell Book House; 1996:3–8.
- 12.Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program; hospital outpatient prospective payment system and CY 2007 payment rates; CY 2007 update to the ambulatory surgical center covered procedures list; Medicare administrative contractors; and reporting hospital quality data for FY 2008 inpatient prospective payment system annual payment update program—HCAHPS survey, SCIP, and mortality. Final rule with comment period and final rule. Fed Regist. 2006;71:67959–68401. [PubMed]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Chu CI, Liu CY, Sun CT, Lin J. The effect of animal assisted activity on inpatients with schizophrenia. J Psychosoc Nurs Ment Health Serv. 2009;47:42–48. doi: 10.3928/02793695-20091103-96. [DOI] [PubMed] [Google Scholar]
- 15.Cole KM, Gawlinski A. Animal-assisted therapy in the intensive care unit: a staff nurse’s dream comes true. Nurs Clin North Am. 1995;30:529–537. [PubMed] [Google Scholar]
- 16.Cole KM, Gawlinski A, Steers N, Kolterman J. Animal-assisted therapy in patients hospitalized with heart failure. Am J Crit Care. 2007;16:575–585. [PubMed] [Google Scholar]
- 17.Coll AM, Ameen JR, Mead D. Postoperative pain assessment tools in day surgery: literature review. J Adv Nurs. 2004;46:124–133. doi: 10.1111/j.1365-2648.2003.02972.x. [DOI] [PubMed] [Google Scholar]
- 18.Duivenvoorden T, Vissers MM, Verhaar JA, Busschbach JJ, Gosens T, Bloem RM, Bierma-Zeinstra SM, Reijman M. Anxiety and depressive symptoms before and after total hip and knee arthroplasty: a prospective multicentre study. Osteoarthritis Cartilage. 2013;21:1834–1840. doi: 10.1016/j.joca.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 19.Elliot D, Tolle SW, Goldberg L, Miller JB. Pet-associated illness. New Engl J Med. 1985;313:985–995. doi: 10.1056/NEJM198510173131605. [DOI] [PubMed] [Google Scholar]
- 20.Ellis HB, Howard KJ, Khaleel MA, Bucholz R. Effect of psychopathology on patient-perceived outcomes of total knee arthroplasty within an indigent population. J Bone Joint Surg Am. 2012;94:e84. doi: 10.2106/JBJS.K.00888. [DOI] [PubMed] [Google Scholar]
- 21.Friedmann E, Thomas SA. Pet ownership, social support, and one-year survival after acute myocardial infarction in the Cardiac Arrhythmia Suppression Trial (CAST) Am J Cardiol. 1995;76:1213–1217. doi: 10.1016/S0002-9149(99)80343-9. [DOI] [PubMed] [Google Scholar]
- 22.Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann Emerg Med. 2001;38:633–638. doi: 10.1067/mem.2001.118863. [DOI] [PubMed] [Google Scholar]
- 23.Havener L, Gentes L, Thaler B, Megel ME, Baun MM, Driscoll FA, Beiraghi S, Agrawal S. The effects of a companion animal on distress in children undergoing dental procedures. Issues Compr Pediatr Nurs. 2001;24:137–152. doi: 10.1080/01460860118472. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann AO, Lee AH, Wertenauer F, Ricken R, Jansen JJ, Gallinat J, Lang UE. Dog-assisted intervention significantly reduces anxiety in hospitalized patients with major depression. Eur J Integr Med. 2009;1:145–148. doi: 10.1016/j.eujim.2009.08.002. [DOI] [Google Scholar]
- 25.Johnson RA, Meadows RL, Haubner JS, Sevedge K. Human–animal interaction: a complementary / alternative medical (CAM) intervention for cancer patients. Am Behav Sci. 2003;47:55–69. doi: 10.1177/0002764203255213. [DOI] [Google Scholar]
- 26.Jorgenson J. Therapeutic use of companion animals in health care. Image J Nurs Sch. 1997;29:249–254. doi: 10.1111/j.1547-5069.1997.tb00993.x. [DOI] [PubMed] [Google Scholar]
- 27.Lang UE, Jansen JB, Wertenauer F, Gallinat J, Rapp MA. Reduced anxiety during dog assisted interviews in acute schizophrenic patients. Eur J Integr Med. 2010;2:123–127. doi: 10.1016/j.eujim.2010.07.002. [DOI] [Google Scholar]
- 28.Laun L. Benefits of pet therapy in dementia. Home Healthc Nurs. 2003;21:49–52. doi: 10.1097/00004045-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Lefebvre SL, Waltner-Toews D, Peregrine A, Reid-Smith R, Hodge L, Weese JS. Characteristics of programs involving canine visitation of hospitalized people in Ontario. Infect Control Hosp Epidemiol. 2006;27:754–758. doi: 10.1086/505099. [DOI] [PubMed] [Google Scholar]
- 30.Lerner-DurJava L. Pet visitation is an infection control issue? Am J Infect Control. 1994;22:112. doi: 10.1016/0196-6553(94)90174-0. [DOI] [Google Scholar]
- 31.Levinson FM. Pet psychotherapy: use of household pets in the treatment of behavior disorder in childhood. Psychol Rep. 1965;17:695–698. doi: 10.2466/pr0.1965.17.3.695. [DOI] [PubMed] [Google Scholar]
- 32.Lust E, Ryan-Haddad A, Coover K, Snell J. Measuring clinical outcomes of animal-assisted therapy: impact on resident medication usage. Consult Pharm. 2007;22:580–585. doi: 10.4140/TCP.n.2007.580. [DOI] [PubMed] [Google Scholar]
- 33.Marcus DA. Complementary medicine in cancer care: adding a therapy dog to the team. Curr Pain Headache Rep. 2012;16:289–291. doi: 10.1007/s11916-012-0264-0. [DOI] [PubMed] [Google Scholar]
- 34.Marcus DA, Bernstein CD, Constantin JM, Kunkel FA, Breuer P, Hanlon RB. Animal-assisted therapy at an outpatient pain management clinic. Pain Med. 2012;13:45–57. doi: 10.1111/j.1526-4637.2011.01294.x. [DOI] [PubMed] [Google Scholar]
- 35.Martin F, Farnum J. Animal-assisted therapy for children with pervasive developmental disorders. West J Nurs Res. 2002;24:657–670. doi: 10.1177/019394502320555403. [DOI] [PubMed] [Google Scholar]
- 36.Merle C, Brendle S, Wang H, Streit MR, Gotterbarm T, Schiltenwolf M. Multidisciplinary treatment in patients with persistent pain following total hip and knee arthroplasty. J Arthroplasty. 2014;29:28–32. doi: 10.1016/j.arth.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Mossello E, Ridolfi A, Mello AM, Lorenzini G, Mugnai F, Piccini C, Barone D, Peruzzi A, Masotti G, Marchionni N. Animal-assisted activity and emotional status of patients with Alzheimer’s disease in day care. Int Psychogeriatr. 2011;23:899–905. doi: 10.1017/S1041610211000226. [DOI] [PubMed] [Google Scholar]
- 38.Odendaal JS, Meintjes RS. Neurophysiological correlates of affiliative behaviour between humans and dogs. Vet J. 2003;165:296–301. doi: 10.1016/S1090-0233(02)00237-X. [DOI] [PubMed] [Google Scholar]
- 39.Orlandi M, Trangeled K, Mambrini A, Tagliani M, Ferrarini A, Zanetti L, Tartarini R, Pacetti P, Cantore M. Pet therapy effects on oncological day hospital patients undergoing chemotherapy treatment. Anticancer Res. 2007;27:4301–4303. [PubMed] [Google Scholar]
- 40.Perna G. Hospital leaders create ‘the culture of always’: hospital leaders look at HCAHPS as a way to improve the patient-centered culture. Healthc Inform. 2013;30:42, 44, 56. [PubMed]
- 41.Pinto PR, McIntyre T, Ferrero R, Almeida A, Araújo-Soares V. Predictors of acute postsurgical pain and anxiety following primary total hip and knee arthroplasty. J Pain. 2013;14:502–515. doi: 10.1016/j.jpain.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 42.Silva K, Correia R, Lima M, Magalhaes A, de Sousa L. Can dogs prime autistic children for therapy? Evidence from a single case study. J Altern Complement Med. 2011;17:655–659. doi: 10.1089/acm.2010.0436. [DOI] [PubMed] [Google Scholar]
- 43.Sobo EJ, Eng B, Kassity-Krich N. Canine visitation (pet) therapy: pilot data on decreases in child pain perception. J Holist Nurs. 2006;24:51–57. doi: 10.1177/0898010105280112. [DOI] [PubMed] [Google Scholar]
- 44.Souter MA, Miller MD. Do animal-assisted activities effectively treat depression: a meta-analysis. Anthrozoos. 2007;20:167–180. doi: 10.2752/175303707X207954. [DOI] [Google Scholar]
- 45.Todd KH, Funk JP. The minimum clinically important difference in physician-assigned visual analog pain scores. Acad Emerg Med. 1996;3:142–146. doi: 10.1111/j.1553-2712.1996.tb03402.x. [DOI] [PubMed] [Google Scholar]
- 46.Westbrook KW, Babakus E, Grant CC. Measuring patient-perceived hospital service quality: validity and managerial usefulness of HCAHPS scales. Health Mark Q. 2014;31:97–114. doi: 10.1080/07359683.2014.907114. [DOI] [PubMed] [Google Scholar]
- 47.Writing Panel of Working Group, LeFebvre SL, Golab GC, Christensen E, Castrodale L, Aureden K, Bialachowski A, Gumley N, Robinson K, Peregrine A, Benoit M, Card ML, Van Horne L, Weese JS. Guidelines for animal-assisted interventions in health care facilities. Am J Infect Control. 2008:36:78–85. [DOI] [PubMed]
- 48.Zusman EE. HCAHPS replaces Press Ganey survey as quality measure for patient hospital experience. Neurosurgery. 2012;71:N21–24. doi: 10.1227/01.neu.0000417536.07871.ed. [DOI] [PubMed] [Google Scholar]