Abstract
Background
The survival of patients who present with nonmetastatic extremity osteosarcoma has dramatically improved, but there are some patients who do not respond to chemotherapy. The ability to identify patients with a poorer prognosis might allow us to target different therapy for these patients. Glucose transporter protein-1 (Glut-1), one of the key factors in glucose metabolism, has been reported to be an independent prognostic factor in various tumors. However, little is known about the role of the Glut-1 pathway in osteosarcoma.
Questions/purposes
We asked (1) if Glut-1 expression is a prognostic marker for survival in patients with osteosarcoma, and (2) if there is a relationship between Glut-1 expression and tumor angiogenesis.
Patients and Methods
Thirty-seven patients with resectable high-grade osteosarcomas treated between 1982 and 2007 were reviewed retrospectively. Patients were excluded if representative biopsy material and followup data were not available. The expression of Glut-1 and the number of CD34-positive microvessels for angiogenic activity were measured immunohistochemically. The median followup was 6 years 6 months (range, 11–211 months). Survival analyses were evaluated using the Kaplan-Meier method and the Cox proportional hazards model. The association between Glut-1 expression and microvessel density was analyzed using Student’s t-test and chi-square test. For 12 (32.4%) of 37 patients with osteosarcoma, the expression of Glut-1 was positive, with four patients (10.8%) showing strong expression of Glut-1 protein.
Results
The expression of Glut-1 correlated with a shorter disease-free survival period (relative risk, 20.13; 95% CI, 1.77–229.3; p = 0.0016). The microvessel density mean value of positive Glut-1 expression (mean ± SD, 26.5 ± 19.4) was lower than that of negative expression (mean ± SD, 46.4 ± 35.3; Student’s t-test, p = 0.038). When more than 50 was defined as a high microvessel density, positive expression of Glut-1 was significantly associated with low microvessel density (chi-square test, p = 0.049).
Conclusions
These findings indicate that Glut-1 is a potential predictor of survival in patients with osteosarcoma and that glucose metabolism may be negatively associated with angiogenesis. If substantiated in larger numbers of patients, these findings might stimulate the development of novel treatments for patients with a poorer prognosis.
Level of Evidence
Level III, prognostic study. See the Instructions for Authors for a complete description of levels of evidence.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-014-3910-5) contains supplementary material, which is available to authorized users.
Introduction
Osteosarcoma is a pleomorphic sarcoma of bone in which the proliferating spindle cells produce osteoid or immature bone. Approximately 10% to 20% of patients present with clinically detectable metastatic disease. The value of perioperative chemotherapy for osteosarcoma is well established [4, 6]. Despite advances in comprehensive treatments, approximately 30% of patients with no evidence of metastases at diagnosis who are treated with wide tumor resection and intensive adjuvant chemotherapy have lung metastases develop [17, 32, 34], which are associated with poor survival. Currently, the most powerful prognostic factors for osteosarcoma survival are the presence or absence of metastases at diagnosis and the histologic response to neoadjuvant chemotherapy, although others such as serum lactate dehydrogenase and tumor location and size have been found to be prognostic [7, 10, 25]. Attention has been given to the molecular genetics of sarcomas to identify genes or proteins that might predict prognosis. We have reported novel prognostic markers for survival in patients with osteosarcoma [20, 30, 31, 45]. Identification of better prognostic factors at diagnosis might allow the potential to treat patients with poorer prognoses with novel treatment strategies.
Glucose transporter protein-1 (Glut-1), one of the key factors in glucose metabolism, originally is purified from human erythrocytes and is a member of the facilitative cell-surface glucose transporter family which includes five other isoforms [24, 35]. Cancer cells are known for displaying an enhanced glucose uptake and consumption [39]. Glucose transporters are deregulated in cancer cells so they consume higher amounts of glucose than normal cells. In addition to its role as a glucose transporter, Glut-1, a downstream target of hypoxia-inducible factor (HIF), also is known to play an important angiogenesis role in its cellular response to hypoxia [43]. The hypoxic conditions in tumors also are considered to cause resistance to radiotherapy and chemotherapy [2, 8]. Glut-1 has been reported to be an independent prognostic factor in various tumors including ovarian, colorectal, and urinary bladder carcinoma [9, 19, 46]. However, little is known regarding the role of the Glut-1 pathway in osteosarcoma.
We therefore asked if (1) Glut-1 expression is a prognostic marker for survival in patients with osteosarcoma, (and 2) there is a relationship between Glut-1 expression and tumor angiogenesis.
Patients and Methods
Thirty-seven patients with resectable high-grade osteosarcomas between 1982 and 2007 were reviewed retrospectively. During this period a total of 63 patients with newly diagnosed primary osteosarcomas were treated. Patients were excluded because of unavailable or missing biopsy material (10 patients), low-grade osteosarcoma (six patients), unresectable primary lesion (five patients), no chemotherapy (two patients), lost to followup (two patients), and less than 2 years of followup (one patient). This retrospective study was approved by the institutional review board. The surgical specimens were obtained at the time of biopsy at our institute between 1982 and 2007. All patients received conventional chemotherapy, which involved high-dose methotrexate, doxorubicin, and cisplatin [23, 26]. The surgical margins were defined using the system described by Enneking et al. [16]. The histologic response to neoadjuvant chemotherapy (histologic necrosis) was determined by the Huvos grading scale [22].
The median age of the patients at the time of surgery was 18 years (range, 10–55 years). There were 18 male and 19 female patients. The femur was the tumor site in 23 patients, the lower leg in 10, and other sites in four. According to the American Joint Committee on Cancer (AJCC), 10 tumors were Stage IIA, 19 were Stage IIB, and two were Stage IV (six were missing tumor size). Two patients presented with lung metastases at diagnosis. The operative treatments consisted of 22 limb salvage operations and 15 ablations. The surgical margins of all operations were wide. Histologic subtypes comprised osteoblastic in 25 tumors, fibroblastic in five, and others in seven. There were 13 good responses to preoperative chemotherapy and 18 poor responses (six were unknown or unavailable). The median followup was 6 years 6 months (range, 11–211 months). Twenty five patients showed no evidence of disease and 12 died of disease. Patients with less than 2 years of followup were included only if they had local or distant relapse within 2 years after treatment. The 5-year actuarial disease-free and overall survival rates were 61.8% and 68.8%, respectively.
To monitor for relapse, the patients were followed every 3 months after completion of treatment with chest CT and MRI of the primary site for 2 years, then every 6 months for at least 5 years.
Biopsy specimens were immunohistochemically stained. Each tissue block was cut in 6-μm sections, transferred to adhesive silane-coated glass slides (Matsunami Glass Industry Ltd, Osaka, Japan; http://www.matsunami-glass.co.jp/english/index.html), deparaffinized in xylene, rehydrated in a graded series of decreasing ethanol concentrations, and then rinsed in Tris-buffered saline with Tween® 20 (Sigma-Aldrich Japan KK, Tokyo, Japan) (50 mmol/L Tris/HCl pH 7.6, containing 0.3 mol/L sodium chloride and 0.1% Tween® 20). Tissue sections were immersed in Target Retrieval Solution (Dako North America Inc, Carpinteria, CA, USA), and subjected to a hot water bath for 20 minutes, cooled for 20 minutes, then incubated with a mouse anti-Glut-1 antibody (1:200 dilution; Abcam Inc, Cambridge, MA, USA) or with CD34 antibody (Ready-to use N-series Qbend 10; Dako Inc) for 30 minutes at room temperature in a moisture chamber. The reaction products were observed by the polymer-peroxidase method (EnVision +/HRP; Dako Inc). Nuclei were lightly counterstained for approximately 10 seconds with Gill’s hematoxylin solution No. 2. Nonspecific reactivity was assessed by omitting the primary antibody.
The extent of Glut-1 staining was scored as: negative, indicating staining in less than 10% of cells; weak, indicating staining in 10% to 50% of cells; or strong, indicating staining in greater than 50% of cells. For analyses, the tumors in which stained tumor cells made up greater than 10% of the tumor were regarded as positive. In 12 (32.4%) of 37 patients with osteosarcoma, the expression of Glut-1 was positive, with four patients (10.8%) showing strong expression of protein (Fig. 1). Angiogenic activity assessed by estimating the number of CD34-positive microvessels was calculated by two independent observers (TK and KA) according to the method described by Weidner et al. [42]. Briefly, hot-spot areas displaying the highest vessel density were identified by scanning tumor sections at low-power magnification (x40). The maximum vessel density was determined from these hot-spot areas at fields under high-power magnification (x400, high power field), and the mean of the counts for the three fields was calculated. Vessel lumens were not necessary for a structure to be defined as a vessel (Fig. 2).
Fig. 1.
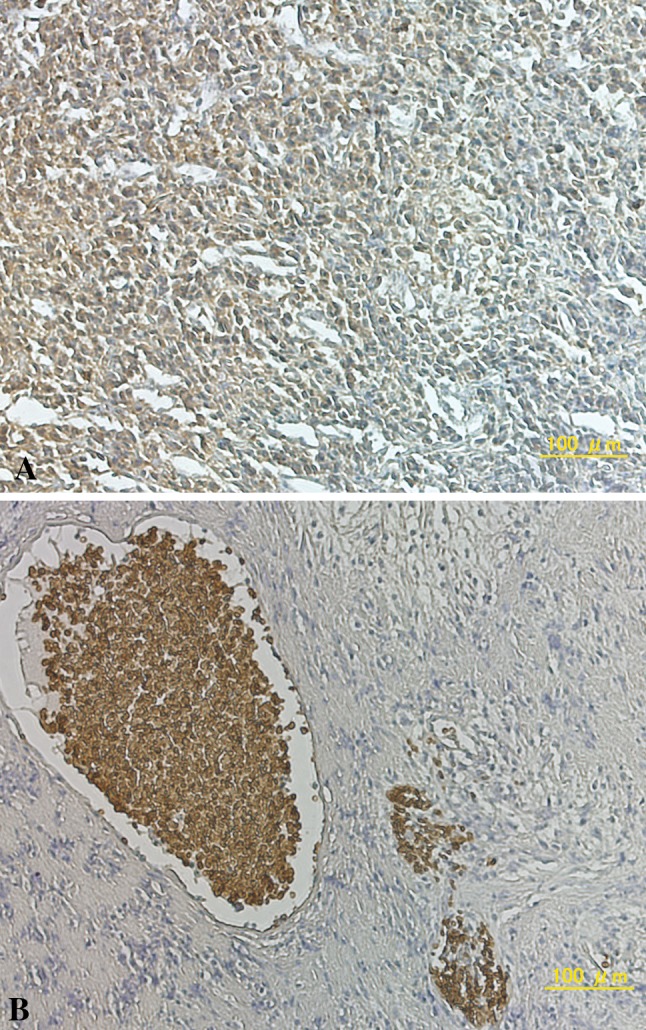
A–B Representative photomicrographs show immunohistochemical staining with anti-Glut-1 antibodies. (A) A strong dark brown staining of the cytoplasm in greater than 50% of cells, and (B) dark brown staining of erythrocyte alone in the negative control were seen (Original magnification, x200)
Fig. 2.
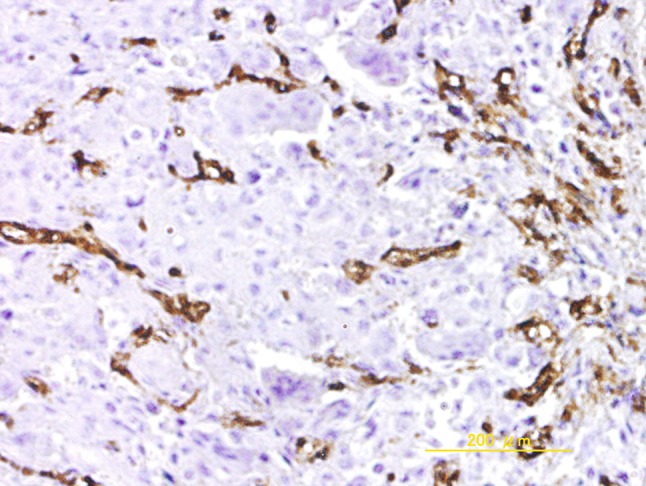
The photomicrograph shown representative immunohistochemical staining in cells with high microvessel density (Original magnification, x100)
The survival results were evaluated using the Kaplan-Meier method and the log-rank test. The Cox proportional hazards model was used for multivariate analysis to determine the relative risk and independent significance of individual factors. The association between Glut-1 expression and microvessel density was analyzed using Student’s t-test and chi-square test. All analyses were performed with Excel 2007 (Microsoft Japan Co, Ltd, Tokyo, Japan) and a statistical analysis add-in for Excel (Excel Statistics 2008; Social Survey Research Information Co, Ltd, Tokyo, Japan; https://www.ssri.com/e/about_us/our_business.html). A probability less than 0.05 was defined as significant.
Age, gender, tumor site, tumor volume, alkaline phosphatase (ALP), lactate dehydrogenase (LDH), surgical stage, histologic type, type of local control (limb salvage versus amputation), response to chemotherapy, and Glut-1 expression were the prognostic factors evaluated by univariate analysis. Of these, high LDH, AJCC tumor Stage IIB, poor histologic response to chemotherapy, and the expression of Glut-1 correlated significantly with a short disease-free survival. These four parameters were evaluated by multivariate analysis.
The statistical power analysis was performed according to power and sample size calculation reported by Dupont and Plummer [12]. To adjust for multiple comparisons in setting the level of statistical significance, we used Holm’s familywise error rate [21] and Benjamini and Hochberg’s false discovery rate [5]. Since these data were underpowered to detect statistically significant associations owing to the small sample size, we considered the p value with respect to multiple hypothesis testing. Univariate analysis after the adjustment for multiple comparisons showed that Glut-1 expression alone was an independent prognostic factor (familywise error rate, p = 0.004; false discovery rate, p = 0.004) (Appendix 1. Supplemental material is available with the online version of CORR®).
Results
After controlling for likely confounding variables, we found that only Glut-1 was associated with an increased likelihood of death in these patients with osteosarcoma (relative risk, 20.13; 95% CI, 1.77–229.3; p = 0.0016) (Table 1).
Table 1.
Multivariate Cox regression analysis
| Variable | Relative risk | 95% CI | p value |
|---|---|---|---|
| Lactase dehydrogenase | |||
| ≤ cutoff | 1.00 | 0.117 | |
| > cutoff | 0.16 | 0.02–1.58 | |
| Surgical stage | |||
| IIA | 1.00 | 0.542 | |
| IIB | 2.09 | 0.20–22.31 | |
| Response to chemotherapy | |||
| Good | 1.00 | 0.236 | |
| Poor | 3.05 | 0.48–19.22 | |
| Glut-1 | |||
| Negative | 1.00 | 0.016 | |
| Positive | 20.13 | 1.77– 229.3 | |
Glut-1 = glucose transporter protein-1
The microvessel density mean value of positive Glut-1 expression (mean ± SD, 26.5 ± 19.4) was lower than that of negative expression (mean ± SD, 46.4 ± 35.3)(Student’s t-test, p = 0.038). When more than 50 was defined as a high microvessel density, positive expression of Glut-1 was significantly associated with low microvessel density (chi-square test, p = 0.049).
Discussion
Upregulation of Glut-1 expression, presumably attributable to enhanced glycolytic metabolism, has been shown to be a relatively common feature in various cancers [9, 19, 46]. However, little is known regarding the role of Glut-1 expression in osteosarcoma. Cifuentes et al. [11] reported that 100% of the human samples exhibited strong positive staining of Glut-1 in seven primary osteosarcoma and two pulmonary metastases. Conversely, Ahrens et al. [1] immunohistochemically studied Glut-1 expression in mesenchymal tumors, finding negative results in two osteosarcomas. Endo et al. [15] reported on Glut-1 expression in a total of 67 patients with bone and soft tissue sarcomas, including 16 osteosarcomas. They did not assess the survival rate in osteosarcoma alone. Of the potential prognostic factors we studied, only Glut-1 expression was an independent prognostic factor for survival in patients with osteosarcoma. In addition to prognostic factors reported by others [7, 10, 25], such as surgical stage and histologic response to chemotherapy, the expression of Glut-1 in biopsy specimens may help to predict a poor prognosis group of patients with osteosarcoma before treatment.
Our study has some limitations. The followup for our study to evaluate the patient survival rate was relatively short. In addition, a small patient cohort tends to introduce an element of selection bias. It is unusual that response to chemotherapy and stage of the tumor did not correlate to disease-free survival. This suggests that we may have an atypical population of patients with osteosarcoma. A multiinstitution study is necessary to gather a tissue array to analyze more specimens and determine if data of Glut-1 expression and microvessel density hold up with a larger patient population. Second, immunohistochemistry for assessment of Glut-1 expression and microvessel density is qualitative and somewhat subjective. Our findings should prompt further investigation into the quantitative analysis of Glut-1 and the molecular mechanism of the hypoxia signaling pathway using fresh osteosarcoma tissues or cell cultures. Third, positron emission tomography (PET) associated with glucose metabolism was not included in this analysis, although expansion of the data in subsequent studies that include PET analysis might be useful. Based on our promising results, additional clinical studies using PET as a predictor for survival and response to chemotherapy would be warranted.
Studies have shown that increased F18-fluorodeoxyglucose (FDG), as quantified by PET, correlates with the tumor grade in patients with bone and soft tissue sarcomas and that the maximal standardized uptake value is the most reliable predictor of high-grade bone and soft tissue sarcoma [14, 36]. A study of osteosarcoma in a rat model has proved that F18-FDG PET predicts a response to neoadjuvant chemotherapy and Glut-1 staining [13]. Tateishi et al. [41] reported that there is amplification of Glut-1 expression when FDG accumulation increases in bone and soft tissue sarcomas, suggesting that glucose transport and metabolism might provide crucial prognostic information. Furthermore, although selective Glut-1 inhibitors are not available in the clinical setting thus far, an in vitro study indicates that RNA interference targeting of Glut-1 inhibits the growth and invasion potential of the osteosarcoma cell line MG63 [18]. Preclinical cancer cell studies regarding potential inhibitors of glucose transport, such as fasentin or apigenin, are in progress [3, 8, 33]. These data encourage us to identify Glut-1 as not only a novel prognostic factor but also a therapeutic target in osteosarcoma.
The Glut-1 expression of osteosarcoma was negatively related to the microvessel density value in the current study. Our results are supported by a large-scale osteosarcoma study by Kreuter et al. [29], who reported that patients with a high microvessel density had a significantly higher overall and relapse-free survival period than patients with a low microvessel density. A good response to chemotherapy correlated significantly with a higher microvessel density. They suggested that the correlation between response to chemotherapy and microvessel density may be partly attributable to improved accessibility of the agents to osteosarcoma cells. The efficacy of drug delivery is much greater in a tumor with a high degree of microvessels than in a tumor with a low degree, especially in a chemotherapy-sensitive tumor such as an osteosarcoma. In tumor progression, hypoxia is an important condition in various pathologic processes. Induction of HIF results in upregulation of various target genes, such as Glut-1 and VEGF, involved in glucose metabolism and angiogenesis [37]. Studies of various tumor types suggest that VEGF and microvessel density are poor prognostic factors [28, 40]. In human osteosarcoma, HIF-1α and VEGF are reported to be predictive of patient survival [44, 47]. However, the relationship between glucose metabolism and angiogenesis is debatable in some malignancies. Shan et al. [38] reported that a higher Glut-1 expression grade was associated with a lower microvessel density value in breast cancer. They speculated that tumors might survive on at least two mechanisms with angiogenesis and glycolysis and that a metabolic shift from oxidative to anaerobic glycolytic metabolism might occur in a tumor microenvironment where oxygen is scarce and glucose consumption is high. In addition, immunohistochemical findings pertaining to hepatocellular carcinoma showed there was an inverse correlation between tumor glucose metabolism and angiogenesis, suggesting metabolic alteration: a complementary pattern of glucose metabolism and tumor angiogenesis in tumors [27]. To clarify the exact pathways and mechanisms involved in hypoxia, our findings should prompt more comprehensive investigations using other parameters, such as HIF and VEGF.
Our study showed that assessment of Glut-1 expression has the potential to be useful in assessing tumor prognosis in osteosarcoma. The results also suggest an inverse correlation between tumor glucose metabolism and angiogenesis. Although these results are preliminary, if confirmed in larger studies they might provide a basis on which to consider novel therapies based on the presence or absence of Glut-1 expression, but more study is required.
Electronic supplementary material
Footnotes
Each author certifies that he has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his institution has approved the reporting of this report, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This study was performed at the Department of Orthopaedic Surgery, Graduate School of Biomedical Sciences, Hiroshima University.
References
- 1.Ahrens WA, Ridenour RV, 3rd, Caron BL, Miller DV, Folpe AL. GLUT-1 expression in mesenchymal tumors: an immunohistochemical study of 247 soft tissue and bone neoplasms. Hum Pathol. 2008;39:1519–1526. doi: 10.1016/j.humpath.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Airley RE, Phillips RM, Evans AE, Double J, Burger AM, Feibig HH, West CM, Stratford IJ. Hypoxia-regulated glucose transporter Glut-1 may influence chemosensitivity to some alkylating agents: results of EORTC (First Translational Award) study of the relevance of tumour hypoxia to the outcome of chemotherapy in human tumour-derived xenografts. Int J Oncol. 2005;26:1477–1484. doi: 10.3892/ijo.26.6.1477. [DOI] [PubMed] [Google Scholar]
- 3.Amann T, Hellerbrand C. GLUT1 as a therapeutic target in hepatocellular carcinoma. Expert Opin Ther Targets. 2009;13:1411–1427. doi: 10.1517/14728220903307509. [DOI] [PubMed] [Google Scholar]
- 4.Bacci G, Briccoli A, Ferrari S, Longhi A, Mercuri M, Capanna R, Donati D, Lari S, Forni C, DePaolis M. Neoadjuvant chemotherapy for osteosarcoma of the extremity: long-term results of the Rizzoli’s 4th protocol. Eur J Cancer. 2001;37:2030–2039. doi: 10.1016/S0959-8049(01)00229-5. [DOI] [PubMed] [Google Scholar]
- 5.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 6.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jürgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.20.3.776. [DOI] [PubMed] [Google Scholar]
- 7.Bramer JA, van Linge JH, Grimer RJ, Scholten RJ. Prognostic factors in localized extremity osteosarcoma: a systematic review. Eur J Surg Oncol. 2009;35:1030–1036. doi: 10.1016/j.ejso.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Calvo MB, Figueroa A, Pulido EG, Campelo RG, Aparicio LA. Potential role of sugar transporters in cancer and their relationship with anticancer therapy. Int J Endocrinol. 2010;2010 pii: 205357. [DOI] [PMC free article] [PubMed]
- 9.Cantuaria G, Fagotti A, Ferrandina G, Magalhaes A, Nadji M, Angioli R, Penalver M, Mancuso S, Scambia G. GLUT-1 expression in ovarian carcinoma: association with survival and response to chemotherapy. Cancer. 2001;92:1144–1150. doi: 10.1002/1097-0142(20010901)92:5<1144::AID-CNCR1432>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Sun MX, Hua YQ, Cai ZD. Prognostic significance of serum lactate dehydrogenase level in osteosarcoma: a meta-analysis. J Cancer Res Clin Oncol. 2014;140:1205–1210. doi: 10.1007/s00432-014-1644-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cifuentes M, García MA, Arrabal PM, Martínez F, Yañez MJ, Jara N, Weil B, Domínguez D, Medina RA, Nualart F. Insulin regulates GLUT1-mediated glucose transport in MG-63 human osteosarcoma cells. J Cell Physiol. 2011;226:1425–1432. doi: 10.1002/jcp.22668. [DOI] [PubMed] [Google Scholar]
- 12.Dupont WD, Plummer WD., Jr Power and sample size calculations: a review and computer program. Control Clin Trials. 1990;11:116–128. doi: 10.1016/0197-2456(90)90005-M. [DOI] [PubMed] [Google Scholar]
- 13.Dutour A, Decouvelaere AV, Monteil J, Duclos ME, Roualdes O, Rousseau R, Marec-Bérard P. 18F-FDG PET SUVmax correlates with osteosarcoma histologic response to neoadjuvant chemotherapy: preclinical evaluation in an orthotopic rat model. J Nucl Med. 2009;50:1533–1540. doi: 10.2967/jnumed.109.062356. [DOI] [PubMed] [Google Scholar]
- 14.Eary JF, Conrad EU, Bruckner JD, Folpe A, Hunt KJ, Mankoff DA, Howlett AT. Quantitative [F-18]fluorodeoxyglucose positron emission tomography in pretreatment and grading of sarcoma. Clin Cancer Res. 1998;4:1215–1220. [PubMed] [Google Scholar]
- 15.Endo M, Tateishi U, Seki K, Yamaguchi U, Nakatani F, Kawai A, Chuman H, Beppu Y. Prognostic implications of glucose transporter protein-1 (glut-1) overexpression in bone and soft-tissue sarcomas. Jpn J Clin Oncol. 2007;37:955–960. doi: 10.1093/jjco/hym125. [DOI] [PubMed] [Google Scholar]
- 16.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–120. [PubMed] [Google Scholar]
- 17.Eselgrim M, Grunert H, Kühne T, Zoubek A, Kevric M, Bürger H, Jürgens H, Mayer-Steinacker R, Gosheger G, Bielack SS. Dose intensity of chemotherapy for osteosarcoma and outcome in the Cooperative Osteosarcoma Study Group (COSS) trials. Pediatr Blood Cancer. 2006;47:42–50. doi: 10.1002/pbc.20608. [DOI] [PubMed] [Google Scholar]
- 18.Fan J, Zhou JQ, Yu GR, Lu DD. Glucose transporter protein 1-targeted RNA interference inhibits growth and invasion of the osteosarcoma cell line MG63 in vitro. Cancer Biother Radiopharm. 2010;25:521–527. doi: 10.1089/cbr.2010.0784. [DOI] [PubMed] [Google Scholar]
- 19.Haber RS, Rathan A, Weiser KR, Pritsker A, Itzkowitz SH, Bodian C, Slater G, Weiss A, Burstein DE. GLUT1 glucose transporter expression in colorectal carcinoma: a marker for poor prognosis. Cancer. 1998;83:34–40. doi: 10.1002/(SICI)1097-0142(19980701)83:1<34::AID-CNCR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 20.Hoang BH, Kubo T, Healey JH, Sowers R, Mazza B, Yang R, Huvos AG, Meyers PA, Gorlick R. Expression of LDL receptor-related protein 5 (LRP5) as a novel marker for disease progression in high-grade osteosarcoma. Int J Cancer. 2004;109:106–111. doi: 10.1002/ijc.11677. [DOI] [PubMed] [Google Scholar]
- 21.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 22.Huvos AG. Osteogenic sarcoma: pathologic assessment of preoperative (neoadjuvant) chemotherapy. Bone Tumors: Diagnosis, Treatment, and Prognosis. 2nd ed. Philadelphia, PA: WB Saunders;1991:122–128.
- 23.Iwamoto Y, Tanaka K, Isu K, Kawai A, Tatezaki S, Ishii T, Kushida K, Beppu Y, Usui M, Tateishi A, Furuse K, Minamizaki T, Kawaguchi N, Yamawaki S. Multiinstitutional phase II study of neoadjuvant chemotherapy for osteosarcoma (NECO study) in Japan: NECO-93 J and NECO-95J. J Orthop Sci. 2009;14:397–404. doi: 10.1007/s00776-009-1347-6. [DOI] [PubMed] [Google Scholar]
- 24.Kasahara M, Hinkle PC. Reconstitution and purification of the D-glucose transporter from human erythrocytes. J Biol Chem. 1977;252:7384–7390. [PubMed] [Google Scholar]
- 25.Kim MS, Lee SY, Cho WH, Song WS, Koh JS, Lee JA, Yoo JY, Jung ST, Jeon DG. Effect of increases in tumor volume after neoadjuvant chemotherapy on the outcome of stage II osteosarcoma regardless of histological response. J Orthop Sci. 2009;14:292–297. doi: 10.1007/s00776-009-1334-y. [DOI] [PubMed] [Google Scholar]
- 26.Kimura H, Tsuchiya H, Shirai T, Nishida H, Hayashi K, Takeuchi A, Ohnari I, Tomita K. Caffeine-potentiated chemotherapy for metastatic osteosarcoma. J Orthop Sci. 2009;14:556–565. doi: 10.1007/s00776-009-1372-5. [DOI] [PubMed] [Google Scholar]
- 27.Kitamura K, Hatano E, Higashi T, Narita M, Seo S, Nakamoto Y, Yamanaka K, Nagata H, Taura K, Yasuchika K, Nitta T, Uemoto S. Proliferative activity in hepatocellular carcinoma is closely correlated with glucose metabolism but not angiogenesis. J Hepatol. 2011;55:846–857. doi: 10.1016/j.jhep.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 28.Kleespies A, Guba M, Jauch KW, Bruns CJ. Vascular endothelial growth factor in esophageal cancer. J Surg Oncol. 2004;87:95–104. doi: 10.1002/jso.20070. [DOI] [PubMed] [Google Scholar]
- 29.Kreuter M, Bieker R, Bielack SS, Auras T, Buerger H, Gosheger G, Jurgens H, Berdel WE, Mesters RM. Prognostic relevance of increased angiogenesis in osteosarcoma. Clin Cancer Res. 2004;10:8531–8537. doi: 10.1158/1078-0432.CCR-04-0969. [DOI] [PubMed] [Google Scholar]
- 30.Kubo T, Piperdi S, Rosenblum J, Antonescu CR, Chen W, Kim HS, Huvos AG, Sowers R, Meyers PA, Healey JH, Gorlick R. Platelet-derived growth factor receptor as a prognostic marker and a therapeutic target for imatinib mesylate therapy in osteosarcoma. Cancer. 2008;112:2119–2129. doi: 10.1002/cncr.23437. [DOI] [PubMed] [Google Scholar]
- 31.Kubo T, Shimose S, Matsuo T, Fujimori J, Arihiro K, Ochi M. Interferon-α/β receptor as a prognostic marker in osteosarcoma. J Bone Joint Surg Am. 2011;93:519–526. doi: 10.2106/JBJS.J.00198. [DOI] [PubMed] [Google Scholar]
- 32.Lewis IJ, Nooij MA, Whelan J, Sydes MR, Grimer R, Hogendoorn PC, Memon MA, Weeden S, Uscinska BM, van Glabbeke M, Kirkpatrick A, Hauben EI, Craft AW, Taminiau AH. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: a randomized phase III trial of the European Osteosarcoma Intergroup. J Natl Cancer Inst. 2007;99:98–99. doi: 10.1093/jnci/djk015. [DOI] [PubMed] [Google Scholar]
- 33.Luo XM, Zhou SH, Fan J. Glucose transporter-1 as a new therapeutic target in laryngeal carcinoma. J Int Med Res. 2010;38:1885–1892. doi: 10.1177/147323001003800601. [DOI] [PubMed] [Google Scholar]
- 34.Meyers PA, Gorlick R, Heller G, Casper E, Lane J, Huvos AG, Healey JH. Intensification of preoperative chemotherapy for osteogenic sarcoma: results of the Memorial Sloan-Kettering (T12) protocol. J Clin Oncol. 1998;16:2452–2458. doi: 10.1200/JCO.1998.16.7.2452. [DOI] [PubMed] [Google Scholar]
- 35.Mueckler M. Facilitative glucose transporters. Eur J Biochem. 1994;219:713–725. Review. [DOI] [PubMed]
- 36.Nieweg OE, Pruim J, van Ginkel RJ, Hoekstra HJ, Paans AM, Molenaar WM, Koops HS, Vaalburg W. Fluorine-18-fluorodeoxyglucose PET imaging of soft-tissue sarcoma. J Nucl Med. 1996;37:257–261. [PubMed] [Google Scholar]
- 37.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shan X, Wang D, Chen J, Xiao X, Jiang Y, Wang Y, Fan Y. Necrosis degree displayed in computed tomography images correlated with hypoxia and angiogenesis in breast cancer. J Comput Assist Tomogr. 2013;37:22–28. doi: 10.1097/RCT.0b013e318279abd1. [DOI] [PubMed] [Google Scholar]
- 39.Szablewski L. Expression of glucose transporters in cancers. Biochim Biophys Acta. 2013;1835:164–169. doi: 10.1016/j.bbcan.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995;55:3964–3968. [PubMed] [Google Scholar]
- 41.Tateishi U, Yamaguchi U, Seki K, Terauchi T, Arai Y, Hasegawa T. Glut-1 expression and enhanced glucose metabolism are associated with tumour grade in bone and soft tissue sarcomas: a prospective evaluation by [18F]fluorodeoxyglucose positron emission tomography. Eur J Nucl Med Mol Imaging. 2006;33:683–691. doi: 10.1007/s00259-005-0044-8. [DOI] [PubMed] [Google Scholar]
- 42.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis: correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 43.Wenger RH. Mammalian oxygen sensing, signalling and gene regulation. J Exp Biol. 2000;203:1253–1263. doi: 10.1242/jeb.203.8.1253. [DOI] [PubMed] [Google Scholar]
- 44.Yang QC, Zeng BF, Dong Y, Shi ZM, Jiang ZM, Huang J. Overexpression of hypoxia-inducible factor-1alpha in human osteosarcoma: correlation with clinicopathological parameters and survival outcome. Jpn J Clin Oncol. 2007;37:127–134. doi: 10.1093/jjco/hyl137. [DOI] [PubMed] [Google Scholar]
- 45.Yang R, Hoang BH, Kubo T, Kawano H, Chou A, Sowers R, Huvos AG, Meyers PA, Healey JH, Gorlick R. Over-expression of parathyroid hormone Type 1 receptor confers an aggressive phenotype in osteosarcoma. Int J Cancer. 2007;121:943–954. doi: 10.1002/ijc.22749. [DOI] [PubMed] [Google Scholar]
- 46.Younes M, Juarez D, Lechago LV, Lerner SP. Glut 1 expression in transitional cell carcinoma of the urinary bladder is associated with poor patient survival. Anticancer Res. 2001;21:575–578. [PubMed] [Google Scholar]
- 47.Zhuang Y, Wei M. Impact of vascular endothelial growth factor expression on overall survival in patients with osteosarcoma: a meta-analysis. Tumour Biol. 2014;35:1745–1749. doi: 10.1007/s13277-014-1692-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


