Abstract
Background
An infraacetabular screw path facilitates the closure of a periacetabular fixation frame to increase the plate fixation strength in acetabular fractures up to 50%. Knowledge of the variance in corridor sizes and axes has substantial surgical relevance for safe screw placement.
Questions/purposes
(1) What proportion of healthy pelvis specimens have an infraacetabular corridor that is 5 mm or larger in diameter? (2) Does a universal corridor axis and specific screw entry point exist? (3) Are there sex-specific differences in the infraacetabular corridor size or axis and are these correlated with anthropometric parameters like age, body weight and height, or the acetabular diameter?
Methods
A template pelvis with a mean shape from 523 segmented pelvis specimens was generated using a CT-based advanced image analyzing system. Each individual pelvis was registered to the template using a free-form registration algorithm. Feasible surface regions for the entry and exit points of the infraacetabular corridor were marked on the template and automatically mapped to the individual samples to perform a measurement of the maximum sizes and axes of the infraacetabular corridor on each specimen. A minimum corridor diameter of at least 5 mm was defined as a cutoff for placing a 3.5-mm cortical screw in clinical settings.
Results
In 484 of 523 pelves (93%), an infraacetabular corridor with a diameter of at least 5 mm was found. Using the mean axis angulations (54.8° [95% confidence interval {CI}, 0.6] from anterocranial to posterocaudal in relation to the anterior pelvic plane and 1.5° [95% CI, 0.4] from anteromedial to posterolateral in relation to the sagittal midline plane), a sufficient osseous corridor was present in 64% of pelves. Allowing adjustment of the three-dimensional axis by another 5° included an additional 25% of pelves. All corridor parameters were different between females and males (corridor diameter, 6.9 [95% CI, 0.2] versus 7.7 [95% CI, 0.2] mm; p < 0.001; corridor length, 96.2 [95% CI, 0.7] versus 106.4 [95% CI, 0.6] mm; p < 0.001; anterior pelvic plane angle, 54.0° [95% CI, 0.9] versus 55.3° [95% CI, 0.8]; p < 0.01; sagittal midline plane angle, 4.3° [95% CI, 0.6] versus −0.3° [95% CI, 0.5]; p < 0.001).
Conclusion
This study provided reference values for placement of a 3.5-mm cortical screw in the infraacetabular osseous corridor in 90% of female and 94% of male pelves. Based on the sex-related differences in corridor axes, the mean screw trajectory is approximately parallel to the sagittal midline plane in males but has to be tilted from medial to lateral in females. Considering the narrow corridor diameters, we suggest an individual preoperative CT scan analysis for fine adjustments in each patient.
Introduction
Acetabular fracture surgery is one of the most demanding procedures performed in orthopaedic trauma. Anatomic reduction is the most challenging goal followed by rigid fracture fixation. Based on systematic fracture analysis on radiographs, and using a standardized classification system [16, 26], approaches and reduction techniques have improved. Fracture fixation is accomplished using reconstruction plates in combination with isolated screws for most fracture patterns [29]. For complex fracture types, the trend is toward less invasive single approaches [29]. When there is a separation of both columns and displacement of a quadrilateral plane fragment, sufficient fragment retention remains demanding, particularly in older patients with osteoporotic bone quality or when single approaches are used [12]. Therefore, several modifications for iliopectineal plate fixation through common anterior approaches (Letournel’s ilioinguinal [21], modified Stoppa-Rives [6], or anterior intrapelvic approach [34]) have been described. Spring plates buttressing the quadrilateral plate [6], infrapectineal plates on the inner side of the pelvic rim [13, 20, 32], placement of additional screws parallel to the quadrilateral plate [21] or in the posterior column [3, 19, 28, 39], and use of cerclage techniques are reported [4, 9, 23, 35].
The recently described infraacetabular screw placement seems to be a promising alternative to increase fracture fixation strength up to 50% by closing the periacetabular fixation frame as shown in two biomechanical studies [11, 24]. In our clinical experience, the infraacetabular corridor of some patients is too narrow or has a bowing shape; this may exclude secure intraosseous screw placement. Knowledge regarding morphologic features of the corridor and potential correlation of shape and size with patient-specific parameters would have substantial surgical relevance; however, to our knowledge, there are no biomorphometric analyses of this osseous corridor.
Therefore, CT data sets of 523 healthy pelves were evaluated to answer the following study questions: (1) What proportion of healthy pelvis specimens have an infraacetabular corridor that is 5 mm or larger in diameter? (2) Does a universal corridor axis and specific screw entry point exist? (3) Are there sex-specific differences of the infraacetabular corridor size or axis and are these correlated with anthropometric parameters like age, body weight and height, or the acetabular diameter?
Materials and Methods
The evaluation was performed on a set of 523 segmented pelvis specimens available as triangulated surface meshes in an advanced image analyzing system developed by a team from Technische Universität München in cooperation with Stryker Trauma GmbH (Kiel, Germany) (Table 1) [38]. All segmentations were performed manually by medical experts based on CT scans acquired exclusively for medical indications: polytrauma (20%), CT angiography (70%), and others (10%). Pelves with fractures, pelvic ring deformity, hip dysplasia, and hardware in situ were excluded.
Table 1.
Characteristics of pelvis specimens
| Variable | Pelves | Females | Males | Significance |
|---|---|---|---|---|
| Number in overall data set | 523 | 208 | 315 | |
| Mean acetabular diameter (mm) | 51 [0.3] | 47 [0.4] | 53 [0.3] | p < 0.001 |
| Range of acetabular diameter (mm) | 40–59 | 40–53 | 47–59 | |
| Number in subgroup analyses | 286 | 115 | 171 | |
| Mean age [95% CI] (years) | 63 [1.9] | 63 [3.3] | 62 [2.3] | NS |
| Range of age (years) | 19–93 | 19–93 | 20–93 | |
| Mean body height [95% CI] (mm) | 171 [1.1] | 165 [1.3] | 176 [1.1] | p < 0.001 |
| Range of body height [95% CI ] (mm) | 142–196 | 142–181 | 154–196 | |
| Mean body weight [95% CI] (kg) | 79 [1.9] | 74 [3.1] | 82 [2.3] | p < 0.001 |
| Range of body weight [95% CI] (kg) | 45–140 | 45–140 | 48–135 |
CI = confidence interval; NS = not significant.
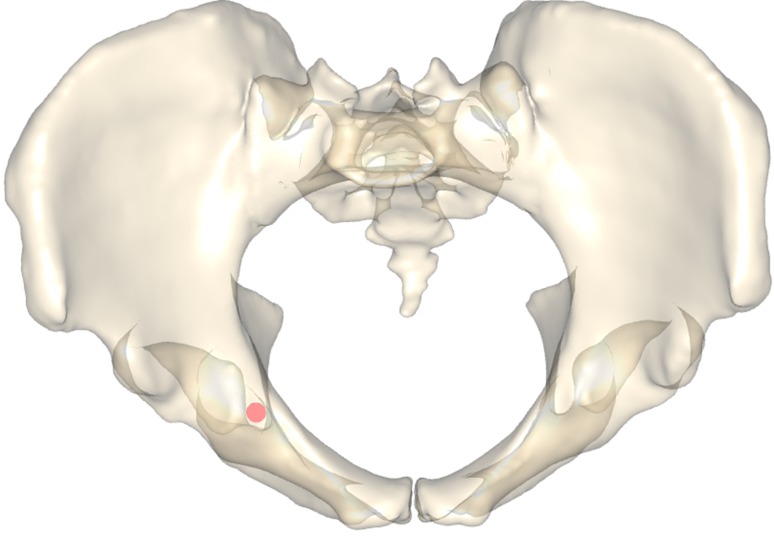
The specimens were processed in two stages, as described subsequently. A mean shape (the template) was generated from all input data sets (Fig. 1). The individual specimens (the samples) were registered to the template using a free-form registration algorithm that yields a dense surface mapping from points located on the template to anatomically corresponding points on the samples. On the template shape, two surface regions were marked manually (gray area in Fig. 1C): the entry region (around the eminentia iliopectinea), which defines the area through which the screw should enter the bone, and the exit region (around the distal part of the os ischii), through which the screw should exit the bone. Both areas allow a screw to pass the infraacetabular region in the fovea acetabuli without penetrating the acetabular cavity or the obturator foramen. Using the surface mapping found in the previous stage, these regions were automatically mapped to the individual samples. A search then was performed to find the maximum-width corridor connecting any two points on the entry and exit regions, respectively. The exact entry and exit point for each corridor are displayed on the template pelvis (green and red dots in Fig. 1C). Except for the manual definition of the regions on the template, all steps on the samples were performed automatically without any user interaction to ensure a highly reproducible and objective anatomic analysis.
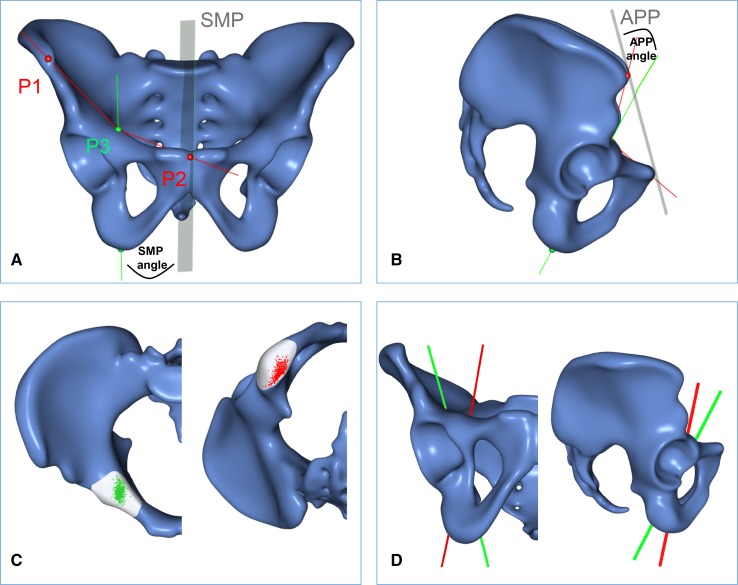
Fig. 1A–D.
The mean shapes of all 523 pelves are shown as templates in (A) front and (B) lateral views. The green line represents the mean corridor axis in relation to the sagittal midline plane (SMP) and the anterior pelvic plane (APP). P1 and P2 represent Distance A (anterosuperior iliac spine to the pubic symphysis). P2 and P3 represent Distance B (pubic symphysis to the screw entry point). The mean ratio of Distance A/B was 1.36 ± 0.16, facilitating determination of the screw entry point for each pelvis based on the intraoperative measurement of Distance A. (C) The cumulative entry (green) and exit (red) points of all 523 optimized osseous corridors are shown. (D) The maximum ranges (green - > red line) of the corridor axes in relation to the sagittal midline plane and the anterior pelvic plane are shown.
Template Generation
The template was generated from the samples using the following procedure: (1) One sample from the input set was selected randomly and designated as the template (this template was preliminary and was refined iteratively); (2) for each sample, the affine transformation (translation, rotation, scaling) minimizing the registration error to the template was determined; (3) the remaining error was minimized using a nonrigid registration algorithm [14]. The composition of the affine and nonrigid transformations maps points on the template to corresponding points on the sample; (4) the mean shape was obtained through averaging the corresponding points found in the previous step over all samples. Steps 2 to 4 were iterated until convergence using the mean shape as a new template in each step; and (5) a final (nonrigid) registration of the final template to each sample was performed. This yielded dense mapping from the template surface to the individual samples and allowed any point defined on the template to be mapped automatically to its corresponding location on the samples [36].
Corridor Detection
On the template surface, the entry and exit regions of the screw were marked manually. Using the mapping found during the template generation, these patches were automatically mapped to all samples and thus defined the set of all potential corridor axes on a given specimen, which were simply the lines connecting an entry point to an exit point. Each corridor axis had an associated diameter, which was defined as the diameter of the largest cylinder that runs along the corridor axis and intersects the bone surface only at the entry and exit regions (ie, it does not penetrate the cortical bone surface anywhere else).
To find the corridor with the largest diameter for each specimen, a search over the space of potential corridor axes was performed as follows: (1) Points were distributed uniformly over the entry and exit regions at a grid distance of 1 mm; (2) for every pairing of (entry, exit) points, the diameter of the associated corridor was found by generating points on the entry-exit line at a distance of 0.5 mm and determining for each of these point the shortest distance to any point on the bone surface (Fig. 2). If any one of these points was found to lie outside the bone, the corresponding corridor was discarded. Otherwise, the minimum of all the distances is the maximum radius of the cylinder along the axis that does not penetrate the bone surface, and therefore the radius of the corridor (or half its diameter); (3) among these corridors, the 25% with the largest diameters was selected, and Steps 1 to 3 were repeated at twice the previous resolution (eg, 1 mm → 0.5 mm); and (4) Steps 1 to 3 were iterated to four levels of refinement, meaning that the location of the entry and exit points of the maximum corridor was determined up to a resolution of 0.125 mm.
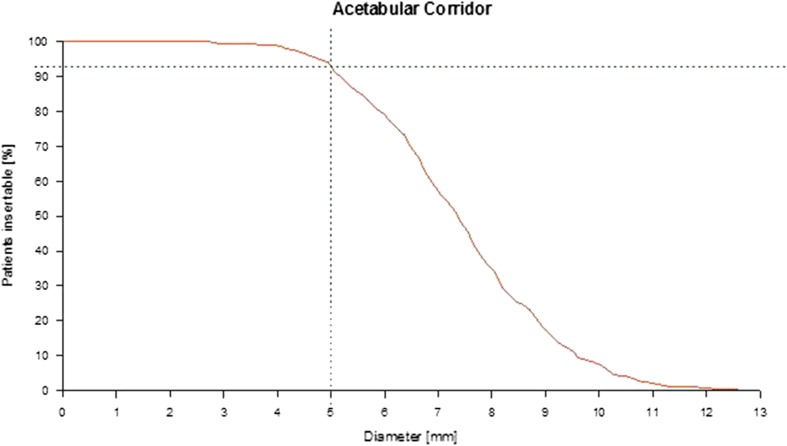
Fig. 2.
The distribution of the infraacetabular corridor diameters is shown. The dotted line represents the prevalence of at least 5-mm corridor diameters in 93% of pelves.
Corridor Size and Axis Measurements
The maximum diameter and length of each optimized corridor were measured in an automatic procedure. A minimum corridor diameter of at least 5 mm was defined as the cutoff for placing a 3.5-mm cortical screw, which does not penetrate the cortical bone in clinical settings. The corridor axis was evaluated in relation to the patient’s specific anatomic reference planes (Fig. 1): anterior pelvic plane (determined by the superoanterior iliac spines and the pubic symphysis); and (2) the sagittal midline plane [22, 30]. Both constructed planes are independent of the patient’s position on the operating table and reduce the interindividual and intraindividual variances compared with the operating table as an alternative external reference.
In the next step, the mean axis parameters of all pelves with at least 5 mm diameter were used as a potential recommendation for a universal screw trajectory. In a reverse evaluation procedure it was analyzed in how many pelves an infraacaetabular corridor with at least 5 mm diameter in this axis orientation (= universal corridor) exists.
Statistical Analyses
For statistical analysis, MS-Excel 2013 (Microsoft Inc, Redmond, WA, USA) and the software program R x64 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria) were used. All data are presented as mean and 95% confidence interval (CI) to determine statistical significance. A p value of < 0.05 was considered significant. The proportions of sex-related differences in corridor sizes (≥ 5 mm) were analyzed with the Pearson’s chi square contingence test. Matrix correlation analyses for all parameters as well as pairwise testing of the corridor parameters (corridor diameter and length, anterior pelvic plane, and sagittal midline plane) with anthropometric data (age, body height and weight, and acetabular diameter) were performed separately for females and males. Pelvic-specific CT-based anatomic parameters and sex correlation could be analyzed for all 523 pelves. Metadata of age, body height, and weight were available for only 286 pelves (Table 1).
Results
Proportion of Infraacetabular Corridors 5 mm or Larger
In all 523 pelves, an infraacetabular corridor with a mean diameter of 7.4 mm (95% CI, 0.2; range, 2.8–12.9 mm) and mean length of 103 mm (95% CI, 0.7; range, 81–122 mm) was found, but only in 484 pelves (93%), the corridor had a diameter of at least 5 mm (Fig. 3).
Fig. 3.
An axial projection image shows the infraacetabular corridor axis (red transsection) showing the limiting borders in the tear drop at the 02:30 and 08:00 o’clock positions.
Corridor Axis and Screw Entry Point
The mean tilt of the corridor axis in relation to the anterior pelvic plane was 54.8° (95% CI, 0.6; range, 32°–75°) from anterocranial to posterocaudal.
The mean tilt of the corridor axis in relation to the sagittal midline plane was 1.5° (95% CI, 0.4 (range, 17.8° medial to 11.3° lateral tilt) from anteromedial to posterolateral (Fig. 1).
A universal corridor, characterized by the mean axis parameters of all specimens with a corridor diameter of at least 5 mm (93% of pelves), was found in 64% of pelves. In 29% of pelves, modification of the three-dimensional (3-D) angulation was necessary (less than 5° in 25% and greater than 5° in 4%). In contrast to the homogenous screw trajectories, the intraoperative determination of the optimized screw entry point in the region of the eminentia iliopectinea is more demanding (Fig. 1C). Therefore, the distance of the entry point from the pubic symphysis was measured. Absolute values with a range of 54 to 91 mm are of less clinical value owing to high interindividual variation of pelvic sizes and shapes. Therefore, the individual ratio of Distance A (anterosuperior iliac spine – pubic symphysis) to Distance B (pubic symphysis – entry point) was calculated (Fig. 1A). This mean ratio of Distance A/B was 1.36 (95% CI, 0.1) and facilitates calculation of the individual distance of the screw entry point from the pubic symphysis for each pelvis based on intraoperative measurement.
Sex-specific Differences of the Infraacetabular Corridor and Correlation With Anthropometric Parameters
The maximum infraacetabular corridor diameter was smaller in females than males (6.9 [95% CI, 0.21] versus 7.7 [95% CI, 0.19]; p = 0.001). In contrast, the overall proportion of corridors with a diameter of at least 5 mm was not different in both groups (90% in females versus 94% in males; not significant).
The mean tilt of the corridor axis in relation to the anterior pelvic plane (from anterocranial to posterocaudal) was smaller in females than males (54.0° [95% CI, 0.9]; range, 32°–75°) versus (55.3° [95% CI, 0.8]; range, 32°–75°; p < 0.01).
The corridor axis in relation to the sagittal midline plane was 4.3° (95% CI, 0.6) tilted from anteromedial to posterolateral in females (range, 16.3° medial to 9.0° lateral) and approximately parallel with −0.3° (95% CI, 0.5) in males (range, 17.8° medial to −11.3° lateral) (p < 0.001).
Only the parameters body height and acetabular diameter were strongly correlated with the corridor length independent of the sex (r > 0.3; p < 0.05). A weaker correlation was found for the age with all corridor parameters in females but not males (Table 2).
Table 2.
Correlation analysis of anthropometric and infraacetabular corridor parameters
| Parameter | Body weight | Body height | Age | Acetabular diameter | ||||
|---|---|---|---|---|---|---|---|---|
| r value | p value | r value | p value | r value | p value | r value | p value | |
| Females | ||||||||
| Corridor diameter | −0.049 | 0.603 | −0.132 | 0.160 | 0.194 | 0.038 | 0.184 | 0.050 |
| Corridor length | 0.221 | 0.017 | 0.353 | 1.08E−04 | 0.267 | 0.004 | 0.570 | 2.84E−11 |
| Corridor axis in relation to the APP | 0.083 | 0.376 | −0.143 | 0.128 | 0.264 | 0.004 | 0.166 | 0.077 |
| Corridor axis in relation to the SMP | −0.035 | 0.714 | −0.015 | 0.873 | 0.266 | 0.009 | 0.145 | 0.122 |
| Males | ||||||||
| Corridor diameter | 0.216 | 0.004 | 0.198 | 0.01 | −0.079 | 0.307 | 0.220 | 0.004 |
| Corridor length | 0.236 | 0.002 | 0.354 | 2.08E−06 | 0.006 | 0.940 | 0.544 | 1.51E−14 |
| Corridor axis in relation to the APP | −0.079 | 0.307 | −0.054 | 0.49 | 0.069 | 0.369 | 0.055 | 0.472 |
| Corridor axis in relation to the SMP | −0.061 | 0.424 | −0.065 | 0.40 | 0.135 | 0.079 | 0.223 | 0.003 |
Bold values indicate strong correlations with statistical significance.
APP = anterior pelvic plane; SMP = sagittal midline plane.
Discussion
Several modifications of the common iliopectineal plate fixation have been reported (spring plates, infrapectineal plates, cerclages, and lag screws in different positions) [3, 4, 6, 9, 13, 19, 20, 23, 28, 32, 35, 39] to increase the fracture fixation strength in acetabular surgery. One promising approach, especially for fractures with separation of both columns, is the use of an additional lag screw in the infraacetabular corridor to close the periacetabular fixation frame [7], but biomorphometric data of the corridor size and axis for a secure screw placement are missing. Based on 523 CT scans of pelves specimens, a corridor with a diameter of at least 5 mm exists in 93% of cases (90% in females versus 94% in males; not significant). Using the mean axes of these optimized infraacetabular corridors as a common recommendation for a universal corridor orientation, such a corridor with a diameter of at least 5 mm could be determined in 64% of cases. Only in 29% of pelves was a modification of the 3-D angulation necessary (less than 5° in 25% and greater than 5° in 4%). The female corridors were significantly smaller in size (diameter and length) and the axis more angulated (in relation to sagittal midline plane) compared with male corridors.
Our study has some limitations. First, CT data sets of different medical investigations (polytrauma, angiography, and others) were segmented, not necessarily representing the cohort group of patients with acetabular fractures. Only bone surfaces were segmented, not facilitating the automatic measurement of the inner diameter of the infraacetabular corridor excluding the cortical thickness. Furthermore, unfractured pelves were used for the biomorphometric CT-based analysis of the infraacetabular corridor, whereas a fracture component exists in clinical settings. In cases of a persistent fracture malreduction, the corridor diameter size may further decrease. Reilly et al. [33] reported the relevant influence of fracture malreduction for secure screw placement in the osseous corridor of the S1 pedicle for sacral fractures. In contrast to the posterior pelvic ring, a persistent fracture gap or step in the articular region is unacceptable owing to the risk of osteoarthritis. The reduction quality correlates with the clinical and radiologic outcomes and determines the risk of posttraumatic osteoarthritis of the hip [25, 27]. On the other side, a partial extraosseous screw position may be tolerated for buttressing the quadrilateral plate with an inside-out-inside placement in the fovea acetabuli region as reported by Letournel and Judet [21].
A further clinical drawback of the proposed infraacetabular screw placement is the too narrow osseous corridor diameter in 7% of patients and the demanding placement technique of a 3.5-mm cortical screw in all other patients despite the reported data in this study. The obturator nerve on its path from the inner pelvic ring through the obturator foramen to the outer side [34, 40] and the femoral head in the acetabular cavity [13] are at risk during periacetabular screw placements and have to be preserved. Whereas the obturator nerve can be protected by direct observation in open procedures, the femoral head is obscured. The best fluoroscopic plane to exclude intraarticular misplacement is the projection of the screw as a dot in the teardrop figure [7] or use of an intraoperative CT- or 3-D c-arm scan [15, 17, 18]. The benefit of CT or 3-D c-arm scan evaluation has to be weighted against additional radiation exposure individually for each patient.
In doubt of a secure extraarticular screw placement, we recommend abandoning infraacetabular screw placement to prevent an iatrogenic femoral head violation. Alternative fixation implants with a similar fixation philosophy of “closing the periacetabular fixation frame” have to be developed.
Numerous biomorphometric studies have investigated different osseous corridors for placement of screws in the supraacetabular region or the anterior or posterior column [5, 8, 30, 31, 37], but data for the infraacetabular corridor are missing. Only one experimental study investigated percutaneous placement of an infraacetabular screw [10]. Compared with the other periacetabular corridors (supraacetabular, anterior column, and posterior column), the navigated screw placement in the infraacetabular corridor was accompanied by the greatest misplacement rate. This seems to be atributed to the overall narrow corridor size with a mean diameter of 7.4 mm (95% CI, 0.2; range, 2.8–12.9 mm) determined in this study.
Furthermore, in most studies, cross-sectional corridor diameters were analyzed, resulting in potentially overestimated oblique tangential measurements compared with a real 3-D volume procedure used in this study. Significant differences of both techniques were reported by Attias et al. for the anterior column corridor [1].
A universal corridor axis with a craniocaudal screw orientation from anteromedial to posterolateral (54.8° angle in relation to the anterior pelvic plane and 1.5° angle in relation to the sagittal midline plane) fits in 64% of pelves with a corridor diameter of at least 5 mm. The screw entry points for all optimized infraacetabular corridors are located in the mediocaudal region of the eminentia iliopectinea (Fig. 1C). Contrary to the initial description of the screw insertion technique by Culemann et al. [7], with more surgical experience and optimized retractors, the area for the screw entry point can be visualized [2] and the infraacetabular screw placed through the anterior intrapelvic approach.
Small sample sizes in previous studies limit the statistical analysis of periacetabular corridor parameters and their correlation with several anthropomorphic parameters [1, 5, 8, 30, 31, 37]. An automatic registration and analyzing algorithm is one strength of this study facilitating large data acquisition of 523 pelves.
The infraacetabular corridor diameter was significantly larger in males compared with females, as reported for the anterior column corridor in several studies [5, 30, 31]. In contradiction to these studies with no sex-related difference in the anterior column corridor axis, different infraacetabular corridor axes in females (4° medial tilt in relation to the sagittal midline plane) and males (almost parallel orientation with a lateral tilt of 0.3° in relation to the sagittal midline plane) were observed in this study. This is in line with data from Puchwein et al. reporting different screw angulations in male versus female pelves for the supraacetabular and posterior column, but not the anterior column [31].
Common anthropometric data like age, body height, body weight, and acetabular diameter were correlated with the infraacetabular corridor parameters to evaluate their potential predictive value for the infraacetabular corridor size and axis. In contrast to the anterior column corridor, for which Ochs et al. found no correlations of corridor sizes and axes with any anthropometric data [30], a strong correlation of body height and acetabular diameter with the corridor length was found in this study. The clinical relevance of this finding seems to be of less value, because the corridor length is the only corridor parameter, which can be easily determined intraoperatively with a reverse ruler. The found weak correlation of age with all corridor parameters in females, but not in males, was surprising and cannot be conclusively explained.
We conclude that an infraacetabular corridor of at least 5 mm exists in 90% of female and 94% of male pelves specimens. The presented corridor sizes and axes may provide the surgeon additional information for safe infraacetabular screw placement in patients with an acetabular fracture with separation of both columns. Nevertheless, evaluation of the individual preoperative CT scan for determination of the corridor diameter and individual fine adjustment of screw trajectory is recommended.
Acknowledgments
We thank Dr Jürgen Schwarz for help with the statistical analysis.
Footnotes
One of the authors (NR) is an employee of Stryker Trauma GmbH, Kiel, Germany.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Friedrich-Schiller University, Jena, Germany, and the Klinikum r.d. Isar, Technische Universität in München, Munich, Germany.
References
- 1.Attias N, Lindsey RW, Starr AJ, Borer D, Bridges K, Hipp JA. The use of a virtual three-dimensional model to evaluate the intraosseous space available for percutaneous screw fixation of acetabular fractures. J Bone Joint Surg Br. 2005;87:1520–1523. doi: 10.1302/0301-620X.87B11.16614. [DOI] [PubMed] [Google Scholar]
- 2.Bible JE, Choxi AA, Kadakia RJ, Evans JM, Mir HR. Quantification of bony pelvic exposure through the modified Stoppa approach. J Orthop Trauma. 2014;28:320–323. doi: 10.1097/BOT.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 3.Chang JK, Gill SS, Zura RD, Krause WR, Wang GJ. Comparative strength of three methods of fixation of transverse acetabular fractures. Clin Orthop Relat Res. 2001;392:433–441. doi: 10.1097/00003086-200111000-00057. [DOI] [PubMed] [Google Scholar]
- 4.Chen CM, Chiu FY, Lo WH, Chung TY. Cerclage wiring in displaced both-column fractures of the acetabulum. Injury. 2001;32:391–394. doi: 10.1016/S0020-1383(00)00243-6. [DOI] [PubMed] [Google Scholar]
- 5.Chen KN, Wang G, Cao LG, Zhang MC. Differences of percutaneous retrograde screw fixation of anterior column acetabular fractures between male and female: a study of 164 virtual three-dimensional models. Injury. 2009;40:1067–1072. doi: 10.1016/j.injury.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Cole JD, Bolhofner BR. Acetabular fracture fixation via a modified Stoppa limited intrapelvic approach. Description of operative technique and preliminary treatment results. Clin Orthop Relat Res. 1994;305:112–123. doi: 10.1097/00003086-199408000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Culemann U, Marintschev I, Gras F, Pohlemann T. Infra-acetabular corridor—technical tip for an additional screw placement to increase the fixation strength of acetabular fractures. J Trauma. 2011;70:244–246. doi: 10.1097/TA.0b013e3181f45f91. [DOI] [PubMed] [Google Scholar]
- 8.Ebraheim NA, Xu R, Biyani A, Benedetti JA. Anatomic basis of lag screw placement in the anterior column of the acetabulum. Clin Orthop Relat Res. 1997;339:200–205. doi: 10.1097/00003086-199706000-00028. [DOI] [PubMed] [Google Scholar]
- 9.Farid YR. Cerclage wire-plate composite for fixation of quadrilateral plate fractures of the acetabulum: a checkrein and pulley technique. J Orthop Trauma. 2010;24:323–328. doi: 10.1097/BOT.0b013e3181c90bbe. [DOI] [PubMed] [Google Scholar]
- 10.Gras F, Marintschev I, Klos K, Muckley T, Hofmann GO, Kahler DM. Screw placement for acetabular fractures: which navigation modality (2-dimensional vs 3-dimensional) should be used? An experimental study. J Orthop Trauma. 2012;26:466–473. doi: 10.1097/BOT.0b013e318234d443. [DOI] [PubMed] [Google Scholar]
- 11.Gras F, Marintschev I, Schwarz CE, Hofmann GO, Pohlemann T, Culemann U. Screw- versus plate-fixation strength of acetabular anterior column fractures: a biomechanical study. J Trauma Acute Care Surg. 2012;72:1664–1670. doi: 10.1097/TA.0b013e3182463b45. [DOI] [PubMed] [Google Scholar]
- 12.Guerado E, Cano JR, Cruz E. Fractures of the acetabulum in elderly patients: an update. Injury. 2012;43(Suppl 2):S33–S41. doi: 10.1016/S0020-1383(13)70177-3. [DOI] [PubMed] [Google Scholar]
- 13.Guy P, Al-Otaibi M, Harvey EJ, Helmy N. The ‘safe zone’ for extra-articular screw placement during intra-pelvic acetabular surgery. J Orthop Trauma. 2010;24:279–283. doi: 10.1097/BOT.0b013e3181bfcebf. [DOI] [PubMed] [Google Scholar]
- 14.Huang X, Paragios N, Metaxas DN. Shape registration in implicit spaces using information theory and free form deformations. IEEE Trans Pattern Anal Mach Intell. 2006;28:1303–1318. doi: 10.1109/TPAMI.2006.171. [DOI] [PubMed] [Google Scholar]
- 15.Huegli RW, Staedele H, Messmer P, Regazzoni P, Steinbrich W, Gross T. Displaced anterior column acetabular fracture: closed reduction and percutaneous CT-navigated fixation. Acta Radiol. 2004;45:618–621. doi: 10.1080/02841850410008199. [DOI] [PubMed] [Google Scholar]
- 16.Judet R, Judet J, Letournel E. Fractures of the acetabulum: classification and surgical approaches for open reduction. preliminary report. J Bone Joint Surg Am. 1964;46:1615–1646. [PubMed] [Google Scholar]
- 17.Kendoff D, Citak M, Gardner MJ, Stubig T, Krettek C, Hufner T. Intraoperative 3D imaging: value and consequences in 248 cases. J Trauma. 2009;66:232–238. doi: 10.1097/TA.0b013e31815ede5d. [DOI] [PubMed] [Google Scholar]
- 18.Kendoff D, Gardner MJ, Citak M, Kfuri M, Jr, Thumes B, Krettek C, Hufner T. Value of 3D fluoroscopic imaging of acetabular fractures comparison to 2D fluoroscopy and CT imaging. Arch Orthop Trauma Surg. 2008;128:599–605. doi: 10.1007/s00402-007-0411-y. [DOI] [PubMed] [Google Scholar]
- 19.Khajavi K, Lee AT, Lindsey DP, Leucht P, Bellino MJ, Giori NJ. Single column locking plate fixation is inadequate in two column acetabular fractures. A biomechanical analysis. J Orthop Surg Res. 2010;5:30. [DOI] [PMC free article] [PubMed]
- 20.Laflamme GY, Hebert-Davies J, Rouleau D, Benoit B, Leduc S. Internal fixation of osteopenic acetabular fractures involving the quadrilateral plate. Injury. 2011;42:1130–1134. doi: 10.1016/j.injury.2010.11.060. [DOI] [PubMed] [Google Scholar]
- 21.Letournel E, Judet R. Operative treatment of specific types of fractures. In: Smith WR, Ziran BH, Morgan SJ, eds. Fractures of the Acetabulum. 2nd ed. Berlin and Heidelberg, Germany; New York, NY, USA; Philadelphia, PA, USA: Lippincott Williams & Wilkins; 1993:436–441.
- 22.Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60:217–220. [PubMed] [Google Scholar]
- 23.Lin HH, Hung SH, Su YP, Chiu FY, Liu CL. Cerclage wiring in displaced associated anterior column and posterior hemi-transverse acetabular fractures. Injury. 2012;43:917–920. doi: 10.1016/j.injury.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Marintschev I, Gras F, Schwarz CE, Pohlemann T, Hofmann GO, Culemann U. Biomechanical comparison of different acetabular plate systems and constructs–the role of an infra-acetabular screw placement and use of locking plates. Injury. 2012;43:470–474. doi: 10.1016/j.injury.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Matta JM. Fractures of the acetabulum: accuracy of reduction and clinical results in patients managed operatively within three weeks after the injury. J Bone Joint Surg Am. 1996;78:1632–1645. [PubMed] [Google Scholar]
- 26.Matta JM, Anderson LM, Epstein HC, Hendricks P. Fractures of the acetabulum. A retrospective analysis. Clin Orthop Relat Res. 1986;205:230–240. [PubMed] [Google Scholar]
- 27.Mayo KA. Open reduction and internal fixation of fractures of the acetabulum. Results in 163 fractures. Clin Orthop Relat Res. 1994;305:31–37. [PubMed] [Google Scholar]
- 28.Mu WD, Wang XQ, Jia TH, Zhou DS, Cheng AX. Quantitative anatomic basis of antegrade lag screw placement in posterior column of acetabulum. Arch Orthop Trauma Surg. 2009;129:1531–1537. doi: 10.1007/s00402-009-0836-6. [DOI] [PubMed] [Google Scholar]
- 29.Ochs BG, Marintschev I, Hoyer H, Rolauffs B, Culemann U, Pohlemann T, Stuby FM. Changes in the treatment of acetabular fractures over 15 years: analysis of 1266 cases treated by the German Pelvic Multicentre Study Group (DAO/DGU). Injury. 2014 Jul 7. pii: S0020-1383(14)00319-2. doi: 10.1016/j.injury.2014.06.026 [Epub ahead of print]. [DOI] [PubMed]
- 30.Ochs BG, Stuby FM, Ateschrang A, Stoeckle U, Gonser CE. Retrograde lag screw placement in anterior acetabular column with regard to the anterior pelvic plane and midsagittal plane—virtual mapping of 260 three-dimensional hemipelvises for quantitative anatomic analysis. Injury. 2014;2014/07/27. [DOI] [PubMed]
- 31.Puchwein P, Enninghorst N, Sisak K, Ortner T, Schildhauer TA, Balogh ZJ, Pichler W. Percutaneous fixation of acetabular fractures: computer-assisted determination of safe zones, angles and lengths for screw insertion. Arch Orthop Trauma Surg. 2012;132:805–811. doi: 10.1007/s00402-012-1486-7. [DOI] [PubMed] [Google Scholar]
- 32.Qureshi AA, Archdeacon MT, Jenkins MA, Infante A, DiPasquale T, Bolhofner BR. Infrapectineal plating for acetabular fractures: a technical adjunct to internal fixation. J Orthop Trauma. 2004;18:175–178. doi: 10.1097/00005131-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Reilly MC, Bono CM, Litkouhi B, Sirkin M, Behrens FF. The effect of sacral fracture malreduction on the safe placement of iliosacral screws. J Orthop Trauma. 2003;17:88–94. doi: 10.1097/00005131-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Sagi HC, Afsari A, Dziadosz D. The anterior intra-pelvic (modified Rives-Stoppa) approach for fixation of acetabular fractures. J Orthop Trauma. 2010;24:263–270. doi: 10.1097/BOT.0b013e3181dd0b84. [DOI] [PubMed] [Google Scholar]
- 35.Schopfer A, Willett K, Powell J, Tile M. Cerclage wiring in internal fixation of acetabular fractures. J Orthop Trauma. 1993;7:236–241. doi: 10.1097/00005131-199306000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Schröder M, Gottschling H, Reimers N, Hauschild M, Burgkart R. Automated morphometric analysis of the femur on large anatomical database with highly accurate correspondence detection. Open Medical Journal. 2014;1:15–22. doi: 10.2174/1874220301401010015. [DOI] [Google Scholar]
- 37.Shahulhameed A, Roberts CS, Pomeroy CL, Acland RD, Giannoudis PV. Mapping the columns of the acetabulum—implications for percutaneous fixation. Injury. 2010;41:339–342. doi: 10.1016/j.injury.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Surup T, Hänler A, Homeier A, Petersik A, Oldenburg G, Burgkart R. Verfahren zur Referenzmodellerstellung für die Evaluierung CT-basierter Segmentierung des Kortikalis-Spongiosa-Überganges im Femur. In: Meinzer HP, Deserno TM, Handels H, Tolxdorff T, editors. Bildverarbeitung für die Medizin. Berlin, Heidelberg, Germany: Springer-Verlag; 2013. [Google Scholar]
- 39.Tannast M, Siebenrock KA. [Operative treatment of T-type fractures of the acetabulum via surgical hip dislocation or Stoppa approach] [in German] Oper Orthop Traumatol. 2009;21:251–269. doi: 10.1007/s00064-009-1803-7. [DOI] [PubMed] [Google Scholar]
- 40.Whiteside JL, Walters MD. Anatomy of the obturator region: relations to a trans-obturator sling. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:223–226. doi: 10.1007/s00192-004-1250-9. [DOI] [PubMed] [Google Scholar]