Abstract
Background
Local delivery can achieve the high antimicrobial concentrations necessary to kill biofilm-related microbes. Degradation times for resorbable carriers are too long. Hydrogels (gels of hydrophilic polymer in water) can degrade faster but release antimicrobials too quickly. We previously developed hydrogels based on the copolymer poly(N-isopropylacrylamide-co-dimethyl-γ-butyrolactone acrylate-co-Jeffamine® M-1000 acrylamide) (PNDJ) with delivery times of several days with complete degradation in less than 6 weeks.
Questions/purposes
We asked: (1) What is the elution profile of gentamicin from PNDJ hydrogels? (2) Is gentamicin released from gentamicin-loaded PNDJ (G-PNDJ) hydrogel effective for treatment of orthopaedic infection? (3) Does local gentamicin delivery from G-PNDJ hydrogel cause renal dysfunction?
Methods
(1) Two formulations of G-PNDJ, lower dose (1.61 wt%) and higher dose (3.14 wt%), five samples each, were eluted in buffered saline under infinite sink conditions. (2) Infections were induced in 16 New Zealand White rabbits by inserting a Kirschner wire in a devascularized radius segment and inoculating with 7.5 × 106 colony-forming units Staphylococcus aureus. At 3 weeks, débridement was performed and a new Kirschner wire was placed in the dead space. Treatment was randomized to higher-dose G-PNDJ or no hydrogel. No systemic antimicrobials were used. Positive culture and acute inflammation on histology were used to determine the presence of infection 4 weeks postdébridement. (3) 3.14 wt% G-PNDJ, 0.75, 1.5, or 3.0 mL, was injected subcutaneously in nine Sprague-Dawley rats, three of each dose. Serum gentamicin, blood urea nitrogen, and creatinine were measured on Days 1, 3, 7, 14, and 28.
Results
(1) Gentamicin release was sustained over 7 days with the higher-dose formulation release profile similar to release from high-dose antimicrobial-loaded bone cement. (2) Four weeks postdébridement, infection was present in eight of eight no-hydrogel rabbits but zero of eight rabbits treated with G-PNDJ hydrogel (p < 0.001). (3) Blood urea nitrogen and creatinine were transiently elevated (p < 0.05) only for the two of three rats receiving the 3.0-mL dose on Days 3 and 7.
Conclusions
Gentamicin is delivered from PNDJ hydrogel with low systemic exposure and decreased treatment failure for orthopaedic infection. Transient renal dysfunction occurs at high doses. Biodistribution and toxicity testing are needed for G-PNDJ to be clinically usable.
Clinical Relevance
Resorbable viscous hydrogels for local antimicrobial delivery may improve outcomes for one-stage management of implant infections when uncemented reconstructions are performed.
Introduction
Bacteria in biofilms exhibit decreased susceptibility to antimicrobials compared with the same organisms in their planktonic (individual, floating) state [5, 20, 25, 28, 32, 36, 38, 55]. In studies of well-characterized bacterial strains [12, 31, 49] and clinical bacterial isolates from biofilm-based infections [6, 45, 48, 54], the antimicrobial concentration required to kill bacteria in biofilms can be 100 to 1000 times greater than the minimum inhibitory concentration for the planktonic form. Because the antimicrobial concentrations required to manage biofilm often exceed the concentrations at which systemic toxicity is prohibitive, surgical débridement and local antimicrobial delivery are necessary [10, 13, 14, 56].
Currently, there is no acceptable strategy for local antimicrobial delivery from a resorbable delivery vehicle at the time of second-stage reconstruction. When antimicrobial-loaded bone cement is used, it cannot be used in its high-dose therapeutic formulation. The antimicrobial load must be low enough that the structural integrity of the cement fixation is not compromised [17, 44]. Low-dose antimicrobial-loaded bone cement releases only a very small fraction of the drug load during the first week when it is needed [42, 52, 62]. Other investigational delivery vehicles, including calcium phosphate cements [30], hydroxyapatite composites [9, 61], antibiotic implant coatings [2, 3], and polymer beads/microparticles [4, 46, 47], also have drawbacks. Prolonged or incomplete degradation of some resorbable delivery vehicles such as poly(lactic-co-glycolic acid) and polyanhydrides has limited their use [34, 46].
With the goal of identifying a resorbable delivery vehicle that controls release of the loaded antimicrobial over many days and fully degrades over a few weeks, we have previously developed viscous, resorbable hydrogels based on the temperature-responsive polymer poly(N-isopropylacrylamide-co-dimethyl-γ-butyrolactone acrylate-co-Jeffamine® M-1000 acrylamide (PNDJ) [50]. Hydrogels are typically composed of natural or synthetic polymers dissolved in water. They are typically 70% or more water by weight. PNDJ hydrogels provide sustained, partition-controlled release of low-molecular-weight hydrophilic drugs such as antibacterials over approximately 3 to 5 days and dissolve in as little as 9 days depending on polymer composition. Published data document controlled release of cefazolin and vancomycin from PNDJ hydrogels [51].
We chose to study gentamicin release from gentamicin-loaded PNDJ (G-PNDJ) hydrogels because gentamicin is a common antimicrobial in commercially prepared antimicrobial-loaded bone cement. Importantly, renal excretion is expected from both PNDJ polymer and gentamicin [8, 19, 43] and could cause nephrotoxicity. It is therefore necessary to determine the level of systemic exposure to gentamicin and to evaluate the renal effects of locally delivered G-PNDJ hydrogels. Furthermore, efficacy of locally delivered antimicrobials to treat orthopaedic infections from any delivery vehicle has not been definitively proven [33].
We therefore designed a study to answer the following questions: (1) What is the elution profile of gentamicin from PNDJ hydrogels? (2) Is gentamicin released from PNDJ hydrogel effective for treatment of orthopaedic infection? (3) Does local gentamicin delivery from G-PNDJ hydrogel cause renal dysfunction?
Materials and Methods
Gel Synthesis and Characterization
PNDJ hydrogels were synthesized and characterized similarly to that reported previously [51]. N-isopropylacrylamide (NIPAAm, recrystallized from hexane) (Sigma Aldrich, St Louis, MO, USA), dimethyl-γ-butyrolactone acrylate (DBLA) (Sigma Aldrich), and Jeffamine® M-1000 acrylamide (JAAm, Jeffamine® M-1000) (Huntsman Corp) were copolymerized to create PNDJ polymer using free radical polymerization in a blend of dioxane/tetrahydrofuran (THF). The feed ratio was 90.1% NIPAAm, 7.0% DBLA, and 2.9% JAAm by mole; this composition was identified in preliminary work as having desirable sustained release and degradation time. PNDJ was recollected by precipitation in 10-fold excess diethyl ether followed by filtering and overnight drying under vacuum. PNDJ was then purified by dialysis (3500 molecular weight cutoff) against deionized water at 4° C for 24 hours with frequent water exchanges and then lyophilized to obtain a dry powder. Successful synthesis and purification was confirmed by 1H nuclear MR (Varian Inova 300 MHz; Varian, Inc, Palo Alto, CA, USA). Molecular weight was determined using size exclusion chromatography and dynamic light scattering (MiniDAWN; Wyatt Technology Corp, Santa Barbara, CA, USA) with THF as the solvent. The lower critical solution temperature (LCST) was determined by a cloud-point method after dissolving PNDJ polymer in 150 mM phosphate-buffered saline (PBS) using a spectrophotometer at 450 nm [50]. PNDJ polymer solution forms a hydrogel at temperatures above the LCST. Hydrolysis over time increases LCST [16]. For in situ PNDJ, when the LCST increases above body temperature, the hydrogel dissolves and is cleared by renal excretion [8].
Gentamicin Release From G-PNDJ
To answer the first research question, the elution profile of gentamicin from G-PNDJ hydrogels was determined by forming the hydrogels from PNDJ polymer powder, gentamicin sulfate powder (New Chemic US, Montvale, NJ, USA), and PBS, then eluting the hydrogels in deionized water under “infinite sink” conditions. Infinite sink refers to the loss of drug into lymph and the bloodstream as opposed to the buildup of drug in a sealed bottle. Lyophilized PNDJ polymer powder and gentamicin sulfate powder were hand-mixed and then dissolved in 150 mM, pH 7.4, PBS in both 30:2.5:70 and 30:5:70 ratios by weights of PNDJ polymer:gentamicin sulfate:PBS. The gentamicin sulfate powder contained 66 wt% gentamicin base, resulting in gentamicin loads of 1.61 wt% (low-dose) or 3.14 wt% (high-dose). The mixture was shaken to thoroughly wet the polymer; then it was allowed to dissolve at 4° C for 24 hours. After dissolution, the G-PNDJ solution was shaken for 10 to 15 seconds (separation of two aqueous phases occurs in storage). One milliliter of PNDJ/gentamicin solution was added into the bottom of 20-mL glass scintillation vials and solidified over 15 minutes in a water bath at 37° C, resulting in a 2-mm thick layer of G-PNDJ hydrogel. The mass of G-PNDJ was weighed. Five replicates of each formulation, total of 10 samples, were tested. After gelling, 20 mL of prewarmed 150 mM, pH 7.4 PBS was added on top of the gels, and the gels were maintained at 37° C throughout elution. Total eluant exchange was performed at 1, 3, 6, 12, 24, 48, 72, 120, and 168 hours. Gentamicin concentration in each eluant aliquot was determined using a ninhydrin-based colorometric assay [24]. Cumulative recovered gentamicin was then calculated.
Rabbit Model for Infection Treatment
To answer the second study question, the efficacy of gentamicin released from G-PNDJ hydrogel in the treatment of orthopaedic infection was determined using a rabbit model for osteomyelitis. All procedures were approved by the Institutional Animal Care and Use Committee at Arizona State University. All procedures were performed under strict sterile surgical conditions. Sixteen female New Zealand White Rabbits (3–4 kg) were divided into two groups: eight were treated by débridement only and eight were treated by débridement plus locally delivered gentamicin from G-PNDJ hydrogel. Rabbits were anesthetized using ketamine/xylazine and maintained free breathing on 2% to 3% isoflurane in oxygen during the course of the procedure. An incision was made along the volar-radial aspect of the left forelimb. The muscles were separated to expose the radius. The periosteum was elevated and osteotomies of the radial shaft were performed 1 cm apart using a fine-toothed hand saw. The 1-cm long segment of radius was removed, a 1-cm 0.045-inch Kirschner wire was placed in the intramedullary canal of the free segment, and was inoculated with 7.5 × 106 colony-forming units (CFU) of Staphylococcus aureus (50 μL of a 108 CFU/mL UAMS-1, ATCC 49230) in tryptic soy broth (Becton Dickinson, Franklin Lakes, NJ, USA) in the intramedullary canal of the free segment [29, 46]. Bacterial concentrations were measured by absorbance versus McFarland standards and adjusted by dilution after overnight growth [29, 46]. The inoculated segment containing the Kirschner wire was placed in the wound dead space. The wound was closed in layers with 3-0 Prolene™ suture (Ethicon, Somerville, NJ, USA). Rabbits were recovered from surgery and given buprenorphine every 8 hours for 24 hours and then twice daily for 3 days to manage postoperative pain. All rabbits were allowed unrestricted cage activity on a normal diet for 3 weeks before débridement of the infection. No systemic antimicrobials were given. Infection was defined as a positive culture for S aureus or acute inflammation on histopathology.
Débridement and Local G-PDNJ Delivery
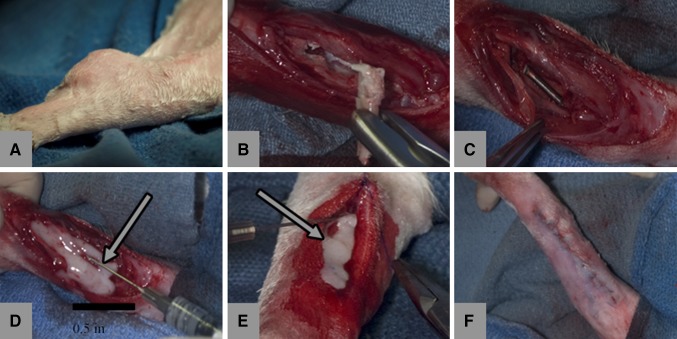
All experimental sites developed culture-positive, purulent abscesses with acute inflammatory cellular response on histopathology. Three weeks after the index procedure, each rabbit underwent complete intralesional débridement, implantation of a sterile Kirschner wire implant, and randomization to ether local gentamicin delivery with G-PNDJ hydrogel or no-antimicrobials control. Anesthesia was again done by ketamine/xylazine induction and maintenance-free breathing on 2% to 3% isoflurane in oxygen. Débridement of all infection sites was performed by an orthopaedic surgeon with extensive experience in surgical management of orthopaedic infections (Fig. 1). The surgeon was blinded to the treatment group during débridement and rabbits were débrided in random order. The scar from the index incision was excised to include the draining sinus when present. Intralesional evacuation of pus and removal of all dead bone, the Kirschner wire, and all adjacent infected tissues were performed consistent with common clinical practice. The wound was then irrigated with 100 mL normal saline. A sterile implant made of 0.045-inch Kirschner wire was placed in the wound dead space, nonstructurally spanning the postdébridement defect in the radius. Introduction of an implant into the postdébridement wound is a modification of the Nelson osteomyelitis model [46] intended to represent the clinical scenario of a one-stage débridement/reconstruction procedure. Adding the implant as a foreign body reliably prevented eradication of infection by the host without any antimicrobial treatment postdébridement. Based on intent to treat, the wound was either closed immediately with 3-0 Prolene™ suture or filled with higher-dose (3.14 wt%) G-PNDJ hydrogel and then closed. The volume of G-PNDJ hydrogel placed in the débrided space ranged from 400 to 1100 μL (mean 760 μL) for a total dose per rabbit of 15 to 43 mg (mean 30 mg) gentamicin. The volume of débrided space was determined by the amount of gel delivered. Rabbits were allowed unrestricted cage activity on a normal diet for 4 weeks after débridement.
Fig. 1A–F.
The course of management is seen in (A) a subcutaneous abscess in the rabbit forelimb, (B) involucrum and Kirschner wire with tissues during débridement after evacuation of pus and removal of granulation tissue, (C) surgical site postdebridement with Kirschner wire, (D) application of G-PNDJ gel (white arrow), (E) closure of the wound with additional gel (white arrow), and (F) postoperatively (arrows indicate hydrogel).
Euthanasia and Histology
Rabbits were euthanized by intravenous injection of 1 mg/kg Beuthanasia D solution (Merck, Summit, NJ, USA) and thoracotomy as the secondary method of euthanasia. After euthanasia, the left forelimb was sterilely prepared. The Kirschner wire implant was exposed and removed under sterile surgical conditions. The Kirschner wire and a biopsy of adjacent tissue were sent to microbiology for culture. The forelimb was then disarticulated at the elbow and wrist, fixed in formalin, and sent for histological processing. Tissues were decalcified, stained with hematoxylin and eosin, and evaluated by a pathologist (AD) for histologic findings related to infection as described by Skinner et al. [57].
Culture
Sterile culture tubes with swabs and transport medium were supplied by IDEXX reference laboratories (Peoria, AZ, USA). Cultures were routinely taken for all subjects at two time points: (1) at débridement and (2) at euthanasia. All samples were sent to the IDEXX reference laboratory for independent culturing. All negative cultures were incubated for 21 days to ensure the absence of small colony variants before they were termed “negative.” Infection was defined as a positive culture for S aureus or acute inflammatory response on histopathology.
Maximum Tolerated Dose
To answer the third study question, systemic exposure to gentamicin released from G-PNDJ hydrogel and renal function were determined using a rat model. The doses given in the rabbit infection model portion of this study were insufficient to cause measurable serum gentamicin levels. Rats are a well-established model for evaluation of the pharmacokinetics and nephrotoxicity of free gentamicin [7, 15, 23, 27]. Nephrotoxicity markers (blood urea nitrogen [BUN] and serum creatinine) were evaluated because both the PNDJ polymer and gentamicin are cleared by renal excretion. Nine female Sprague-Dawley rats (approximately 300 g), three in each of three dosage groups (0.75 mL, 1.5 mL, and 3 mL), received subcutaneous injections of higher-dose G-PNDJ hydrogel loaded with 3.14% gentamicin. G-PNDJ hydrogel was injected through an 18-G needle with rats briefly anesthetized with 2% to 3% isoflurane in oxygen. These rats had 400 µL of blood drawn from the saphenous vein while restrained, before the G-PNDJ injection and at 1, 3, 7, 14, and 28 days postinjection. Serum was assayed for gentamicin, BUN, and creatinine by independent laboratories. Serum gentamicin levels were measured at the Auburn University Clinical Pharmacology Laboratory by turbidimetric inhibition immunoassay (PETINIA) on a Siemens Dimension Xpand Plus General Chemistry Analyzer (Siemens Healthcare Diagnostics Inc, Deerfield, IL, USA). BUN and creatinine levels were measured by IDEXX reference laboratories.
Statistical Analysis
Differences in elution rate were analyzed with repeated measures analysis of variance and t-test. Differences in infection rate were analyzed with Fisher’s exact test for two-by-two tables. Differences in BUN, creatinine, serum gentamicin, and eluted gentamicin in vitro were analyzed by t-test and relative to baseline. Statistical analysis was performed in Minitab 15 (Minitab Inc, State College, PA, USA).
Results
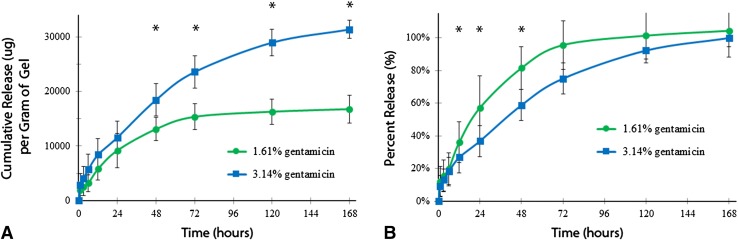
Gentamicin release from G-PNDJ hydrogel was greater (p = 0.01) and over a longer time period (p < 0.001) for the higher-dose (3.14%) formulation compared with the lower dose (1.61%) (Fig. 2). Both formulations released approximately 10,000 µg gentamicin/g of hydrogel in the first 24 hours: 9210 ± 3158 µg/g of hydrogel for the lower dose and 11,584 ± 2990 µg/g of hydrogel for the higher dose. Sustained release from the higher-dose formulation delivered 15,000 µg more gentamicin/g of hydrogel than the lower-dose formulation between 24 and 120 hours. Under infinite sink conditions, 96% of the contained gentamicin load was recovered by 72 hours for the lower-dose formulation, whereas the higher dose delivered 75% of the contained gentamicin at 3 days and took 7 days to release 100% of its contained load.
Fig. 2A–B.
Gentamicin release (n = 5) from G-PNDJ hydrogel containing 1.61 wt% or 3.14 wt% gentamicin is expressed as (A) cumulative mass and (B) fraction of total gentamicin load. Release from higher-dose G-PNDJ gel is greater than the release from the lower-dose G-PNDJ gel after 48 hours (p < 0.05) Data points are the mean and error bars represent SD.
Continued infection after débridement was lower in the G-PNDJ treatment group than in the control group that received no G-PNDJ hydrogel (p < 0.001). Postdébridement, eight of eight rabbits that underwent débridement and insertion of a Kirschner wire implant alone had positive cultures with gross findings of swelling, granulation tissue, abscess formation, and fluid or pus adjacent to the Kirschner wire implant consistent with active infection. Culture results ranged from 2 + to 4 + for S aureus (referring to the number of quadrants exhibiting growth) [35]. Eight of eight rabbits that received local gentamicin delivery in G-PNDJ hydrogel after débridement and Kirschner wire implantation had wounds with minimal fluid present, no swelling, granulation tissue, or abscess formation and were culture-negative (Fig. 3). Histological examination of the specimens found normal wound healing in both groups, consistent with status 4 weeks postdébridement [57] (Fig. 4A). Acute inflammation consistent with active infection was seen only in the rabbits not treated with G-PNDJ hydrogel (Fig. 4B). None of the specimens from wounds treated with G-PNDJ hydrogel had histologic findings consistent with an adverse response to foreign material or persistent nondegraded hydrogel, although benign fibrous encapsulation of the metallic rod was seen (Fig. 4A).
Fig. 3A–B.
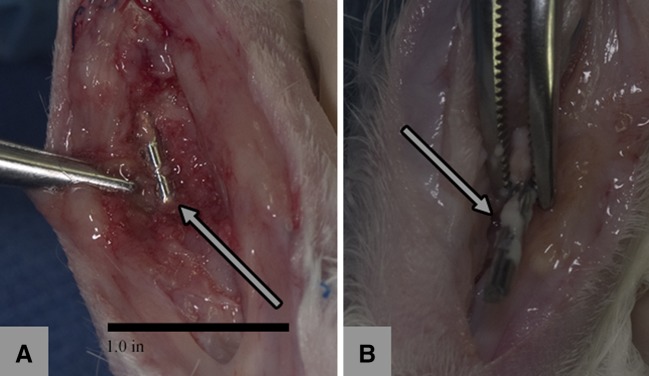
At necropsy the tissue appearance was consistent with (A) normal healing in rabbits that received G-PNDJ (arrow = normal tissue adjacent to the Kirschner wire) and (B) acute inflammation in rabbits that did not receive G-PNDJ (arrow = pus and lack of tissue apposition on the Kirschner wire).
Fig. 4A–B.
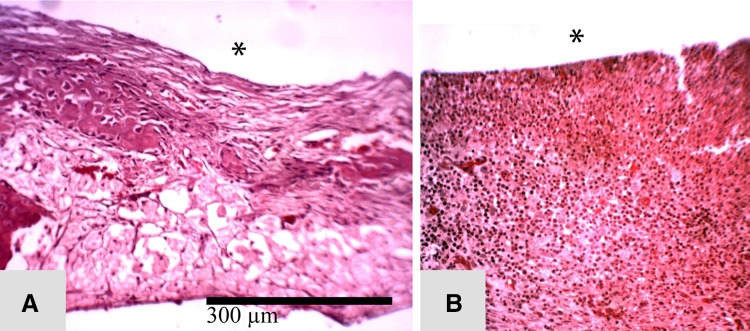
Histological features of the tissue response at 4 weeks postdébridement are seen in the following representative sections. (A) Healthy fibrous tissue is seen in the débridement site of a rabbit that received G-PNDJ (*débrided surface). (B) Thicker fibrous tissue invaded with acute inflammatory cells is seen in the débridement site of a rabbit that did not receive G-PNDJ. This reaction extends deep into the tissue, and there is new bone formation adjacent to the ulna (Stain, hematoxylin and eosin; original magnification, × 100) (*débrided surface).
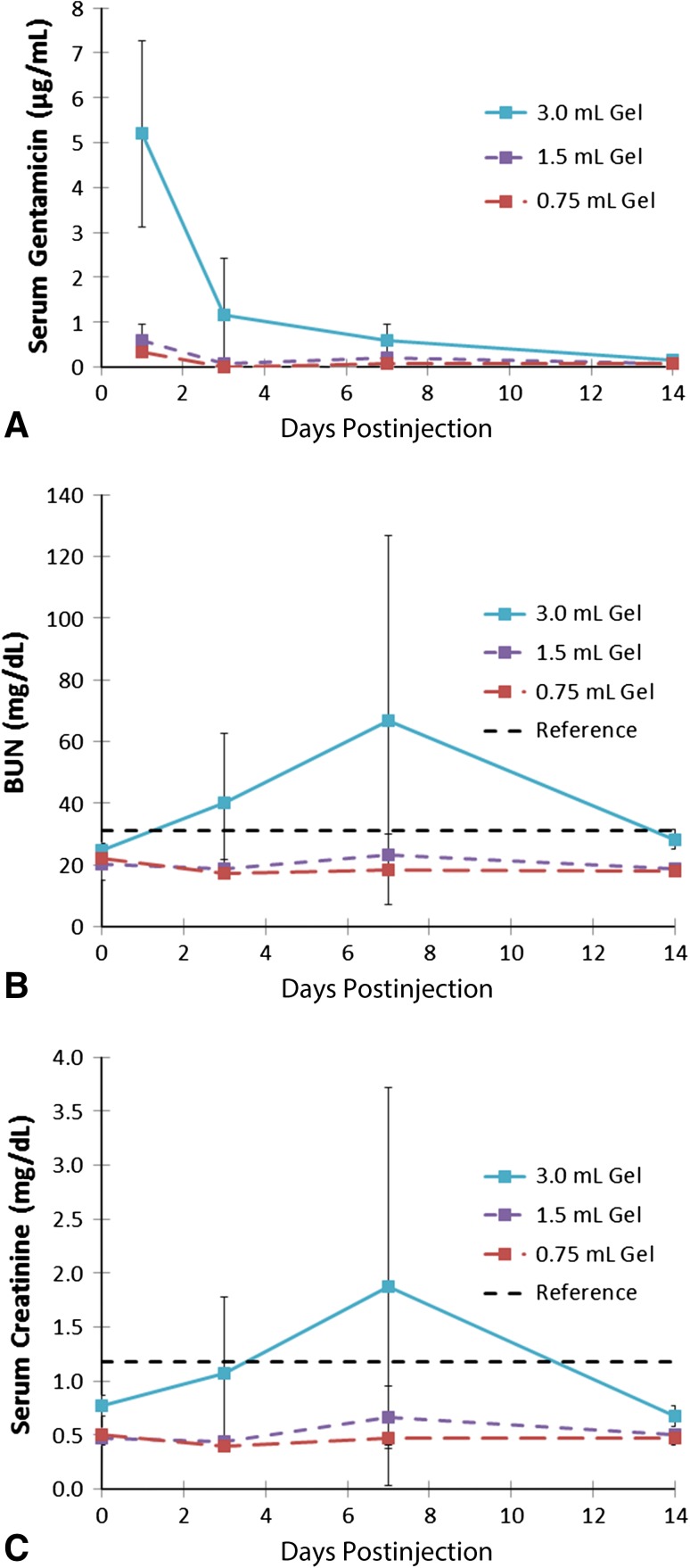
The systemic exposure to gentamicin from local delivery from G-PNDJ hydrogels in the rats lasted less than 14 days. Peak gentamicin concentration in the rat serum in the 3.0-mL dose group was 5 ± 2 µg/mL on the first day after injection, decreasing to 1.15 µg/mL on Day 3, 0.6 µg/mL on Day 7, and to an unmeasurable level by Day 14 (Fig. 5). Renal function was decreased transiently for the highest dose only. In all rats that received 0.75-mL and 1.5-mL doses and one of three rats that received 3.0 mL, BUN and creatinine were not elevated. Two of three rats that received the 3.0-mL subcutaneous dose of G-PNDJ hydrogel had transient elevation of BUN and creatinine levels that returned to baseline by Day 14. The average BUN and creatinine for the 3.0-mL dose group increased to 67 ± 60 and 1.9 ± 1.8 mg/dL, respectively, by Day 7, returning to preinjection baseline by Day 14 (Fig. 5; Table 1). BUN and creatinine values were not elevated relative to baseline at 14 or 28 days postdose in any group (smallest p value is 0.07, 3.0-mL group, 28.3 mg/dL versus 24.8 mg/dL) (Table 1).
Fig. 5A–C.
Laboratory data associated with G-PNDJ hydrogel (n = 3) doses of 0.75 mL, 1.5 mL, and 3.0 mL are displayed on the following graphs: (A) serum gentamicin concentration, (B) BUN, and (C) serum creatinine. BUN and creatinine are elevated on Day 7 for the 3.0-mL dose (118-mg gentamicin load). Data points are the mean and error bars represent SD. The horizontal line is the upper limit of normal [37].
Table 1.
BUN and creatinine (mg/dL) in rat serum on Days 0, 3, 7, 14, and 28 after receiving 0.75, 1.5, or 3.0 mL of G-PNJD hydrogel subcutaneously
| G-PNDJ dose | Test | Baseline | Day 3 | Day 7 | Day 14 | Day 28 | p value baseline versus 14 days |
|---|---|---|---|---|---|---|---|
| 0.75 mL | BUN | 22.0 ± 1.0 | 17.3 ± 1.53 | 18.3 ± 0.06 | 18.0 ± 1.0 | 18.3 ± .6 | 0.008 |
| Creatinine | 0.5 ± 0.01 | 0.4 ± 0.01 | 0.5 ± 0.06 | 0.5 ± 0.06 | 0.5 ± 0.06 | 1.00 | |
| 1.5 mL | BUN | 20.3 ± 5.5 | 18.7 ± 3.2 | 23.2 ± 6.8 | 18.7 ± 6.8 | 18.7 ± 3.1 | .772 |
| Creatinine | 0.5 ± 0.06 | 0.4 ± 0.06 | 0.7 ± 0.29 | 0.5 ± 0.01 | 0.4 ± 0.06 | 1.0 | |
| 3.0 mL | BUN | 24.8 ± 2.4 | 40.3 ± 22.4 | 67 ± 60 | 28.3 ± 3.1 | 20.3 ± 4.2 | .220 |
| Creatinine | 0.8 ± 0.1 | 1.1 ± 0.70 | 1.9 ± 1.8 | 0.7 ± 0.1 | 0.5 ± 0.06 | .021 |
Values are mean ± SD; normal upper limit for BUN is 30.1 mg/dL and creatinine 1.2 mg/dL [37]; abnormal values are in bold. Some specimens show reduced BUN or creatinine relative to baseline, which may be related to hydration; BUN = blood urea nitrogen; G-PNJD = gentamicin-loaded poly(N-isopropylacrylamide-co-dimethyl-γ-butyrolactone acrylate-co-Jeffamine® M-1000 acrylamide).
Discussion
Bacteria in biofilm are difficult to treat, requiring antimicrobial concentrations that are hundreds of times higher than the levels needed to kill the respective planktonic phenotype. Local delivery is required to achieve these high levels. Clinical application has not produced consistent data on outcomes [33], which we believe may be attributable in part to variability in treatment protocols that can lead to subtherapeutic levels in some regions or all of the postdébridement surgical site. G-PNDJ hydrogels may represent a reliable way to provide high-dose local delivery that can be distributed throughout a surgical site and can be used when antimicrobial-loaded bone cement is not used for implant fixation. However, the release characteristics of gentamicin from PNDJ hydrogels, efficacy of antimicrobial delivery from PNDJ hydrogels for the treatment of orthopaedic infections, and systemic toxicity of PNDJ hydrogels were all unknown. In this study, we asked: (1) What is the elution profile of gentamicin from PNDJ hydrogels? (2) Is gentamicin released from PNDJ hydrogel effective for treatment of orthopaedic infection? (3) Does local gentamicin delivery from G-PNDJ hydrogel cause renal dysfunction?
This study has a number of limitations. First, the toxicity studies were performed in a different animal model than the infection treatment studies so they cannot be directly related to each other. Rats are more frequently used for toxicity studies with more published data. The methods used in this study are consistent with FDA and industry guidelines for establishing no observed adverse effect levels [22]. Comparison between rabbit and rat data is far less important than establishing the validity of each model for its clinical applicability. Second, in vitro elution data do not represent release kinetics observed in vivo because infinite sink conditions (low gentamicin concentrations in the surrounding fluid that allow continued release by diffusion) are not maintained in a postdébridment surgical wound [40]. Buildup of gentamicin in the surgical site leads to high concentrations, which is the goal of local delivery, and may slow release compared with in vitro elution studies. Release studies are designed to specifically determine the maximum release capability of a delivery vehicle as a metric to compare delivery capability between vehicles and therefore deduce what relative performance of different vehicles might be in vivo. The infection treatment part of this study did not measure in vivo concentration levels or distribution or duration of gentamicin in the postdébridement surgical site, but the concentrations that did occur were sufficient to improve the outcome of treatment. Third, the model used to study effectiveness of G-PNDJ for the treatment of orthopaedic infections was a rabbit osteomyelitis model. The primary drawback of the model is the small volume of tissue in the rabbit forelimb. The dimensions of the surgical site and dead space and volume of distribution for local delivery are limited compared with the dimensions of surgical sites and dead space experienced clinically. Although scalability to human dimensions has not yet been shown, this model is well established in the literature. This model produces osteomyelitis reliably, provides surfaces for biofilm formation, and has been used to study various treatment protocols with discriminatory sensitivity to variations in treatment protocols [29, 46, 58]. Another weakness of the model is the capability of rabbits, on occasion, to clear infection postdébridement without local or systemic antimicrobials, confounding the analysis for response to treatment. We modified the model by adding a metallic implant to the postdébridement wound, leading to continued infection in every case that antimicrobials were not used. Fourth, the number of animals was too small to identify all the potential risks or establish clinical safety. Renal dysfunction is the primary indicator of clinical toxicity for gentamicin in toxicology studies [64]. PNDJ is composed of, and degrades to, components that are generally felt to be nontoxic but are cleared through the kidneys. We believe renal function is by far the greatest risk if toxicity to G-PNDJ does occur and therefore is a good initial toxicity metric. Fifth, we studied only gentamicin as a single locally delivered antibacterial. We chose gentamicin as the single antimicrobial for local delivery for several reasons. Aminoglycosides are the dominant antibacterial in commercially prepared antibacterial-loaded bone cement available in the United States. Very high efficacy rates have been reported in animal experiments using locally delivered gentamicin with Nelson’s [46] reported 15 of 16 cured with high-dose local gentamicin. Gentamicin has a broad spectrum of activity against Gram-negative and Gram-positive microbes encountered in orthopaedic infection. Although gentamicin is not commonly used clinically as the primary parenteral agent to manage Gram-positive infection, roughly 94% of antibiotic-naïve orthopaedic infections are caused by gram-negative or gram-positive bacteria which are susceptible to gentamicin at concentrations achievable with local delivery [11, 53]. Finally, susceptibility to aminoglycosides, both concentration- and duration-dependent, is reduced but not eliminated in microbes that have organized into biofilms in vivo [63]. Sustained high concentrations overcome concentration-dependent susceptibility [39]. Small water-soluble molecules (like aminoglycosides) have been shown to penetrate biofilms, whereas larger molecules like antibodies have limited transport in biofilms [18, 63].
We found consistent and high levels of release from both formulations examined in this study. The higher-dose formulation released more gentamicin over a longer duration. Release from the higher-dose G-PNDJ formulation is very similar to the release characteristics of high-dose antimicrobial-loaded bone cement over the first week [41] but G-PNDJ does not have the long-duration subtherapeutic release that occurs with antimicrobial-loaded bone cement because it is fully degraded by 4 weeks [50]. It is generally accepted that high-dose antimicrobial-loaded bone cement is indicated for treatment of established infection. From recent work we reported that high-dose formulations do provide measurably higher concentrations throughout a larger volume of distribution over a longer duration than low-dose formulations in surgical sites after local delivery [40]. Therapeutic levels are only achieved for 1 day over a limited volume of distribution with low-dose formulations in bone cement (1–2 g/batch), but high concentrations are achieved as long as 7 days over a larger volume of distribution with high-dose formulations in bone cement (10 g/batch or greater) [40]. Similar postdelivery concentration and distribution studies are required for G-PNDJ hydrogel to confirm that its in vivo performance is similar to high-dose antimicrobial-loaded bone cement.
The local delivery of gentamicin from G-PNDJ was effective in the treatment of infection postdébridement in this rabbit model. The status, infected or noninfected, for all 16 postdébridement wounds was clear with 100% concordance of gross appearance and both metrics: histology and culture. Eight of eight infections that received the higher-dose local delivery had no infection after treatment, whereas all eight rabbits that did not receive G-PNDJ had active infection after treatment. The débridements were intralesional with certain retained contamination by S aureus biofilm. When G-PNDJ was not placed in the wound, the infection was likely propagated from these fragments of biofilm that remained in the wound. The 24-hour in vitro minimum biofilm eradication concentration (MBEC) for gentamicin versus ATCC 49230 (S aureus, UAMS-1) as measured by our laboratory is 1000 µg/mL using a resazurin assay [21] and static culture. When G-PNDJ was placed in the wound, it is likely that the gentamicin concentration achieved throughout the postdébridement surgical site was at least the MBEC of 1000 µg/mL because continuation of the infection was prevented, even with an implant placed in the contaminated dead space. In the rabbits that received G-PNDJ hydrogel, no evidence of hydrogel was observed 4 weeks after débridement on gross observation or histologically, consistent with in vitro degradation of 28 days for the hydrogel formulation used in this study.
The highest dose of gentamicin in our rabbit model was roughly 13 mg/kg. Systemic exposure to locally delivered gentamicin was studied at higher doses in a rat model from 0.75-mL and 1.5-mL doses of higher-dose G-PNDJ hydrogel containing 98 and 196 mg/kg gentamicin, respectively, which did not result in detectable change in renal function in rats. Two of three rats that received the highest dose of 3.0 mL G-PNDJ containing 393 mg/kg gentamicin had a transient rise in BUN and creatinine during the first 14 days. We did not determine whether the gentamicin, the hydrogel, or the combination was responsible for the renal dysfunction. Our data are consistent with reported nephrotoxicity to gentamicin exposure [64]. Serum levels of gentamicin in the rat data were low with a maximum level of 6 µg/mL that occurred on the first day, which is considerably less than the peak serum levels of 40 to 100 µg/mL reported by Bennett et al. [7] after intravenous administration of gentamicin. To scale-up to the equivalent human dose in mg/kg, the rat dose is divided by six [22] based on conversion of mg/kg to mg/m2 surface area [64]. Three milliliters of higher-dose (3.14 wt%) G-PNDJ hydrogel contains 118 mg of active gentamicin (3.14% × 3750 mg [3 mL * 1.25 g/mL]). Based on rats in our study that weighed approximately 300 g, the rat mg/kg dose is 393 mg/kg divided by six gives the human dose equivalent of 65.5 mg/kg. For a 70-kg adult, this would be 4600 mg, or approximately 117 mL of gel. The effect of variations in area of contact between the hydrogel and host tissue and patient-to-patient variation in renal clearance also need to be considered but likely have a small effect [65]. We estimate the volume of G-PNDJ hydrogel that might be used in a human case would be approximately 15 to 20 mL, one-sixth the volume that led to renal toxicity in rats. When similar amounts of gentamicin are delivered using a collagen sponge as the delivery vehicle, the release is more rapid [59] and this is reported to be well tolerated in human patients [60], understanding that pharmacokinetics of gentamicin in human patients are highly variable [65].
We agree with the concepts that (1) established orthopaedic infections are associated with mature biofilm; (2) several days of exposure to high antimicrobial concentrations, above the MBEC, from local delivery is required to kill resistant bacteria in mature biofilm; (3) there are fragments of biofilm retained throughout the entire surgical site after complete intralesional debridement; (4) all débrided surfaces and implant surfaces are at risk to establish continuation of the infection; and (5) local delivery vehicles that do not provide a secondary structural function should totally resorb or incorporate soon after the contained antimicrobial is released [1, 26, 40, 53]. Gentamicin release from G-PNDJ hydrogel in vitro follows the release pattern expected under infinite sink conditions with performance similar to high-dose antimicrobial-loaded bone cement. In vivo G-PNDJ delivers sufficient gentamicin to generate and sustain concentrations exceeding MBEC for sufficient time to kill residual bacteria in biofilm after débridement in the rabbit model we studied. There was no evidence of residual hydrogel at 4 weeks. Renal impairment from local injection of G-PNDJ occurred only transiently in the rat model at doses six times greater than are expected for clinical use. Although G-PNDJ characteristics are consistent with the concepts related to management of established orthopaedic infections, robust evaluations in a Good Laboratory Practice-compliant environment for biodistribution and adverse effects and Phase I human trials are needed before clinical application can occur. A clinically usable, therapeutically effective antimicrobial delivery in a high-viscosity resorbable vehicle could have a meaningful impact on treatment and outcomes for orthopaedic infections where a nonstructural delivery vehicle is desirable, like in uncemented one-stage reconstruction for prosthetic joint infections.
Acknowledgments
We acknowledge Dr Allan Dovigi for histopathological interpretation and the Arizona State University Department of Animal Care and Technologies for their assistance with this work.
Footnotes
Research reported in this manuscript was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number R41AR064080. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. One or more of the authors (DO, AM, BV, RM) have ownership in Sonoran Biosciences.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Arizona State University, Tempe, AZ, USA.
References
- 1.Adams K, Couch L, Cierny G, Calhoun J, Mader JT. In vitro and in vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethylmethacrylate beads. Clin Orthop Relat Res. 1992;278:244–252. [PubMed] [Google Scholar]
- 2.Alt V, Bitschnau A, Böhner F, Heerich KE, Magesin E, Sewing A, Pavlidis T, Szalay G, Heiss C, Thormann U, Hartmann S, Pabst W, Wenisch S, Schnettler R. Effects of gentamicin and gentamicin-RGD coatings on bone ingrowth and biocompatibility of cementless joint prostheses: an experimental study in rabbits. Acta Biomater. 2011;7:1274–1280. doi: 10.1016/j.actbio.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Alt V, Bitschnau A, Osterling J, Sewing A, Meyer C, Kraus R, Meissner SA, Wenisch S, Domann E, Schnettler R. The effects of combined gentamicin-hydroxyapatite coating for cementless joint prostheses on the reduction of infection rates in a rabbit infection prophylaxis model. Biomaterials. 2006;27:4627–4634. doi: 10.1016/j.biomaterials.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 4.Ambrose CG, Gogola GR, Clyburn TA, Raymond AK, Peng AS, Mikos AG. Antibiotic microspheres: preliminary testing for potential treatment of osteomyelitis. Clin Orthop Relat Res. 2003;415:279–285. doi: 10.1097/01.blo.0000093920.26658.ae. [DOI] [PubMed] [Google Scholar]
- 5.Anderson GG, O’Toole GA. Innate and induced resistance mechanisms of bacterial biofilms. Curr Top Microbiol Immunol. 2008;322:85–105. doi: 10.1007/978-3-540-75418-3_5. [DOI] [PubMed] [Google Scholar]
- 6.Arciola CR, Campoccia D, Gamberini S, Donati ME, Pirini V, Visai L, Speziale P, Montanaro L. Antibiotic resistance in exopolysaccharide-forming Staphylococcus epidermidis clinical isolates from orthopaedic implant infections. Biomaterials. 2005;26:6530–6535. doi: 10.1016/j.biomaterials.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 7.Bennett WM, Plamp CE, Gilbert DN, Parker RA, Porter GA. The influence of dosage regimen on experimental gentamicin nephrotoxicity: dissociation of peak serum levels from renal failure. J Infect Dis. 1979;140:576–580. doi: 10.1093/infdis/140.4.576. [DOI] [PubMed] [Google Scholar]
- 8.Bertrand N, Fleischer JG, Wasan KM, Leroux J-C. Pharmacokinetics and biodistribution of N-isopropylacrylamide copolymers for the design of pH-sensitive liposomes. Biomaterials. 2009;30:2598–2605. doi: 10.1016/j.biomaterials.2008.12.082. [DOI] [PubMed] [Google Scholar]
- 9.Buranapanitkit B, Oungbho K, Ingviya N. The efficacy of hydroxyapatite composite impregnated with amphotericin B. Clin Orthop Relat Res. 2005;437:236–241. doi: 10.1097/01.blo.0000165851.81386.6a. [DOI] [PubMed] [Google Scholar]
- 10.Cabrita HB, Croci AT, de Camargo OP, de Lima ALLM. Prospective study of the treatment of infected hip arthroplasties with or without the use of an antibiotic-loaded cement spacer. Clin São Paulo Braz. 2007;62:99–108. doi: 10.1590/s1807-59322007000200002. [DOI] [PubMed] [Google Scholar]
- 11.Campoccia D, Montanaro L, Arciola CR. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials. 2006;27:2331–2339. doi: 10.1016/j.biomaterials.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 12.Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol. 1999;37:1771–1776. doi: 10.1128/jcm.37.6.1771-1776.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cierny G. Surgical treatment of osteomyelitis. Plast Reconstr Surg. 2011;127(Suppl 1):190S–204S. doi: 10.1097/PRS.0b013e3182025070. [DOI] [PubMed] [Google Scholar]
- 14.Cierny G, Mader JT, Penninck JJ. The Classic: A clinical staging system for adult osteomyelitis. Clin Orthop Relat Res. 2003;414:7–24. doi: 10.1097/01.blo.0000088564.81746.62. [DOI] [PubMed] [Google Scholar]
- 15.Cohen L, Lapkin R, Kaloyanides GJ. Effect of gentamicin on renal function in the rat. J Pharmacol Exp Ther. 1975;193:264–273. [PubMed] [Google Scholar]
- 16.Cui Z, Lee BH, Vernon BL. New hydrolysis-dependent thermosensitive polymer for an injectable degradable system. Biomacromolecules. 2007;8:1280–1286. doi: 10.1021/bm061045g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies JP, O’Connor DO, Burke DW, Harris WH. Influence of antibiotic impregnation on the fatigue life of Simplex P and Palacos R acrylic bone cements, with and without centrifugation. J Biomed Mater Res. 1989;23:379–397. doi: 10.1002/jbm.820230402. [DOI] [PubMed] [Google Scholar]
- 18.de Beer D, Stoodley P, Lewandowski Z. Measurement of local diffusion coefficients in biofilms by microinjection and confocal microscopy. Biotechnol Bioeng. 1997;53:151–158. doi: 10.1002/(SICI)1097-0290(19970120)53:2<151::AID-BIT4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 19.DeNardo SJ, Yao Z, Lam KS, Song A, Burke PA, Mirick GR, Lamborn KR, O’Donnell RT, DeNardo GL. Effect of molecular size of pegylated peptide on the pharmacokinetics and tumor targeting in lymphoma-bearing mice. Clin Cancer Res. 2003;9:3854S–3864S. [PubMed] [Google Scholar]
- 20.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elkhatib WF, Khairalla AS, Ashour HM. Evaluation of different microtiter plate-based methods for the quantitative assessment of Staphylococcus aureus biofilms. Future Microbiol. 2014;9:725–735. doi: 10.2217/fmb.14.33. [DOI] [PubMed] [Google Scholar]
- 22.FDA Center for Drug Evaluation and Research (CDER). Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Rockville, MD, USA: Office of Training and Communications, Division of Drug Information, HFD-240 Center for Drug Evaluation and Research, Food and Drug Administration; 2005.
- 23.Frame PT, Phair JP, Watanakunakorn C, Bannister TW. Pharmacologic factors associated with gentamicin nephrotoxicity in rabbits. J Infect Dis. 1977;135:952–956. doi: 10.1093/infdis/135.6.952. [DOI] [PubMed] [Google Scholar]
- 24.Frutos P, Torrado S, Perez-Lorenzo ME, Frutos G. A validated quantitative colorimetric assay for gentamicin. J Pharm Biomed Anal. 2000;21:1149–1159. doi: 10.1016/S0731-7085(99)00192-2. [DOI] [PubMed] [Google Scholar]
- 25.Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005;13:34–40. doi: 10.1016/j.tim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Giers MB, McLaren AC, Plasencia JD, Frakes D, McLemore R, Caplan MR. Spatiotemporal quantification of local drug delivery using MRI. Comput Math Methods Med. 2013;2013:149608. doi: 10.1155/2013/149608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilbert DN, Plamp C, Starr P, Bennett WM, Houghton DC, Porter G. Comparative nephrotoxicity of gentamicin and tobramycin in rats. Antimicrob Agents Chemother. 1978;13:34–40. doi: 10.1128/AAC.13.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilbert P, Maira-Litran T, McBain AJ, Rickard AH, Whyte FW. The physiology and collective recalcitrance of microbial biofilm communities. Adv Microb Physiol. 2002;46:202–256. [PubMed] [Google Scholar]
- 29.Gillaspy AF, Hickmon SG, Skinner RA, Thomas JR, Nelson CL, Smeltzer MS. Role of the accessory gene regulator (AGR) in pathogenesis of staphylococcal osteomyelitis. Infect Immun. 1995;63:3373–3380. doi: 10.1128/iai.63.9.3373-3380.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ginebra MP, Traykova T, Planell JA. Calcium phosphate cements as bone drug delivery systems: a review. J Control Release. 2006;113:102–110. doi: 10.1016/j.jconrel.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Girard LP, Ceri H, Gibb AP, Olson M, Sepandj F. MIC versus MBEC to determine the antibiotic sensitivity of Staphylococcus aureus in peritoneal dialysis peritonitis. Perit Dial Int. 2010;30:652–656. doi: 10.3747/pdi.2010.00010. [DOI] [PubMed] [Google Scholar]
- 32.Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Iarikov D, Demian H, Rubin D, Alexander J, Nambiar S. Choice and doses of antibacterial agents for cement spacers in treatment of prosthetic joint infections: review of published studies. Clin Infect Dis. 2012;55:1474–1480. doi: 10.1093/cid/cis735. [DOI] [PubMed] [Google Scholar]
- 34.Koort JK, Suokas E, Veiranto M, Mäkinen TJ, Jalava J, Törmälä P, Aro HT. In vitro and in vivo testing of bioabsorbable antibiotic containing bone filler for osteomyelitis treatment. J Biomed Mater Res A. 2006;78:532–540. doi: 10.1002/jbm.a.30766. [DOI] [PubMed] [Google Scholar]
- 35.Levine NS, Lindberg RB, Mason AD, Pruitt BA. The quantitative swab culture and smear: a quick, simple method for determining the number of viable aerobic bacteria on open wounds. J Trauma. 1976;16:89–94. doi: 10.1097/00005373-197602000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Lewis K. Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol. 2008;322:107–131. doi: 10.1007/978-3-540-75418-3_6. [DOI] [PubMed] [Google Scholar]
- 37.Loeb WF, Quimby F. Clinical Chemistry of Laboratory Animals. 2. Philadelphia, PA, USA: CRC Press; 1999. [Google Scholar]
- 38.Mah TF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39. doi: 10.1016/S0966-842X(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 39.Mayhall CG, Medoff G, Marr JJ. Variation in the susceptibility of strains of Staphylococcus aureus to oxacillin, cephalothin, and gentamicin. Antimicrob Agents Chemother. 1976;10:707–712. doi: 10.1128/AAC.10.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McLaren A, Giers MB, Fraser J, Hosack L, Caplan MR, McLemore R. Antimicrobial distribution from local delivery depends on dose: a pilot study with MRI. Clin Orthop Relat Res. 2014 Feb 8 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 41.Miller R, McLaren A, Leon C, McLemore R. Mixing method affects elution and strength of high-dose ALBC: a pilot study. Clin Orthop Relat Res. 2012;470:2677–2683. doi: 10.1007/s11999-012-2351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller RB, McLaren AC, Leon CM, Vernon BL, McLemore R. Surfactant-stabilized emulsion increases gentamicin elution from bone cement. Clin Orthop Relat Res. 2011;469:2995–3001. doi: 10.1007/s11999-011-1934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mingeot-Leclercq M-P, Tulkens PM. Aminoglycosides: nephrotoxicity. Antimicrob Agents Chemother. 1999;43:1003–1012. doi: 10.1128/aac.43.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moran JM, Greenwald AS, Matejczyk M-B. Effect of gentamicin on shear and interface strengths of bone cement. Clin Orthop Relat Res. 1979;141:96–101. [PubMed] [Google Scholar]
- 45.Moskowitz SM, Foster JM, Emerson J, Burns JL. Clinically feasible biofilm susceptibility assay for isolates of Pseudomonas aeruginosa from patients with cystic fibrosis. J Clin Microbiol. 2004;42:1915–1922. doi: 10.1128/JCM.42.5.1915-1922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson CL, Hickmon SG, Skinner RA. Treatment of experimental osteomyelitis by surgical débridement and the implantation of bioerodable, polyanhydride-gentamicin beads. J Orthop Res. 1997;15:249–255. doi: 10.1002/jor.1100150214. [DOI] [PubMed] [Google Scholar]
- 47.Nie L, Nicolau DP, Tessier PR, Kourea HP, Browner BD, Nightingale CH. Use of a bioabsorbable polymer for the delivery of ofloxacin during experimental osteomyelitis treatment. J Orthop Res. 1998;16:76–79. doi: 10.1002/jor.1100160113. [DOI] [PubMed] [Google Scholar]
- 48.Nishimura S, Tsurumoto T, Yonekura A, Adachi K, Shindo H. Antimicrobial susceptibility of Staphylococcus aureus and Staphylococcus epidermidis biofilms isolated from infected total hip arthroplasty cases. J Orthop Sci. 2006;11:46–50. doi: 10.1007/s00776-005-0968-7. [DOI] [PubMed] [Google Scholar]
- 49.Olson ME, Ceri H, Morck DW, Buret AG, Read RR. Biofilm bacteria: formation and comparative susceptibility to antibiotics. Can J Vet Res. 2002;66:86–92. [PMC free article] [PubMed] [Google Scholar]
- 50.Overstreet DJ, Huynh R, Jarbo K, McLemore RY, Vernon BL. In situ forming, resorbable graft copolymer hydrogels providing controlled drug release. J Biomed Mater Res A. 2013;101:1437–1446. doi: 10.1002/jbm.a.34443. [DOI] [PubMed] [Google Scholar]
- 51.Overstreet DJ, McLemore RY, Doan BD, Farag A, Vernon BL. Temperature-responsive graft copolymer hydrogels for controlled swelling and drug delivery. Soft Mater. 2013;11:294–304. doi: 10.1080/1539445X.2011.640731. [DOI] [Google Scholar]
- 52.Penner MJ, Duncan CP, Masri BA. The in vitro elution characteristics of antibiotic-loaded CMW and Palacos-R bone cements. J Arthroplasty. 1999;14:209–214. doi: 10.1016/S0883-5403(99)90128-6. [DOI] [PubMed] [Google Scholar]
- 53.Ramage G, Tunney MM, Patrick S, Gorman SP, Nixon JR. Formation of Propionibacterium acnes biofilms on orthopaedic biomaterials and their susceptibility to antimicrobials. Biomaterials. 2003;24:3221–3227. doi: 10.1016/S0142-9612(03)00173-X. [DOI] [PubMed] [Google Scholar]
- 54.Saginur R, Stdenis M, Ferris W, Aaron SD, Chan F, Lee C, Ramotar K. Multiple combination bactericidal testing of staphylococcal biofilms from implant-associated infections. Antimicrob Agents Chemother. 2006;50:55–61. doi: 10.1128/AAC.50.1.55-61.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schierholz JM, Beuth J, König D, Nürnberger A, Pulverer G. Antimicrobial substances and effects on sessile bacteria. Zentralbl Bakteriol. 1999;289:165–177. doi: 10.1016/S0934-8840(99)80101-7. [DOI] [PubMed] [Google Scholar]
- 56.Simpson AH, Deakin M, Latham JM. Chronic osteomyelitis. The effect of the extent of surgical resection on infection-free survival. J Bone Joint Surg Br. 2001;83:403–407. doi: 10.1302/0301-620X.83B3.10727. [DOI] [PubMed] [Google Scholar]
- 57.Skinner R, Hickmon S, Nelson C, Evans R. Correlation of histology with culture to more accurately determine experimental osteomyelitis. Orthop Trans. 1994:18598–99.
- 58.Smeltzer MS, Thomas JR, Hickmon SG, Skinner RA, Nelson CL, Griffith D, Parr TR, Jr, Evans RP. Characterization of a rabbit model of staphylococcal osteomyelitis. J Orthop Res. 1997;15:414–421. doi: 10.1002/jor.1100150314. [DOI] [PubMed] [Google Scholar]
- 59.Sorensen TS, Sorensen AI, Merser S. Rapid release of gentamicin from collagen sponge. Acta Orthop Scand. 1990;61:353–356. doi: 10.3109/17453679008993535. [DOI] [PubMed] [Google Scholar]
- 60.Swieringa AJ, Goosen JHM, Jansman FGA, Tulp NJA. In vivo pharmacokinetics of a gentamicin-loaded collagen sponge in acute periprosthetic infection: serum values in 19 patients. Acta Orthop. 2008;79:637–642. doi: 10.1080/17453670810016650. [DOI] [PubMed] [Google Scholar]
- 61.Tang W, Zhao J, Sha B, Liu H. Adsorption and drug release based on β-cyclodextrin-grafted hydroxyapatite composite. J Appl Polym Sci. 2013;127:2803–2808. doi: 10.1002/app.37607. [DOI] [Google Scholar]
- 62.VV Van de Belt H, Neut D, Schenk W, van Horn JR, van der Mei HC, Busscher HJ. Staphylococcus aureus biofilm formation on different gentamicin-loaded polymethylmethacrylate bone cements. Biomaterials. 2001;22:1607–1611. [DOI] [PubMed]
- 63.Walters MC, 3rd, Roe F, Bugnicourt A, Franklin MJ, Stewart PS. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother. 2003;47:317–323. doi: 10.1128/AAC.47.1.317-323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walton K, Dorne JLCM, Renwick AG. Species-specific uncertainty factors for compounds eliminated principally by renal excretion in humans. Food Chem Toxicol. 2004;42:261–274. doi: 10.1016/j.fct.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 65.Zaske DE, Cipolle RJ, Rotschafer JC, Solem LD, Mosier NR, Strate RG. Gentamicin pharmacokinetics in 1,640 patients: method for control of serum concentrations. Antimicrob Agents Chemother. 1982;21:407–411. doi: 10.1128/AAC.21.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]