Abstract
Background
Heart failure hospitalizations (HFHs) cost the US health care system ~$20 billion annually. Identifying patients at risk of HFH to enable timely intervention and prevent expensive hospitalization remains a challenge. Implantable cardioverter defibrillators (ICDs) and cardiac resynchronization devices with defibrillation capability (CRT-Ds) collect a host of diagnostic parameters that change with HF status and collectively have the potential to signal an increasing risk of HFH. These device-collected diagnostic parameters include activity, day and night heart rate, atrial tachycardia/atrial fibrillation (AT/AF) burden, mean rate during AT/AF, percent CRT pacing, number of shocks, and intrathoracic impedance. There are thresholds for these parameters that when crossed trigger a notification, referred to as device observation, which gets noted on the device report. We investigated if these existing device observations can stratify patients at varying risk of HFH.
Methods
We analyzed data from 775 patients (age: 69 ± 11 year, 68% male) with CRT-D devices followed for 13 ± 5 months with adjudicated HFHs. HFH rate was computed for increasing number of device observations. Data were analyzed by both excluding and including intrathoracic impedance. HFH risk was assessed at the time of a device interrogation session, and all the data between previous and current follow-up sessions were used to determine the HFH risk for the next 30 days.
Results
2276 follow-up sessions in 775 patients were evaluated with 42 HFHs in 37 patients. Percentage of evaluations that were followed by an HFH within the next 30 days increased with increasing number of device observations. Patients with 3 or more device observations were at 42× HFH risk compared to patients with no device observation. Even after excluding intrathoracic impedance, the remaining device parameters effectively stratified patients at HFH risk.
Conclusion
Available device observations could provide an effective method to stratify patients at varying risk of heart failure hospitalization.
Keywords: Implantable device diagnostics, Heart failure, Ambulatory monitoring, Heart failure hospitalization, Intrathoracic impedance
Introduction
Implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy (CRT) devices have become the mainstay of treating persistent systolic heart failure in addition to guideline directed medical therapy.1,2 Many of these devices are implanted in patients with congestive heart failure with New York Heart Association (NYHA) class status of II to IV. While these devices considerably ameliorate patient morbidity and mortality, heart failure remains a significant economic burden costing the US health care system ~$30 billion annually. Heart failure hospitalizations (HFHs) account for two-thirds of the total expense.3 Identifying patients at risk of worsening heart failure to allow timely intervention has the potential to prevent hospitalizations and improve long-term patient outcomes while reducing costs of care.
In addition to providing life-saving therapies, implantable devices collect a host of continuous physiological patient data (e.g. activity, day and night heart rate, AT/AF burden, heart rate during AT/AF, percent CRT pacing, number of shocks, and intrathoracic impedance). However, the data collected vary by manufacturer. For example, not all manufacturers have devices with intrathoracic impedance capability. Also, while all devices include a single or multi-axis accelerometer, proprietary algorithms to derive daily activity from accelerometer signals vary (see Methods for details). Many of the diagnostic variables have been shown to be prognostic markers of worsening heart failure and/or mortality risk. For example, NHR is a marker of autonomic tone, and an elevated NHR is associated with higher HFH risk.4 Activity is a reflection of patient functional capacity, and decreasing activity is associated with worsening HF status.4–7 A loss of CRT pacing compromises cardiac hemodynamics and hence leads to worsening patient status.8 And finally, a decrease in intrathoracic impedance is associated with an increase in wedge pressure, elevated pre-load, and risk of fluid extravasation into the lungs.9 Risk stratification models combining various device diagnostic parameters have been proposed.10–12 The diagnostic performance of these models is better than each parameter alone. However, the clinical adoption of these risk stratification models has been slow because they use thresholds and measurement schemes not yet implemented in the implantable devices. Some of these approaches in fact use a fixed 30-day look back window requiring manual sifting of data to identify trends, thus making it cumbersome to use them in day-to-day practice.
All CRT-D devices have thresholds for various parameters that when crossed trigger a notification, referred to as device observation. The purpose of this study was to examine the performance of the existing device observations for stratifying patients at HFH risk. Since the impedance observation is not available in all devices [e.g. OptiVol observation is not available on CareLink (Medtronic Inc. MN) in the US], we performed the analysis with and without impedance observation. Furthermore, we investigated the relationship between the number of device observations triggered and risk of HFH.
Methods
We performed retrospective analysis using patient data from FAST13 and PARTNERS-HF10 clinical trials using Medtronic devices. Both study protocols were approved by institutional review boards and all patients provided written informed consent. FAST was a prospective double-blinded observational study in CRT-D and ICD patients (n = 109) with EF ≤ 35% and NYHA class III or IV. PARTNERS-HF was a prospective observational study in CRT-D patients (n = 1024) with EF ≤ 35%, NYHA class III or IV, and QRS duration 130 ms. The two studies combined had 1133 patients and 220 HFHs. Only patients with an OptiVol capable CRT-D device were included in this analysis. Follow-up sessions with less than 7 days of data before and less than 30 days of data after the evaluation were excluded. Furthermore, if there was another follow-up session within 30 days of a previous session, the second session was excluded. After applying above criteria, 186 HFHs in 775 patients and a total of 2276 follow-up sessions were available for analysis. Mean follow-up was 13 ± 5 months. HFH associated with signs and symptoms of pulmonary congestion was used as the endpoint. All HFHs were adjudicated by an independent committee. The HFH event rate of 22.2% per year in this cohort is comparable to that in NYHA III and IV device patients.14,15
Diagnostic parameters and thresholds
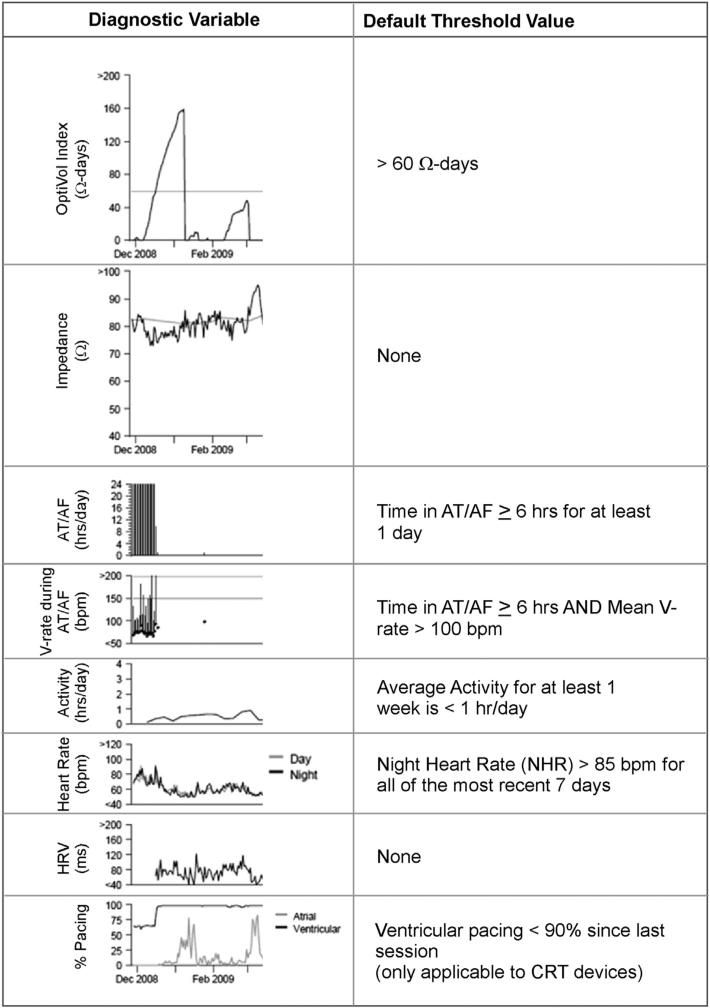
The following diagnostic parameters in Medtronic devices have an observation: OptiVol index, AT/AF burden, ventricular rate during AT/AF (VRAF), activity, night heart rate (NHR), and percent pacing (% CRT pacing) (Fig. 1). In addition, an observation is noted if defibrillation shocks are delivered and this was also included in our analysis. Briefly, the measurement scheme for various parameters was as follows. Impedance (Z) is measured intrathoracically across the right ventricular (RV) coil and device-can by injecting a small current pulse (I) and measuring the developed voltage (V; Z = I/V). OptiVol index is derived as the cumulative difference between expected and actual Zs for the duration when expected Z is higher than actual Z. When actual Z exceeds expected Z, OptiVol index is set to zero. Since OptiVol index is integration of Z over certain duration, it is measured in units of ohm-days (Ω-days). A higher value of OptiVol index has been shown to be associated with HFH.9 Several electrophysiological parameters including NHR, AF burden, and VRAF are derived from atrial and ventricular electrograms (egms) acquired by the device at 10 ms resolution. Device algorithms, such as PR Logic,16 are applied to discriminate among different rhythms and derive these electrophysiological parameters. NHR is the average heart rate between midnight and 4 am and is a measure of resting heart rate. AF burden is measured as total duration of fast atrial rate during a 24-h period associated with atrio-ventricular conduction ratio ≤2:1. VRAF is the average ventricular rate during AF over a 24-h duration. Activity is a quantitative measure of active duration and is a surrogate of functional capacity. It is measured by a single axis accelerometer in the device that is used to detect patient motion and convert it into discrete electrical signals. An algorithm then converts these electrical signals to number of minutes active for the entire 24-h duration during a day (day and night time activities are not reported separately), where a minute is considered active if accelerometer registers signal equivalent to 70 steps/min or greater.4 The device recorded activity has been shown to have a strong intra-individual correlation with activity measured using a validated external sensor.5 However, details of the algorithm differ between implantable and external devices (e.g. pedometers and external accelerometers) and absolute duration of reported activity between the two may differ.
Fig. 1.
Various device diagnostic parameters and corresponding default threshold values that trigger a device observation. The left column shows the various device parameters and their representative trend. The right column shows the corresponding default threshold values (see Methods for more details). Except for raw impedance and HRV, all other parameters have an observation that is triggered when the corresponding threshold is crossed. However, the availability of OptiVol observation varies with geography (refer to text for details).
All of the above parameters have a threshold value, which when exceeded triggers a device observation. Fig. 1 shows empirically derived nominal threshold values for various parameters that can be tailored on a patient basis. All nominals (or values close to the nominals) have been shown to be associated with greater HFH or mortality risk. An OptiVol observation is noted on the device report when a value of 60 Ω-days is exceeded, a threshold shown to predict HFH with optimal sensitivity and false alert rate.9 AT/AF burden of ≤6 h/day for at least one day within the last 30-days is associated with 2× risk of an HF event.17 This risk is further exacerbated with poor rate control with V-rate > 90 bpm.17 NHR of >90 bpm discriminates between hospitalized and non-hospitalized patients.18 And finally, CRT pacing <90% is associated with increased mortality.19
The availability of OptiVol observations varies by geography and mode of device interrogation. For example, while there is an OptiVol observation on the programmer report in the United States, no such observation exists on the CareLink HF management report. Outside the United States, the OptiVol observation is available in both the programmer and CareLink reports. Thus, we performed our analysis by both including and excluding the OptiVol observation.
Analysis scheme
We used the analysis scheme depicted in Fig. 2. At the time-point of each follow-up a look back was performed until the previous follow-up to evaluate the number of device observations triggered. Also, a 30-day look forward was performed to assess if an HFH had occurred within that period. For example, for the follow-up labeled as FU2 in Fig. 2, number of observations triggered was evaluated for the duration labeled as ‘Risk Assessment 2’, and a 30-day HFH risk assessment was performed for the duration labeled as ‘Risk Prediction 2’. This was repeated for all the follow-ups for a given patient, and then for all the patients. These data from all ‘Risk Assessment’ and ‘Risk Prediction’ pairs were then used to compute the relationship between number of observations triggered and 30-day HFH event rate. Specifically, raw event rate for 0, 1, 2, and ≤3 observations was computed as:
Fig. 2.
The schematic for diagnostic evaluation and risk assessment framework. The device observations occurring during the entire duration between two successive follow-ups (FUs) sessions were noted. Various follow-ups are indicated as FU1, FU2 etc. The look-back time window for evaluating device observations is labeled as ‘Risk Assessment’. The corresponding 30-day look-forward time window is labeled as ‘Risk Prediction’. Refer to text under the section Analysis Scheme for more details.
Statistical analysis
The HFH event rates and odds ratios were estimated using a Generalized Estimating Equations (GEE) model for the groups with different number of observations. A GEE model adjusts the estimate to account for multiple evaluations within a patient.20 We made no adjustment for baseline variables (age, gender, NYHA, history of coronary artery disease, MI, AF, diabetes, and hypertension) and baseline medications (ACE-I/ARB, diuretics, b-blockers, and anti-arrhythmic drugs). This reflects the real world since device data are not yet fully integrated with clinical and demographic data.
A sensitivity and specificity analysis was performed to characterize our scheme’s performance. Sensitivity is defined as the number of evaluations with a given number of device observations in the preceding evaluation window and HFH event in next 30-days divided by the total number of evaluations with HFH in next 30-days. Specificity is defined as the number of evaluations without a given number of device observations in the preceding evaluation period and no HFH event in next 30 days divided by the total number of evaluations with no HFH in next 30 days. The sensitivity and specificity computations are adjusted for multiple evaluations in patients using a GEE model. All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Patient demographics and follow-up
Table 1 summarizes clinical and demographic data for the 775 patients in the FAST and PARTNERS-HF trials selected for our analysis. All patients had a CRT-D device, and the majority (87%) of the patients had a heart failure status of NYHA III. These characteristics are similar to the characteristics of a patient population receiving CRT therapy.14,15,21 A total of 2276 in-clinic follow-up sessions were available for analysis. Forty-two follow-up sessions in 37 unique patients had an associated HFH in the following 30 days. Mean inter follow-up duration was 78 days.
Table 1.
Patient clinical and demographic data of combined FAST and PARTNERS-HF trials used for the present analysis.
| Total (n = 775) | |
|---|---|
| Mean age (SD) | 69 (11) |
| Male gender | 524 (68%) |
| Ethnic origin | |
| Caucasian | 655 (85%) |
| African American | 85 (11%) |
| Other/Unknown | 35 (5%) |
| NYHA | |
| I | 9 (1%) |
| II | 59 (8%) |
| III | 674 (87%) |
| IV | 33 (4%) |
| Ischemic | 485 (63%) |
| Coronary artery disease | 524 (68%) |
| Myocardial infarction | 360 (46%) |
| Hypertension | 552 (71%) |
| Diabetes | 324 (42%) |
| History of AF | 219 (28%) |
| LVEF ≤35%a | 676 (100%) |
| Baseline medications | |
| ACE/ARB | 641 (83%) |
| Beta-blockers | 696 (90%) |
| Diuretics | 642 (83%) |
| Digoxin | 279 (36%) |
| Aldosterone antagonist | 257 (33%) |
| Hydralazine | 44 (6%) |
| Nitrates | 215 (28%) |
| Anti-arrhythmic drugs | 138 (18%) |
| Warfarin | 183 (24%) |
LVEF was only available for 676 patients.
Risk stratification performance of device parameters excluding OptiVol
Risk stratification performance of various parameters excluding OptiVol is shown in Table 2. The rate of HFH increased with increasing number of observations. For zero device observation, the 30-day event rate was 0.9% and increased to 13.6% for three or more device observations. The odds ratio for three or more observations versus no observation was 17.9 (see Table 2 for other odds ratios). Also, noteworthy from Table 2 is that a vast majority (~71%) of the total follow-up sessions had no device observation. The proportion of follow-up sessions decreased with increasing number of observations (23.5%, 4.3% and 1.3% for 1, 2 and ≤3 observations, respectively).
Table 2.
Performance of device observations excluding OptiVol in stratifying patients at risk of heart failure hospitalization (HFH).
| Number of device observation(s) | Number of follow-ups (Number of patients) | Number of HFHs (%) | GEE adjusted HFHs (95% CI) | Odds ratio versus 0 observation (95% CI) |
|---|---|---|---|---|
| 0 | 1614 (631) | 14 (0.9) | 0.9% (0.5-1.6) | Reference group |
| 1 | 535 (284) | 17 (3.2) | 3.0% (1.8-5.0) | 3.6 (1.6-7.8) |
| 2 | 98 (71) | 7 (7.1) | 7.0% (3.4-13.8) | 8.5 (3.3-22.3) |
| ≥3 | 29 (24) | 4 (13.8) | 13.6% (5.5-30.0) | 17.9 (5.6-57.2) |
Based on univariate analysis (Table 3), HFH rate varied from 3.5% (for OptiVol) to 11.2% (for VRAF). Activity, AF Burden, and Decrease in CRT Pacing triggered observations during a large proportion of follow-up sessions (~10% or more) and the corresponding event rates were 5.1%, 4.7% and 4.6%, respectively.
Table 3.
Univariate analysis and risk of various device parameters for HFH.
| Device observation | Number of follow-ups | Number of HFHs (%) | GEE (95% CI) |
|---|---|---|---|
| Activity | |||
| Yes | 277 | 14 (5.1%) | 5.1% (3.0-8.4) |
| No | 1999 | 28 (1.4%) | 1.4% (0.9-2.1) |
| NHR | |||
| Yes | 28 | 2 (7.1%) | 7.2% (2.2-21.7) |
| No | 2248 | 40 (1.8%) | 1.8% (1.3-2.5) |
| AF burden | |||
| Yes | 235 | 11 (4.7%) | 4.7% (2.5-8.5) |
| No | 2041 | 31 (1.5%) | 1.5% (1.0-2.2) |
| VRAF | |||
| Yes | 26 | 3 (11.5%) | 11.2% (3.8-28.5) |
| No | 2250 | 39 (1.7%) | 1.7% (1.2-2.4) |
| Decrease in CRT pacing | |||
| Yes | 228 | 11 (4.8%) | 4.6% (2.4-8.4) |
| No | 2048 | 31 (1.5%) | 1.5% (1.0-2.2) |
| Shock | |||
| Yes | 26 | 2 (7.7%) | 7.1% (1.5-27.3) |
| No | 2250 | 40 (1.8%) | 1.8% (1.3-2.5) |
| OptiVol | |||
| Yes | 783 | 28 (3.6%) | 3.5% (2.4-5.2) |
| No | 1493 | 14 (0.9%) | 0.9% (0.6-1.6) |
Risk stratification performance of device parameters including OptiVol
Risk stratification performance with OptiVol included is shown in Table 4. Similar to the case of OptiVol excluded, the HFH rate increased with increasing number of observations. The HFH rate for zero device observation was 0.4% and increased to 13.6% for ≤3 observations with an odds ratio of 42.4 (see Table 4 for other odds ratios). Follow-up sessions with zero observation constituted the largest proportion (48.5%) of all follow-up sessions, and the proportion declined with increasing number of observations (36.4%, 12.3% and 2.9% for 1, 2 and ≤3 observations, respectively).
Table 4.
Performance of device observations including OptiVol in stratifying patients at risk of heart failure hospitalization (HFH).
| Number of device observation(s) | Number of follow-ups (number of patients) | Number of HFHs (%) | GEE adjusted HFHs (95% CI) | Odds ratio versus 0 observation (95% CI) |
|---|---|---|---|---|
| 0 | 1103 (554) | 4 (0.4) | 0.4% (0.1-1.0) | Reference group |
| 1 | 828 (514) | 14 (1.7) | 1.7% (0.9-3.0) | 4.6 (1.4-14.5) |
| 2 | 279 (190) | 15 (5.4) | 5.3% (3.1 -8.8) | 14.9 (5.2-43.1) |
| ≥3 | 66 (50) | 9 (13.6) | 13.6% (7.2-24.3) | 42.4 (12.6-142.1) |
Event rate during 30 days post-evaluation
Fig. 3 shows HFH event rate during 30 days post-evaluation with OptiVol excluded (Panel A) and included (Panel B). The increase in event rate with increasing number of observations is evident in both plots. For example, with OptiVol excluded, the 30-day HFH rate was less than 1% for ‘0 observation’. The 30-day HFH event rate increased to ~3%, ~7% and ~14% for 1, 2 and ≤3 observations, respectively. With OptiVol included, a greater separation between the ‘ 3 observations’ trace and ‘0 observation’ and ‘1 observation’ traces was observed suggesting a better risk stratification performance.
Fig. 3.
Kaplan Meier curves for time to first HF hospitalization. Panel A shows HFH rate in the next 30-days following an evaluation for varying number of observations with OptiVol excluded from the device parameter set. Panel B shows an analogous plot with OptiVol included in the device parameter set.
Sensitivity and specificity of predicting HFH
Tables 5 and 6 show the sensitivity and specificity in predicting HFH for the parameter set excluding and including OptiVol for ≤1, ≤2 and ≤3 device observations. With OptiVol excluded, the sensitivity for ≤1 observation was 68.9% and decreased to 9.5% for ≤3 observations. The corresponding specificity for ≤1 observation was 71.2% and increased to 98.8% for ≤3 observations. Similarly, with OptiVol included, the sensitivity for ≤1 observation was 90.5% and decreased to 21.6% for 3 observations. The corresponding specificity increased from 49.1% (≤1 observation) to 97.4% (≤3 observations). With OptiVol included, the relative increase in sensitivity for ≤3 observations was significant (21.6% versus 9.5%; see bottom most rows in Tables 5 and 6) compared to the decrease in specificity (97.4% versus 98.8%).
Table 5.
Sensitivity versus specificity in a 30-day evaluation framework for ≥ 1, ≥2 and ≥3 observations for the parameter set excluding OptiVol.
| Number of device observation(s) | Sensitivity |
Specificity |
||
|---|---|---|---|---|
| Unadjusted | GEE adjusted (95% CI) | Unadjusted | GEE adjusted (95% CI) | |
| ≥ 1 observation(s) | 28/42 (66.7%) | 68.9% (52.8-81.5) | 1600/2234 (71.6%) | 71.2% (68.4-73.9) |
| ≥ 2 observations | 11/42 (26.2%) | 27.0% (15.2-43.3) | 2118/2234 (94.8%) | 94.5% (93.1-95.7) |
| ≥ 3 observations | 4/42 (9.5%) | 9.5% (3.7-22.5) | 2209/2234 (98.9%) | 98.8% (98.2-99.3) |
Table 6.
Sensitivity versus specificity in a 30-day evaluation framework for ≥1, ≥2 and ≥3 device observations for the parameter set including OptiVol.
| Number of device observations | Sensitivity |
Specificity |
||
|---|---|---|---|---|
| Unadjusted | GEE adjusted (95% CI) | Unadjusted | GEE adjusted (95% CI) | |
| ≥1 observation(s) | 38/42 (90.5%) | 90.5% (77.5-96.3) | 1099/2234 (49.2%) | 49.1% (46.5-51.7) |
| ≥2 observations | 24/42 (57.1%) | 58.0% (42.0-72.4) | 1913/2234 (85.6%) | 85.5% (83.5-87.4) |
| ≥3 observations | 9/42 (21.4%) | 21.6% (11.2-37.6) | 2177/2234 (97.4%) | 97.4% (96.5-98.1) |
Discussion
In this study we presented a novel scheme to stratify patients at risk of HFH using diagnostic parameters available in Medtronic's CRT-D devices. The thresholds and corresponding device observations were unmodified from what is available in the device. In addition, the look back period for assessing diagnostic parameters was the entire duration between the follow-up sessions to mirror the real world clinical practice. We found that this relatively simple and easy to implement scheme can stratify patients quite effectively. The risk of an HFH event increases with increasing number of device observations, and a patient with three or more observations is at 18× (OptiVol excluded) to 42× (OptiVol included) risk compared to a patient with zero observation (Tables 2 and 4). The sensitivity and specificity exhibit a typical trade-off. As the specificity improves with increasing numbers of observations the sensitivity worsens. For example, sensitivity for ≤3 device observations is lower than that for ≤2 observations while the specificity for ≤3 device observations is better. Inclusion of OptiVol improves overall performance. With OptiVol excluded for ≤3 device observations, sensitivity and specificity are 9.5% and 98.8%, respectively. With OptiVol included sensitivity improves significantly to 21.6% while the specificity drops slightly to 97.4%.
Several non-device data based HF risk stratification strategies have recently been developed.22–25 In contrast to our dynamic risk assessment scheme, these models are static and use a one-time snapshot of laboratory measurements. Furthermore, since the device data are continuously collected, our scheme is amenable to be applied in an ambulatory setting. For example, the device data can be transmitted automatically to a clinic upon an alert (referred to as CareAlert in Medtronic's CareLink system) or on a predetermined schedule.
It is instructive to compare the performance of device diagnostics with patient weight in predicting HFH. Patient weight increases steadily for ~2 weeks before HFH26 and is routinely used in clinical practice. However, in a head-to-head comparison, patient weight performed significantly worse than OptiVol. While OptiVol had a sensitivity of 76% and an unexplained detection rate of 1.9 per patient-year, patient weight had a sensitivity of mere 20% and an unexplained detection rate of 4.3 per patient-year.13 Given that our scheme combines several device parameters, its performance is better than OptiVol alone and hence superior to that of weights [e.g. for ≤1 observation, sensitivity with OptiVol included is 90.5% (Table 6) and an unexplained detection rate at a specificity of 49.1% is 1.5 per patient-year].
Various device diagnostic parameters reflect different underlying physiological processes, and a deviation beyond a certain range may signal a compromise in physiological homeostasis and hence be a marker of patient risk. For example, impedance is an indicator of fluid status.9,27 A drop in impedance and accompanying rise in OptiVol is indicative of possible fluid overload, while an excessive rise in impedance and drop in OptiVol might signal dehydration. Similarly, elevated NHR is a potential marker of imbalance in autonomic tone, and lower activity can signal compromised functional capacity. While each diagnostic parameter is a risk marker, univariate analysis shows that performance of a single parameter is modest (Table 3). For example, 30-day HFH rate following an OptiVol observation is 3.5%, and HFHs occur even in the absence of an OptiVol observation at a rate of 0.9% (Table 3). Corresponding numbers for VRAF, the one with highest HFH rate, are 11.2% and 1.5%. Prognostic value of device diagnostics is significantly improved when observations from all the parameters are combined.
The utility of combining device diagnostic variables for HF risk stratification has been shown earlier. Whellan et al10 combined device diagnostics for the previous 30 days using a heuristic approach to assess next 30-day HF risk. When two or more parameters exceeded preset thresholds or OptiVol alone exceeded a very high threshold, the risk was found to be 5.5× higher compared to diagnostic criterion not met. Our scheme differs in a few important aspects. First, while they segmented patients into two risk categories (i.e. high and low), we use a graded approach with 4 risk categories (i.e. 0, 1, 2, ≤3 observations) in which risk gradually increases with increasing number of device observations. Second, while we also use a clinically relevant 30-day risk prediction window, our look back period spans the entire duration between current and previous follow-up. Finally, while they modified threshold values for a few parameters and used parameters without an existing observation (e.g. HRV), we only selected parameters with available device observations. These last two differences make our scheme readily implementable (e.g. on CareLink) since any alterations in threshold values and ways to combine them makes the implementation cumbersome for health care providers.
Recently a more sophisticated methodology using a probabilistic Bayesian Belief Network approach has been presented to categorize patients into low, medium, and high risk statuses.12 While this approach is elegant and more rigorous, it cannot be readily applied by health care providers using existing device diagnostics.
To improve outcomes using integrated device data has challenges similar to those faced by other management strategies involving a single device parameter (e.g. OptiVol9,13,18,28) or other diagnostic modalities (e.g. intra-cardiac pressure29,30). Foremost among these the diagnostic information provided needs to be actionable and must be acted upon. Provided device diagnostic data are combined with an appropriate intervention algorithm that is adhered to, it has the potential to improve patient outcomes as shown for intrathoracic pressure.29 However, given telemonitoring trials have had mixed results,31–34 no assertions can be made regarding effectiveness of any novel risk assessment scheme in absence of a prospective study.
Similar to other risk assessment models,24,35,36 our scheme does not have perfect performance. Thus, as is the case for other approaches to manage patients (e.g. weight, blood pressure, temperature, ECG, etc.), it is imperative that a patient's overall health status be taken into account to devise a management strategy.
While an odds ratio for our risk stratification scheme is superior to several clinically used risk stratification tools (Tables 2 and 4), it is apparent that the absolute 30-day HF hospitalization rate is relatively low (e.g. ~14% for ≤3 observations, Tables 2 and 4). These low numbers are reflective of HFH being a relatively rare event with a rate of ~1–2% over a 30-day period. Thus, greater number of device observations alone should not trigger an action as it can potentially lead to an increase in health care utilization and hospitalization rate.37 Rather they should be used in conjunction with other clinical data to identify a subset of high risk patients and judiciously allocate resources. The actions may include diet and medication counseling for non-adherence, more frequent tele-monitoring, or medication change.
Since our proposed scheme uses unmodified device observations, they are already available to clinicians for use. However, whether device diagnostics are integrated into a clinic's workflow is influenced by several factors such as expertise of staff, proven or perceived value of device diagnostics, resources needed, and ease of use. Varying approaches can be used to integrate device diagnostics into a clinic's workflow, and are influenced by factors such as volume of patients, skill level of staff, and availability of resources.38,39 In all cases, ease of use could potentially lower the hurdle for adoption. Presently, HF related observations can only be viewed at a patient level (e.g. clinician must open and view HF management report for each patient in CareLink to assess various observations) or they are mingled with other device and electrophysiological observations (e.g. lead failure, low battery voltage). This makes using HF related observations a bit cumbersome. A clinic level view dedicated to HF related observations and the ability to sort patients by the number of these observations could facilitate the triaging process. Streamlining the CareLink system and then demonstrating that clinic efficiencies, time to clinical action, and perhaps patient outcomes are improved by using a device observations based tri-aging scheme remain topics of future research. Although studies demonstrating improvement in time to clinical action with use of device diagnostics are available,40,41 none are available demonstrating improvement in outcomes.
Limitations
This analysis is based on data from only two studies. All patients included in our analysis had a CRT-D device. The device diagnostic parameters and their significance for an ICD and CRT-D are slightly different. For example, % CRT pacing is irrelevant for an ICD. Instead, a lower RV pacing is desirable. Furthermore, since characteristics of ICD and CRT-D patients differ (class II and III for the former versus class III and IV for the latter), our findings may not translate to an ICD patient population. Secondly, our results apply to only Medtronic devices. Since type of diagnostic parameters, collection method (e.g. time and frequency of sampling), and thresholds for observations can vary among manufacturers, our results are not generalizable to non-Medtronic devices. Finally, to reflect the current practice of using device data in a standalone fashion, we only used device data and did not include demographic, medication or clinical data (e.g. weight, blood pressure and brain natriuretic peptide) that clinicians have access to. Additionally, a clinician may have intimate knowledge of a patient's psychosocial status. We did not address incremental value of device parameters to the clinical and psychosocial variables.
Conclusions
We developed a novel and simplified scheme for stratifying patients at risk of HF hospitalization using existing diagnostic observations available in a CRT-D device. The scheme can be readily implemented on a remote management system such as CareLink (Medtronic Inc., MN) and could potentially be another tool in a clinician's repertoire to help quickly identify patients at risk of HF events. However, whether an appropriately devised intervention strategy when coupled with our stratification scheme improves patient outcomes will require prospective evaluation.
Acknowledgments
The authors would like to thank all investigators involved in the clinical trials included in this data analysis.
Funding source: This study was supported by Medtronic Inc. (MN).
Abbreviations
- AF
atrial fibrillation
- AT
atrial tachycardia
- CRT
cardiac resynchronization therapy
- EF
ejection fraction
- GEE
generalized estimating equation
- HF
heart failure
- HFH
heart failure hospitalization
- HRV
heart rate variability
- ICD
implantable cardioverter defibrillator
- NHR
night heart rate
- NYHA
New York Heart Association
- VRAF
ventricular rate during AF
Footnotes
Conflict of interest: Vinod Sharma, Jodi Koehler, and Eduardo Warman: Salary and stock awards from Medtronic Inc. Lisa D. Rathman, Roy S. Small, David J. Whellan, and William T Abraham: Consultation fees from Medtronic Inc.
References
- 1.Hohnloser SH, Israel CW. Current evidence base for use of the implantable cardioverter-defibrillator. Circulation. 2013;128:172–183. doi: 10.1161/CIRCULATIONAHA.112.000547. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg I, Moss AJ. Implantable device therapy. Prog Cardiovasc Dis. 2008;50:449–474. doi: 10.1016/j.pcad.2007.09.002. http://dx.doi.org/10.1016/j.pcad.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adamson PB, Smith AL, Abraham WT, et al. Continuous autonomic assessment in patients with symptomatic heart failure: prognostic value of heart rate variability measured by an implanted cardiac resynchronization device. Circulation. 2004;110:2389–2394. doi: 10.1161/01.CIR.0000139841.42454.78. [DOI] [PubMed] [Google Scholar]
- 5.Pressler A, Danner M, Esefeld K, et al. Validity of cardiac implantable electronic devices in assessing daily physical activity. Int J Cardiol. 2013;168:1127–1130. doi: 10.1016/j.ijcard.2012.11.050. [DOI] [PubMed] [Google Scholar]
- 6.Conraads VM, Spruit MA, Braunschweig F, et al. Physical activity measured with implanted devices predicts patient outcome in chronic heart failure. Circ Heart Fail. 2014;7:279–287. doi: 10.1161/CIRCHEARTFAILURE.113.000883. [DOI] [PubMed] [Google Scholar]
- 7.Walsh JT, Charlesworth A, Andrews R, Hawkins M, Cowley AJ. Relation of daily activity levels in patients with chronic heart failure to long-term prognosis. Am J Cardiol. 1997;79:1364–1369. doi: 10.1016/s0002-9149(97)00141-0. [DOI] [PubMed] [Google Scholar]
- 8.Koplan BA, Kaplan AJ, Weiner S, Jones PW, Seth M, Christman SA. Heart failure decompensation and all-cause mortality in relation to percent biventricular pacing in patients with heart failure: is a goal of 100% biventricular pacing necessary? J Am Coll Cardiol. 2009;53:355–360. doi: 10.1016/j.jacc.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 9.Yu CM, Wang L, Chau E, et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112:841–848. doi: 10.1161/CIRCULATIONAHA.104.492207. [DOI] [PubMed] [Google Scholar]
- 10.Whellan DJ, Ousdigian KT, Al-Khatib SM, et al. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients With Heart Failure) study. J Am Coll Cardiol. 2010;55:1803–1810. doi: 10.1016/j.jacc.2009.11.089. http://dx.doi.org/10.016/j.jacc.2009.11.089. [DOI] [PubMed] [Google Scholar]
- 11.Sarkar S, Koehler J. A dynamic risk score to identify increased risk for heart failure decompensation. IEEE Trans Biomed Eng. 2013;60:147–150. doi: 10.1109/TBME.2012.2209646. http://dx.doi.org/10.1109/TBME.2012.2209646. Epub 2012 Jul 20. [DOI] [PubMed] [Google Scholar]
- 12.Sharma V, Sarkar S, Koehler J. Integrated device diagnostics can identify patients at significantly elevated risk of heart failure hospitalization. Heart Rhythm. 2012;9(5) [May Supplement] [Google Scholar]
- 13.Abraham WT, Compton S, Haas G, et al. Intrathoracic impedance vs daily weight monitoring for predicting worsening heart failure events: results of the Fluid Accumulation Status Trial (FAST). Congest Heart Fail. 2011;17:51–55. doi: 10.1111/j.1751-7133.2011.00220.x. http://dx.doi.org/10.1111/j.751-7133.2011.00220.x. Epub 2011 Mar 21. [DOI] [PubMed] [Google Scholar]
- 14.Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. Epub 2005 Mar 7. [DOI] [PubMed] [Google Scholar]
- 15.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N. Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 16.Gold MR, Ahmad S, Browne K, Berg KC, Thackeray L, Berger RD. Prospective comparison of discrimination algorithms to prevent inappropriate ICD therapy: primary results of the Rhythm ID Going Head to Head Trial. Heart Rhythm. 2012;9:370–377. doi: 10.1016/j.hrthm.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Sarkar S, Koehler J, Crossley GH, et al. Burden of atrial fibrillation and poor rate control detected by continuous monitoring and the risk for heart failure hospitalization. Am Heart J. 2012;164:616–624. doi: 10.1016/j.ahj.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Perego GB, Landolina M, Vergara G, et al. Implantable CRT device diagnostics identify patients with increased risk for heart failure hospitalization. J Interv Card Electrophysiol. 2008;23:235–242. doi: 10.1007/s10840-008-9303-5. [DOI] [PubMed] [Google Scholar]
- 19.Ousdigian KT, Borek PP, Koehler JL, Heywood JT, Ziegler PD, Wilkoff BL. The epidemic of inadequate biventricular pacing in patients with persistent or permanent atrial fibrillation and its association with mortality. Circ Arrhythm Electrophysiol. 2014;7:370–376. doi: 10.1161/CIRCEP.113.001212. [DOI] [PubMed] [Google Scholar]
- 20.Liang K-Y, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 21.Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 22.Kannel WB, D'Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159:1197–1204. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 23.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 24.Levy WC, Mozaffarian D, Linker DT, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 25.Peterson PN, Rumsfeld JS, Liang L, et al. A validated risk score for in-hospital mortality in patients with heart failure from the American Heart Association get with the guidelines program. Circulation Cardiovasc Qual Outcomes. 2010;3:25–32. doi: 10.1161/CIRCOUTCOMES.109.854877. [DOI] [PubMed] [Google Scholar]
- 26.Chaudhry SI, Wang Y, Concato J, Gill TM, Krumholz HM. Patterns of weight change preceding hospitalization for heart failure. Circulation. 2007;116:1549–1554. doi: 10.1161/CIRCULATIONAHA.107.690768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Small RS. Integrating device-based monitoring into clinical practice: insights from a large heart failure clinic. Am J Cardiol. 2007;99:17Ge22G. doi: 10.1016/j.amjcard.2007.02.038. Epub 2007 Mar 9. [DOI] [PubMed] [Google Scholar]
- 28.Small RS, Wickemeyer W, Germany R, et al. Changes in intrathoracic impedance are associated with subsequent risk of hospitalizations for acute decompensated heart failure: clinical utility of implanted device monitoring without a patient alert. J Card Fail. 2009;15:475–481. doi: 10.1016/j.cardfail.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 30.Bourge RC, Abraham WT, Adamson PB, et al. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. J Am Coll Cardiol. 2008;51:1073–1079. doi: 10.1016/j.jacc.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 31.Cleland JG, Louis AA, Rigby AS, Janssens U, Balk AH, TEN-HMS Investigators Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: the Trans-European Network-Home-Care Management System (TEN-HMS) study. J Am Coll Cardiol. 2005;45:1654–1664. doi: 10.1016/j.jacc.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 32.Jaarsma T, van der Wal MH, Lesman-Leegte I, et al. Effect of moderate or intensive disease management program on outcome in patients with heart failure: Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure (COACH). Arch Intern Med. 2008;168:316–324. doi: 10.1001/archinternmed.2007.83. [DOI] [PubMed] [Google Scholar]
- 33.Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363:2301–2309. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koehler F, Winkler S, Schieber M, et al. Telemedicine in heart failure: pre-specified and exploratory subgroup analyses from the TIM-HF trial. Int J Cardiol. 2012;161:143–150. doi: 10.1016/j.ijcard.2011.09.007. http://dx.doi.org/10.1016/j.ijcard.2011.09.007. Epub Oct 8. [DOI] [PubMed] [Google Scholar]
- 35.Crandall MA, Horne BD, Day JD, et al. Atrial fibrillation significantly increases total mortality and stroke risk beyond that conveyed by the CHADS2 risk factors. Pacing Clin Electrophysiol. 2009;32:981–986. doi: 10.1111/j.1540-8159.2009.02427.x. [DOI] [PubMed] [Google Scholar]
- 36.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 37.van Veldhuisen DJ, Braunschweig F, Conraads V, et al. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation. 2011;124:1719–1726. doi: 10.1161/CIRCULATIONAHA.111.043042. [DOI] [PubMed] [Google Scholar]
- 38.Samara MA, Tang WH. Device monitoring strategies in acute heart failure syndromes. Heart Fail Rev. 2011;16:491–502. doi: 10.1007/s10741-011-9236-4. [DOI] [PubMed] [Google Scholar]
- 39.Dierckx R, Houben R, Goethals M, et al. Integration of remote monitoring of device diagnostic parameters into a multidisciplinary heart failure management program. Int J Cardiol. 2014;172:606–607. doi: 10.1016/j.ijcard.2014.01.088. [DOI] [PubMed] [Google Scholar]
- 40.Crossley GH, Boyle A, Vitense H, Chang Y, Mead RH. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol. 2011;57:1181–1189. doi: 10.1016/j.jacc.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Boriani G, Da Costa A, Ricci RP, et al. The MOnitoring Resynchronization dEvices and CARdiac patiEnts (MORE-CARE) randomized controlled trial: phase 1 results on dynamics of early intervention with remote monitoring. J Med Internet Res. 2013;15:e167. doi: 10.2196/jmir.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]