Abstract
Computational models of complex systems, such as signaling networks and biological systems, can be used to explain the behavior of such systems under various conditions. The large number of integrated processes and variables, and the nonlinearities inherent in the fundamental processes, make it difficult for scientists unassisted by computer simulations to effectively predict the consequences of a particular intervention. For this reason, computer simulation has become an important tool for generating hypotheses about the behavior of these systems that can then be tested in the laboratory and clinic. A dynamic data-driven application simulation (DDDAS) was designed by Biospherics to model complex metabolic disease pathways by testing potential binary therapies in simulations at various combinations of two points in the pathways. Since DDDAS chooses the most effective pair-wise combinations, this data-driven system allows for the implementation of real-time data to model or predict a measurement or event. By incorporating data dynamically rather than statically, the predictions and measurements become more reliable.
Dyslipidemia, a common precursor to atherosclerosis, can be manifested by high triglycerides, increased apolipoprotein (Apo) B, high levels of LDL, and low levels of HDL. SPX106 and D-tagatose is a combination drug therapy composed of a carbohydrate (D-tagatose) and SPX106. D-tagatose has been studied for the treatment of diabetes for several years, and has the ability to lower blood insulin levels and to decrease glycogen formation. SPX106 is a natural substance that accelerates lipid catabolism and inhibits dyslipidemia. In apolipoprotein E knockout mice (ApoE−/−), this drug combination has been shown to significantly lower both the amount of atherosclerosis and blood cholesterol levels.
This study used 26 male ApoE−/− mice (n=13 in each group, control and treated). The control group received the normal “Western” diet (Harlan TD88137) and the treatment group received a modified version in which the sucrose was replaced with D-tagatose and 1g of SPX106 was added for every kilogram of chow. Mice were fed the diet for 8 weeks and then sacrificed via cardiac puncture. Blood serum was analyzed for cholesterol concentration. A significant difference was observed between the control and treated groups for total cholesterol levels. FPLC separations were done on fractions from both control and treated groups. A significant difference between VLDL and HDL levels was found between the treated and control mice (p<0.05 for both). Aortas were also taken and preserved in formalin to be quantified for atherosclerosis. Aortic sinuses were frozen in OCT and sectioned using a cryostat and then quantified for atherosclerosis. Treated mice showed statistically significant reduction in atherosclerosis in the aortic arch (p<0.01), the thoracic aorta (p<0.05), and the aortic sinus (p<0.05) as well as a reduction of cholesterol (p<0.05).
Keywords: Atherosclerosis, Dyslipidemia, SPX106, D-tagatose, ApoE mice
Introduction
Computational models of complex systems, such as signaling networks and biological systems, can be used to explain the behavior of such systems under various conditions. The large number of integrated processes and variables, and the nonlinearities inherent in the fundamental processes, make it difficult for scientists unassisted by computer simulations to effectively predict the consequences of a particular intervention. For this reason, computer simulation has become an important tool for generating hypotheses about the behavior of these systems that can then be tested in the laboratory and clinic. A Dynamic Data-Driven Application Simulation (DDDAS) was designed by Biospherics to model complex metabolic disease pathways by testing potential binary therapies in simulations at various combinations of two points in the pathways [1]. DDDAS chooses the most effective pair-wise combinations, allowing the data-driven system to incorporate real-time data to model or predict a measurement or event. By incorporating data dynamically rather than statically, the predictions and measurements become more reliable. Consider weather forecasting: if predictions are made based on static data collected from sparsely distributed sensors, then rapidly changing conditions often make a prediction obsolete shortly after it is made. A more reliable forecasting system continuously incorporates real-time changes from many sensors into its predictions so that the forecast is always built around current conditions. As the conditions change, so does the forecast, in real-time.
Data driven applications have the ability to guide their measurement processes and refocus their resources, much as forecasts guide US Air Force 53rd Weather Reconnaissance Squadron aircraft away from calm seas and into the eyes of hurricanes to concentrate their data collection. The information collected makes possible advance warning of hurricanes, and increases the accuracy of hurricane predictions and warnings by as much as 30 percent [2]. Dyslipidemia and metabolic syndrome have been growing problems in the United States for several years now. It is estimated more than 15% of Americans have high cholesterol. Because dyslipidemia is one of the main risk factors for coronary heart disease, and one of the leading causes of death in both men and women [3], it would be beneficial to use a data-driven system to predict drugs’ effect on lipid levels and atherosclerosis.
Dyslipidemia is generally characterized by high cholesterol or triglyceride levels and can be broken down into 5 types [4,5]. Type I is characterized by elevated cholesterol and extremely elevated triglycerides. Chylomicronemia is also present, even on a normal diet. Post-Heparin Lipolytic Activity (PHLA) is low. Type II is characterized by elevated cholesterol with normal triglycerides. Atherosclerosis and xanthelasmas of the tendons, joints, around the eyes, and other parts of the body are also present. Type III is characterized the same as type II except it also has elevated triglycerides and an abnormal tolerance to glucose. Type IV is similar to type I with normal or slightly elevated cholesterol levels and elevated triglycerides, but has xanthelasmas, hepatosplenomegaly, normal PHLA, and abnormal glucose tolerance. Type V resembles type IV, but also presents with a family history of diabetes and low or normal PHLA. With all types of dyslipidemia, being homozygous increases the severity of the disease and sometimes the speed of its progression [5]. Type V dyslipidemia is one of the most studied types of dyslipidemia, and is more commonly referred to as familial hypercholesterolemia (FH). This hereditary form of dyslipidemia can result from any of over 300 mutations on the low density lipoprotein (LDL) receptor gene. Heterozygous FH is estimated to affect 1 in 500; while homozygous FH is very rare (1 in 1 million) [6]. Many individuals with high cholesterol use statins or other low density lipoprotein (LDL) lowering drugs. However, current cholesterol drugs are not effective on all patients. It is estimated that only 39% of patients receiving drug therapy reach their recommended lipoprotein levels [3]. Most drugs used for dyslipidemia only treat one aspect of dyslipidemia: either high cholesterol or high triglycerides [3]. This causes a need to use two or more drugs for patient treatment, increasing the cost of medications to the patient as well as the risk for potential side effects [3]. Biospherics Inc. has been investigating a combination of two drugs, SPX106T (a stilbene derivative) and D-tagatose, to treat high cholesterol as well as atherosclerosis, another disease associated with metabolic syndrome.
Atherosclerosis may form due to “response to injury” but conclusive in vivo evidence to support that hypothesis in man is not present in all cases [7]. Injury results in the accumulation of LDL, which is then oxidized by macrophages. This accumulation of LDL engorged macrophages leads to foam cell formation and the first visual signs of atherosclerosis (fatty streaks). Macrophages then release enzymes and toxic substances that lead to endothelial denudation and the adhesion of platelets to the site of injury. The maturation of the plaque causes the migration and proliferation of smooth muscle cells and fibroblasts, which is provoked by macrophages. Fibrotic lesions or fibrous caps form over the lipid rich core at the site of injury. If the plaque continues to grow, blood flow is limited and the vessel lumen is compromised. The accumulation of lipids leads to cell necrosis. Plaques can be prone to rupture if collagen formation is inhibited, which will, in turn, cause fibrous caps to be weak and possibly rupture [8].
SPX106T is a combination therapy that is composed of two components, SPX106 (polydatin) and D-tagatose. SPX106 is an antioxidant that appears to promote lipid catabolism. It is also a derivative of trans-resveratrol and has multiple probable mechanisms of action [9]. In a study using Syrian golden hamsters, a derivative of trans-resveratrol known as polydatin was found to decrease total cholesterol levels and total triglyceride levels decreased by 47% and 63%, respectively, when the hamsters were treated with polydatin vs. control [9]. D-tagatose is an epimer of fructose and has been approved as a low-calorie sweetener. D-tagatose works by competitive inhibition in glucose and fructose metabolic pathways, through the dihydroxyacetone-phosphate and glyceraldehydes-3-phosphate pathways, but at a much slower rate [10–14]. Due to its slower rate of metabolism, D-tagatose causes faster glycogen synthesis and decreases usage of glycogen [10,11]. D-tagatose has been shown to inhibit sucrose activity, which is thought to prevent blood glucose and insulin levels from increasing [10,15,16]. Blood uric acid concentrations also increase with D-tagatose ingestion [17,18], while other studies suggest D-tagatose consumption can induce weight loss [19,20]. Research in human subjects with type II diabetes showed a decrease in HbA1c [20] after 12 months of D-tagatose (8 subjects completed the study dropped from 11.2% ± 2.0% to 9.5% ± 2.0%, but after the 3 subjects in whom additional antidiabetic medications were added or increased during the study were excluded from analysis, a nonsignificant decrease in HbA1c was seen (10.6% ± 1.9% vs 9.6% ± 2.3%, p = 0.08)). Research in normal human subjects showed a lowering of serum triglycerides [14], increased high density lipoprotein cholesterol [20], and lower postprandial increases in blood glucose and insulin levels [21].
Apolipoprotein E (ApoE) mediates transport of very low-density lipoproteins (VLDL), as well as high density lipoproteins (HDL), from the blood to the liver [22]. Removal or mutation of ApoE causes HDL and VLDL to accumulate in the blood, leading to hyperlipidemia [22,23]. ApoE is also involved in metabolism of triglyceride rich lipoproteins, and is the apolipoprotein for intermediate density lipoproteins and chylomicrons. Mutations in ApoE in humans have been associated with Alzheimer’s disease, genetic hyperlipoproteinemia, and atherosclerosis. There are three main variations: E2, E3, and E4. E3 is considered normal, E4 is associated with Alzheimer’s and atherosclerosis, and E2 is associated with hyperlipoproteinemia [24–27]. There are two points of mutation that cause the 3 main isoforms: residues 112 and 158. ApoE3 is Cys112 and Arg158, ApoE2 Cys112 and Cys158, and ApoE4 Arg112 and Arg158 [25,27].
The purpose of this study was to test the hypothesis that SPX106T lowers serum triglycerides and cholesterol in ApoE−/− mice. Additionally, this study tested the hypothesis that SPX106T reduces the extent of atherosclerosis in apoE−/− mice fed a Western diet. ApoE knockout (ApoE−/−) mice have been shown to be hyperlipidemic and spontaneously develop atherosclerosis [22,23,28]. Based on previous experiments with D-tagatose, atherosclerosis was expected to decrease along with cholesterol [19].
Methods and Procedures
Mice
28 male Jax ApoE−/− mice 14 to 18 weeks of age that are 12X backcrossed to C57BL/6J, were obtained from an in-house breeding colony at the University of Kentucky. The mice were given acidified water ad libitum, kept on a 12 hours light/dark cycle, and were housed in sterile microisolator cages (Lab Products, Maywood, NJ). The animal use protocol was approved by the University of Kentucky Institutional Animal Care and Use Committee.
Diet
At the start of the study, mice were weighed and randomized based on weight into two treatment groups, control and SPX106T. At this time 2 animals were excluded due to teeth malocclusions. Mice receiving SPX106T treatment were placed on a D-tagatose run-in chow for 2 weeks, where the amount of D-tagatose was increased from 0% to 34% by 7.1% daily over two weeks until the total percentage of D-tagatose in the chow reached 34% (lead-in phase). The percentage of D-tagatose then remained constant throughout the rest of the study (treatment phase). All mice received ground TD2016 (Harlan Teklad) a 16% protein diet-for the 2 week run-in period. The group receiving D-tagatose had increasing amounts of D-tagatose added to the chow each day. The containers with the powdered food were emptied and refilled daily for each cage, even if the mice were on the standard TD2016. Each cage was given 5 grams of chow per mouse in the cage. At the end of the 14 days, the mice were switched to pelleted TD 88137 Western Diet (Harlan Teklad) for the control mice, or TD 110527 (Harlan Teklad) for the treated mice (Table 1 for the nutritional breakdown of the diets). The only differences between TD 88137 and TD 110527 were: TD 110527 had a gram for gram replacement of sucrose with D-tagatose and an added 1 g of SPX106 per kilogram of chow. Mice remained on the pelleted chow for 8 weeks and were given an excess of food, which was checked every other day for mold and was changed as needed. Body weight was measured weekly.
Table 1.
Comparison of Control and Treatment chows, TD 88137 and TD 110527 respectively.
| TD 88137 (Control Diet) |
TD 110527 (Treatment Diet) |
|
|---|---|---|
| Kcal/g of chow | 4.5 | 3.7 |
| Energy Sources (% Kcal) | ||
| Protein | 15.2 | 18.8 |
| Carbohydrate | 42.7 | 29.4 |
| Fat | 42.0 | 51.8 |
| Constituents (g/Kg chow) | ||
| Casein | 195 | 195 |
| DL-Methionine | 3 | 3 |
| Sucrose | 341.46 | 0 |
| D-tagatose | 0 | 341.46 |
| Corn Starch | 150 | 150 |
| Anhydrous Milk Fat | 210 | 210 |
| Cholesterol | 1.5 | 1.5 |
| Cellulose | 50 | 49 |
| Mineral Mix, AIN-76 | 35 | 35 |
| Calcium Carbonate | 4 | 4 |
| Vitamin Mix, Teklad (40060) | 10 | 10 |
| Ethoxyquin. antioxidant | 0.04 | 0.04 |
| SPX-106 | 0 | 1 |
Blood analysis
Total cholesterol and total triglyceride levels were determined using enzymatic assay kits (Wako Pure Chemical, Richmond, VA). Lipoprotein distributions were determined based on the average of four individual samples from each treatment group. The samples were separated into fractions by fast protein liquid chromatography (FPLC) and the area under the curve of the A280 values was analyzed along with the concentration of total cholesterol (TC) in the individual samples. Matlab (MathWorks, Natick, Massachusetts) was used to calculate the individual lipoprotein concentrations. Assuming the TC was equal to the sum of the VLDL, LDL, and HDL concentrations, and the area of the TC would be equal to the sum of the areas of the VLDL, LDL, and HDL peaks, the area under the curve for each of the individual lipoproteins (VLDL, LDL, and HDL) was found using the trapz command in Matlab and correlated to concentration.
Atherosclerosis measurements
Aortas were prepared for atherosclerosis measurements via en face presentation, whereby the entire length of the aorta was removed from the animal, the entire intimal surface and greater curvature of the aortic arch exposed, and the resulting tissue pinned to a dark surface. The atherosclerotic plaques were then traced and quantified by 2 blinded observers with Nikon NIS Elements software [28]. Sinus of Valsalva measurements were done as previously described [28–30]. Briefly, 10 micrometer frozen sections were cut and placed on a single slide at 80 micron intervals. This placement enabled visualization of the entire aortic root for lesion analysis.
Oil red-O staining
Aortas were stained with a 0.14% solution of oil red-O dissolved in isopropyl alcohol. Fixed tissues were washed for 5 min with 60% isopropyl alcohol and then the liquid was decanted off. Next, the samples were rinsed with deionized water and the liquid decanted. The oil red-O solution was added for 10 minutes at room temperature and the liquid decanted. The samples were then placed in 60% isopropyl alcohol for 2 minutes at room temperature and the liquid decanted, followed by a rinse with deionized water and the liquid decanted. The samples were then stained with hematoxylin for 10 seconds and the liquid decanted, followed by a rinse with automation buffer and a final rinse with deionized water. Pictures were taken of the individual aortas.
The same procedure was followed for the sinus of Valsalva slides, except a 0.05% oil red-O solution was used instead. The samples were also fixed for 5 minutes at room temperature in 4% paraformaldehyde/PBS prior to addition of the first isopropyl alcohol rinse to allow the tissues to fix. Warmed glycerol gelatin was used to mount a cover slip over the fixed tissues.
Statistics
Student’s t test was utilized for analyses. Values of p<0.05 were considered to be statistically significant. All statistical analyses were performed using Microsoft Excel.
Results
The purpose of this experiment was to determine the effects of SPX106T on lipid levels and atherosclerosis in ApoE−/− mice. The experiment ran a total of 10 weeks (two weeks of D-tagatose run-in plus eight weeks of SPX106T treatment phase). Body masses were measured weekly. The results showed treatment with SPX106T lowered lipid levels vs Western diet and arrested development of atherosclerosis in ApoE−/− mice.
Table 1 shows a comparison of the diets used in the study. TD 110527 (treatment chow) differs from TD 88137 (control chow) through a gram-by-gram replacement of D-tagatose for sucrose and addition of 1 gram of SPX106 for every kg of chow (Table 1). Because sucrose is a dimer of glucose and fructose, this replacement is also effectively a ring-by-ring replacement. The mice put on TD 88137 gained a significant amount of weight compared to the mice put on TD 110527. Control group mice showed a significant difference in weight starting at day 14 and continuing throughout the experiment in comparison to SPX106T treated mice (Figure 1). Total serum cholesterol levels between control and SPX106T groups differed significantly at the end of the study (p=0.025). Control serum cholesterol levels were 930 ± 330 mg/dl where the SPX106T group levels were 630 ± 310 mg/dl (Figure 2A).
Figure 1.
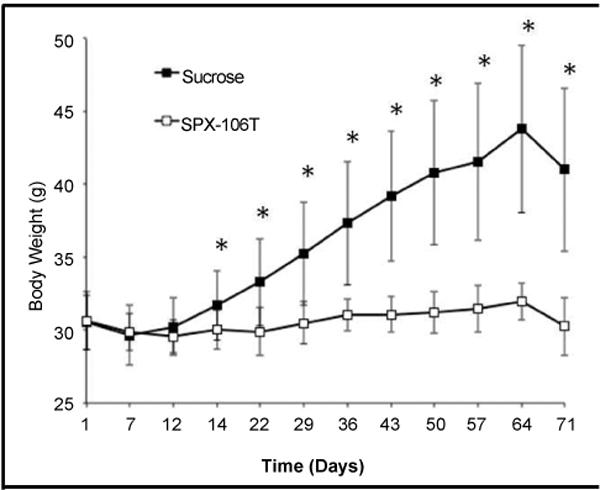
Body weights of mice throughout the experiment broken up into control and SPX-106T treated groups.
*indicates time points with p<0.05 between average body weights.
Figure 2.
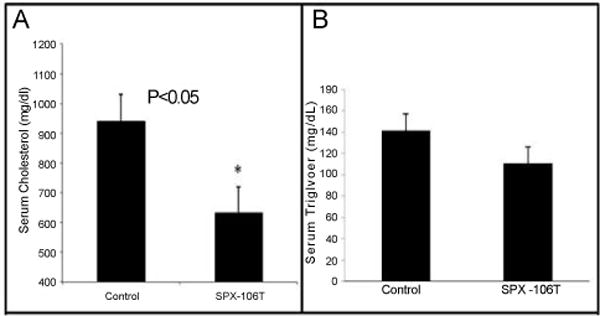
A) Total serum cholesterol and B) total serum triglyceride levels. Serum Cholesterol and Triglyceride levels were measured using enzymatic assay kits for cholesterol and triglycerides respectively. The control group had an average serum cholesterol level of 930 ± 330 mg/dl while the SPX-106T treated group was 630 ± 310 mg/dl giving a p=0.025. Serum triglyceride levels for control and SPX-106T groups were 141.7 ± 43.1 mg/dl and 110.8 ± 6205 mg/dl respectively with a p=0.16.
The difference in serum triglyceride levels at the endpoint was not significant (p=0.16), but without apo E, that was expected because triglyceride levels were not particularly elevated. Nevertheless, there was a slight drop in triglycerides in the treated group. Control triglyceride levels were 141.7 ± 43.1 mg/dl and SPX106 and D-tagatose levels were 110.8 ± 62.5 mg/dl (Figure 2B). VLDL, LDL, and HDL levels were compared between 4 control mice and 5 SPX106 and D-tagatose mice. The Matlab area under the curve analysis of the FPLC graphs showed a significant difference of VLDL and HDL serum levels between control and SPX106T groups (p<0.05). The VLDL levels were 315 ± 29 mg/dl and 201 ± 78 mg/dl (p=0.028). LDL levels were 268 ± 30 mg/dl and 201 ± 70 mg/dl (p = 0.10). HDL levels were 312 ± 53 mg/dl and 208 ± 55 mg/dl (p=0.024) for control and SPX106 and D-tagatose, respectively (Table 2).
Table 2.
VLDL, LDL and HDL averages for a subset of control and SPX-106T groups. Values were found using area under the curve analysis in Matlab.
| Control (mg/dl) | SPX-106T (mg/dl) | P | |
|---|---|---|---|
| VLDL | 315 ± 29 | 201 ± 78 | 0.028 |
| LDL | 268 ± 30 | 201 ± 70 | 0.105 |
| HDL | 312 ± 53 | 208 ± 55 | 0.024 |
SPX106T-treated mice showed significantly less atherosclerosis development compared to control group mice in the sinus of Valsalva (p=0.011), aortic arch (p=0.0027), and thoracic aorta (p=0.049) (Figure 3).
Figure 3.
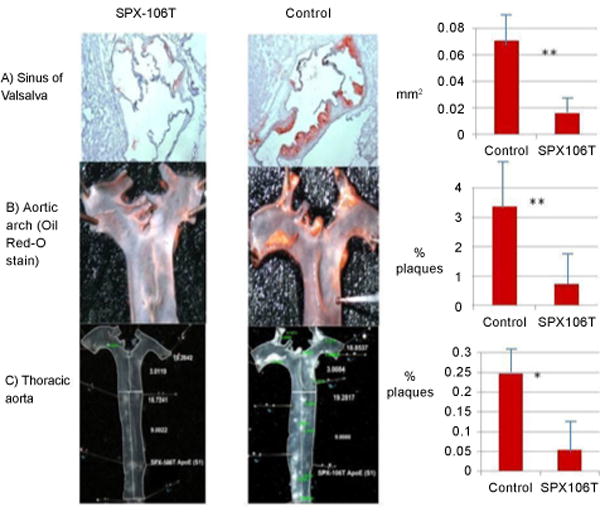
Atherosclerosis measurements in SPX-106T and control mice A) sinus of valsalva, B) aortic arch, and C) thoracic aorta. All atherosclerosis measurements showed significant differences between SPX-106T and control groups. Sinus of valsalva and aortic arch had p<0.01 while thoracic aorta had a p<0.05. Enface measurements of aortic arch and thoracic aorta were done by 2 separate blinded individuals and seconded by the investigator in the same manner.
Mice treated with SPX106T also showed decreased fat pad mass (epididymal, retroperitoneal, and subcutaneous) compared to control mice (p<0.01) (Figure 4). Control mice had epididymal fat pad (EF) mass of 1.997 ± 0.683 g, while SPX106T mice had EF mass of 0.300 ± 0.093 g (p=9.72×10−7). Retroperitoneal fat pad (RPF) mass for control mice was 0.623 ± 0.237 g, and RPF of SPX106T mice was 0.062 ± 0.034 g (p=1.67×10−6). Subcutaneous fat pad (SubQ) mass was found to be 0.942 ± 0.314 g for control mice, and 0.220 ± 0.059 g for SPX106T mice (p=1.98×10−6).
Figure 4.
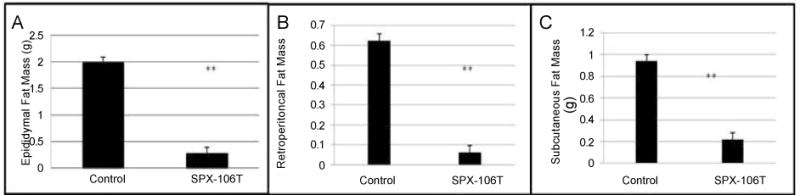
Fat pad analysis. A) SPX-106T mice showed significantly less epididymal fat (p=9.72×10−7) than control mice. B) Retroperitoneal fat pads from each group were weighed giving a p=1.67×10−6 between control and SPX-106T groups. C) Subcutaneous fat pads from each group were weighed giving a p=1.98×10−6 between control and SPX-106T groups. Fat pads were measured at the time of sacrifice.
Discussion
A dynamic data-driven application simulation (DDDAS) is an important tool for generating hypotheses about the behavior of complex systems that can then be tested in the laboratory and clinic. It is an invaluable resource for designing therapies to be used in treating multifactorial conditions such as diabetes, heart disease, and cancer. In the case of cancer, even with the location and molecular composition of the tumor known, unless real-time data are available, the tumor has the ability to evolve while treatment is being administered. With DDDAS, a molecular analysis can be conducted during the administration of treatment in case the therapy needs to be immediately modified. Similarly, real-time information is needed to effectively determine drug doses needed to treat other conditions such as atherosclerosis. For the current study, the DDDAS allowed us to enter values for several reactions associated with lipid metabolism, and correctly predicted that D-tagatose would reduce lipoprotein levels and atherosclerosis (Figure 5). The figure depicts 2 types of modeling that were done with the DDDAS program: (1) toxicokinetic modeling of drug levels in the blood, and (2) pharmacokinetic modeling of lipid levels as a result of drug administration. Indeed, when SPX106T was administered to ApoE−/− mice, the lipoprotein levels of the treated group were significantly reduced.
Figure 5. Toxicokinetics of SPX106T in rats.
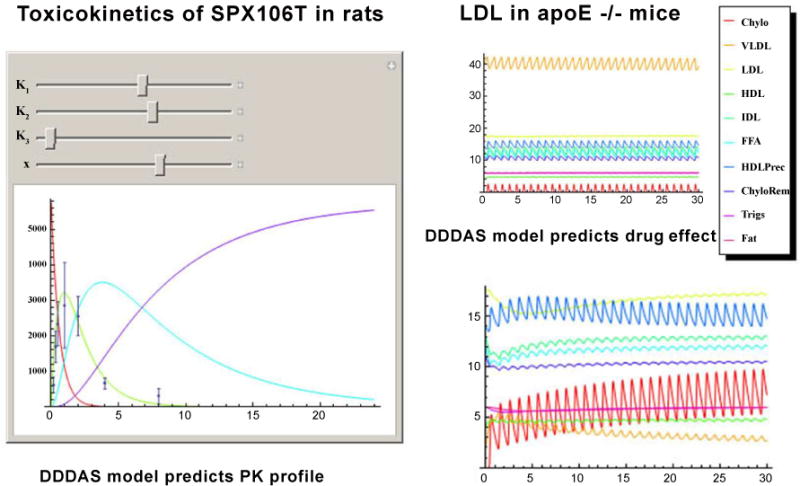
DDDAS model results appear as solid lines. Actual laboratory values for blood serum measurements appear as black dots with error bars. The red line represents disappearance of SPX106T from the gut. The green line represents blood levels of SPX106T. The blue line represents bladder concentrations of SPX106T. The violet line represents elimination of SPX106T (measured externally after elimination). The horizontal axis values are given in hours. The vertical axis units are arbitrary because of the large number of chemicals represented in the graph. Scaling factors were applied to the concentration values in the curves to make them all easily visible in the same graph.
LDL in apo E−/− mice
DDDAS model results appear as solid lines. Compound names are given next to line colors in the legend. Chylomicrons concentrations appear on the red line. VLDL concentrations appear on the orange line. LDL concentrations appear on the pale green line. HDL concentrations appear in a light blue line. IDL concentrations appear in the teal line. Free fatty acid concentrations (FFA) appear with a sky blue line. HDL precursor appears in a dark blue line. Chylomicrons remnants appear in a violent line. Triglycerides appear in a dark red line. Stored fat appears as an orange/red line. The upper right panel labeled “LDL in apo E−/− mice” depicts a steady state in the mice on Western diet. The horizontal axis is given in days. The vertical axis is given in arbitrary units because of the scaling factors that were applied to the concentration values to represent many chemicals on that axis.
DDDAS model predicts drug effect
DDDAS model results appear as solid lines. Compound names are given next to line colors in the legend. Chylomicrons concentrations appear on the red line. VLDL concentrations appear on the orange line. LDL concentrations appear on the pale green line. HDL concentrations appear in a light blue line. IDL concentrations appear in the teal line. Free fatty acid concentrations (FFA) appear with a sky blue line. HDL precursor appears in a dark blue line. Chylomicrons remnants appear in a violent line. Triglycerides appear in a dark red line. Stored fat appears as an orange/red line. This graph shows the effect of a single dose of SPX 106T on the blood values represented in the steady-state model above (upper right panel). The horizontal axis is given in days. The vertical axis is given in arbitrary units because of the scaling factors that were applied to the concentration values to represent many chemicals on that axis.
SPX106T is a novel drug-drug combination of D-tagatose (the T in SPX106T, IND 70971) and trans piceid (polydatin, or SPX106), designed using dynamic data-driven application simulations (DDDAS), and intended to reduce serum triglycerides, VLDL and LDL. The DDDAS code was developed as part of NSF-funded computational mathematics collaboration between Karl Franzen University (Graz, Austria), Yale University, and the University of Kentucky. The code was applied to modeling of complex multifactorial diseases, one of which was the metabolic syndrome, leading to SPX106T.
The purpose of this study was to determine the efficacy of the combination drug SPX106T on atherosclerosis, blood cholesterol, and triglyceride levels in male ApoE−/− mice. Western diet has been shown to increase atherosclerosis, as well as blood cholesterol and triglyceride levels in ApoE−/− mice. By replacing the sucrose in Western diet with D-tagatose and adding SPX106, the effects of SPX106 and D-tagatose can be studied in tandem against Western diet. Mice on the Western diet showed increased atherosclerosis, obesity, blood cholesterol and triglyceride levels. Mice treated with SPX106T showed a significant decrease in atherosclerosis and blood cholesterol and some reduction in triglyceride levels. SPX106T-treated mice also did not show an increase in obesity. There was a significant difference in the amount of atherosclerosis in the sinus of Valsalva, aortic arch, and thoracic aorta between the control group on the Western diet and the treated group receiving SPX106 and D-tagatose. Body weights differed significantly starting at day 14 between the two groups.
The results are consistent with previous studies using D-tagatose that found lipid lowering effects in LDL receptor knockout mice on a high carbohydrate diet [19]. The addition of SPX106 resulted in further decreased atherosclerosis.
Police et al. found that mice treated with a D-tagatose diet did not show an increase in body weight as their counterparts treated with a sucrose diet [19]. Similarly, this study showed mice treated with both SPX106 and D-tagatose did not gain as much weight as the mice on the normal Western diet. Since both D-tagatose and SPX106 have been shown to decrease body weight gain in animals, the results of this study are consistent with previous findings, although previous findings were not statistically significant [9].
The present study detected a significant change in cholesterol levels between the control and SPX106T-treated groups. Police et al. also noted a decrease in serum cholesterol with male D-tagatose treated mice versus mice given sucrose [19]. Likewise, Du et al. saw similar results in hamsters treated with polydatin with various treatment dosages (25, 50, and 100 mg/kg). Du et al. also reported a significant change in LDL in the 50 and 100 mg/kg groups [9], while our study reports a significant change in both LDL and HDL between control and SPX106T groups.
This study found a significant decrease in the development of atherosclerosis in ApoE−/− mice treated with SPX106T compared with controls. Cholesterol was also significantly decreased with this treatment. A Dynamic Data-Driven Application System was able to simulate metabolic reactions in cholesterol pathways, and produce a range of scenarios from stable (healthy) situations to situations that were prone to produce disease. The DDDAS also modeled changing metabolic levels due to changes in activity or time intervals between meals. The DDDAS system allowed scientists to estimate drug doses to use and dosing schedules, by making it possible to predict how the body might respond to different doses. The findings suggest the SPX106T therapeutic combination is a potential candidate for prevention of atherosclerosis and lowering of high cholesterol.
References
- 1.Reminder: spherix to hold business update conference call. Spherix; 2013. [Google Scholar]
- 2.http://www.403wg.afrc.af.mil/photos/mediagallery.asp?galleryID=819&page=6.
- 3.Kopin L, Lowenstein C. In the clinic. Dyslipidemia. Ann Intern Med. 2010;153:ITC21. doi: 10.7326/0003-4819-153-3-201008030-01002. [DOI] [PubMed] [Google Scholar]
- 4.Braun LT. Cholesterol and triglyceride management: “if I take my medication, can I eat what I want?”. J Cardiovasc Nurs. 2010;25:241–246. doi: 10.1097/JCN.0b013e3181cec6d1. [DOI] [PubMed] [Google Scholar]
- 5.Fredrickson DS, Lees RS. A system for phenotyping hyperlipoproteinemia. Circulation. 1965;31:321–327. doi: 10.1161/01.cir.31.3.321. [DOI] [PubMed] [Google Scholar]
- 6.Shafiq N, Singh M, Kaur S, Khosla P, Malhotra S. Dietary treatment for familial hypercholesterolaemia. Cochrane Database Syst Rev. 2010:CD001918. doi: 10.1002/14651858.CD001918.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andreoli TE. CECIL Essentials of Medicine. 6. Philadelphia, WB Saunders; USA: 2004. [Google Scholar]
- 9.Du J, Sun LN, Xing WW, Huang BK, Jia M, et al. Lipid-lowering effects of polydatin from Polygonum cuspidatum in hyperlipidemic hamsters. Phytomedicine. 2009;16:652–658. doi: 10.1016/j.phymed.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Bar A, Lina BA, de Groot DM, de Bie B, Appel MJ. Effect of D-tagatose on liver weight and glycogen content of rats. Regul Toxicol Pharmacol. 1999;29:S11–S28. doi: 10.1006/rtph.1998.1266. [DOI] [PubMed] [Google Scholar]
- 11.Ciudad CJ, Massague J, Salavert A, Guinovart JJ. Synthesis of glycogen from fructose in the presence of elevated levels of glycogen phosphorylase a in rat hepatocytes. Mol Cell Biochem. 1980;30:33–38. doi: 10.1007/BF00215303. [DOI] [PubMed] [Google Scholar]
- 12.Totton EL, Lardy HA. Phosphoric esters of biological importance; the synthesis and biological activity of D-tagatose-6-phosphate. J Biol Chem. 1949;181:701–706. [PubMed] [Google Scholar]
- 13.Koerner TA, Jr, Voll RJ, Ashour AL, Younathan ES. The fructose 6-phosphate site of phosphofructokinase. Epimeric specificity. J Biol Chem. 1976;251:2983–2986. [PubMed] [Google Scholar]
- 14.Buemann B, Toubro S, Astrup A. D-tagatose, a steroisomer of D-fructose, increases hydrogen production in humans without affecting 24-hour energy expenditure or respiratory exchange ratio. J Nutr. 1998;128:1481–1486. doi: 10.1093/jn/128.9.1481. [DOI] [PubMed] [Google Scholar]
- 15.Levin GV, Zehner LR, Saunders JP, Beadle JR. Sugar substitutes: their energy values, bulk characteristics, and potential health benefits. Am J Clin Nutr. 1995;62:1161S–1168S. doi: 10.1093/ajcn/62.5.1161S. [DOI] [PubMed] [Google Scholar]
- 16.Donner TW, Wilber JF, Ostrowski D. D-tagatose, a novel hexose: acute effects on carbohydrate tolerance in subjects with and without type 2 diabetes. Diabetes Obes Metab. 1999;1:285–291. doi: 10.1046/j.1463-1326.1999.00039.x. [DOI] [PubMed] [Google Scholar]
- 17.Boesch C, Ith M, Jung B, Bruegger K, Erban S, et al. Effect of oral D-tagatose on liver volume and hepatic glycogen accumulation in healthy male volunteers. Regul Toxicol Pharmacol. 2001;33:257–267. doi: 10.1006/rtph.2001.1470. [DOI] [PubMed] [Google Scholar]
- 18.Buemann B, Toubro S, Holst JJ, Rehfeld JF, Bibby BM, et al. D-tagatose, a stereoisomer of D-fructose, increases blood uric acid concentration. Metabolism. 2000;49:969–976. doi: 10.1053/meta.2000.7724. [DOI] [PubMed] [Google Scholar]
- 19.Police SB, Harris JC, Lodder RA, Cassis LA. Effect of diets containing sucrose vs. D-tagatose in hypercholesterolemic mice. Obesity (Silver Spring) 2009;17:269–275. doi: 10.1038/oby.2008.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donner TW, Magder LS, Zarbalian K. Dietary supplementation with d-tagatose in subjects with type 2 diabetes leads to weight loss and raises high-density lipoprotein cholesterol. Nutr Res. 2010;30:801–806. doi: 10.1016/j.nutres.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Madenokoji N, Iino H, Shimizu T, Hayakawa J, Sakashita M. Blunting effect of D-tagatose on blood glucose when administered orally with glucose in volunteer donors of boundary glycemic level. J Jap Soc Clin Nutr. 2003;25:21–28. [Google Scholar]
- 22.Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci U S A. 1992;89:4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang YX. Cardiovascular functional phenotypes and pharmacological responses in apolipoprotein E deficient mice. Neurobiol Aging. 2005;26:309–316. doi: 10.1016/j.neurobiolaging.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 24.Breslow JL, Zannis VI, SanGiacomo TR, Third JL, Tracy T, et al. Studies of familial type III hyperlipoproteinemia using as a genetic marker the apoE phenotype E2/2. J Lipid Res. 1982;23:1224–1235. [PubMed] [Google Scholar]
- 25.Civeira F, Pocovi M, Cenarro A, Casao E, Vilella E, et al. Apo E variants in patients with type III hyperlipoproteinemia. Atherosclerosis. 1996;127:273–282. doi: 10.1016/s0021-9150(96)05969-2. [DOI] [PubMed] [Google Scholar]
- 26.Feussner G, Feussner V, Hoffmann MM, Lohrmann J, Wieland H, et al. Molecular basis of type III hyperlipoproteinemia in Germany. Hum Mutat. 1998;11:417–423. doi: 10.1002/(SICI)1098-1004(1998)11:6<417::AID-HUMU1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Poirier J, Davignon J, Bouthillier D, Kogan S, Bertrand P, et al. Apolipoprotein E polymorphism and Alzheimer’s disease. Lancet. 1993;342:697–699. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- 28.Daugherty A, Rateri DL. Development of experimental designs for atherosclerosis studies in mice. Methods. 2005;36:129–138. doi: 10.1016/j.ymeth.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Daugherty A, Rateri DL, Lu H, Inagami T, Cassis LA. Hypercholesterolemia stimulates angiotensin peptide synthesis and contributes to atherosclerosis through the AT1A receptor. Circulation. 2004;110:3849–3857. doi: 10.1161/01.CIR.0000150540.54220.C4. [DOI] [PubMed] [Google Scholar]
- 30.Daugherty A, Whitman SC. Quantification of atherosclerosis in mice. Methods Mol Biol. 2003;209:293–309. doi: 10.1385/1-59259-340-2:293. [DOI] [PubMed] [Google Scholar]


