SUMMARY
In mammals, few retinal ganglion cells (RGCs) survive following axotomy and even fewer regenerate axons. This could reflect differential extrinsic influences or the existence of subpopulations that vary in their responses to injury. We tested these alternatives by comparing responses of molecularly distinct subsets of mouse RGCs to axotomy. Survival rates varied dramatically among subtypes, with alpha-RGCs (αRGCs) surviving preferentially. Among survivors, αRGCs accounted for nearly all regeneration following down-regulation of PTEN, which activates the mTOR pathway. αRGCs have uniquely high mTOR signaling levels among RGCs and also selectively express osteopontin (OPN) and receptors for the growth factor, insulin-like growth factor 1 (IGF-1). Administration of OPN plus IGF-1 promotes regeneration as effectively as down-regulation of PTEN; however, regeneration is still confined to αRGCs. Our results reveal dramatic subtype-specific differences in the ability of RGCs to survive and regenerate following injury, and they identify promising agents for promoting axonal regeneration.
INTRODUCTION
Regeneration following injury to the mammalian brain or spinal cord is notoriously poor: few survivors extend axons beyond the injury site (Ramon y Cajal, 1928) and in some cases, many of the axotomized neurons die (Mansour-Robaey et al., 1994; Conta Steencken et al., 2011). Limited regeneration can be explained in at least two different ways. First, non-genetic differences among neurons could account for differences in outcome – for example, stochastic variation, history of activity or proximity to environmental cues that modulate growth. Alternatively or in addition, distinct subpopulations within a seemingly homogeneous population could regenerate preferentially, owing to preexisting qualities that improve their lot. Distinguishing among these and other alternatives is important both in guiding searches for protective factors and in assessing interventions designed to enhance regeneration.
Here, we analyzed retinal ganglion cells (RGCs) to address this issue. All visual information is conveyed from the eye to the brain by RGC axons, which run though the optic nerve to retinorecipient areas such as the superior colliculus and lateral geniculate nucleus. Although all RGCs share numerous attributes, they can be divided into ~30 distinct subpopulations, based on morphological, physiological and molecular criteria (Masland, 2012; Sanes and Masland, 2015; Yamagata and Sanes, 2010). Following damage to the mouse optic nerve, >80% of RGCs die, and <1% of the survivors extend axons past the site of damage (Mansour-Robaey et al., 1994; Park et al., 2008). Regeneration of a substantial number of RGC axons can be elicited, however, by manipulations of the neurons themselves or the environment through which they grow (Aguayo et al., 1991; Benowitz and Popovich, 2011; Liu et al., 2011; Maier and Schwab, 2006; Park et al., 2008; Smith et al., 2009; Sun et al., 2011). Thus, one can ask whether specific subsets of RGCs differ in their abilities to survive following nerve crush and/or regenerate axons following treatment.
In the first part of this study, we assessed survival of eleven RGC subtypes following transection of the optic nerve in mice. Subtypes differed dramatically in susceptibility to damage, with the largest RGC types, alpha-RGCs (αRGCs), surviving preferentially but not exclusively. We then promoted regeneration by suppressing expression of PTEN, which acts, at least in part, by enhancing mTOR activity (Jaworski et al., 2005; Kim et al., 2009; Park et al., 2008; Zukor et al., 2013). We found that αRGCs accounted for nearly all of the regenerating axons in this paradigm.
Based on these results, we sought features of αRGCs that might account for their regenerative ability and found three: they have high endogenous levels of mTOR activity, they selectively express a secreted phosphoprotein, osteopontin (OPN) (Bellahcene et al., 2008; Wang and Denhardt, 2008), which is capable of stimulating mTOR activity (Ahmed and Kundu, 2010), and they selectively express receptors for insulin-like growth factor 1 (IGF-1), which promotes regeneration of some neuronal types (Dupraz et al., 2013). Ectopic expression of OPN in combination with IGF-1 promotes regeneration of αRGCs as effectively as PTEN suppression. Together, our work identifies a neuronal-intrinsic factor that can promote regeneration and provides a strategy to identify additional regeneration-promoting factors.
RESULTS
Differential survival of RGC subtypes
We used immunohistochemical or transgenic approaches to mark molecularly distinct subsets of RGCs in mice:
There are four groups of ON-OFF direction-selective RGCs (ooDSGCs), each tuned to motion in a single direction: ventral, dorsal, nasal and temporal. Antibodies to the neuropeptide cocaine- and amphetamine-regulated transcript (CART) label all four groups (Kay et al., 2011) while a transgenic line, HB9-GFP, labels the subset tuned to ventral motion (Trenholm et al., 2011).
W3 RGCs are labeled with YFP in the TYW3 mouse line (Kim et al., 2010). W3 RGCs are among the smallest RGCs in terms of soma size and dendritic diameter and are among the most numerous RGCs. They comprise at least two populations: W3B, which are motion-sensitive but not direction-selective, and W3D, which remain physiologically uncharacterized (Zhang et al., 2012).
Antibodies to melanopsin label M1- and M2-RGCs, two subsets of intrinsically photosensitive RGCs that can be distinguished by the sublaminae of the inner plexiform layer (IPL) within which their dendrites arborize (Berson et al., 2010; Ecker et al., 2010).
The RGCs with the largest somata are αRGCs. In mice, they comprise at least three subtypes, which differ in physiological properties as well as dendritic stratification (Estevez et al., 2012; Pang et al., 2003; Schubert et al., 2005; van Wyk et al., 2009; Volgyi et al., 2005). We recently generated and characterized a mouse line in which Cre recombinase is inserted into the locus encoding a potassium channel modulator, kcng4. When crossed to a reporter line (Buffelli et al., 2003), subsets of bipolar cells and RGCs were YFP-positive in double-transgenic offspring (Kcng4-cre;Thy1-stop-YFP line 1; called Kcng4-YFP here) (Duan et al., 2014). Further analysis revealed that the labeled RGCs had large somata and dendrites and were rich in a neurofilament-associated epitope, SMI32 (Fig. S1). These features identified them as αRGCs (Berson, 2008; Peichl, 1991). Morphological and physiological analysis revealed that labeled RGCs included all three types of αRGCs, and no other RGCs (Fig. 1B and M.Q., X.D., J.R.S., B. Krieger and M. Meister, in preparation). Thus, the Kcng4-cre line provides selective genetic access to αRGCs.
Figure 1. Differential survival of RGC subtypes following axotomy.
A. Whole-mount views of retinas. Top panels show retina labeled with antibody Tuj1, which marks all RGCs. Lower panels show retinas from Kcng4-YFP, TYW3 and HB9-GFP mice, in which αRGCs, W3-RGCs, and ooDSGCs, respectively, are labeled. dpc, days post-crush. Scale bar, 50μm.
B. Retinal sections labeled with anti-melanopsin in wild-type mice to label M1 and M2 cells, and YFP in Kcng4-YFP mice to label αRGCs. 1 and 2 indicate M1 and M2 RGCs, which can be distinguished by dendritic lamination; only M1 RGCs survive. 3–5 indicate examples of ON-, OFF-transient and OFF-sustained αRGCs, which can be distinguished by dendritic lamination; all 3 survive axotomy. Scale bar, 20μm.
C, D. Fraction of RGCs of each subtype that survive axotomy at 14dpc (C) or 28dpc (D); data from preparations such as those shown in A and B. n=2–3 retinas per type.
E. Fraction of all RGCs comprised by each subtype in intact retina, 14dpc, and 28dpc. (See also Figure S1.)
Together, these markers allowed us to assay the survival of 11 RGC subtypes (4 ooDSGCs, 2 W3-RGCs, 3 αRGCs, and 2 melanopsin-positive RGCs).
We crushed the optic nerves of wild-type or transgenic mice and then assessed RGC survival 14 days post crush (dpc). Consistent with previous reports (Park et al., 2008), ~20% of RGCs survived, as assessed by staining for class III beta-tubulin (Tuj1), a pan-RGC marker (Fig. 1A, C). The survival rate varied greatly among RGC subtypes. Over 80% of the αRGCs (Kcng4-YFP RGCs) and over 70% of the M1 RGCs survived, whereas few if any M2 RGCs or ooDSGCs (HB9-GFP and CART+ RGCs) survived (Fig. 1A-C). Survival of W3 RGCs was intermediate (~10%). Examination of Kcng4-YFP retinal cross-sections, in which the three αRGC subtypes can be distinguished by dendritic lamination, indicated that all three subtypes survived (Fig. 1B). As a consequence, αRGCs and M1-RGCs, which comprise ~6% and ~3% of all RGCs in the normal retina, respectively, accounted for 23% and 11% of surviving RGCs by 14 dpc (Fig. 1E).
To ask whether the apparently preferential survival of αRGCs represented a delayed cell loss, we examined animals after two additional weeks (28 dpc). Although ~10% of the RGCs were lost between 14 dpc and 28 dpc, αRGCs and M1 RGCs were still preferentially spared, and comprised ~25% and ~15% of all surviving RGCs, respectively (Fig. 1D, E). Thus, αRGCs and M1 RGCs survive preferentially but not exclusively following nerve crush.
Selective regeneration of αRGCs
Next, we compared the ability of RGC subtypes to extend axons following injury. To promote regeneration, we injected an adeno-associated virus (AAV) encoding a previously-validated short hairpin RNA directed against PTEN (shPTEN) (Zukor et al., 2013), a negative regulator of mTOR signaling. We used immunostaining for a phosphorylated form (Ser235/236) of ribosome protein S6 (pS6) to assay the efficacy of shPTEN, based on previous studies showing that pS6 provides a reliable estimate of mTOR activity (Laplante and Sabatini, 2012; Park et al., 2008). Levels of pS6 were increased in ~60% of RGCs in optimally infected areas by 2 weeks following infection with AAV-shPTEN (Fig. 2A). Thus, AAV infects, and PTEN restricts mTOR signaling in, most if not all RGC subtypes. A control AAV (AAV-GFP) infected a similar fraction of RGCs but had no effects on pS6. AAV-shPTEN had no detectable effect on retinal structure or integrity (data not shown).
Figure 2. Selective regeneration of αRGCs following axotomy.
A. Sections from control retina, and a retina infected 2 weeks previously with AAV-shPTEN. Sections were stained with anti-pS6 and Tuj1. PTEN knockdown leads to increased mTOR signaling, revealed by increased levels of pS6. Scale bar, 20μm.
B. Sections from optic nerves of Kcng4-YFP, TYW3 and HB9-GFP mice at 14dpc and 28 day after injection of AAV-shPTEN. * marks lesion site. Scale bar, 200μm.
C. Section from Kcng4-YFP mouse, as in B but injected with cholera toxin B (CTB) to label all regenerated axons. All CTB-positive axons are YFP+ αRGCs. Scale bar, 200μm.
D. Number of labeled regenerating axons 0.5mm distal to the lesion site at 14dpc, based on counts from sections such as those shown in (C), mean+/- S.D., n=3–5 optic nerves per type.
*, p<0.05 (Two-ANOVA with Bonferroni Posttests).
(See also Figure S2.)
To assess regeneration, we crushed the optic nerve Kcng4-YFP, HB9-GFP and TYW3 mice two weeks after AAV-shPTEN administration, then analyzed retina and optic nerve 14 dpc. Selective survival of αRGCs following depletion of PTEN was similar to that observed in control retinas after crush (Fig. S2A, compared to Fig. 1A). Regeneration was detected by counting YFP or GFP-positive axons in longitudinal sections through the optic nerves. We observed substantial regeneration of αRGC axons but no detectable regeneration of ooDSGC or W3-RGC axons (Fig. 2B).
To visualize all regenerating axons, we injected the anterograde tracer cholera toxin subunit B (CTB) into the retina 2–3 days before sacrifice. In all lines, approximately 150 axons per retina regenerated at least 0.5mm past the crush site, accounting for ~2.5% of surviving RGCs (Fig. 2C, D). Importantly, >90% of the CTB-positive axons in the Kcng4-YFP retina were also YFP-positive (Fig. 2D). No markers were available to label M1-RGC axons, but the near-complete overlap of CTB- and YFP-positive axons in the Kcng4-YFP line suggests that few if any M1-RGCs or other RGC subtypes regenerate. Thus, αRGC account for the vast majority of the regenerating axons following down-regulation of PTEN.
αRGCs have high mTOR activity and are rich in osteopontin
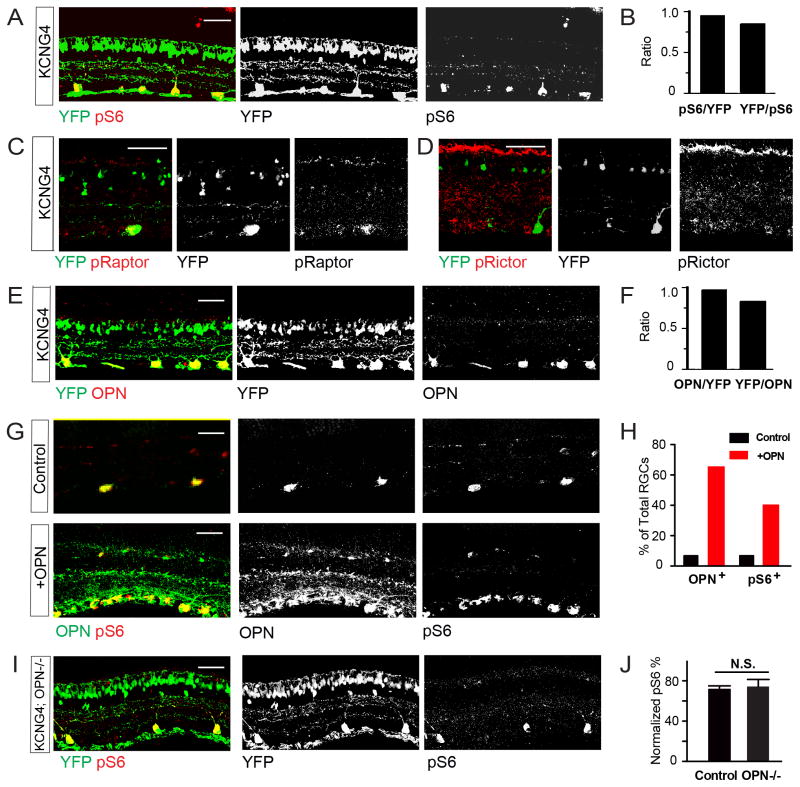
In adult retina, ~10% of RGCs are stained intensely with anti-pS6, and therefore have high mTOR activity (Park et al., 2008). We asked whether the pS6-rich cells are αRGCs. In initial studies, we found variable pS6 levels in RGCs of adult mice (data not shown). We reasoned that neuronal activity, which is known to stimulate phosphorylation of pS6 (Knight et al., 2012), contributed to this variability. We therefore dark-adapted mice overnight to decrease activity of RGCs, then stained retinas of Kcng4-YFP mice for pS6. In dark-adapted retina, ~6% of RGCs were clearly pS6-rich, and the others were not detectably pS6-positive (Fig. 3A). The pS6-rich RGCs were αRGCs: >90% of Kcng4-YFP+ RGCs neurons were rich in pS6 and >85% of pS6-rich neurons were YFP+ (Fig. 3B). In contrast, few if any M1 RGCs were pS6-rich (data not shown).
Figure 3. Selective mTOR activity and OPN expression in αRGCs.
A, B. Section of Kcng4-YFP retina labeled with antibodies to pS6 plus YFP (A) and quantification of their overlap (B).
C,D. Sections of Kcng4-YFP retina labeled with antibodies to pRaptor (C) or pRictor (D) plus YFP.
E, F. Section of Kcng4-YFP retina labeled with antibodies to OPN and YFP (E) and quantification of their overlap (F).
G, H. Section of control retina (top) and retina infected with AAV-OPN (bottom) 2 weeks previously, labeled with antibodies to OPN and pS6 (G). (H) shows fraction of OPN+ and pS6+ cells in both conditions.
I, J. Section of Kcng4-YFP;OPN−/− retina labeled with antibodies to pS6 and YFP (I) and fraction of YFP+ cells that were pS6+
(J). n.s., not significant. n=3 retinas per condition. Scale bars are 50μm.
mTOR acts through two signaling complexes called mTORC1 and mTORC2 (Laplante and Sabatini, 2012). Phosphorylation of S6 is downstream of mTORC1, indicating that mTORC1 activity is enhanced in αRGCs. To test this idea, we stained retinas with antibodies specific to phosphorylated-Raptor (pRaptor) and phosphorylated-Rictor (pRictor), which are mTORC1- and mTORC2-specific signaling components, respectively. pRaptor was concentrated in αRGCs, whereas pRictor was present at low levels in non-αRGCs and barely detectable in αRGCs (Fig. 3C,D). Thus, mTORC1 is active in αRGCs.
We then asked whether αRGCs express other genes that could play roles in their selective survival and regeneration. In initial studies, we detected expression of osteopontin (OPN; gene symbol Spp1 for secreted phosphoprotein-1) in large RGCs in adult retina (M. Yamagata and J.R.S, unpublished). We selected this candidate for further analysis because OPN is expressed by a subset of RGCs in rats (Ju et al., 2000), can enhance mTOR activity (Ahmed and Kundu, 2010) and has been implicated in injury responses of other neuronal types (see Discussion). Immunostaining in the Kcng4-YFP line revealed that >90% of αRGCs were OPN+, and 84% of OPN+ RGCs were αRGCs (Fig. 3E, F). Likewise, OPN and neurofilament SMI32 staining overlapped by >90% (Fig. S1B).
To ask whether OPN regulates mTOR activity in the retina, we used AAV-mediated gene transfer to express OPN in multiple RGC subtypes. Over 60% of RGCs were strongly OPN+ in optimally infected areas two weeks after infection, and levels of pS6 were high in ~60% of OPN-rich RGCs (Fig. 3G,H). Since αRGCs comprise only 6% of RCGs, OPN is clearly able to stimulate mTOR signaling in non-αRGCs We also used OPN null mutant mice (OPN−/−) to ask whether the high mTOR levels of αRGCs require expression of OPN. Levels of pS6 in αRGCs did not differ detectably between controls and OPN−/− mice (Fig. 3I, J). Thus, OPN stimulates mTOR activity, but is presumably not the only factor that maintains high levels of mTOR activity in αRGCs.
Osteopontin promotes RGC growth of non-αRGCs
Before assessing the ability of OPN to affect regeneration, we investigated its role in the normal development of αRGCs. We found that the difference in size between αRGC and non-αRGC somata arose during the first postnatal week, whereas OPN was not detectable in RGCs until the second postnatal week (Fig. 4A, B). These results suggested that OPN is dispensable for the initial growth of RGCs. Consistent with this idea, we found no significant difference in size between control and OPN−/− αRGCs during the period of peak growth or in adulthood (Fig. S3A and data not shown). Moreover, the majority of RGCs were rich in pS6 during the first postnatal week and pS6 immunoreactivity did not become restricted to αRGCs until the third postnatal week (Park et al., 2008). Thus, neither selective mTOR signaling or selective expression of OPN is required for αRGCs to reach their normal size.
Figure 4. Role of OPN in developing retina.
A. Sections from retinas from TYW7 mice of indicated ages stained with antibodies to YFP and OPN. TYW7 labels OFF αRGCs (Kim et al. 2010 and B. Krieger, M.Q, X.D., J.R.S. and M. Meister, in preparation). OPN+ YFP− cells are presumably ON αRGCs. Staining is absent in retinas from OPN−/− mice. Scale bar, 50μm.
B. Soma sizes of developing αRGCs and non-αRGCs, and OPN levels in αRGCs in developing retina. OPN levels were measured from sections such as those in (A), as described in Methods.
C. Retinas from Thy1-cre mice infected 4 weeks previously with Cre-dependent AAV-YFP, with or without Cre-dependent AAV-OPN. Sections were labeled with antibodies to YFP and OPN. Bar, 20μm
D. RGC soma area, calculated from images such as those in C.
E. Soma size increase of W3-RGCs and αRGCs, measured from sections such as those in Fig. S3B. ** p<0.01.
(See also Figure S3.)
We also asked whether OPN can promote growth of RGCs in adults using AAV-mediated gene transfer of OPN, as described above. RGC soma size (measured by area in sections) increased by ~30% over controls 4 weeks after introduction of OPN to a broad range of RGC types using Thy1-cre mice (Fig. 4C, D). Analysis with subtype-specific markers indicated that small W3 RGCs were affected disproportionately (80% increase in area), whereas the size of αRGCs did not increase in the presence of supernormal levels of OPN (Fig. 4E and S3B); this is consistent with the presence of additional growth-promoting factors in these neurons. Together, these results indicate that OPN does not play an essential role in the development of αRGCs but can elicit RGC growth and mTOR signaling in adult retina.
Osteopontin plus IGF-1 promotes axonal regeneration
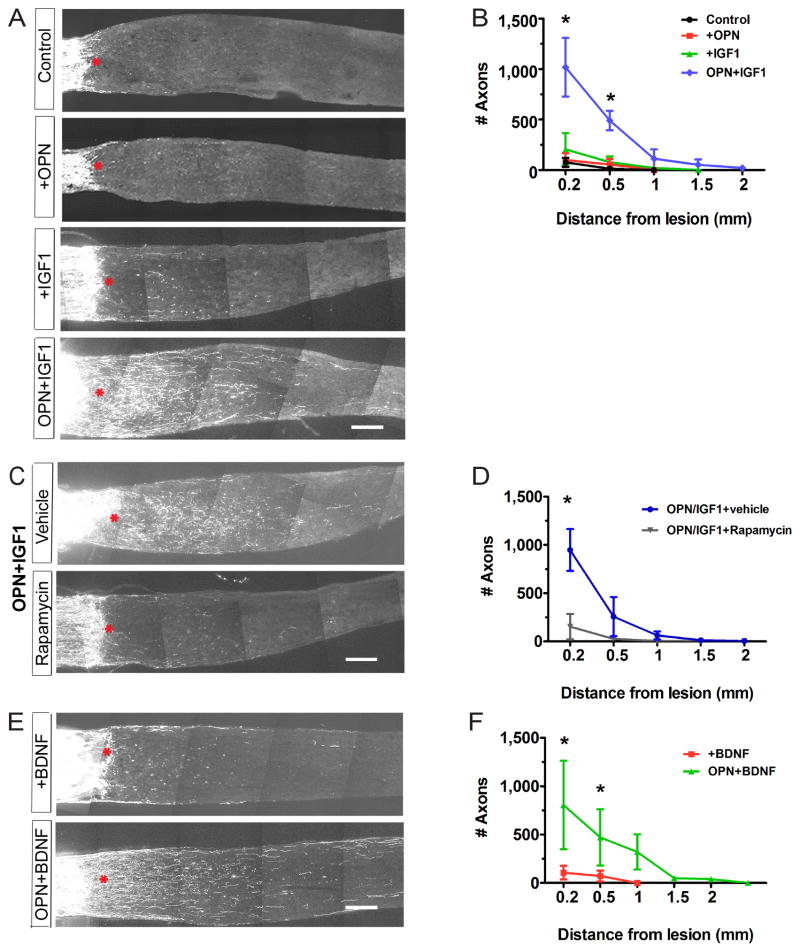
To ask whether OPN could promote axonal regeneration following nerve injury, we used the optic nerve crush protocol described above but infected retinal cells with AAV expressing OPN instead of shPTEN. Immunostaining showed expression of OPN in ~80% of RGCs in injured retina (data not shown) but regeneration was not significantly more effective in its presence than in control retinas (Fig. 5A, B). We therefore combined AAV-OPN administration with intravitreal injection of a growth factor. We chose IGF-1 because it has neuroprotective and regeneration-promoting abilities in other contexts (Dupraz et al., 2013; Hollis et al., 2009) and because its receptor, IGF1R, is expressed by RGCs (Bu et al., 2013; Tagami et al., 2009; and see below). IGF-1 alone had no detectable effect on regeneration, but the combination promoted regeneration as effectively as shPTEN (Fig. 5A, B). In some cases, axons regenerated and extended more than 2 mm (Fig. S4A). OPN and IGF-1 promoted RGC survival to a modest extent (Fig. S5A, B), but the effect on survival was insufficient to account for the effect on axonal regeneration.
Figure 5. OPN promotes regeneration of axotomized RGCs.
A. Sections of optic nerves at 14dpc. Retinas were untreated, infected with AAV-OPN, injected with IGF-1 or BDNF, or both infected and injected.
B. Numbers of regenerating fibers at indicated distances from lesion site, measured from sections such as those in (A).
C. Sections of optic nerves from Kcng4-YFP mice injected with (AAV-OPN+IGF-1) with or without rapamycin.
D. Numbers of regenerating fibers, measured from sections such as those in (C) and Fig. S5C. n = 3–5 optic nerves per condition.
E. Sections of optic nerves from Kcng4-YFP mice injected with BDNF with or without AAV-OPN.
F. Numbers of regenerating fibers measured from sections such as those in (E).
Scale Bar for A, C, E, 200μm. *, p<0.05.
(See also Figures S4 and S5.)
We then tested the relationship of OPN, IGF-1 and mTOR activation as promoters of regeneration. IGF-1 on its own had no detectable effect of mTOR signaling in RGCs following axotomy, but expression of OPN, with or without IGF-1 enhanced mTOR signaling, following axotomy as it did in normal retina; (Fig. S6A, B). Moreover, rapamycin, a potent and specific inhibitor of mTOR (Laplante and Sabatini, 2012), blocked axonal regeneration promoted by OPN plus IGF-1 (Fig. 5C,D) as well as the OPN-induced increase in pS6 levels (Fig. S6C) with minimal effects on neuronal survival (Fig. S5C). Conversely, regeneration promoted by shPTEN is affected little if at all in OPN−/− mice (data not shown). These data place mTOR signaling downstream of OPN in a molecular pathway that promotes regeneration.
We also tested the combination of OPN and brain-derived neurotrophic factor (BDNF), because BDNF promotes RGC branching and its receptor, TrkB, is expressed by RGCs (Cui et al., 2002; Sawai et al., 1996). Like IGF-1, BDNF was ineffective on its own at promoting regeneration and had a modest effect on survival. However, OPN plus BDNF stimulated regeneration to a similar extent as OPN plus IGF-1 (Figs. 5E,F and S5B). Thus the stimulatory role of IGF-1 was not unique and OPN can promote substantial axon regeneration when paired with growth factors.
Osteopontin plus IGF-1 promotes selective regeneration of αRGCs
We expected that administration of OPN plus IGF-1 to most or all RGCs would promote regeneration in multiple RGC types. To test this idea, we introduced AAV-OPN and IGF-1 intravitreally in Kcng4-YFP, HB9-GFP, and TYW3 mice to label αRGCs, ooDSGCs and W3-RGCs, respectively. Surprisingly, using protocols and criteria described above (Fig. 2), we found that nearly all regenerating axons arose from αRGCs (Fig. 6A, B).
Figure 6. Osteopontin promotes selective regeneration of αRGCs.
A. Sections of optic nerves of Kcng4-YFP, TYW3, and HB9-GFP mice at 14dpc treated with AAV-OPN and IGF-1 and injected with CTB at 12dpc. Right panels show region boxed in top, left panel.
B. Numbers of regenerating fibers 0.5 mm from lesion site from sections such as those in F. n = 3–4 optic nerves per type. *, p<0.05.
C. Section of optic nerves from Kcng4-YFP mice injected with Cre-dependent AAV-OPN plus IGF-1.
D. Numbers of regenerating fibers 0.5 mm from lesion sites, measured from sections such as those in (C). P<0.05 (Two-ANOVA with Bonferroni Posttests).
(See also Figure S6.)
The ability to selectively target αRGCs provides an opportunity to ask whether the delivery of OPN directly to αRGCs promote regeneration. To this end, we used an AAV in which expression of OPN was Cre-dependent, and limited over-expression to αRGCs by infecting retinas of Kcng4-YFP mice. αRGC-specific expression of OPN promoted αRGC regeneration following nerve crush (Fig. 6C, D).
Selective IGF1R expression and mTOR signaling in axotomized αRGCs
The result that AAV-OPN and IGF-1 are insufficient to promote regeneration of other RGC subtypes to which they are delivered implies that αRGCs differ from non-α-RGCs in some quality that enhances their regenerative responses following injury. The restriction of shPTEN-induced regeneration to αRGCs (Fig. 2) leads to the same conclusion. The difference could be in the ability of RGC subtypes to respond to IGF-1, to up-regulate mTOR signaling, or to respond appropriately to IGF-1- and mTOR-initiated signals. As a first step in distinguishing these alternatives, we compared levels of the IGF-1 receptor, IGF1R in αRGCs and non-αRGCs. IGF1R was selectively expressed in αRGCs in both control adult retina, and in retinas 3 dpc and 7 dpc, although some expression in non-αRGCs and Müller glial cells was observed following axotomy (Fig. 7A). TrkB showed a similar expression pattern, although the expression was less selective and the neuropil was also intensely stained (Fig. S7). Thus, one factor contributing to selective responsiveness of αRGCs to OPN plus growth factors is selective expression of the growth factor receptors.
Fig. 7. Selective expression of IGF1R and activation of mTOR signaling in axotomized αRGCs.
A. IGF1R expression in αRGCs (labeled with OPN) in control retina, 3dpc and 7dpc.
B–D. Sections from mice injected with control vector, AAV-shPTEN or AAV-OPN plus IGF-1. Optic nerves were crushed 14 days later and retinas analyzed 14dpc with anti-pS6 (B), anti-pRaptor (C) or anti-pRictor (D). Scale bars are 50μm.
E. Fraction of αRGC and other RGCs (YFP+ and YFP−, respectively in Kcng4-YFP) that are pS6+, from sections such as those in (B). n=4-6 retinas per treatment.
F. Model showing pathways by which PTEN knock-down or exogenous OPN expression could promote regeneration. a,b,c indicate steps at which differences between αRGC and non-αRGCs could affect their regenerative abilities. Results in A–E implicate steps “a” and “b” as critical differences (see Discussion).
(See also Figure S7.)
We also compared levels of mTOR signaling in axotomized αRGCs and non-αRGCs, using pS6 as a marker. Levels of pS6 fell dramatically in RGCs following axotomy, as shown previously (Park et al., 2008), and introduction of shPTEN or OPN plus IGF-1 restored its levels. These treatments were 9-fold and 3-fold more effective, respectively in increasing pS6 levels in axotomized αRGCs than in neighboring non-αRGCs (Fig. 7B,E). Moreover, even though αRGCs comprise only 20% of surviving RGCs at 14 dpc, they accounted for ~90% of pS6-rich RGCs following shPTEN treatment and ~60% of pS6-RGCs following OPN plus IGF-1 treatment. As in control retina (Fig. 3A), levels of pRaptor paralleled those of pS6, αRGCs accounted for >90% of pRaptor-rich RGCs following either shPTEN treatment or OPN plus IGF-1 administration (Fig. 7C and data not shown). However, the levels of pRictor were low, with <5% of total αRGCs being pRictor-positive. The expression level did not differ detectably between αRGCs and non-αRGCs, and did not change detectably following shPTEN treatment or OPN plus IGF-1 administration(Fig. 7D). (The ability of the pRictor antibody to detect mTORC2 signaling is demonstrated by staining of non-neuronal cells in injured but not intact retina; compare Figs. 3D and 7D.) Thus, a second property of αRGCs that can help account for their ability to regenerate is their ability to maintain or restore mTORC1 levels following axotomy.
DISCUSSION
Following damage to the optic nerve, few RGCs survive and even fewer can be coaxed to extend new axons (Aguayo et al., 1991; Liu et al., 2011). Using markers of eleven RGC subtypes, we found that limited survival and regeneration does not reflect uniformly low vigor of many subtypes; instead it results from selective survival and regeneration of specific subtypes. We then analyzed factors that promote regeneration of those RGCs that survive, and identified OPN as a promoter of RGC growth and regeneration. Together, our results provide new insights into both the cellular and molecular bases of axon regeneration in the mammalian CNS.
Selective survival and regeneration of αRGCs
We found dramatic differences among RGC subtypes in their ability to survive axotomy. Some populations, such as ooDSGCs, are almost completely eliminated within two weeks, whereas most αRGCs and M1-RGCs survive. These results are consistent with previous reports that M1 RGCs preferentially survive axotomy in rats, and that alpha-like RGCs selectively regenerate in cats (Perez de Sevilla Muller et al., 2014; Robinson and Madison, 2004; Watanabe et al., 1993; Watanabe and Fukuda, 2002; Watanabe et al., 1995). Differential susceptibility to injury has also been reported in a mouse model of glaucoma, although the subtypes affected were not molecularly identified (Della Santina et al., 2013). As a consequence of selective survival, the repertoire of visual features that the retina could potentially report to the brain is fundamentally altered (Fig. 1D), a change that will need to be taken into account if efforts to promote regeneration of RGC survivors succeed.
Among surviving RGCs, the ability to regenerate is specific to αRGCs, an evolutionarily conserved RGC type characterized by large somata, smooth dendrites, high levels of neurofilaments and large receptive fields (Berson, 2008; Peichl, 1991). αRGCs comprise ~6% of all RGCs in intact retina and ~25% of the RGCs that survive axotomy, but give rise to >90% of the axons that extend >0.5mm beyond the site of nerve crush following down-regulation of PTEN. In that αRGCs and non-αRGCs are intermingled in the retina, and their axons are intermingled in the optic nerve, it is almost certain that the differences in their regenerative abilities reflect intrinsic differences rather than differences in their environments.
One caveat to the conclusion that αRGCs regenerate selectively is that we tested only two regeneration-promoting treatments, PTEN knock-down and OPN plus a growth factor. It will be important to ask whether other interventions, such as deletion of suppressor of cytokine signaling 3 (SOCS3) or provoking an inflammatory response (Benowitz and Popovich, 2011; Morgan-Warren et al., 2013; Park et al., 2008; Smith et al., 2009; Sun et al., 2011; Watkins et al., 2013) can promote regeneration of additional RGC subtypes.
Osteopontin as a promoter of axon regeneration
OPN promotes RGC regeneration when introduced in combination with either IGF-1 or BDNF, neither of which promotes significant regeneration on its own. OPN is a secreted, glycosylated phosphoprotein; it was discovered as a component of bone matrix but has since been shown to be synthesized by many cell types and to affect multiple cellular processes, including adhesion, proliferation and survival (Kahles et al., 2014; Kazanecki et al., 2007; Wang and Denhardt, 2008). It is expressed by subsets of neurons as well as several classes of glial cells including Schwann cells, Muller glia and microglia. Levels are affected by neural injury in several systems, and OPN is reported to exert both pro- and anti-inflammatory roles that can promote neuronal survival and regeneration (Carecchio and Comi, 2011; Chidlow et al., 2008; Del Rio et al., 2011; Hashimoto et al., 2007; Misawa et al., 2012; Wright et al., 2014). In most cases, these effects have been ascribed to depots in glial cells; for OPN-stimulated regeneration of injured motor axons, elegant transplantation experiments have demonstrated that this is the case (Wright et al., 2014). In the retina, in contrast, directed delivery reveals that neuron-derived OPN promotes regeneration.
OPN may be useful for promoting regeneration for several reasons. First, PTEN is a tumor suppressor. Whereas OPN, like PTEN knockdown, acts in part by elevating mTOR, PTEN inhibition also activates many additional pathways that likely contribute to its tumor suppressor activity. OPN may circumvent this potentially dangerous activity. Second, soluble protein therapeutics are clearly promising for treatment of a variety of neural injuries and neurological diseases (Thoenen and Sendtner, 2002). In other systems, OPN acts as an extracellular cytokine (Kahles et al., 2014). Therefore, a soluble form of OPN, together with a growth factor, might represent a therapeutic method of transiently activating regenerative ability in mature neurons.
Growth promoting capabilities of αRGCs
Regeneration promoted by OPN plus IGF-1 or shPTEN is restricted to αRGCs. This surprising result raises two questions. First, why are endogenous OPN and mTOR, both of which are enriched in adult αRGCs, insufficient to promote their regeneration? Second, why is regeneration restricted to αRGCs even when OPN plus IGF-1 or shPTEN is supplied to most RGCs?
One answer to the first question is that mTOR signaling decreases dramatically following axon damage (Park et al., 2008). We hypothesized that OPN might also decrease following axotomy, but detected no striking changes in OPN levels by immunohistochemistry or in OPN mRNA levels by RT-PCR (data not shown). However, our immunohistochemical methods are non-quantitative, and decreases in OPN mRNA abundance in RGCs would have been masked by known increases in microglia (Chidlow et al., 2008). Moreover, OPN is heterogeneous in several respects: there are several alternatively spliced isoforms (at least in humans), many forms of post-translational modification, and an alternative translation product that remains intracellular and mediates activities distinct from those of the secreted isoform (Gimba and Tilli, 2013; Inoue and Shinohara, 2011; Kazanecki et al., 2007). It remains to be determined which forms promote regeneration and whether the active forms, which may be a small fraction of the total, are affected by axotomy.
In considering the second question, we note that at least two signaling pathways are activated by the interventions we have assayed here (Fig. 7F). The first is mTOR signaling, as indicated by the sensitivity of PTEN shRNA and OPN-induced regeneration to the mTOR-specific blocker, rapamycin (Park et al., 2008) (“a” in Fig. 7F). For reasons we do not yet understand, OPN upregulates mTOR signaling selectively in αRGCs following axotomy, even though it can upregulate mTOR signaling in most RGCs in uninjured retina.
mTOR signaling alone is insufficient for robust regeneration, however. PTEN regulates several pathways other than mTOR (Hill and Wu, 2009; Manning and Cantley, 2007; Morgan-Warren et al., 2013) and interventions that more selectively up-regulate mTOR signaling, such as deletion of TSC1, are significantly less effective in eliciting regeneration than down-regulation of PTEN (Park et al., 2008). Likewise, OPN can up-regulate mTOR signaling in the presence or absence of a growth factor, but does not induce regeneration unless accompanied by a growth factor. Thus, mTOR-dependent and independent pathways must be co-activated for optimal regeneration. We suggest that IGF-1, which does not detectably enhance mTOR signaling, activates the mTOR-independent pathway (“b” in Fig. 7F). Selective activation of this pathway in αRGCs is likely based on the selective expression of IGF1R by αRGCs. Other salient differences between αRGCs and non-αRGCs may well exist, for example at later steps in the signaling pathway (“c” in Fig. 7F). Molecular comparison of subtypes refractory and susceptible to the effects of injury will be a promising approach for identifying these and other factors that promote survival and regeneration.
EXPERIMENTAL PROCEDURES
Animals
OPN mutant mice were produced by inserting CreER into the translation start codon of the spp1 gene using lambda phage-mediated recombineering (Chan et al., 2007), followed by homologous recombination in embryonic stem cells. Chimeras were produced by the Harvard University Genome Modification Facility. High percentage chimeras transmitting the knock-in alleles were bred to animals expressing FLP recombinase from the beta-actin promoter (Rodriguez et al., 2000) to remove the PGK-NEO cassette. Primers used for genotyping OPNCreER are: OPN Common Forward Primer (TTGGTGGTGATCTAGTGGTGCCAA), CreER Reverse Primer (CATCGACCGGTAATGCAGGCAAAT), OPN wild-type Reverse Primer (CAAGGAAATGCGTGTGAGTGTGCT). The primers amplify fragments of 500bp for the knock-in allele and 225bp for the wild-type allele. Insertion of CreER led to generation of a null allele (Fig. 4A and data not shown). However, we detected very low levels of CreER expression and activity in this line, so it was not useful for marking OPN-expressing cells.
HB9-GFP transgenic mice (Trenholm et al., 2011) were obtained from K. Eggan (Stem Cell and Regenerative Biology Department, Harvard) and Thy1-cre transgenic mice (Dewachter et al., 2002) were obtained from Jackson Laboratories. Other lines were generated and characterized in our laboratory as described previously: Kcng4-Cre (Duan et al.,2014); TWY3-YFP (Kim et al., 2010; Zhang et al., 2012); TWY7-YFP (Kim et al., 2010); and Thy1-STOP-YFP (Buffelli et al., 2003). Mice were maintained on a C57/BL6 background and experiments were done according to protocols approved by both the Harvard University Standing Committee on the Use of Animals in Research and Teaching and IACUC at Boston Children’s Hospital.
Gene transfer and surgical methods
A cDNA encoding OPN was cloned from a mouse retina cDNA library (Kay et al, 2012) and inserted into AAV plasmids for ubiquitous or Cre-dependent expression (Cardin et al., 2009). The coding sequence corresponds to that in accession number BC020355 (NCBI-Nucleotide). AAV-U6-shPTEN-CMV-mCherry was modified from a previously characterized vector AAV-U6-shPTEN-CMV-GFP (Zukor et al. 2013) by changing the fluoresecent protein. The targeting sequence was AGGTGAAGATATATTCCTCCAA. AAV serotype 2/2 was produced at Boston Children’s Hospital Viral Core. AAV2 was titered to >1×1012 genome copies per ml.
Detailed surgical methods were described by Park et al. (2008). For injection, adult animals were anesthetized with ketamine/xylazine (100/10 mg/kg). AAV (~3 μl) was injected intravitreally with a fine glass pipette. Optic nerves were crushed with a pair of Dumont #5 forceps (Roboz) 2 weeks after injection. IGF-1 or BDNF (1 μl, 1 μg/μL, Peprotech) was injected into the vitreal space of the eye at 0 dpc and 7 dpc. 1 μl of Alexa-conjugated CTB568 or 647 (Invitrogen) was injected intravitreally 2–3 days before euthanasia to label all regenerating axons. Rapamycin (6 mg/kg, LC Laboratories) was delivered intraperitoneally every two days from the time of AAV injection.
Histology
Anesthetized mice were transcardially perfused with 4% paraformaldehyde (PFA). Eyes and optic nerves were dissected out and post-fixed in 4% PFA at 4°C overnight. For frozen sections, tissues were immersed in 30% sucrose for two days before sectioning in a cryostat (20 μm for retina, 10 μm for optic nerve). For some experiment, eyes were fixed in 4% PFA at 4°C by immersion for ~30–60 mins, immediately after the mice were sacrificed by a lethal overdose of anesthesia.
For immunohistochemistry, sections were incubated in PBS with 3% donkey serum and 0.3% Triton X-100 for blocking, followed by primary antibodies overnight at 4° C and secondary antibodies for ~2 hrs at room temperature. Wholemounts were incubated in PBS with 5% donkey serum and 0.5% Triton X-100 for blocking, followed by primary antibodies for 48 hrs at 4°C and secondary antibodies for 16 hrs at 4°C. Finally, sections were washed with PBS and mounted in Vectashield (Vectorlabs).
Primary antibodies used were: rabbit anti-GFP (1:1000, Millipore), chicken anti-GFP (1:500, Abcam); rabbit anti-RFP (1:500, Abcam); rabbit anti-CART (1:2500, Phoenix Peptide); rabbit anti-melanopsin (1:1000, gift from I. Provencio, University of Virginia); rabbit anti-phosphorylated S6 Ser235/236 (1:200, Cell Signaling Technology); mouse anti-neurofilament (SMI32, 1:1000, Convance); rabbit anti-IGF1R (1:1000, Sigma); goat anti-mouse TrkB (1:500, R&D systems); rabbit anti-phosphorylated-Raptor (Ser792) (1:200, Cell Signaling Technology), rabbit anti-phosphorylated-Rictor (Thr1135) (1:200, Cell Signaling Technology) and goat anti-osteopontin (1:1000, R&D Systems); Nuclei were labelled with NeuroTrace Nissl 435/455 (1:1000, Invitrogen). Secondary antibodies were conjugated to DyLight 649 (Jackson ImmunoResearch), Alexa Fluor 568 or Alexa Fluor 488 (Invitrogen) and used at 1:500.
Imaging and quantification
For whole mounts of retinas, at least eight areas (~0.5 × 0.5 mm) across the whole retinas were imaged with a standard epi-fluorescence microscope (Nikon) focusing on the retinal ganglion layer. Cells were counted and the counts obtained from all areas were averaged to generate a single value for each retina.
For retina sections, images were taken with a confocal microscope (Olympus FV1000 or Zeiss LSM-710) using 440/488 515/568 and 647 lasers with a step size of 0.5μm and a 40X (NA 1.3) lens. Images were analyzed using ImageJ (NIH) software. One field from at least eight sections per sample were imaged and analyzed. The numbers from all sections were averaged to generate a single value for each retina.
For soma size measurement, Z stacks were projected onto a single plane and the largest area was measured with ImageJ. To measure level of immunoreactivity, slides were stained, mounted and imaged in parallel, and the signals were imaged within a linear range. Fluorescent intensity was measured in ImageJ to indicate the relative expression level.
For nerve sections, regenerating axons were identified and counted as described by (Park et al., 2008). In analyzing YFP+ axons, signals from fluorescent or autofluorescent tissue debris was excluded.
Only contrast and brightness were adjusted for all images. Caution was taken not to over-saturate the images and only brightly-stained cells were counted when positive staining was to be identified.
Supplementary Material
Acknowledgments
We thank Masa Yamagata and Terrance Kummer for providing the first evidence that osteopontin is expressed by αRGCs, members of the Sanes and He laboratories for advice and discussions, and M. Evarts for technical assistance. AAV was generated by C. Wang in the Boston Children’s Hospital Viral Core, supported by NIH P30EY012196. Mice were generated at the Harvard Genome Modification Core supported by NIH P30NS062685. This work was supported by grants from NIH to J.R.S (NS029169, EY022073), and Z.H. (EY021342, EY021526), and a HHMI-LSRF Fellowship to X.D.
Footnotes
AUTHOR CONTRIBUTIONS
X.D., M.Q and F.B. planned and performed all experiments and analyzed data. I-J. K. performed initial studies showing that osteopontin is expressed in αRGCs. Z.H. and J.R.S planned the study and analyzed data. X.D. and J.R.S. wrote the paper with input from all authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguayo AJ, Rasminsky M, Bray GM, Carbonetto S, McKerracher L, Villegas-Perez MP, Vidal-Sanz M, Carter DA. Degenerative and regenerative responses of injured neurons in the central nervous system of adult mammals. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 1991;331:337–343. doi: 10.1098/rstb.1991.0025. [DOI] [PubMed] [Google Scholar]
- Ahmed M, Kundu GC. Osteopontin selectively regulates p70S6K/mTOR phosphorylation leading to NF-kappaB dependent AP-1-mediated ICAM-1 expression in breast cancer cells. Molecular cancer. 2010;9:101. doi: 10.1186/1476-4598-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellahcene A, Castronovo V, Ogbureke KU, Fisher LW, Fedarko NS. Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nature reviews Cancer. 2008;8:212–226. doi: 10.1038/nrc2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz LI, Popovich PG. Inflammation and axon regeneration. Current opinion in neurology. 2011;24:577–583. doi: 10.1097/WCO.0b013e32834c208d. [DOI] [PubMed] [Google Scholar]
- Berson DM. Retinal ganglion-cell types and their central projections. In: Allan AK, Basbaum I, Shepard Gordon M, Westheimer Gerald, editors. The Senses: A Comprehensive Reference. San Diego: Academic Press; 2008. pp. 491–520. [Google Scholar]
- Berson DM, Castrucci AM, Provencio I. Morphology and mosaics of melanopsin-expressing retinal ganglion cell types in mice. The Journal of comparative neurology. 2010;518:2405–2422. doi: 10.1002/cne.22381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu SY, Yu GH, Xu GX. Expression of insulin-like growth factor 1 receptor in rat retina following optic nerve injury. Acta ophthalmologica. 2013;91:e427–431. doi: 10.1111/aos.12096. [DOI] [PubMed] [Google Scholar]
- Buffelli M, Burgess RW, Feng G, Lobe CG, Lichtman JW, Sanes JR. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424:430–434. doi: 10.1038/nature01844. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carecchio M, Comi C. The role of osteopontin in neurodegenerative diseases. Journal of Alzheimer’s disease : JAD. 2011;25:179–185. doi: 10.3233/JAD-2011-102151. [DOI] [PubMed] [Google Scholar]
- Chan W, Costantino N, Li R, Lee SC, Su Q, Melvin D, Court DL, Liu P. A recombineering based approach for high-throughput conditional knockout targeting vector construction. Nucleic Acids Res. 2007;35:e64. doi: 10.1093/nar/gkm163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidlow G, Wood JP, Manavis J, Osborne NN, Casson RJ. Expression of osteopontin in the rat retina: effects of excitotoxic and ischemic injuries. Investigative ophthalmology & visual science. 2008;49:762–771. doi: 10.1167/iovs.07-0726. [DOI] [PubMed] [Google Scholar]
- Conta Steencken AC, Smirnov I, Stelzner DJ. Cell survival or cell death: differential vulnerability of long descending and thoracic propriospinal neurons to low thoracic axotomy in the adult rat. Neuroscience. 2011;194:359–371. doi: 10.1016/j.neuroscience.2011.05.052. [DOI] [PubMed] [Google Scholar]
- Cui Q, Tang LS, Hu B, So KF, Yip HK. Expression of trkA, trkB, and trkC in injured and regenerating retinal ganglion cells of adult rats. Investigative ophthalmology & visual science. 2002;43:1954–1964. [PubMed] [Google Scholar]
- Del Rio P, Irmler M, Arango-Gonzalez B, Favor J, Bobe C, Bartsch U, Vecino E, Beckers J, Hauck SM, Ueffing M. GDNF-induced osteopontin from Muller glial cells promotes photoreceptor survival in the Pde6brd1 mouse model of retinal degeneration. Glia. 2011;59:821–832. doi: 10.1002/glia.21155. [DOI] [PubMed] [Google Scholar]
- Della Santina L, Inman DM, Lupien CB, Horner PJ, Wong RO. Differential progression of structural and functional alterations in distinct retinal ganglion cell types in a mouse model of glaucoma. Journal of Neuroscience. 2013;33:17444–17457. doi: 10.1523/JNEUROSCI.5461-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewachter I, Reverse D, Caluwaerts N, Ris L, Kuiperi C, Van den Haute C, Spittaels K, Umans L, Serneels L, Thiry E, et al. Neuronal deficiency of presenilin 1 inhibits amyloid plaque formation and corrects hippocampal long-term potentiation but not a cognitive defect of amyloid precursor protein [V717I] transgenic mice. Journal of Neuroscience. 2002;22:3445–3453. doi: 10.1523/JNEUROSCI.22-09-03445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Krishnaswamy A, De la Huerta I, Sanes JR. Type II cadherins guide assembly of a direction-selective retinal circuit. Cell. 2014;158:793–807. doi: 10.1016/j.cell.2014.06.047. [DOI] [PubMed] [Google Scholar]
- Dupraz S, Grassi D, Karnas D, Nieto Guil AF, Hicks D, Quiroga S. The insulin-like growth factor 1 receptor is essential for axonal regeneration in adult central nervous system neurons. PloS one. 2013;8:e54462. doi: 10.1371/journal.pone.0054462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez ME, Fogerson PM, Ilardi MC, Borghuis BG, Chan E, Weng S, Auferkorte ON, Demb JB, Berson DM. Form and function of the M4 cell, an intrinsically photosensitive retinal ganglion cell type contributing to geniculocortical vision. Journal of Neuroscience. 2012;32:13608–13620. doi: 10.1523/JNEUROSCI.1422-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimba ER, Tilli TM. Human osteopontin splicing isoforms: known roles, potential clinical applications and activated signaling pathways. Cancer letters. 2013;331:11–17. doi: 10.1016/j.canlet.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Sun D, Rittling SR, Denhardt DT, Young W. Osteopontin-deficient mice exhibit less inflammation, greater tissue damage, and impaired locomotor recovery from spinal cord injury compared with wild-type controls. Journal of Neuroscience. 2007;27:3603–3611. doi: 10.1523/JNEUROSCI.4805-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R, Wu H. PTEN, stem cells, and cancer stem cells. The Journal of biological chemistry. 2009;284:11755–11759. doi: 10.1074/jbc.R800071200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis ER, 2nd, Lu P, Blesch A, Tuszynski MH. IGF-I gene delivery promotes corticospinal neuronal survival but not regeneration after adult CNS injury. Experimental neurology. 2009;215:53–59. doi: 10.1016/j.expneurol.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Shinohara ML. Intracellular osteopontin (iOPN) and immunity. Immunologic research. 2011;49:160–172. doi: 10.1007/s12026-010-8179-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski J, Spangler S, Seeburg DP, Hoogenraad CC, Sheng M. Control of dendritic arborization by the phosphoinositide-3′-kinase-Akt-mammalian target of rapamycin pathway. Journal of Neuroscience. 2005;25:11300–11312. doi: 10.1523/JNEUROSCI.2270-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju WK, Kim KY, Cha JH, Kim IB, Lee MY, Oh SJ, Chung JW, Chun MH. Ganglion cells of the rat retina show osteopontin-like immunoreactivity. Brain research. 2000;852:217–220. doi: 10.1016/s0006-8993(99)02140-x. [DOI] [PubMed] [Google Scholar]
- Kahles F, Findeisen HM, Bruemmer D. Osteopontin: A novel regulator at the cross roads of inflammation, obesity and diabetes. Molecular metabolism. 2014;3:384–393. doi: 10.1016/j.molmet.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay JN, De la Huerta I, Kim IJ, Zhang Y, Yamagata M, Chu MW, Meister M, Sanes JR. Retinal ganglion cells with distinct directional preferences differ in molecular identity, structure, and central projections. Journal of Neuroscience. 2011;31:7753–7762. doi: 10.1523/JNEUROSCI.0907-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanecki CC, Uzwiak DJ, Denhardt DT. Control of osteopontin signaling and function by post-translational phosphorylation and protein folding. Journal of cellular biochemistry. 2007;102:912–924. doi: 10.1002/jcb.21558. [DOI] [PubMed] [Google Scholar]
- Kim IJ, Zhang Y, Meister M, Sanes JR. Laminar restriction of retinal ganglion cell dendrites and axons: subtype-specific developmental patterns revealed with transgenic markers. Journal of Neuroscience. 2010;30:1452–1462. doi: 10.1523/JNEUROSCI.4779-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Duan X, Liu CY, Jang MH, Guo JU, Powanpongkul N, Kang E, Song H, Ming GL. DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron. 2009;63:761–773. doi: 10.1016/j.neuron.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight ZA, Tan K, Birsoy K, Schmidt S, Garrison JL, Wysocki RW, Emiliano A, Ekstrand MI, Friedman JM. Molecular profiling of activated neurons by phosphorylated ribosome capture. Cell. 2012;151:1126–1137. doi: 10.1016/j.cell.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Tedeschi A, Park KK, He Z. Neuronal intrinsic mechanisms of axon regeneration. Annual review of neuroscience. 2011;34:131–152. doi: 10.1146/annurev-neuro-061010-113723. [DOI] [PubMed] [Google Scholar]
- Maier IC, Schwab ME. Sprouting, regeneration and circuit formation in the injured spinal cord: factors and activity. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2006;361:1611–1634. doi: 10.1098/rstb.2006.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proceedings of the National Academy of Sciences USA. 1994;91:1632–1636. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH. The neuronal organization of the retina. Neuron. 2012;76:266–280. doi: 10.1016/j.neuron.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misawa H, Hara M, Tanabe S, Niikura M, Moriwaki Y, Okuda T. Osteopontin is an alpha motor neuron marker in the mouse spinal cord. Journal of neuroscience research. 2012;90:732–742. doi: 10.1002/jnr.22813. [DOI] [PubMed] [Google Scholar]
- Morgan-Warren PJ, Berry M, Ahmed Z, Scott RA, Logan A. Exploiting mTOR signaling: a novel translatable treatment strategy for traumatic optic neuropathy? Investigative ophthalmology & visual science. 2013;54:6903–6916. doi: 10.1167/iovs.13-12803. [DOI] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Wu SM. Light-evoked excitatory and inhibitory synaptic inputs to ON and OFF alpha ganglion cells in the mouse retina. Journal of Neuroscience. 2003;23:6063–6073. doi: 10.1523/JNEUROSCI.23-14-06063.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichl L. Alpha ganglion cells in mammalian retinae: common properties, species differences, and some comments on other ganglion cells. Visual neuroscience. 1991;7:155–169. doi: 10.1017/s0952523800011020. [DOI] [PubMed] [Google Scholar]
- Perez de Sevilla Muller L, Sargoy A, Rodriguez AR, Brecha NC. Melanopsin ganglion cells are the most resistant retinal ganglion cell type to axonal injury in the rat retina. PloS one. 2014;9:e93274. doi: 10.1371/journal.pone.0093274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon y Cajal S. Degeneration and Regeneration of the Nervous System. New York: Oxford Univ. Press; 1928. Reprinted 1991. [Google Scholar]
- Robinson GA, Madison RD. Axotomized mouse retinal ganglion cells containing melanopsin show enhanced survival, but not enhanced axon regrowth into a peripheral nerve graft. Vision research. 2004;44:2667–2674. doi: 10.1016/j.visres.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Rodriguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25:139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- Sanes J, Masland R. The types of retinal ganglion cells: current status and implications for neuronal classifcation. Annual Review Neuroscience. 2015 doi: 10.1146/annurev-neuro-071714-034120. (In Press) [DOI] [PubMed] [Google Scholar]
- Sawai H, Clarke DB, Kittlerova P, Bray GM, Aguayo AJ. Brain-derived neurotrophic factor and neurotrophin-4/5 stimulate growth of axonal branches from regenerating retinal ganglion cells. Journal of Neuroscience. 1996;16:3887–3894. doi: 10.1523/JNEUROSCI.16-12-03887.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert T, Degen J, Willecke K, Hormuzdi SG, Monyer H, Weiler R. Connexin36 mediates gap junctional coupling of alpha-ganglion cells in mouse retina. The Journal of comparative neurology. 2005;485:191–201. doi: 10.1002/cne.20510. [DOI] [PubMed] [Google Scholar]
- Smith PD, Sun F, Park KK, Cai B, Wang C, Kuwako K, Martinez-Carrasco I, Connolly L, He Z. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron. 2009;64:617–623. doi: 10.1016/j.neuron.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Park KK, Belin S, Wang D, Lu T, Chen G, Zhang K, Yeung C, Feng G, Yankner BA, et al. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature. 2011;480:372–375. doi: 10.1038/nature10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagami Y, Kurimoto T, Miyoshi T, Morimoto T, Sawai H, Mimura O. Axonal regeneration induced by repetitive electrical stimulation of crushed optic nerve in adult rats. Japanese journal of ophthalmology. 2009;53:257–266. doi: 10.1007/s10384-009-0657-8. [DOI] [PubMed] [Google Scholar]
- Thoenen H, Sendtner M. Neurotrophins: from enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nature neuroscience. 2002;5(Suppl):1046–1050. doi: 10.1038/nn938. [DOI] [PubMed] [Google Scholar]
- Trenholm S, Johnson K, Li X, Smith RG, Awatramani GB. Parallel mechanisms encode direction in the retina. Neuron. 2011;71:683–694. doi: 10.1016/j.neuron.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyk M, Wassle H, Taylor WR. Receptive field properties of ON- and OFF-ganglion cells in the mouse retina. Visual neuroscience. 2009;26:297–308. doi: 10.1017/S0952523809990137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgyi B, Abrams J, Paul DL, Bloomfield SA. Morphology and tracer coupling pattern of alpha ganglion cells in the mouse retina. Journal of comparative neurology. 2005;492:66–77. doi: 10.1002/cne.20700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine & growth factor reviews. 2008;19:333–345. doi: 10.1016/j.cytogfr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Fukuda Y. Survival and axonal regeneration of retinal ganglion cells in adult cats. Progress in retinal and eye research. 2002;21:529–553. doi: 10.1016/s1350-9462(02)00037-x. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Sawai H, Fukuda Y. Number, distribution, and morphology of retinal ganglion cells with axons regenerated into peripheral nerve graft in adult cats. Journal of Neuroscience. 1993;13:2105–2117. doi: 10.1523/JNEUROSCI.13-05-02105.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Sawai H, Fukuda Y. Number and dendritic morphology of retinal ganglion cells that survived after axotomy in adult cats. Journal of neurobiology. 1995;27:189–203. doi: 10.1002/neu.480270206. [DOI] [PubMed] [Google Scholar]
- Watkins TA, Wang B, Huntwork-Rodriguez S, Yang J, Jiang Z, Eastham-Anderson J, Modrusan Z, Kaminker JS, Tessier-Lavigne M, Lewcock JW. DLK initiates a transcriptional program that couples apoptotic and regenerative responses to axonal injury. Proceedings of the National Academy of Sciences USA. 2013;110:4039–4044. doi: 10.1073/pnas.1211074110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MC, Mi R, Connor E, Reed N, Vyas A, Alspalter M, Coppola G, Geschwind DH, Brushart TM, Hoke A. Novel roles for osteopontin and clusterin in peripheral motor and sensory axon regeneration. Journal of Neuroscience. 2014;34:1689–1700. doi: 10.1523/JNEUROSCI.3822-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Synaptic localization and function of Sidekick recognition molecules require MAGI scaffolding proteins. Journal of Neuroscience. 2010;30:3579–3588. doi: 10.1523/JNEUROSCI.6319-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kim IJ, Sanes JR, Meister M. The most numerous ganglion cell type of the mouse retina is a selective feature detector. Proceedings of the National Academy of Sciences USA. 2012;109:E2391–2398. doi: 10.1073/pnas.1211547109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukor K, Belin S, Wang C, Keelan N, Wang X, He Z. Short hairpin RNA against PTEN enhances regenerative growth of corticospinal tract axons after spinal cord injury. Journal of Neuroscience. 2013;33:15350–15361. doi: 10.1523/JNEUROSCI.2510-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.