Abstract
Statins are widely used drugs to lower cholesterol levels and to reduce the risk of cardiovascular disease. However, it has been reported that statins are associated with adverse side effects of skeletal myopathy. Statin treatment can impair mitochondrial function and induce apoptosis in skeletal muscle in both human and animal models. Ubiquinone plays an essential role in transferring electrons in the mitochondrial electron transfer chain for oxidative phosphorylation. However, statin treatment reduces ubiquinone levels in the cholesterol synthesis pathway, which may be associated with mitochondrial dysfunction. In addition, reactive oxygen species (ROS) production and apoptosis induced by statins may provide cellular and molecular mechanisms in skeletal myopathy. Exercise is the most effective therapy to prevent metabolic and cardiovascular diseases. However, whether exercise provides a benefit to or exacerbation of statin-induced myopathy in skeletal muscle remains poorly investigated. This review will briefly provide a comprehensive summary regarding the effects of statins on skeletal myopathy, and discuss the potential mechanisms of statin-induced myopathy and the role of exercise in statin-induced myopathy in skeletal muscle.
Keywords: Statins, Myopathy, Exercise, Skeletal muscle
INTRODUCTION
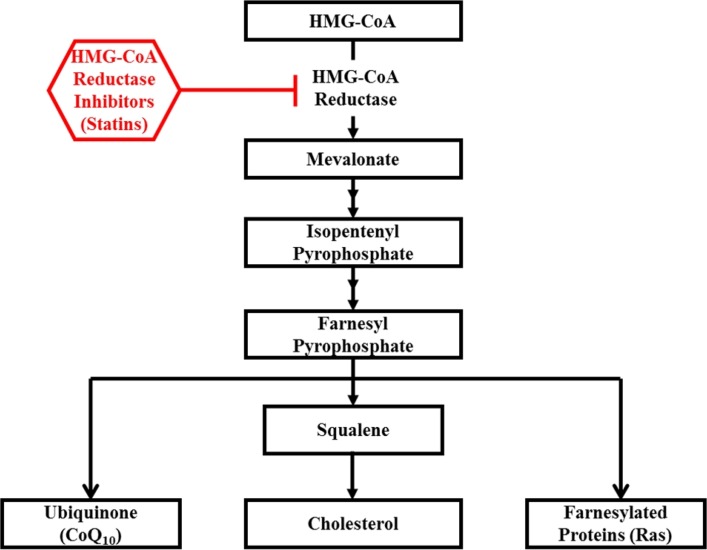
3-hydroxy-3-methylgutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) are cholesterol-lowering drugs which work by blocking the rate-limiting step in the cholesterol synthesis pathway (Fig. 1). Stains are the most frequently and widely used medication in the treatment of cardiovascular disease, diabetes, and cancer to reduce cholesterol levels (e.g., LDL-cholesterol) by inhibiting the formation of mevalonate (a precursor to cholesterol), ubiquinone (coenzyme Q), and other compounds [1,2]. Although statins have a number of beneficial effects including a lipid-lowering effect, improved endothelial function, anti-inflammation, and insulin sensitivity [1,3], statins, particularly lipophilic statins (e.g., simvastatin, atorvastatin, cerivastatin, and lovastatin), also cause adverse side effects in skeletal muscle ranging from mild to moderate muscle fatigue, weakness, and pain to fatal rhabdomyolysis [4–6]. In fact, considering that the occurrence of less adverse side effects is not reported, the incidence of statin-induced myopathy may be 5–10%, and concerns about the safety of statins on skeletal muscle are expected to increase [7]. However, the underlying mechanisms by which statins induce skeletal muscle side effects have not been clearly determined. Therefore, this review primarily focuses on statin-induced myopathy and the potential mechanisms of statin-associated myopathy. In addition, this review provides an overview of the role of exercise in stain-induced myopathy.
Fig. 1.
Cholesterol synthesis pathway and inhibition of statins.
EFFECTS OF STATINS ON SKELETAL MYOPATHY
Statins, widely prescribed cholesterol-lowering drugs for the treatment of dyslipidemia and cardiovascular disease, are associated with skeletal muscle-related complaints or myopathies. Apoptosis is programmed cell death that is highly regulated and executed via the activation of caspase dependent or independent signaling. In general, apoptosis plays an important role in governing development, growth, and repair in cells [8]. However, excessive apoptosis may be associated with dysfunction, disease, and myopathy in skeletal muscle. It has been reported that statin treatment can induce apoptosis in skeletal muscle in both human [9–12] and rodent [13–16] models. For example, simvastatin treatment (5 μM) during 48 hours increased protein levels of proapoptotic protein Bax and apoptosis marker TUNEL-positive nuclei in primary human skeletal muscle cells [12]. Furthermore, Kobayashi et al. [11] showed that cerivastatin treatment (100 μM) during 24–72 hours elevated apoptosis in rhabdomyosarcoma cells from human subjects.
Mitochondria play a central role in regulating homeostasis as well as inducing apoptosis in skeletal muscle. Therefore, mitochondrial dysfunction is associated with the increase in the susceptibility to apoptosis and oxidative stress in skeletal muscle. Previous studies showed that statins might impair mitochondrial function in the skeletal muscles of humans [17–23] and animals [15,24], leading to myopathy. For example, patients with hypercholesterolemia taking simvastatin (80 mg/day) for 8 weeks displayed a decrease in mitochondrial respiratory chain enzyme and citrate synthase activities [20]. Stains also inhibit the synthesis of ubiquinone (coenzyme Q10), a major electron carrier in the mitochondrial respiratory chain [5,17]. However, statin treatment does not appear to consistently affect mitochondrial function in the whole body. Chung et al. [25] showed that fat oxidation and respiratory exchange ratio (RER) did not change in patients with hypercholesterolemia taking atorvastatin (40 mg/day) for 8 weeks. Table 1 summarizes the effects of statins on the whole body and skeletal myopathy.
Table 1.
Effects of statins on whole body and skeletal myopathy
| Subject or animal | Sex | Types of statins (doses) | Treatment | Duration | Tissues | Results | References |
|---|---|---|---|---|---|---|---|
| Patients with hypercholesterolemia | Both | Simvastatin Pravastatin Fluvastatin |
Oral intake | 8 weeks | Serum | ↓ Ubiquinone ↑ Lactate/pyruvate ratio |
Pinieux et al., 1996 [17] |
| Patients with hypercholesterolemia | Both | Simvastatin (80 mg/day) Lovastatin (40 mg/day) Atorvastatin (20 mg/day) |
Oral intake | 2–4 years | Muscle biopsy | ↓ Muscle strength ↓ Cytochrome oxidase activity |
Phillips et al., 2002 [18] |
| Healthy subjects | - | Simvastatin (30 μM) | Cell culture | 24 hours | Primary skeletal muscle cells from muscle biopsy | ↑Apoptosis | Sacher et al., 2005 [9] |
| Healthy subjects | Male | Simvastatin (200 μM) | Fiber incubation | Acute | Muscle biopsy (quadriceps) | ↑ Mitochondrial membrane depolarization ↑ Cytoplasmic Ca2+ |
Sirvent et al., 2005 [19] |
| Patients with hypercholesterolemia | Both | Simvastatin (80 mg/day) | Oral intake | 8 weeks | Muscle biopsy (quadriceps femoris) | ↓ Respiratory chain enzyme ↓ Citrate synthase activity |
Paiva et al., 2005 [20] |
| Patients with heart disease | - | Simvastatin (5 μM) | Cell culture | 96 hours | Cardiac myocytes | ↓ Mcl-1(inhibitor of apoptosis) ↔ Bax ↑ DNA fragmentation |
Demyanets et al., 2006 [10] |
| Healthy subjects | - | Cerivastatin (100 μM) | Cell culture | 24–72 hours | Rhabdomyosarcoma cells | ↑ Apoptosis | Kobayashi et al., 2007 [11] |
| Patients with hypercholesterolemia | Both | Simvastatin (80 mg/day) | Oral intake | 8 weeks | Muscle biopsy (quadriceps) | ↓ Mitochondrial DNA ↓ LDL |
Schick et al., 2007 [21] |
| Patients with hypercholesterolemia | Female | Atorvastatin (40 mg/day) | Oral intake | 8 weeks | - Whole body - Plasma |
↔ RER & anaerobic threshold ↔ Fat oxidation |
Chung et al., 2008 [25] |
| Patients with hypercholesterolemia | Both | Simvastatin (10–80 mg/day) Atorvastatin (10–80 mg/day) |
Oral intake | 4 months | Muscle biopsy (vastus lateralis) | ↓ Oxidative phosphorylation | Hubal et al., 2011 [22] |
| Patients with statin-induced myopathy | Both | Simvastatin (20 mg/day) Atorvastatin (20 mg/day) |
Oral intake | 24–48 months | Muscle biopsy (deltoid) | ↑ ROS ↓ mRNA of SOD1,2 |
Bouitbir et al., 2012 [23] |
| Healthy subjects | Male | Simvastatin (5 μM) | Cell culture | 48 hours | Primary skeletal muscle cells from muscle biopsy | ↓ O2 consumption ↑ Oㆍ−2 & H2O2 ↑ Apoptosis |
Kwak et al., 2012 [12] |
| Rats | Male | Atorvastatin (100 µM) | Cell culture | 24 hours | Vascular smooth muscle cells | ↑ Apoptosis | Guijarro et al., 1998 [13] |
| Rats & Humans | - | Cerivastatin (50, 100 nM) | Cell culture | 24 hours | - L-6 cells - Human (fetal thigh) myotubes |
↑ Apoptosis | Johnson et al., 2004 [14] |
| Rats | Male | Fluvastatin (20 mg/kg/day) Atorvastatin (10 mg/kg/day) |
Oral intake | 2 months | Muscle biopsy (EDL, TA) | ↑ Myoglobinemia | Pierno et al., 2006 [26] |
| Mice | Both | Lovastatin (100 mg/kg/day) | Oral intake | 15 days | Mitochondria from muscle and liver | ↑ Mitochondrial permeability transition | Velho et al., 2006 [24] |
| Rats | - | Cerivastatin (100 µM) Fluvastatin (100 µM) Atorvastatin (100 µM) Simvastatin (100 µM) |
Cell culture | 24 hours | L-6 cells | ↑ Cell death (apoptosis) ↓ Mitochondrial membrane potential ↓ O2 consumption & beta-oxidation |
Kaufmann et al., 2006 [15] |
| Rats | Female | Simvastatin (88 mg/kg/day) | Oral intake | 12 days | Muscle biopsy (biceps femoris) | ↑ Necrosis ↑ PDK4 & MAFbx |
Mallinson et al., 2012 [16] |
POTENTIAL MECHANISMS OF STATIN-INDUCED MYOPATHY
Although numerous studies on statin-associated myopathy have been reported in animals and humans, the molecular mechanisms of statin-induced myopathy have not been completely elucidated. A variety of hypotheses regarding potential mechanisms of statin-induced myopathy have been proposed to gain insight into myopathy in skeletal muscle, including (a) deficiency of ubiquinone, (b) reactive oxygen species (ROS) production, and (c) induction of apoptosis.
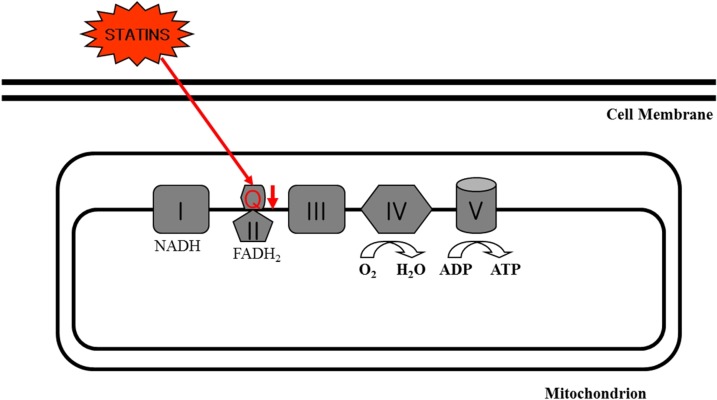
Ubiquinone is located in the mitochondrial respiratory chain, where it plays an essential role in transferring electrons from complex I and II to complex III associated with oxidative phosphorylation and energy production [27]. In addition, ubiquinone acts as a potent antioxidant in the inner mitochondrial membrane by scavenging free radicals [28]. However, it has been shown that statins reduced levels of ubiquinone in muscle and blood (Fig. 2). The rationale of statin-induced decrease in ubiquinone is the fact that statins can inhibit the biosynthesis of ubiquinone as well as cholesterol in the cholesterol synthesis pathway as shown in Fig. 1. For example, blood and muscle concentrations of ubiquinone were decreased after short- and long-term treatment with statins [20,29], which suggests that deficiency of ubiquinone in mitochondria may impair cellular respiration resulting in skeletal myopathy and that supplementation with ubiquinone may be an appropriate therapy to counteract adverse side effects of statin treatment.
Fig. 2.
Ubiquinone (Q) inhibition by statins in the mitochondrial electron transfer chain.
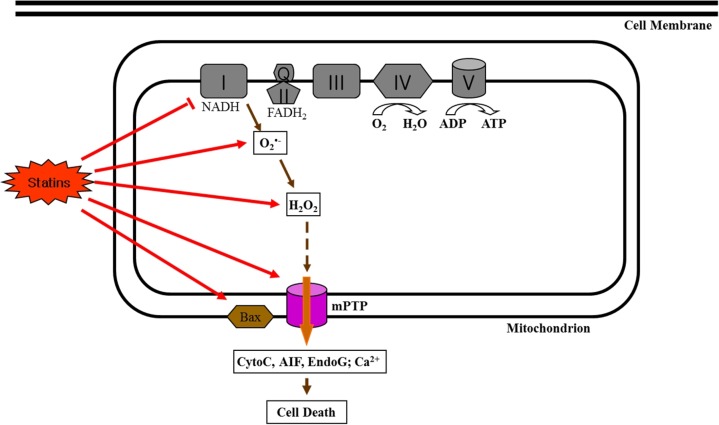
Impaired mitochondrial function is involved in the production of oxidative stress in cells. Most oxidative stress, such as ROS, is generated in the mitochondria. In particular, superoxide (O2ㆍ−) free radicals are generated from complex I (mainly) and complex III in the electron transport system and changed to hydrogen peroxide (H2O2). It has been recently reported that statin treatment increased oxidative stress in human skeletal muscle cells [12] and fibers [23] (Fig. 3). For example, we recently found that simvastatin treatment induced mitochondrial oxidative stress as indicated by increases in O2ㆍ− and H2O2 production as well as impaired oxygen consumption supported by complex I substrates (glutamate + malate) [12].
Fig. 3.
Effects of statins on reactive oxygen species (ROS) production and apoptotic signaling.
In addition, it has been suggested that statin-induced myopathy is associated with apoptosis in skeletal muscle [5,9,12,30]. As mentioned above, statins induce apoptosis in skeletal muscle, which may be an essential factor causing myopathy experienced by patients taking stains. In general, apoptosis is induced through three major apoptotic signaling pathways: the (a) mitochondrial-driven pathway, (b) cytokines/Fas-driven pathway, and (c) endoplasmic reticulum (ER)/Ca2+-driven pathway [31]. However, statin-induced apoptosis in skeletal muscle may be mitochondrial-mediated as indicated by an increase in Bax, release of cytochrome c, active caspase-9, and caspase-3 by statin treatment [12,30]. In particular, the increase in ROS (e.g., O2ㆍ− and H2O2) generation with statin treatment may play an important role in opening the mitochondrial permeability transition pore (mPTP), which results in caspase dependent (e.g., cytochrome c and caspase-9) or independent (e.g., apoptosis inducing factor [AIF] and EndoG) apoptosis in skeletal muscle (Fig. 3), suggesting that statin-induced oxidative stress triggers mitochondrial-mediated apoptosis. For example, Kwak et al. [12] demonstrated that simvastatin treatment induced apoptosis as well as oxidative stress in differentiated skeletal muscle cells.
ROLE OF EXERCISE IN STAIN-INDUCED MYOPATHY: FRIEND OR FOE?
Exercise is regarded as one of the most cost effective ways to prevent metabolic and cardiovascular diseases and is recommended to patients as a lifestyle intervention to supplement drug therapy. However, the benefit/risk of exercise with statin therapy has not been thoroughly investigated. To date, the effects of exercise frequency, intensity, time or type on the risk of statin-induced myopathy have not been well studied. Most studies of the interactions of exercise and statin therapy include an acute/single exercise and indirect measures of muscle damage (i.e., blood creatine kinase [CK] levels). In contrast to statin-induced myopathy, chronic exercise training has the potential to counteract statin-induced side effects in skeletal muscle. For example, endurance exercise training increases mitochondrial biogenesis and mitochondrial respiration, and decreases oxidative stress and apoptosis in skeletal muscle [32].
However, previous studies have shown inconsistent findings regarding the effects of exercise on statin-induced myopathy. While some studies reported that exercise seemed to increase the risk of statin-induced myopathy [33–37], others suggested that exercise did not affect statin-induced myopathy [33,38–42]. For example, 12 weeks of aerobic exercise training in combination with simvastatin (40 mg/day) decreased cardiorespiratory fitness and muscle citrate synthase activity in obese subjects [36]. In addition, 2 weeks of treadmill exercise increased muscle damage in rats taking cerivastatin (0.5–1.0 mg/kg/day) for 2 weeks [37]. In contrast, 10 weeks of endurance and resistance exercise training did not affect serum CK in hypercholesterolemic patients taking rosuvastatin (10 mg/day) for 20 weeks [40]. Furthermore, Meador and Huey [42] showed that 4 weeks of wheel running exercise with cerivastatin treatment (1 mg/kg/day) for 2 weeks prevented statin-associated force loss and increased fatigability in mice, suggesting that exercise prior to statin treatment can protect against statin-induced muscle dysfunction. Table 2 shows a summary of studies examining the effects of exercise on statin-induced myopathy in human and animal models.
Table 2.
Effects of exercise on statin-induced myopathy
| Subject or animal | Sex | Types of exercise (Duration) | Types of statins (doses) | Duration of statin treatment | Tissues | Results | References |
|---|---|---|---|---|---|---|---|
| Healthy subjects | Male | Acute eccentric treadmill exercise (1 hour) | Lovastatin (40 mg/day) | 30 days | Serum | ↔ CK | Reust et al., 1991 [38] |
| Healthy subjects | Both | Acute maximal treadmill exercise | Lovastatin (20 mg/day) | 4 weeks | Serum | ↔ CK | Thompson et al., 1991 [39] |
| Healthy subjects | Male | -Acute downhill treadmill walking (45 min) -Acute biceps curl exercise (10 RM, 4 sets) |
Lovastatin (40 mg/day) | 5 weeks | Serum | - Downhill treadmill: ↑ CK - Biceps exercise: ↔ CK |
Thompson et al., 1997 [33] |
| Healthy subjects | Male | Acute eccentric contractions (30 min) | Atorvastatin (80 mg/day) | 4 weeks | Muscle biopsy (vastus lateralis) | ↑ Ubiquitin proteasome pathway & catabolism | Urso et al., 2005 [34] |
| Patients with hypercholesterolemia | Both | Endurance and resistance exercise (10 weeks) | Rosuvastatin (10 mg/day) | 20 weeks | Serum | ↔ CK | Coen et al., 2009 [40] |
| Athletes with hypercholesterolemia | Both | Acute marathon | All statins (various doses) | 6 months | Plasma | ↑ Statin-related muscle injury (CK) | Parker et al., 2012 [35] |
| A healthy subject | Male | Acute aerobic exercise (1 h 42 min) | Simvastatin (10 mg/day) | 6 months | Blood | ↔ Lipoprotein & white blood cell concentrations | Semple, 2012 [41] |
| Obese subjects | Both | Aerobic exercise (12 weeks) | Simvastatin (40 mg/day) | 12 weeks | -Whole body -Muscle biopsy (vastus lateralis) |
↓ Cardiorespiratory fitness ↓ Muscle citrate synthase activity |
Mikus et al., 2013 [36] |
| Rats | Female | Treadmill exercise (2 weeks) | Cerivastatin (0.5, 1.0 mg/kg/day) | 2 weeks | Muscles | ↑ Muscle damage | Seachrist et al., 2005 [37] |
| Mice | Male | Wheel running (4 weeks) | Cerivastatin (1 mg/kg/day) | 2 weeks | Whole body | ↓ Statin-associated force loss & increased fatigability | Meandor and Huey, 2011 [42] |
CONCLUSIONS
Statins are common cholesterol-lowering drugs for treating cardiovascular disease. However, adverse side effects of statins include skeletal muscle myopathy. Although the mechanisms of statin-induced skeletal myopathy have not been determined, the mechanisms may be associated with ubiquinone deficiency, oxidative stress, and apoptosis. However, the underlying molecular and cellular mechanism by which statins affect mitochondrial function and apoptosis in skeletal muscle remains unknown. Furthermore, it is not clear whether exercise exacerbates statin-associated myopathy in skeletal muscle. Therefore, further studies of patients taking statins with different kinds of exercise are warranted to develop new strategies for statin-associated mitochondrial dysfunction and apoptosis leading to skeletal myopathy.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A1042383) and an Inha University Research Grant.
REFERENCES
- 1.Jasinska M, Owczarek J, Orszulak-Michalak D. Statins: a new insight into their mechanisms of action and consequent pleiotropic effects. Pharmacol Rep. 2007;59:483–99. [PubMed] [Google Scholar]
- 2.Thompson PD, Clarkson P, Kara RH. Statin-associated myopathy. JAMA. 2003;289:1681–90. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- 3.Koh KK, Sakuma I, Quon MJ. Differential metabolic effects of distinct statins. Atherosclerosis. 2011;215:1–8. doi: 10.1016/j.atherosclerosis.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 4.Bellosta S, Corsini A. Statin drug interactions and related adverse reactions. Expert Opin Drug Saf. 2012;11:933–46. doi: 10.1517/14740338.2012.712959. [DOI] [PubMed] [Google Scholar]
- 5.Sirvent P, Mercier J, Lacampagne A. New insights into mechanisms of statin-associated myotoxicity. Curr Opin Pharmacol. 2008;8:333–8. doi: 10.1016/j.coph.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Vaklavas C, Chatzizisis YS, Ziakas A, Zamboulis C, Giannoglou GD. Molecular basis of statin-associated myopathy. Atherosclerosis. 2009;202:18–28. doi: 10.1016/j.atherosclerosis.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Sewright KA, Clarkson PM, Thompson PD. Statin myopathy: incidence, risk factors, and pathophysiology. Curr Atheroscler Rep. 2007;9:389–96. doi: 10.1007/s11883-007-0050-3. [DOI] [PubMed] [Google Scholar]
- 8.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–6. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 9.Sacher J, Weigl L, Werner M, Szegedi C, Hohenegger M. Delineation of myotoxicity induced by 3-hydroxy-3-methylglutaryl CoA reductase inhibitors in human skeletal muscle cells. J Pharmacol Exp Ther. 2005;314:1032–41. doi: 10.1124/jpet.105.086462. [DOI] [PubMed] [Google Scholar]
- 10.Demyanets S, Kaun C, Pfaffenberger S, Hohensinner PJ, Rega G, Pammer J, Maurer G, Huber K, Wojta J. Hydroxymethylglutaryl-coenzyme A reductase inhibitors induce apoptosis in human cardiac myocytes in vitro. Biochem Pharmacol. 2006;71:1324–30. doi: 10.1016/j.bcp.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi M, Kaido F, Kagawa T, Itagaki S, Hirano T, Iseki K. Preventive effects of bicarbonate on cerivastatin-induced apoptosis. Int J Pharm. 2007;341:181–8. doi: 10.1016/j.ijpharm.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Kwak HB, Thalacker-Mercer A, Anderson EJ, Lin CT, Kane DA, Lee NS, Cortright RN, Bamman MM, Neufer PD. Simvastatin impairs ADP-stimulated respiration and increases mitochondrial oxidative stress in primary human skeletal myotubes. Free Radic Biol Med. 2012;52:198–207. doi: 10.1016/j.freeradbiomed.2011.10.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guijarro C, Blanco-Colio LM, Ortego M, Alonso C, Ortiz A, Plaza JJ, Díaz C, Hernández G, Egido J. 3-Hydroxy-3-methylglutaryl coenzyme a reductase and isoprenylation inhibitors induce apoptosis of vascular smooth muscle cells in culture. Circ Res. 1998;83:490–500. doi: 10.1161/01.RES.83.5.490. [DOI] [PubMed] [Google Scholar]
- 14.Johnson TE, Zhang X, Bleicher KB, Dysart G, Loughlin AF, Schaefer WH, Umbenhauer DR. Statins induce apoptosis in rat and human myotube cultures by inhibiting protein geranylgeranylation but not ubiquinone. Toxicol Appl Pharmacol. 2004;200:237–50. doi: 10.1016/j.taap.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann P, Torok M, Zahno A, Waldhauser KM, Brecht K, Krahenbuhl S. Toxicity of statins on rat skeletal muscle mitochondria. Cell Mol Life Sci. 2006;63:2415–25. doi: 10.1007/s00018-006-6235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mallinson JE, Constantin-Teodosiu D, Glaves PD, Martin EA, Davies WJ, Westwood FR, Sidaway JE, Greenhaff PL. Pharmacological activation of the pyruvate dehydrogenase complex reduces statin-mediated upregulation of FOXO gene targets and protects against statin myopathy in rodents. J Physiol. 2012;590:6389–402. doi: 10.1113/jphysiol.2012.238022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinieux G, Chariot P, Ammi-Saïd M, Louarn F, Lejonc JL, Astier A, Jacotot B, Gherardi R. Lipid-lowering drugs and mitochondrial function: effects of HMG-CoA reductase inhibitors on serum ubiquinone and blood lactate/pyruvate ratio. Br J Clin Pharmacol. 1996;42:333–7. doi: 10.1046/j.1365-2125.1996.04178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips PS, Haas RH, Bannykh S, Hathaway S, Gray NL, Kimura BJ, Vladutiu GD, England JD, Scripps Mercy Clinical Research Center Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med. 2002;137:581–5. doi: 10.7326/0003-4819-137-7-200210010-00009. [DOI] [PubMed] [Google Scholar]
- 19.Sirvent P, Mercier J, Vassort G, Lacampagne A. Simvastatin triggers mitochondria-induced Ca2+ signaling alteration in skeletal muscle. Biochem Biophys Res Commun. 2005;329:1067–75. doi: 10.1016/j.bbrc.2005.02.070. [DOI] [PubMed] [Google Scholar]
- 20.Paiva H, Thelen KM, Van Coster R, Smet J, De Paepe B, Mattila KM, Laakso J, Lehtimaki T, von Bergmann K, Lutjohann D, Laaksonen R. High-dose statins and skeletal muscle metabolism in humans: a randomized, controlled trial. Clin Pharmacol Ther. 2005;78:60–8. doi: 10.1016/j.clpt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Schick BA, Laaksonen R, Frohlich JJ, Paiva H, Lehtimaki T, Humphries KH, Cote HC. Decreased skeletal muscle mitochondrial DNA in patients treated with high-dose simvastatin. Clin Pharmacol Ther. 2007;81:650–3. doi: 10.1038/sj.clpt.6100124. [DOI] [PubMed] [Google Scholar]
- 22.Hubal MJ, Reich KA, De Biase A, Bilbie C, Clarkson PM, Hoffman EP, Thompson PD. Transcriptional deficits in oxidative phosphorylation with statin myopathy. Muscle Nerve. 2011;44:393–401. doi: 10.1002/mus.22081. [DOI] [PubMed] [Google Scholar]
- 23.Bouitbir J, Charles AL, Echaniz-Laguna A, Kindo M, Daussin F, Auwerx J, Piquard F, Geny B, Zoll J. Opposite effects of statins on mitochondria of cardiac and skeletal muscles: a ‘mitohormesis’ mechanism involving reactive oxygen species and PGC-1. Eur Heart J. 2012;33:1397–407. doi: 10.1093/eurheartj/ehr224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Velho JA, Okanobo H, Degasperi GR, Matsumoto MY, Alberici LC, Cosso RG, Oliveira HC, Vercesi AE. Statins induce calcium-dependent mitochondrial permeability transition. Toxicology. 2006;219:124–32. doi: 10.1016/j.tox.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Chung J, Brass EP, Ulrich RG, Hiatt WR. Effect of atorvastatin on energy expenditure and skeletal muscle oxidative metabolism at rest and during exercise. Clin Pharmacol Ther. 2008;83:243–50. doi: 10.1038/sj.clpt.6100264. [DOI] [PubMed] [Google Scholar]
- 26.Pierno S, Didonna MP, Cippone V, De Luca A, Pisoni M, Frigeri A, Nicchia GP, Svelto M, Chiesa G, Sirtori C, Scanziani E, Rizzo C, De Vito D, Conte Camerino D. Effects of chronic treatment with statins and fenofibrate on rat skeletal muscle: a biochemical, histological and electrophysiological study. Br J Pharmacol. 2006;149:909–19. doi: 10.1038/sj.bjp.0706917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Littarru GP, Langsjoen P. Coenzyme Q10 and statins: biochemical and clinical implications. Mitochondrion. 2007;7:S168–74. doi: 10.1016/j.mito.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Kettawan A, Takahashi T, Kongkachuichai R, Charoenkiatkul S, Kishi T, Okamoto T. Protective effects of coenzyme Q10 on decreased oxidative stress resistance induced by simvastatin. J Clin Biochem Nutr. 2007;40:194–202. doi: 10.3164/jcbn.40.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laaksonen R, Ojala JP, Tikkanen MJ, Himberg JJ. Serum ubiquinone concentrations after short- and long-term treatment with HMG-CoA reductase inhibitors. Eur J Clin Pharmacol. 1994;46:313–7. doi: 10.1007/BF00194398. [DOI] [PubMed] [Google Scholar]
- 30.Dirks AJ, Jones KM. Statin-induced apoptosis and skeletal myopathy. Am J Physiol Cell Physiol. 2006;291:C1208–12. doi: 10.1152/ajpcell.00226.2006. [DOI] [PubMed] [Google Scholar]
- 31.Pollack M, Leeuwenburgh C. Apoptosis and aging: role of the mitochondria. J Gerontol A Biol Sci Med Sci. 2001;56:475–82. doi: 10.1093/gerona/56.11.B475. [DOI] [PubMed] [Google Scholar]
- 32.Hood DA, Uguccioni G, Vainshtein A, D’souza D. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle: implications for health and disease. Compr Physiol. 2011;1:1119–34. doi: 10.1002/cphy.c100074. [DOI] [PubMed] [Google Scholar]
- 33.Thompson PD, Zmuda JM, Domalik LJ, Zimet RJ, Staggers J, Guyton JR. Lovastatin increases exercise-induced skeletal muscle injury. Metabolism. 1997;46:1206–10. doi: 10.1016/S0026-0495(97)90218-3. [DOI] [PubMed] [Google Scholar]
- 34.Urso ML, Clarkson PM, Hittel D, Hoffman EP, Thompson PD. Changes in ubiquitin proteasome pathway gene expression in skeletal muscle with exercise and statins. Arterioscler Thromb Vasc Biol. 2005;25:2560–6. doi: 10.1161/01.ATV.0000190608.28704.71. [DOI] [PubMed] [Google Scholar]
- 35.Parker BA, Augeri AL, Capizzi JA, Ballard KD, Troyanos C, Baggish AL, D’Hemecourt PA, Thompson PD. Effect of statins on creatine kinase levels before and after a marathon run. Am J Cardiol. 2012;109:282–7. doi: 10.1016/j.amjcard.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 36.Mikus CR, Boyle LJ, Borengasser SJ, Oberlin DJ, Naples SP, Fletcher J, Meers GM, Ruebel M, Laughlin MH, Dellsperger KC, Fadel PJ, Thyfault JP. Simvastatin impairs exercise training adaptations. J Am Coll Cardiol. 2013;62:709–14. doi: 10.1016/j.jacc.2013.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seachrist JL, Loi CM, Evans MG, Criswell KA, Rothwell CE. Roles of exercise and pharmacokinetics in cerivastatin-induced skeletal muscle toxicity. Toxicol Sci. 2005;88:551–61. doi: 10.1093/toxsci/kfi305. [DOI] [PubMed] [Google Scholar]
- 38.Reust CS, Curry SC, Guidry JR. Lovastatin use and muscle damage in healthy volunteers undergoing eccentric muscle exercise. West J Med. 1991;154:198–200. [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson PD, Gadaleta PA, Yurgalevitch S, Cullinane E, Herbert PN. Effects of exercise and lovastatin on serum creatine kinase activity. Metabolism. 1991;40:1333–6. doi: 10.1016/0026-0495(91)90039-Y. [DOI] [PubMed] [Google Scholar]
- 40.Coen PM, Flynn MG, Markofski MM, Pence BD, Hannemann RE. Adding exercise training to rosuvastatin treatment: influence on serum lipids and biomarkers of muscle and liver damage. Metabolism. 2009;58:1030–8. doi: 10.1016/j.metabol.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 41.Semple SJ. Statin therapy, myopathy and exercise--a case report. Lipids Health Dis. 2012;11:40. doi: 10.1186/1476-511X-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meador BM, Huey KA. Statin-associated changes in skeletal muscle function and stress response after novel or accustomed exercise. Muscle Nerve. 2011;44:882–9. doi: 10.1002/mus.22236. [DOI] [PubMed] [Google Scholar]