Abstract
Non-alcoholic fatty liver disease (NAFLD) is a hepatic presentation of obesity and metabolic syndrome. NAFLD includes a large spectrum of hepatic pathologies that range from simple steatosis and non-alcoholic steatohepatitis (NASH), to liver cirrhosis without an all-encompassing approved therapeutic strategy. Mitochondrial dysfunction is a key component of many metabolic diseases, such as obesity, type 2 diabetes, cancer, NAFLD, and aging. Sirtuin 3 (SIRT3) is a NAD+-dependent deacetylase that regulates many of the mitochondrial proteins that are involved with metabolic homeostasis, oxidative stress, and cell survival. This review discusses the association between mitochondrial dysfunction and insulin resistance and later explore the possibility that SIRT3 plays a protective role against NAFLD by improving mitochondrial dysfunction.
Keywords: Sirtuin 3, Mitochondrial dysfunction, Insulin resistance, Nonalcoholic fatty liver disease
INTRODUCTION
The combination of easily available high-calorie food and physical inactivity is driving the rising prevalence of obesity and metabolic syndrome. Non-alcoholic fatty liver disease (NAFLD) is more than simply a “hepatic disease.” Rather, it is a hepatic presentation of obesity with metabolic impairment by the metabolically active organ triad that comprises the liver, adipose tissue, and skeletal muscle. NAFLD covers a large spectrum of hepatic pathologies, which range from simple steatosis and non-alcoholic steatohepatitis (NASH) to liver cirrhosis. By definition, NAFLD affects 10–24% of the general population in many countries[1]. Because of its association with obesity and insulin resistance, the prevalence of NAFLD is increasing worldwide, including in Korea. However, there is currently no broadly approved therapeutic strategy for treating NAFLD.
There is growing evidence that mitochondrial dysfunction is a common underlying feature of obesity [2], type 2 diabetes [3], NAFLD [4,5], and cancers [6]. Identification of the causes of mitochondrial dysfunction, as well as of potential target molecules that protect cells from mitochondrial dysfunction, will be crucial for treating mitochondria-mediated diseases. Sirtuin 3 (SIRT3) is a NAD+-dependent protein deacetylase that regulates numerous mitochondrial proteins involved in metabolic homeostasis, oxidative stress, and cell survival. SIRT3 is emerging as a promising therapeutic target against mitochondrial dysfunction.
This review discusses mitochondrial dysfunction as an underlying feature of NAFLD and evaluate the potential for SIRT3 to enhance mitochondrial function and, thus, act as a therapeutic target against NAFLD.
MITOCHONDRIAL DYSFUNCTION AND INSULIN RESISTANCE
Mitochondria are the main source of cellular energy production, mostly in the form of ATP and intracellular reactive oxygen species (ROS); they are encased in a double membrane and contain their own unique DNA [7–9]. Increasingly, mitochondria are being seen as the hearts of cells because they regulate various homeostatic processes, such as cell proliferation, apoptosis, oxidative stress, and calcium homeostasis. They are also capable of inherent morphological and metabolic plasticity and can respond to cellular stresses and nutrient demand [10–13].
A large and growing body of literature has demonstrated that long-chain fatty acids (LCFAs) induce mitochondrial dysfunctions that lead to insulin resistance and type 2 diabetes [11,14–17]. Generally, LCFAs induce excessive mitochondrial ROS production by partial inhibition of mitochondrial respiratory chain activities and depolarization of mitochondrial inner membranes (weak uncouplers) [18]. High LCFA intake may lead to an accumulation of ROS, lipotoxicity, alteration of mitochondrial gene expression, and activation of inflammatory signaling in peripheral tissues, resulting in mitochondrial dysfunction [11]. There are also reports that free fatty acids can induce hepatic lipotoxicity and insulin resistance through mitochondrial dysfunction [19–21].
Another hypothesis is that mitochondrial dysfunction causes insulin resistance; consequently, the defects in mitochondrial beta-oxidation induce an increase in intracellular fatty acid metabolites that disrupt insulin signaling [22–24]. Petersen et al. found that skeletal insulin resistance in the insulin-resistant offspring of patients with type 2 diabetes was accompanied by an increase of intramyocellular fatty-acid content, compared with insulin-sensitive control subjects. This may be because of an inherited defect associated with mitochondrial oxidative phosphorylation [3]. Another study showed that intrinsic mitochondrial dysfunction at the level of both electron-transport chain capacity and the oxidative phosphorylation system is implicated as the etiology of type 2 diabetes [25]. There is an impairment of mitochondrial function and structure in the skeletal muscle of patients with type 2 diabetes and obesity [24], suggesting that impaired mitochondrial function in muscle and other tissue can lead to lipid accumulation, which in turn can induce insulin resistance. As a consequence of defective mitochondrial fatty acid oxidation, intracellular levels of lipid metabolites (long-chain fatty acyl-coenzyme A and diacylglycerol) are increased in skeletal muscle, which disrupts insulin signaling [22].
Growing evidence has demonstrated an association between mitochondrial dysfunction and insulin resistance. Nevertheless, the overall picture of insulin resistance remains murky. Herein, mitochondrial dysfunction is postulated as a causal factor for the regulation of insulin resistance.
SIRTUINS IMPROVE MITOCHONDRIAL FUNCTION
The silent information regulation-2 (SIR2) gene has been found to promote longevity in Saccharomyces cerevisiae [26] and SIR2 was identified as a NAD+-dependent deacetylase for histone proteins that induces calorie restriction and life-span extension in yeast [27].
The mammalian sirtuins are a family of NAD+-dependent deacetylases and/or ADP-ribosyltransferases that are homologous to the Saccharomyces cerevisiae gene, SIR2 [28]. Humans have seven sirtuins, SIRT1-SIRT7 [28,29], that share a catalytic domain with SIR2 and act as cellular energy sensors that modulate metabolic processes [28].
The mammalian sirtuins all have different subcellular distributions, with a subset of sirtuins residing predominantly in nuclear (SIRT1, SIRT6, and SIRT7), cytosolic (SIRT2), or mitochondrial (SIRT3, SIRT4, and SIRT5) compartments [30,31]. Defects in the pathways controlled by SIRT1 and SIRT3 are known to result in various metabolic and neurodegenerative diseases, such as obesity, type 2 diabetes, nonalcoholic fatty liver disease, Alzheimer disease, and cancer [8,32–35].
SIRT1 and SIRT3 are known to increase mitochondrial biogenesis and improve mitochondrial function [36–39]. SIRT3 is a major regulator of mitochondrial protein acetylation levels and its biological activities [40]. Consistent with this framework, mice lacking SIRT3 showed increased hyperacetylation of mitochondrial proteins and, in contrast, mice lacking either SIRT4 or SIRT5 showed no obvious changes in mitochondrial protein acetylation [41]. SIRT4 regulates ATP homeostasis via adenine nucleotide translocator 2 (ANT2) and a feedback loop involving AMP-activated protein kinase (AMPK) [42] and resveratrol induces a mitochondrial NADH oxidation via SIRT3 in hepG2 cells [43]. Take collectively, these reports suggested that sirtuins may improve mitochondrial dysfunction.
SIRT3 AND MITOCHONDRIAL FUNCTION IN NAFLD
SIRT3 is the most studied member of the mitochondrial sirtuins family; it is nuclear encoded and expressed as a 45-kDa protein containing an N-terminal mitochondrial targeting sequence that is cleaved off after import into the mitochondria, leaving an enzymatically active 28-kDa protein [44].
SIRT3 is highly expressed in the brain, heart, kidney, brown adipose tissue, and liver with high oxidative capacity and is preferentially localized to the mitochondrial matrix [28]. SIRT3 plays an important role in mitochondrial metabolism through a reversible acetylation process of mitochondrial proteins [28,41,45]. SIRT3 expression in the liver increases after fasting [46] and SIRT3 expression in muscle tissue increases after exercise [47], fasting, and caloric restriction and it decreases with chronic high fat eating [48].
The germline knockout of the Sirt3 mouse model has been utilized extensively to elucidate the physiological role of SIRT3 in metabolism. Heber et al. quantified 1,578 mitochondrial acetyl sites during caloric restriction and observed loss of SIRT3 using mass spectrometry, suggesting that SIRT3 is an important regulator in caloric restriction adaptation [49]. Additionally, it was recently discovered that Sirt3 can regulate amino acid metabolism and mitochondrial integrity, including mtDNA transcription and translation, compared to wild-type mice [49].
One particular observational study demonstrated that Sirt3-deficient mice are metabolically unremarkable under both fed and fasted conditions and that Sirt3 increased global hyperacetylation of mitochondrial proteins [41]. Another study demonstrated that mice lacking Sirt3 had diminished fatty acid oxidation during fasting, hyperacetylated long-chain acyl CoA dehydrogenase (LCAD) [46], reduced basal levels of ATP in the heart and liver and increased acetylation of mitochondrial proteins, including Complex I [50].
Mice fed a chronic high-fat diet had low Sirt3 activity, impaired mitochondrial function, and hyperacetylation of proteins in their livers [51]. In another study, mice lacking Sirt3 who were fed a chronic high-fat diet developed accelerated obesity, insulin resistance, and steatohepatitis, compared to wild-type mice [52]. However, mice with liver- or muscle-specific Sirt3 deficiency showed no significant metabolic differences to wild-type mice, except that, even after being fed a high-fat diet, wild-type mice still experienced hyperacetylation of mitochondrial proteins [53]. In yet another study of a transgenic mouse with muscle-specific Sirt3 expression increased oxygen consumption, lipid utilization, and reduced muscle strength were observed compared to wild-type mice [54]. Further studies are needed to clarify the tissue-specific actions of Sirt3.
The study data from Sirt3-deficient mice suggest that target mitochondrial proteins, which are in a hyperacetylated form possess sufficient activity to achieve metabolic homeostasis under basal normal conditions, however, under conditions of oxidative stress, such as having a high-fat diet, SIRT3-target enzyme activity required for protection cannot be sufficiently increased to meet the demand resulting from metabolic derangement due to obesity and steatohepatitis [8].
SIRT3 regulates carbohydrate metabolism, ketogenesis [55], β-oxidation [46], and amino-acid metabolism [41] by reversible enzyme deacetylation and activity of specific mitochondrial complexes [50] and stress-related pathways [13].
Overall these studies indicate that SIRT3 acts as a master switch that is an adaptive response to energy shortages (fasting) in the catabolic pathway and plays a key role in protecting against mitochondrial stress through regulation of acetylation status (Fig. 1). These observations suggest that SIRT3 could be a potential therapeutic target of metabolic syndrome, including NAFLD.
Fig. 1.
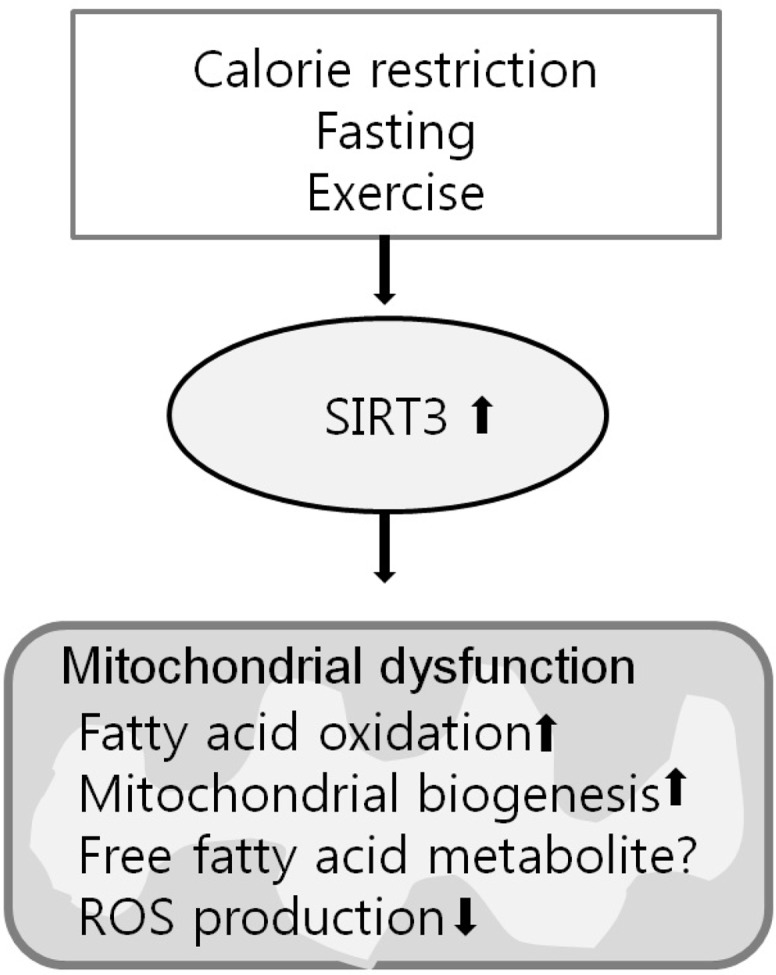
Schematic representation of SIRT3’s primary regulation pathway for mitochondrial dysfunction.
However, there has been little investigation of SIRT3’s gain-of-function effect. It is also unclear which specific tissues play a dominant role in mediating the whole-body effects of SIRT3 [13]. In this regard, liver- and muscle-specific Sirt3-knockout animals show no detectable changes in their metabolic phenotype in response to a high-fat diet [53], suggesting new questions to explore. During the period during which test animals were fed a high-fat diet (8 weeks or 12 months), the genetic background of the animals and the developmental onset of SIRT3 deletion may have led to disparate findings [13,53].
Additionally, compared to caloric restriction, relatively little is known about the role of SIRT3 and NAFLD under conditions of caloric excess, such as receiving a high-fat diet. Palmitate modulates oxygen consumption and enhanced ROS levels and apoptosis in the primary hepatocytes of Sirt3 deficient mice and Sirt3 siRNA-depleted hepatocytes [56].
Overall, these studies demonstrate that there is still much to learn about the metabolic role of SIRT3, including the tissue-specific (liver, muscle, and adipose tissue) and condition-specific (caloric restriction, fasting, and high-fat diet) role of SIRT3 in model animals, as well as the beneficial effects of sirtuin enhancers that have been shown to treat metabolic diseases.
CONCLUSIONS
SIRT3 has emerged as a pivotal therapeutic target in the regulation of mitochondrial dysfunction and signaling that arise, via protein deacetylation, in response to changes in nutrient flux. However, the exact tissue-specific and condition-specific metabolic role of SIRT3 and the beneficial effects of SIRT3 for treating metabolic diseases are still not completely understood. Additionally, there are still concerns about the long-term effects of chronic SIRT3 activation and the yet undiscovered SIRT3-specific activators.
Acknowledgments
This study was supported by a research grant through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Science, ICT, and Future Planning (NRF-2013R1A1A1058962).
REFERENCES
- 1.Ibrahim MA, Kelleni M, Geddawy A. Nonalcoholic fatty liver disease: current and potential therapies. Life Sci. 2013;92:114–8. doi: 10.1016/j.lfs.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Bell JA, Reed MA, Consitt LA, Martin OJ, Haynie KR, Hulver MW, Muoio DM, Dohm GL. Lipid partitioning, incomplete fatty acid oxidation, and insulin signal transduction in primary human muscle cells: effects of severe obesity, fatty acid incubation, and fatty acid translocase/CD36 overexpression. J Clin Endocrinol Metab. 2010;95:3400–10. doi: 10.1210/jc.2009-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–71. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Ruiz C, Baulies A, Mari M, Garcia-Roves PM, Fernandez-Checa JC. Mitochondrial dysfunction in non-alcoholic fatty liver disease and insulin resistance: cause or consequence? Free Radic Res. 2013;47:854–68. doi: 10.3109/10715762.2013.830717. [DOI] [PubMed] [Google Scholar]
- 5.Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW, Ibdah JA. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol. 2010;52:727–36. doi: 10.1016/j.jhep.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaude E, Frezza C. Defects in mitochondrial metabolism and cancer. Cancer Metab. 2014;2:10. doi: 10.1186/2049-3002-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pirinen E, Lo Sasso G, Auwerx J. Mitochondrial sirtuins and metabolic homeostasis. Best Pract Res Clin Endocrinol Metab. 2012;26:759–70. doi: 10.1016/j.beem.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weir HJ, Lane JD, Balthasar N. SIRT3: A Central Regulator of Mitochondrial Adaptation in Health and Disease. Genes Cancer. 2013;4:118–24. doi: 10.1177/1947601913476949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raha S, Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci. 2000;25:502–8. doi: 10.1016/S0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- 10.Giorgi C, Agnoletto C, Bononi A, Bonora M, De Marchi E, Marchi S, Missiroli S, Patergnani S, Poletti F, Rimessi A, Suski JM, Wieckowski MR, Pinton P. Mitochondrial calcium homeostasis as potential target for mitochondrial medicine. Mitochondrion. 2012;12:77–85. doi: 10.1016/j.mito.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hafizi Abu Bakar M, Kian Kai C, Wan Hassan WN, Sarmidi MR, Yaakob H, Zaman Huri H. Mitochondrial dysfunction as a central event for mechanisms underlying insulin resistance: the roles of long chain fatty acids. Diabetes Metab Res Rev. 2014 doi: 10.1002/dmrr.2601. [DOI] [PubMed] [Google Scholar]
- 12.Sinha K, Das J, Pal PB, Sil PC. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol. 2013;87:1157–80. doi: 10.1007/s00204-013-1034-4. [DOI] [PubMed] [Google Scholar]
- 13.Osborne B, Cooney GJ, Turner N. Are sirtuin deacylase enzymes important modulators of mitochondrial energy metabolism? Biochim Biophys Acta. 2014;1840:1295–302. doi: 10.1016/j.bbagen.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Saini V. Molecular mechanisms of insulin resistance in type 2 diabetes mellitus. World J Diabetes. 2010;1:68–75. doi: 10.4239/wjd.v1.i3.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraegen EW, Cooney GJ. Free fatty acids and skeletal muscle insulin resistance. Curr Opin Lipidol. 2008;19:235–41. doi: 10.1097/01.mol.0000319118.44995.9a. [DOI] [PubMed] [Google Scholar]
- 16.Abdul-Ghani MA, DeFronzo RA. Mitochondrial dysfunction, insulin resistance, and type 2 diabetes mellitus. Curr Diab Rep. 2008;8:173–8. doi: 10.1007/s11892-008-0030-1. [DOI] [PubMed] [Google Scholar]
- 17.Hoeks J, Schrauwen P. Muscle mitochondria and insulin resistance: a human perspective. Trends Endocrinol Metab. 2012;23:444–50. doi: 10.1016/j.tem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Schonfeld P, Wojtczak L. Fatty acids decrease mitochondrial generation of reactive oxygen species at the reverse electron transport but increase it at the forward transport. Biochim Biophys Acta. 2007;1767:1032–40. doi: 10.1016/j.bbabio.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Egnatchik RA, Leamy AK, Jacobson DA, Shiota M, Young JD. ER calcium release promotes mitochondrial dysfunction and hepatic cell lipotoxicity in response to palmitate overload. Mol Metab. 2014;3:544–53. doi: 10.1016/j.molmet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egnatchik RA, Leamy AK, Noguchi Y, Shiota M, Young JD. Palmitate-induced activation of mitochondrial metabolism promotes oxidative stress and apoptosis in H4IIEC3 rat hepatocytes. Metabolism. 2014;63:283–95. doi: 10.1016/j.metabol.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura S, Takamura T, Matsuzawa-Nagata N, Takayama H, Misu H, Noda H, Nabemoto S, Kurita S, Ota T, Ando H, Miyamoto K, Kaneko S. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J Biol Chem. 2009;284:14809–818. doi: 10.1074/jbc.M901488200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–7. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 23.Kim EH, Koh EH, Park JY, Lee KU. Adenine nucleotide translocator as a regulator of mitochondrial function: implication in the pathogenesis of metabolic syndrome. Korean Diabetes J. 2010;34:146–53. doi: 10.4093/kdj.2010.34.3.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–50. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 25.Phielix E, Schrauwen-Hinderling VB, Mensink M, Lenaers E, Meex R, Hoeks J, Kooi ME, Moonen-Kornips E, Sels JP, Hesselink MK, Schrauwen P. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes. 2008;57:2943–9. doi: 10.2337/db08-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–80. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 28.Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT, Tschop MH. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev. 2012;92:1479–514. doi: 10.1152/physrev.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun. 2000;273:793–8. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- 30.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13:225–38. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onyango P, Celic I, McCaffery JM, Boeke JD, Feinberg AP. SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc Natl Acad Sci U S A. 2002;99:13653–8. doi: 10.1073/pnas.222538099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giblin W, Skinner ME, Lombard DB. Sirtuins: guardians of mammalian healthspan. Trends in Genetics. 2014;30:271–86. doi: 10.1016/j.tig.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newsom SA, Boyle KE, Friedman JE. Sirtuin 3: A major control point for obesity-related metabolic diseases? Drug Discov Today Dis Mech. 2013;10:e35–e40. doi: 10.1016/j.ddmec.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giralt A, Villarroya F. SIRT3, a pivotal actor in mitochondrial functions: metabolism, cell death and aging. Biochem J. 2012;444:1–10. doi: 10.1042/BJ20120030. [DOI] [PubMed] [Google Scholar]
- 35.Colak Y, Ozturk O, Senates E, Tuncer I, Yorulmaz E, Adali G, Doganay L, Enc FY. SIRT1 as a potential therapeutic target for treatment of nonalcoholic fatty liver disease. Med Sci Monit. 2011;17:Hy5–9. doi: 10.12659/MSM.881749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohamed JS, Hajira A, Pardo PS, Boriek AM. MicroRNA-149 inhibits PARP-2 and promotes mitochondrial biogenesis via SIRT-1/PGC-1alpha network in skeletal muscle. Diabetes. 2014;63:1546–59. doi: 10.2337/db13-1364. [DOI] [PubMed] [Google Scholar]
- 37.Khader A, Yang WL, Kuncewitch M, Jacob A, Prince JM, Asirvatham JR, Nicastro J, Coppa GF, Wang P. Sirtuin 1 activation stimulates mitochondrial biogenesis and attenuates renal injury after ischemia-reperfusion. Transplantation. 2014;98:148–56. doi: 10.1097/TP.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 38.Samant SA, Zhang HJ, Hong Z, Pillai VB, Sundaresan NR, Wolfgeher D, Archer SL, Chan DC, Gupta MP. SIRT3 deacetylates and activates OPA1 to regulate mitochondrial dynamics during stress. Mol Cell Biol. 2014;34:807–19. doi: 10.1128/MCB.01483-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papa L, Germain D. SirT3 regulates the mitochondrial unfolded protein response. Mol Cell Biol. 2014;34:699–710. doi: 10.1128/MCB.01337-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirschey MD, Shimazu T, Huang JY, Schwer B, Verdin E. SIRT3 regulates mitochondrial protein acetylation and intermediary metabolism. Cold Spring Harb Symp Quant Biol. 2011;76:267–77. doi: 10.1101/sqb.2011.76.010850. [DOI] [PubMed] [Google Scholar]
- 41.Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807–14. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ho L, Titus AS, Banerjee KK, George S, Lin W, Deota S, Saha AK, Nakamura K, Gut P, Verdin E, Kolthur-Seetharam U. SIRT4 regulates ATP homeostasis and mediates a retrograde signaling via AMPK. Aging (Albany NY) 2013;5:835–49. doi: 10.18632/aging.100616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desquiret-Dumas V, Gueguen N, Leman G, Baron S, Nivet-Antoine V, Chupin S, Chevrollier A, Vessieres E, Ayer A, Ferre M, Bonneau D, Henrion D, Reynier P, Procaccio V. Resveratrol induces a mitochondrial complex I-dependent increase in NADH oxidation responsible for sirtuin activation in liver cells. J Biol Chem. 2013;288:36662–75. doi: 10.1074/jbc.M113.466490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwer B, North BJ, Frye RA, Ott M, Verdin E. The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase. J Cell Biol. 2002;158:647–7. doi: 10.1083/jcb.200205057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi T, Wang F, Stieren E, Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem. 2005;280:13560–7. doi: 10.1074/jbc.M414670200. [DOI] [PubMed] [Google Scholar]
- 46.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–5. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hokari F, Kawasaki E, Sakai A, Koshinaka K, Sakuma K, Kawanaka K. Muscle contractile activity regulates Sirt3 protein expression in rat skeletal muscles. J Appl Physiol (1985) 2010;109:332–40. doi: 10.1152/japplphysiol.00335.2009. [DOI] [PubMed] [Google Scholar]
- 48.Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL, 3rd, Goodyear LJ, Tong Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Albany NY) 2009;1:771–83. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hebert AS, Dittenhafer-Reed KE, Yu W, Bailey DJ, Selen ES, Boersma MD, Carson JJ, Tonelli M, Balloon AJ, Higbee AJ, Westphall MS, Pagliarini DJ, Prolla TA, Assadi-Porter F, Roy S, et al. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol Cell. 2013;49:186–99. doi: 10.1016/j.molcel.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447–52. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choudhury M, Jonscher KR, Friedman JE. Reduced mitochondrial function in obesity-associated fatty liver: SIRT3 takes on the fat. Aging (Albany NY) 2011;3:175–8. doi: 10.18632/aging.100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, Stancakova A, Goetzman E, Lam MM, Schwer B, Stevens RD, Muehlbauer MJ, Kakar S, Bass NM, Kuusisto J, et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell. 2011;44:177–90. doi: 10.1016/j.molcel.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fernandez-Marcos PJ, Jeninga EH, Canto C, Harach T, de Boer VC, Andreux P, Moullan N, Pirinen E, Yamamoto H, Houten SM, Schoonjans K, Auwerx J. Muscle or liver-specific Sirt3 deficiency induces hyperacetylation of mitochondrial proteins without affecting global metabolic homeostasis. Sci Rep. 2012;2:425. doi: 10.1038/srep00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin L, Chen K, Khalek WA, Ward JL, 3rd, Yang H, Chabi B, Wrutniak-Cabello C, Tong Q. Regulation of skeletal muscle oxidative capacity and muscle mass by SIRT3. PLoS One. 2014;9:e85636. doi: 10.1371/journal.pone.0085636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimazu T, Hirschey MD, Hua L, Dittenhafer-Reed KE, Schwer B, Lombard DB, Li Y, Bunkenborg J, Alt FW, Denu JM, Jacobson MP, Verdin E. SIRT3 deacetylates mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase 2 and regulates ketone body production. Cell Metab. 2010;12:654–61. doi: 10.1016/j.cmet.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bao J, Scott I, Lu Z, Pang L, Dimond CC, Gius D, Sack MN. SIRT3 is regulated by nutrient excess and modulates hepatic susceptibility to lipotoxicity. Free Radic Biol Med. 2010;49:1230–37. doi: 10.1016/j.freeradbiomed.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


