Abstract
Epidemiological studies suggest that fine particulate matter (PM2.5) may increase the risk for developing diabetes mellitus (DM). To evaluate possible mechanisms explaining these associations, we investigated if sub-acute ambient-level exposures can impair insulin sensitivity. Twenty-five healthy adults living in rural Michigan were transported to an urban location for 5 consecutive days (exposure-block) of daily 4- to 5-hour-long ambient air pollution exposures. Health outcomes, including the homeostasis model assessment of insulin resistance (HOMA-IR) the primary outcome of insulin sensitivity, were measured at 3 time points in relation to exposure-blocks: 7 days prior to start; on the last exposure-day; and 7 days after completion. PM2.5 was monitored at the urban exposure site and at community monitors near subjects’ residences. We calculated 3 “sub-acute” exposure periods (approximately 5-days-long) starting retrospective from the time of health outcome measurements (PM2.5 ranges: 9.7±3.9 to 11.2±3.9 µg•m−3). A 10 µg•m−3 increase in sub-acute PM2.5 exposures was associated with increased HOMA-IR (+0.7, 95% confidence interval (CI) 0.1 to 1.3; p=0.023) and reduced heart rate variability (standard deviation of normal-to-normal intervals [−13.1 msec, 95%CI −25.3 to −0.9; p=0.035]). No alterations in other outcomes (inflammatory markers, vascular function) occurred in relation to PM2.5 exposures. Our findings suggest that ambient PM2.5, even at low levels, may reduce metabolic insulin sensitivity supporting the plausibility that air pollution could potentiate the development of DM.
Keywords: Metabolic syndrome, insulin sensitivity, PM2.5, heart rate variability, endothelial function
1. Introduction
Exposure to fine particulate matter < 2.5µm in diameter (PM2.5) air pollution is associated with increased cardiovascular (CV) morbidity and mortality (Brook, et al 2010). Numerous experiments have elucidated biological mechanisms whereby brief exposures could trigger CV events (Brook, et al 2010). Additional studies have also begun to illustrate how longer-term exposures could further increase CV risk, such as by the promotion of atherosclerosis and hypertension (Brook, Rajagopalan, 2009; Brook, Rajagopalan 2010). In this regard, we have recently demonstrated a novel adverse health effect of long-term exposures. In an experimental animal model of diet-induced obesity, PM2.5 was shown to potentiate metabolic insulin resistance (Sun, 2009). In this construct, adverse systemic responses such as inflammation, oxidative stress, autonomic imbalance and endothelial dysfunction may represent “a common soil” that promotes the development of insulin resistance contemporaneously with a pro-atherogenic state. Indeed, a few epidemiological studies suggest that there may be an association between air pollutants and diabetes mellitus (DM) (Brook, et al 2008; Chuang, et al 2010; Kelishadi, et al 2009; Kramer, et al 2010; Pearson, et al 2010; Puett, et al 2011).
DM is a growing worldwide epidemic promoted by many aspects of modern society (Cornier, et al 2008). We have speculated that an under-recognized societal risk factor may be air pollution (Brook, et al 2009). Given the billions of people continuously exposed and the rapid industrial/urban growth among developing nations (Narayan et al 2010), even a modest causal-association would be of major public health importance (Nawrot, et al 2011). Hence, our aim was to evaluate mechanisms potentially explaining this relationship in humans by investigating if commonly-encountered PM2.5 levels are capable of reducing metabolic insulin sensitivity, which is fundamentally involved in the genesis of DM. We designed this study to discern the impact of 5-day-long cumulative exposures given that slowly changing metabolic parameters of insulin sensitivity (i.e. the homeostasis model assessment of insulin resistance (HOMA-IR)) are more likely to be altered within a subject over this “sub-acute” period (Kelishadi, et al 2009). In addition, we sought to explore several underlying mechanisms potentially involved in the putative instigation of metabolic insulin resistance (i.e. systemic inflammation, autonomic imbalance, endothelial dysfunction).
2. Materials and Methods
We enrolled 25 healthy (age 18–50 years) non-smoking subjects from non-smoking households without known CV disease or risk factors (blood pressure < 140/90 mm Hg; fasting glucose < 126 mg/dL; without diagnosis or treatment for hyperlipidemia during screening visits). Subjects with impaired fasting glucose (100–125 mg/dL) could be included. Subjects were not taking any known prescription (e.g. statin, anti-hypertensives) or over-the-counter drugs (e.g. anti-oxidants, fish oil) that could alter CV function. The study was approved by the Institutional Review Board of the University of Michigan and each subject provided written informed consent.
2.2 Study Outline
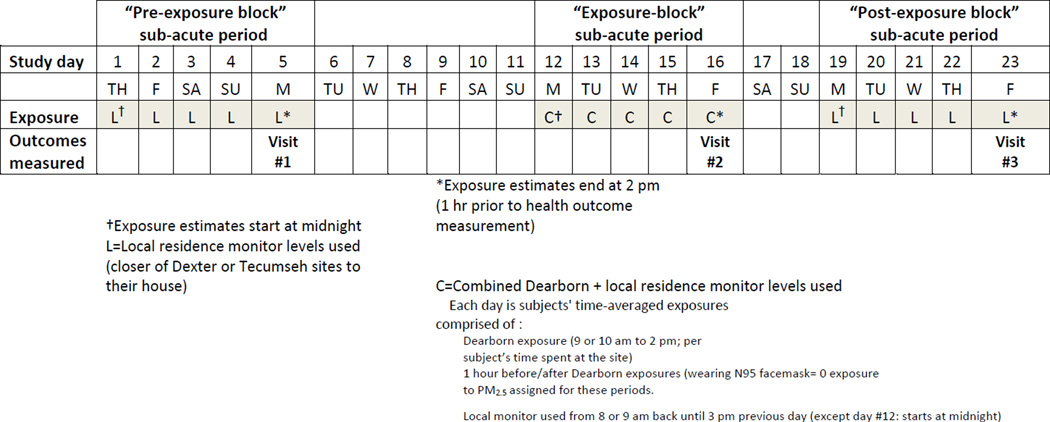
Figure 1 outlines the details of the study that took place during summer months only (June–August) in 2009 and 2010. The primary aim of this study was to investigate if “sub-acute” exposures (defined as 3 separate integrated approximately 5-day-long periods) to ambient PM2.5 are associated with HOMA-IR. Other parameters were measured as secondary outcomes. For the purposes of this study, the rationale for the daily transporting of subjects to an urban location during the exposure block period was to enhance within-subject variability in sub-acute exposure PM2.5 levels. In addition, it was anticipated that this would also alter the characteristics of exposures. The effects of specific PM components will be evaluated in subsequent analyses and is beyond the scope of this first paper focusing on PM2.5 mass alone.
Figure 1. Outline of the study design.
Overview of the study flow and of the sub-acute exposure estimate methods.
The integrated sub-acute exposures start at 2 pm on each of the 3 visit days (1 hour before health outcomes were measured at 3 pm) as described above (L or C) retrospectively until midnight 4 days previous (day #1, #12, #19) for a total of 4 days and 14 hours (110 hours). We had subjects wear the N95 facemask during car travel to the exposure site (1 hour before and 1 hour after daily exposures); hence a uniform zero level PM mass exposure was estimated for these 2 hours each day during the exposure-block. In the GEE, the health outcomes measured at each of the 3 time points were evaluated in relation to their temporally corresponding sub-acute PM2.5 exposure ending 1 hour prior to measurement.
Each subject lived in a location (within 20 kilometers (Km) of Dexter or Tecumseh, Michigan PM monitoring sites and >400 meters (m) from a major highway) that typically exhibits background levels of PM2.5 from 5–10 µg•m−3 lower than the selected urban exposure site (USEPA, website). Subjects were instructed to remain within the region of their residence throughout the study periods. During the “exposure-block” period they were transported for 5 consecutive days (Monday–Friday) to an urban site located in Dearborn Michigan with PM2.5 levels among the highest in the state (Oakes, et al 2010). Each day subjects rested at the same spot (under an open-air shelter if needed) for 4 to 5 hours. Subjects were instructed to remain seated in a chair for the exposure time; however, for brief periods they were permitted to have a low level of activity (e.g., walk to restroom, stand to stretch). Exposures began between 9 or 10 am and ended at 2 pm each day. Subjects were driven to and from this location by a research assistant. During the 1 hour-long transportation each way subjects wore a properly fitted N-95 face mask (3M™ model 1860). A similar mask has recently proven effective in removing almost all PM down to a few nanometers during exposure testing to diesel and traffic-related PM (Langrisk, et al 2009). Subjects wore the facemask during car travel because it would not be possible to accurately assign a PM exposure during this period without personal monitors; hence we could assign a zero level PM exposure uniformly during travel to all subjects when they wore the mask. On the final day (Friday) of the exposure-block period, subjects ate breakfast at 8 AM but remained fasting until completion of the day (~7 hours). On this day subjects were transported to the University of Michigan Research Vascular Laboratory (UMRVL), where they had all the health outcomes and blood drawn starting at 3 PM. On the previous Monday (7 days prior to undergoing the exposure-block) and on the following Friday (7 days after completing the exposure-block) subjects also had health outcomes and blood drawn at the UMRVL starting at 3 PM. They were told to remain fasting (~7 hours) on each of those days after eating similar breakfasts at 8 AM.
2.2 Exposure Measurement and Assignment Methods
The exposure assignment method for each subject over the 3 time periods is outlined in Figure 1. Details regarding PM2.5 measurement methods and aspects of the 3 exposure monitoring site locations are provided in the online supplement methods.
2.3 Health Outcomes Evaluated
All health outcomes assessments were performed on 3 separate visits (Figure 1; study days: 5, 16, 23) at the UMRVL starting at 3 PM. Subjects were instructed to fast for approximately 7 hours (since 8 AM) on each day. The order of testing was the same for each patient and all visits.
Subjects had automated seated blood pressure and heart rate measured (Omron 780) in triplicate on their right arm resting at heart level. After resting supine for 10–15 minutes, subjects had 6 minutes of resting supine heart rate variability recorded and analyzed for the standard deviation of normal-to-normal intervals (SDNN) as well as frequency domain metrics (evo Holter monitor; Pathfinder software; Spacelabs). Afterward, right radial artery tonometry pulse wave analyses for aortic hemodynamic profiles followed by arterial pulse wave velocity for arterial compliance were performed (SyphgmoCor; AtCor Medical). Microvascular endothelial function and nitroglycerin-mediated dilatation were next performed on the right hand using the EndoPAT2000 (Itamar Medical). Further details of the individual protocols are provided in the online supplement methods.
Blood outcomes were drawn last. Insulin and glucose values were drawn in triplicate (5 minute intervals) per optimal HOMA-IR protocol (Wallace, et al 2004; Muniyappa, et al 2008). Along with adiponectin, these blood aliquots were stored in −70C freezer for batched analyses at the Michigan Diabetes Research and Training Center Laboratory (http://www.med.umich.edu/mdrtc/cores/ChemCore/index.html). HOMA-IR was calculated from the average of the 3 measurements at each time period using the following formula: [glucose (mg/dL) × insulin (µU•ml−1)]/405. Lower values correspond to greater insulin sensitivity. HOMA-IR is a validated metric of insulin sensitivity used in repeated measures studies to demonstrate changes across time periods within individuals (Walace et al, 2004; Muniyappa, et al 2008).
All other aliquots of blood were sent to the laboratory of Dr Rajagopalan at Ohio State University for analyses of inflammatory markers. These included sub-types of peripheral blood mononuclear cells (PBMC) and an array of circulating blood cytokine levels. Details regarding these methods are outlined in the online supplement methods.
2.4 Statistical Methods
Data are presented as mean ± standard deviation unless otherwise stated. Comparisons across the mean values from the 3 time periods in Table 1 were performed by repeated measures analysis of variance. Models for determining the overall associations between PM2.5 exposure across 3 periods and each temporally-corresponding health outcomes (including HOMA-IR as the primary outcome) were performed using generalized estimating equations (GEE) to account for the within-subject correlation due to repeated measures. The best fit GEE model was selected based on quasilikelihood independence criteria. Models were performed unadjusted and secondarily adjusted for a few predetermined parameters that alter HOMA-IR (body mass index, age, sex) given the limited sample size. We started with adding BMI and age and excluded either variable if it was individually non-significant (p>0.1) to provide the most parsimonious model.
Table 1.
Cardio-metabolic health outcomes measured among 25 Michigan residents in relation to fine particulate matter air pollution exposures in an urban environment
| Pre-exposure block period (Visit 1: Study day #5) |
Exposure block period (Visit 2: Study day #16) |
Post-exposure block period (Visit 3: Study day #23) |
p† | ||
|---|---|---|---|---|---|
| Microvascular Function | |||||
| Reactive Hyperemia Index (RHI) | 2.20 ± 0.72 | 2.05 ± 0.43 | 1.85 ± 0.40 | 0.14 | |
| Arterial Compliance and Hemodynamics | |||||
| Brachial Systolic Blood Pressure (mm Hg) | 116 ± 13 | 118 ± 14 | 120 ± 14 | 0.13 | |
| Brachial Diastolic Blood Pressure (mm Hg) | 75 ± 10 | 76 ± 9 | 77 ± 9 | 0.17 | |
| Heart Rate (beats•min−1) | 72 ± 12 | 68 ± 10 | 72 ± 11 | 0.08 | |
| Central Aortic Systolic Blood Pressure (mm Hg) | 106.9 ±12.6 | 107.5 ±13.3 | 109.1 ± 14.5 | 0.25 | |
| Augmentation Index @ HR 75 (%) | 13.8 ± 13.5 | 14.1 ± 11.2 | 14.6 ± 10.7 | 0.83 | |
| Pulse Wave Velocity (m•sec−1) | 6.7 ± 1.3 | 6.5 ± 1.3 | 6.7 ± 1.4 | 0.96 | |
| Heart Rate Variability | |||||
| Standard Deviation of Normal-to-Normal Beats (SDNN) (msec) | 57.3 ± 34.6 | 65.1 ± 29.2 | 61.1 ± 29.7 | 0.30 | |
| High Frequency Peak (msec2) | 1217 ± 2274 | 1544 ± 3762 | 1225 ± 2014 | 0.73 | |
| Low Frequency Peak (msec2) | 996 ± 1420 | 1168 ± 1364 | 1120 ± 1020 | 0.71 | |
| Low/High Frequency ratio | 2.1 ± 2.2 | 2.4 ± 2.6 | 2.2 ± 2.2 | 0.38 | |
| Metabolic Blood Biomarkers | |||||
| Glucose (mg•dl−1)* | 84.0 ± 8.0, n=24 | 74.3 ± 6.3, n=23 | 78.5 ± 7.1, n=23 | 0.001 | |
| Insulin (µU•ml−1)* | 15.1 ± 5.8, n=24 | 12.5 ± 4.6, n=24 | 15.1 ± 6.4, n=24 | 0.08 | |
| Homeostasis model assessment of insulin resistance (HOMA-IR)* | 3.26 ± 1.53, n=24 | 2.39 ± 0.98, n=24 | 2.79 ± 1.24, n=23 | 0.05 | |
| Adiponectin (ng•ml−1) | 7021 ± 3539 | 6652 ± 4082 | 6705 ± 3274 | 0.46 | |
All data are presented as mean ± SD, n=25
Data <25 samples in Table 1 values represent the results excluding extreme outliers for insulin and glucose (changes > 2 SD from mean probably representing incomplete fasting state)
P value represents significance of differences between the three measures determined using a general linear model repeated measures analysis of variance.
3. Results
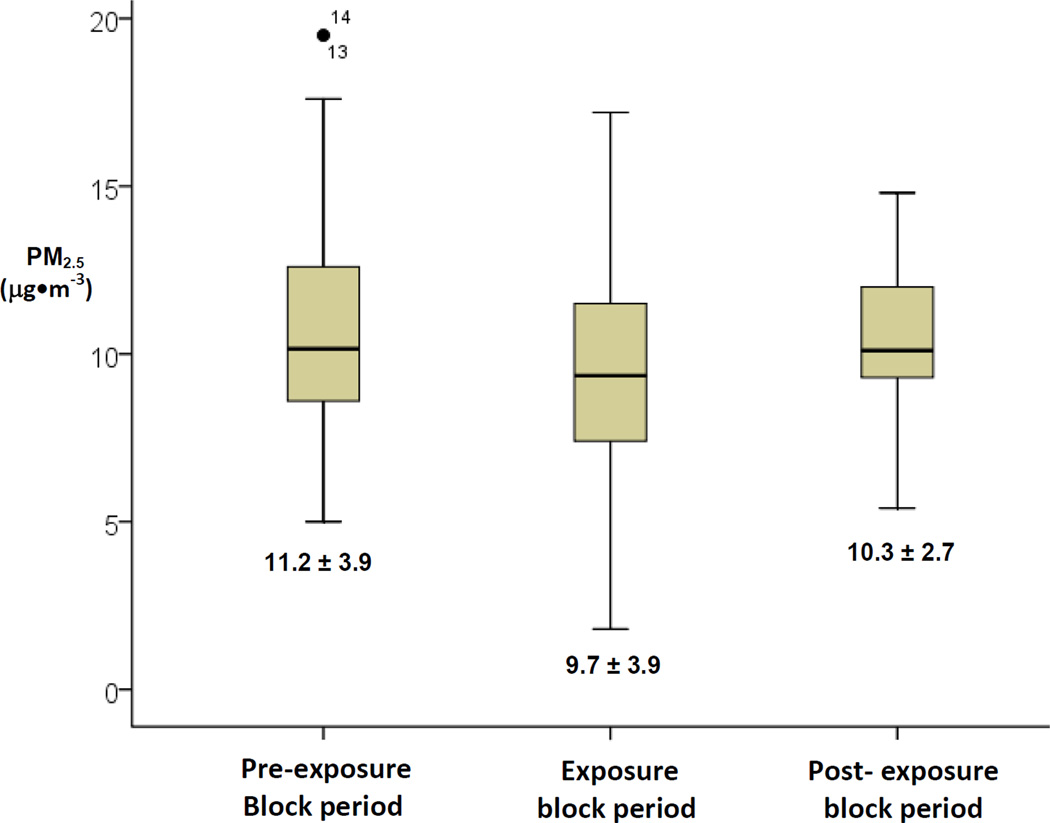
We enrolled a total of 25 subjects (14 subjects during 2009 and 11 during 2010). The mean age and body mass index (BMI) were 38 ± 12 years and 25.7 ± 4.5 kg·m−2, respectively. The cohort consisted of 17 females and 8 males. Other subject-related parameters are presented in Table 1 with the mean results from each of the 3 separate weeks presented separately. The average exposure duration each day at the Dearborn urban site was 239.5 ± 2.3 and 300.4 ± 0.3 minutes for summers 1 and 2, respectively. The mean PM2.5 level that occurred during just the 4–5 hour-long acute exposures at the Dearborn urban site was 11.5 ± 4.8 µg•m−3. The PM2.5 values for the 3 sub-acute integrated 5-day-long exposure periods are presented in Figure 2.
Figure 2. Time-averaged fine particulate matter levels during 5-day periods prior to each outcome measurement.
Box and whiskers represent the inter-quartile ranges and 2 standard deviations of PM2.5 (µg•m−3), respectively
Lines in the box are medians and text data in the figure are the means and standard deviations Individual points represent outliers with individual patient numerical identifier
Table 1 presents the results of the major health outcomes measured at the end of the 3 separate visit periods. There were no significant differences in microvascular endothelial function (reactive hyperemia index), blood pressures, aortic hemodynamics, arterial stiffness (pulse wave velocity), and heart rate variability metrics between the 3 time points. However, glucose and HOMA-IR were significantly lower during the exposure-block week visit when PM2.5 levels also trended lower (lower HOMA-IR implies greater insulin sensitivity). Among all other CV endpoints and biomarkers measured, the only significant differences were found for interleukin (IL)-6, IL-1β, and IL-8 levels that were actually higher during the exposure-block week when HOMA-IR was lower. (Online supplement: Supplement Table 1). All additional outcomes outlined in the methods section are available in the online supplement.
Table 2 presents the sum results for all of the significant associations between the sub-acute PM2.5 exposure levels (all 3 time periods together) and the temporally-corresponding health outcomes. A 10 µg•m−3 increase in sub-acute 5-day PM2.5 level was associated with a reduction in SDNN, consistent with lower overall heart rate variability primarily due to reduced parasympathetic activity (adjusted for age and body mass index), as well as an increase in insulin and HOMA-IR (adjusted and unadjusted models). This means that higher pollution levels were related to lower insulin sensitivity. For an approximate 1 standard deviation change in sub-acute PM2.5 exposure level (3.5 µg•m−3), the adjusted HOMA-IR would increase by 0.25 (95% CI 0.04–0.46). No other CV outcome or blood biomarker (cytokines, PBMC) beyond HOMA-IR and SDNN was associated with the 5-day PM2.5 exposure levels (Table 2).
Table 2.
Associations between cardio-metabolic parameters and sub-acute fine particulate matter air pollution exposures measured among 25 Michigan residents
| OUTCOME | β† | 95% CI | p | β* | 95% CI | p |
|---|---|---|---|---|---|---|
| SDNN (msec) | −11.60 | −24.0 to 0.9 | 0.070 | −13.1 | −25.3 to −0.9 | 0.035 |
| Glucose (mg•dl−1) | 4.6 | −1.0 to 10.3 | 0.109 | 5.4 | 0.5 to 10.3 | 0.029 |
| Insulin (µU•ml−1) | 3.1 | 0.2 to 6.1 | 0.036 | 2.9 | 0.2 to 5.6 | 0.034 |
| HOMA-IR | 0.8 | 0.11 to 1.5 | 0.024 | 0.7 | 0.1 to 1.3 | 0.023 |
Associations of outcomes per 10 µg•m−3 increase in PM2.5 level:
β estimate from the GEE (unadjusted)
β estimate from the GEE adjusted for age + body mass index (SNDD); age (glucose); body mass index (insulin, HOMA-IR). The parameters included in adjusted models were pre-selected as stated in methods and thereafter included because they showed a trend for associations with the health outcome of interest and/or modified the association with exposure. Given the limited sample size (n=25) we adjusted for only up to 2 variables in a model. No major outcome differences were found adjusting for other factors.
No changes in GEE associations occurred between HOMA-IR and PM2.5 exposures whether outlying values for insulin or glucose (thus calculated HOMA-IR) were included or excluded (per Table 1). The association results presented in Table 2 includes all values.
95%CI, 95% confidence interval; SDNN, Standard Deviation of Normal-to-Normal Beats
SDNN was also associated with changes in HOMA-IR across all 3 time periods (β=−0.13 per 10 msec decrease, p=0.035), suggesting that reduced overall heart rate variability (principally lower parasympathetic activity) was related to lower insulin sensitivity. When SDNN and 5-day PM2.5 were jointly considered as predictors of HOMA-IR in unadjusted models, SDNN was significant (β=−0.14 per 10 msec increase, p=0.022) while the effect of 5-day PM2.5 level was reduced (β=0.64 per 10 µg•m−3 increase, p=0.063). Hence, a portion of the effect of PM2.5 on HOMA-IR was possibly mediated via changes in SDNN. Importantly, PM2.5 levels during the post-exposure period time window alone were significantly related to HOMA-IR values measured at the corresponding 3rd time point visit (β=0.43 per 10 µg•m−3 increase, p=0.03). Exposure levels during period 2 (the exposure block period alone) showed a strong similar trend of an association with HOMA-IR values (β=0.28 per 10 µg•m−3 increase, p=0.19). There were no other significant associations between any other health outcomes (e.g. vascular and inflammatory mediators) and sub-acute exposures or HOMA-IR changes.
4. Discussion
Exposures to PM2.5 over sub-acute periods even at relatively low concentrations were associated with reduced metabolic insulin sensitivity among healthy adults. This is the first study to show that this occurs even in relation to PM2.5 levels meeting daily (<35 µg•m−3) U.S. National Ambient Air Quality Standards (NAAQS) (Brook, et al 2010). These results support the emerging evidence that PM2.5 might contribute to the risk for developing DM (Brook, et al 2008; Chuang, et al 2010; Kelishadi, et al 2009; Kramer, et al 2010; Pearson, et al 2010; Puett, et al 2011), given the fundamental role of insulin resistance in its etiology (Cornier, et al 2008). Even if the physiological effect is small compared to traditional risk factors (e.g. obesity), since billions of people are continuously exposed (Narayan, et al 2010) this health effect has the potential to translate into significant population-attributable risks for DM (Nawrot, et al 2011).
4.1 Previous Studies
Few epidemiological studies have explored the relationship between air pollutants and DM (Brook, et al 2008; Chuang, et al 2010; Kelishadi, et al 2009; Kramer, et al 2010; Pearson, et al 2010; Puett, et al 2011). We demonstrated that NO2, a pollutant often associated with traffic pollution, was associated with DM among women in Ontario (Brook, et al 2008). Studies from Germany (traffic pollutants) (Kramer, et al 2010) and a large cross-sectional analysis of DM prevalence in the U.S. (PM2.5 levels) (Pearson, et al 2010) also supported a positive association. On the other hand, another recent study reported mixed results and less consistent relationships (Puett, et al 2011).
Given the small number of studies and mixed results, the presence of a true biological relationship could be more strongly supported by the demonstration of an accompanying mechanistic basis. Only 2 studies have evaluated metrics of insulin sensitivity in relation to PM exposures in humans (Kelishadi, et al 2008); Chuang, et al 2010). In Taiwan, elevations in annual PM2.5 levels (mean 35.3 ± 15.9 µg•m−3) were related to increases in glucose and hemoglobin-A1c (Chuang, et al 2010). Among adolescents living in Isfahan Iran, very high weekly PM10 concentrations (mean 111–157 µg•m−3) were positively associated with HOMA-IR levels (Kelishadi, et al 2008). Though perhaps of importance for developing nations, these concentrations are almost never encountered in the U.S. (4–10 fold higher than typical daily levels) (Brook, et al 2010). These 2 reports showing adverse alterations in HOMA-IR therefore do not directly support the epidemiological associations between PM and DM found in Europe and the U.S. (Brook, et al 2008; Kramer, et al 2010; Pearson, et al 2010; Puett, et al 2011) given that the PM levels were extremely high. Our findings substantively add to these 2 previous papers by demonstrating that PM2.5, even low concentrations meeting NAAQS and encountered by billions of people, are still associated with adverse perturbations in insulin sensitivity. Moreover, our study was designed to specifically investigate the association between PM2.5 and HOMA-IR.
4.2 Potential Mechanisms
Pathways whereby PM2.5 could potentially instigate insulin resistance include the consequences of systemic inflammation (cellular- or cytokine-mediated), a hypothalamic-adrenal stress response, tissue-level oxidative stress altering insulin signaling cascades, direct actions on insulin signaling by pollutant constituents reaching the circulation, reduced nitric oxide bioavailability, blunted tissue perfusion due to endothelial dysfunction or vasoconstriction, altered high density lipoprotein particle function, and/or relative augmentation of sympathetic activity (Brook, et al 2010; Brook, Rajagopalan 2009; Brook, Rajagopalan 2010). At present, the precise mechanism for our findings must remain speculative. Nonetheless, we have previously demonstrated in an animal model an adverse effect of PM2.5 exposure over several weeks on insulin sensitivity by promoting systemic and cellular inflammatory responses in tissues such as the visceral adipose cells (Sun, et al 2009). In addition to direct actions of cytokines (Cornier, et al 2008), inflammatory mechanisms may also involve activation of pattern recognition receptors such as Toll-like Receptor 4 by PM constituents (e.g. LPS) or oxidized fatty acids/phospholipids (Kampfrath, et al 2011; Shi, et al 2006). However, all the circulating inflammatory markers or modulators we evaluated (monocyte sub-types, cytokines, and adiponectin) were not related to PM2.5 exposures or changes in HOMA-IR. While a role for other mediators or longer durations of exposures cannot be excluded, this does not support systemic inflammation as the likely etiology for our findings.
The duration of this study was abbreviated compared to the chronic exposures in our animal studies (Sun, et al 2009). This suggests that alternative biological mechanisms capable of more quickly altering insulin signaling might be involved. The lack of effect of PM exposures on endothelial function argues against an important mechanistic role played by impaired nitric oxide signaling or vasoconstrictive changes. It remains possible that the methodology utilized did not capture the endothelial or hemodynamic changes relevant to perturbations in wholebody insulin sensitivity (i.e. perfusion of large skeletal muscle beds) (Cornier, et al 2008). On the other hand, changes in SDNN (i.e. reduced overall heart rate variability primarily reflecting lower parasympathetic nervous system activity) across the 3 time periods were inversely related to insulin sensitivity. Sub-acute PM2.5 exposure was concomitantly associated with a decrease in SDNN. In a multivariate model, the statistical association between sub-acute PM2.5 levels and HOMA-IR was partially explained by lower SDNN. The results suggest that air pollution-mediated parasympathetic withdrawal (i.e. autonomic imbalance favoring sympathetic activity) may be a contributing mechanism to the rapid worsening of insulin sensitivity. Indeed, alterations in autonomic balance are well-known to mediate changes in insulin sensitivity (Cornier et al 2008; Egan 2003). However, we cannot exclude the possibility that the changes in HOMA-IR and SDNN occurred in parallel and were not mechanistically interlinked. Further studies will be needed to better elucidate the mechanisms whereby PM2.5 could alter metabolic insulin sensitivity in humans. Employing more sophisticated measures of insulin sensitivity (e.g. glucose clamp, glucose tolerance tests) could more firmly corroborate our initial findings and better establish a hepatic versus muscle source of the metabolic derangement.
4.3 Strengths and Limitations
While many panel studies have reported the health effects of different exposure lag periods during post hoc analyses, we believe this to be the first protocol that was a priori designed to investigate the effect of an integrated cumulative 5 day-long exposure to ambient PM2.5 on CV responses and insulin sensitivity. Hence, this represents one of the first attempts to evaluate the physiological effects of “sub-acute” periods of exposure. The reason for our selection of this sub-acute period was that changes in HOMA-IR were thought to likely require several days to occur in response to higher PM exposures.
Mean sub-acute PM2.5 levels were slightly non-significantly lower during the exposure-block period. This occurred due to lower regional levels near the subjects’ residences due to temporal variances (these integrated averages were heavily influenced by the 17 to 18 hours per day of local regional monitor concentrations) and as an effect of the zero level of exposure while wearing the facemask for 2 hours/day. Regardless, this had no consequence upon our primary aim or statistical methods which was to evaluate the effect of 3 pooled sub-acute time periods of PM2.5 exposures upon insulin sensitivity. The travel to the urban location was a method employed in this study design solely to foster greater within-subject variability in PM exposures across the 3 time periods. Moreover, PM2.5 levels measured during the post-exposure period alone were significantly associated with HOMA-IR. These secondary observations add internal validity and support for the authenticity of the overall study findings.
The present paper does not identify the PM2.5 components and sources responsible for the observed effects; though detailed data in this regard and other pollutants (e.g. ozone) were collected as part of the study. These data will be used in future analyses to investigate the role of PM composition and interactions with other co-pollutant on outcomes. Interestingly, several recent studies have reported on this issue; for example, a recent animal inhalation study in a nearby exposure site location in Detroit indicated that modulation of cardiac function was most strongly linked to local industrial sources including iron/steel manufacturing (Rohr, et al 2011). Similarly, determining if there are factors altering subjects’ susceptibilities (e.g. obesity) will require future studies. Details regarding the time course, the dose-response, any interaction with other time-varying parameters (e.g. personal-level ambient temperature), and the effect of other particle sizes (coarse PM10-2.5) will also require further experiments.
We cannot exclude the possibility for exposure estimation errors which is an inherent limitation to all panel studies. We believe there is no evidence for a systematic flaw given that levels during the post-exposure period alone were significantly associated with HOMA-IR. It is also possible that particle constituent differences between the exposure periods, particularly at the urban site, may have had additional unaccounted for effects upon health outcomes including HOMA-IR. Future examination of the same-day health effects (which were acquired on a daily basis during the exposure block period only) that occurred in relation to a wider variety of air pollutants (which were measured in detail only at the exposure site) may help elucidate this issue further. We also cannot exclude the potential for significant correlations of unmeasured air pollutants with PM and hence that some of the health actions attributed to PM were (at least partially) mediated by other co-varying pollutants. However, we believe it is unlikely that this accounts for the majority of our reported associations, particularly since many of the gaseous pollutants (e.g., NO2) are more spatio-temporally heterogeneous than our metric of PM2.5 exposure. Whilst some pollutants are indeed known to be correlated with PM levels, we believe their correlation with 5-day long PM2.5 is unlikely to be robust enough to account for the majority of the positive association between PM2.5 mass concentration and HOMA-IR. We acknowledge that further studies investigating the sources, components and effects of other co-pollutants on insulin sensitivity are warranted. Regardless, the possible effect of unmeasured co-pollutants does not diminish the veracity and importance of the observed association between higher PM2.5 (mass concentration per se whether or not it is the primary or only responsible pollutant) and reduced insulin sensitivity.
Finally, though HOMA-IR is a reasonable measure of insulin sensitivity for epidemiological studies and can be used to evaluate for within-subject metabolic alterations (Wallace, et al 2004; Muniyappa, et al 2008), we acknowledge that superior tests for assessing insulin sensitivity are available. This was the first study we have undertaken to investigate the metabolic perturbations induced by PM2.5 in humans. Hence, the metric of insulin sensitivity necessarily provided preliminary mechanistic evidence. Given these supportive findings, we are now performing studies using a more invasive and sophisticated metric in our ongoing air pollution exposure studies (i.e., frequently sampled intravenous glucose tolerance test). The results of these studies will help corroborate and further add to our current findings. In the current study we did not have detailed time-activity and dietary assessments of the subjects each day of study participation. Ongoing studies will gather this information which may be important by impacting basal insulin sensitivity and/or by interacting with air pollution to affect metabolic outcomes.
5. Conclusions
Sub-acute exposures over 5 days to PM2.5, even at low concentrations commonly-encountered throughout the world, were associated with reduced insulin sensitivity. This metabolic perturbation was most likely explained by unfavorable alterations in autonomic balance. Our findings support the contention that in addition to the traditionally-implicated factors of modern society (e.g. obesity, poor diet); exposure to PM2.5 may be an independent contributing risk factor for developing DM.
Supplementary Material
Highlights.
Sub-acute exposure to low levels of ambient PM2.5 for approximately 5 days was associated with an increase in HOMA-IR, suggestive of a worsening of metabolic insulin sensitivity.
The decrease in heart rate variability related to sub-acute PM2.5 exposures was associated with the reduction in metabolic insulin sensitivity, suggesting that autonomic imbalance may be an important underlying biological mechanism.
Sub-acute PM2.5 exposure at these low levels did not promote systemic inflammation, an increase in blood pressure or alter vascular function.
These findings offer support for the biological plausibility that PM2.5 could potentiate the development of diabetes mellitus over chronic periods of exposure consistent with observations from epidemiological studies.
Acknowledgements
We would like to acknowledge Dr. Mary Ann Heindorf and colleagues from the Michigan Department of Environmental Quality for the data regarding continuous PM measurements at the monitoring sites in Dearborn, Dexter and Tecumseh, Michigan.
Sources of Funding:
This study was funded by grants from the National Institutes of Health (NIH - R01 ES01514) and the NIH CTSA grant (UL1RR024986). The study was also supported by a grant from the Environmental Protection Agency (EPA) RD83479701. This study was also supported by the Electric Power Research Institute and by Mr. and Mrs. Craig Sincock of Avfuel Corporation.
Abbreviations
- PM2.5
Fine particulate matter
- DM
diabetes mellitus
- HOMA-IR
homeostasis model assessment of insulin resistance
- CV
cardiovascular
- UMRVL
University of Michigan Research Vascular Laboratory
- SDNN
standard deviation of normal-to-normal intervals
- PBMC
peripheral blood mononuclear cells
- GEE
generalized estimating equations
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
No relevant conflict of interest
Author Contributions:
1. researched data 2.wrote the manuscript 3.reviewed/edited the manuscript 4. contributed to the discussion 5. contributed to research design
Robert D Brook 1,2,3,4,5
Xiaohua Xu 2,3,4,5
Robert L Bard 1,2,3
J Timothy Dvonch 1,2,3,4,5
Masako Morishita 1,2,3,4,5
Niko Kaciroti 1,3,5
Qinghua Sun 1,2,3,4
Jack Harkema 3,4,5
Sanjay Rajagopalan 3,4,5
References
- Anenberg SC, Horowitz LW, Tong DQ, West JJ. An estimate of the global burden of anthropogenic ozone and fine particulate matter on premature human mortality using atmospheric modeling. Environ Health Perspect. 2010;118:1189–1195. doi: 10.1289/ehp.0901220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Jerrett M, Brook JR, Finkelstein M. The relationship between diabetes mellitus and traffic-related air pollution. J Occup Environ Med. 2008;50:32–38. doi: 10.1097/JOM.0b013e31815dba70. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S. Particulate matter air pollution and blood pressure. J Am Soc Hypertens. 2009;3:332–350. doi: 10.1016/j.jash.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, III, Brook JR, Bhatnagar A, Diez-Roux A, Holguin F, Hong Y, Luepker RV, Mittleman M, Peters A, Siscovich D, Smith SC, Jr, Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease. An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan Particulate Matter Air Pollution and Atherosclerosis. Curr Atherosclerosis Rep. 2010;12:291–300. doi: 10.1007/s11883-010-0122-7. [DOI] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knaug C, Bastelica D, Neyrinck AM, Fava F, Touhy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti J-F, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Chuang K-J, Yan Y-H, Chiu S-Y, Cheng T-J. Long-term air pollution exposure and risk factors for cardiovascular diseases among the elderly in Taiwan. Occup Environ Med. 2010 doi: 10.1136/oem.2009.052704. [DOI] [PubMed] [Google Scholar]
- Cornier M-A, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. The metabolic syndrome. Endocrine Reviews. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan BM. Insulin resistance and the sympathetic nervous system. Curr Hypertens Rep. 2003;5:247–254. doi: 10.1007/s11906-003-0028-7. [DOI] [PubMed] [Google Scholar]
- Kelishadi R, Mirghaffari N, Pourasfa P, Gidding SS. Lifestyle and environmental factors associated with inflammation, oxidative stress and insulin resistance in children. Atherosclerosis. 2009;203:311–319. doi: 10.1016/j.atherosclerosis.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Krämer U, Herder C, Surgiri D, Strassburger K, Schikowski T, Ranft U, Rathmann W. Traffic-related air pollution and incident type 2 diabetes: Results from the SALIA cohort study. Environ Health Perspect. 2010;118:1273–1279. doi: 10.1289/ehp.0901689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampfrath T, Moiseev A, Shah Z, Ying Z, Deiuliis JA, Kongara RM, Kherada N, Padture NP, Chen LC, Moffatt-Bruce S, Parthasarathy S, Sun Q, Morawietz H, Brook RD, Rajagopalan S. Chronic Fine Particulate matter Exposure Induces Systemic Vascular Dysfunction via NADPH Oxidase and TLR4 Pathways. Circulation Research. 2011;108:716–726. doi: 10.1161/CIRCRESAHA.110.237560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish JP, Mills NL, Chan JKK, Leseman DLAC, Aitken RJ, Fokkens PHB, LIi J, Cassess FR, Donaldson K, Newby DE, Jiang L. Beneficial cardiovascular effects of reducing exposure to particulate air pollution with a simple face mask. Particle Fibre Toxicol. 2009;6:8. doi: 10.1186/1743-8977-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrine Metab. 2008;294:E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- Narayan KMV, Ali MK, Koplan JP. Global noncommuicable diseases – where worlds meet. N Engl J Med. 2010;363:1196–1198. doi: 10.1056/NEJMp1002024. [DOI] [PubMed] [Google Scholar]
- Nawrot TS, Perez L, Kunzli N, Munters E, Nemery B. Public health importance of triggers of myocardial infarction: A comparative risk assessment. Lancet. 2011;377:732–740. doi: 10.1016/S0140-6736(10)62296-9. [DOI] [PubMed] [Google Scholar]
- Oakes M, Rastogi N, Majestic BJ, Shafer M, Schauer JJ, Edgerton ES, Weber RJ. Characterization of soluble iron in urban aerosols using near-real time data. J Geophys Res. 2010;115:D15302. [Google Scholar]
- Pearson JF, Bachireddy C, Shyamprasad S, Goldfine AB, Brownstein JS. Association between fine particulate matter and diabetes prevalence in the U.S. Diabetes Care. 2010;33:2196–2201. doi: 10.2337/dc10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puett RC, Hart JE, Schwartz J, Hu FB, Liese AD, Laden F. Are particulate matter exposures associated with risk of type 2 diabetes? Environ Health Perspect. 2011;119:384–389. doi: 10.1289/ehp.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr AC, Kamal A, Morishita M, Mukherjee B, Keeler GJ, Harkema JR, Wager JG. 2010. Altered Heart Rate Variability in Spontaneously Hypertensive Rats is Associated with Specific Particulate Matter Components in Detroit, Michigan. Environ Health Perspect. 2011;119:474–480. doi: 10.1289/ehp.1002831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Kokoeva M, Inouye K, Tzameili I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Yue P, Deiuliis JA, Lumerng CN, Kampfrath T, Mikolaj MB, Cai Y, Ostrowski MC, Parthasarathy S, Brook RD, Moffatt-Bruce SD, Chen LC, Rajagopalan S. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet induced obesity. Circulation. 2009;119:538–546. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA. EPA Clean Air Markets Division; Clean Air Status and Trends Network (CASTNET) http://java.epa.gov/castnet/viewsiteinfo.do?siteId=ANA115. [Google Scholar]
- Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.