Abstract
The movement of water between the air space and capillary compartments is important for the maintenance of air space hydration during respiration and for reabsorption of excess alveolar fluid. We have obtained immunocytochemical and functional evidence that plasma-membrane water channels are responsible for water transport in the intact lung. Northern and quantitative immunoblot analysis showed high expression of CHIP28 (channel-forming integral membrane protein of 28 kDa) water channels in rat lung; immunocytochemistry showed CHIP28 localization to epithelial cell plasma membranes. Stopped-flow light scattering measurements of osmotic water permeability (Pf) in freshly isolated rat alveolar type II epithelial cells indicated a high Pf of 0.015 +/- 0.002 cm/s (10 degrees C) that was weakly temperature-dependent (activation energy, 4 kcal/mol) and reversibly inhibited by 78 +/- 4% by 0.5 mM HgCl2. An in situ-perfused sheep lung model was used to determine the route for water movement in intact lung. Blood-to-air-space water transport was measured by sampling air space fluid after instillation into distal air spaces of hyperosmolar saline (900 mOsm) containing radioiodinated albumin and [14C]mannitol. In seven sets of experiments, air space osmolality and radioiodinated albumin equilibrated with a t1/2 of 0.85 +/- 0.1 min. In the contralateral lung perfused with 0.5 mM HgCl2, t1/2 increased to 2.7 +/- 0.4 min; the inhibitory effect of HgCl2 was fully reversed by 5 mM 2-mercaptoethanol. These results provide direct evidence for transcellular movement of water across the alveolar epithelium in intact lung through mercury-sensitive water channels.
Full text
PDF
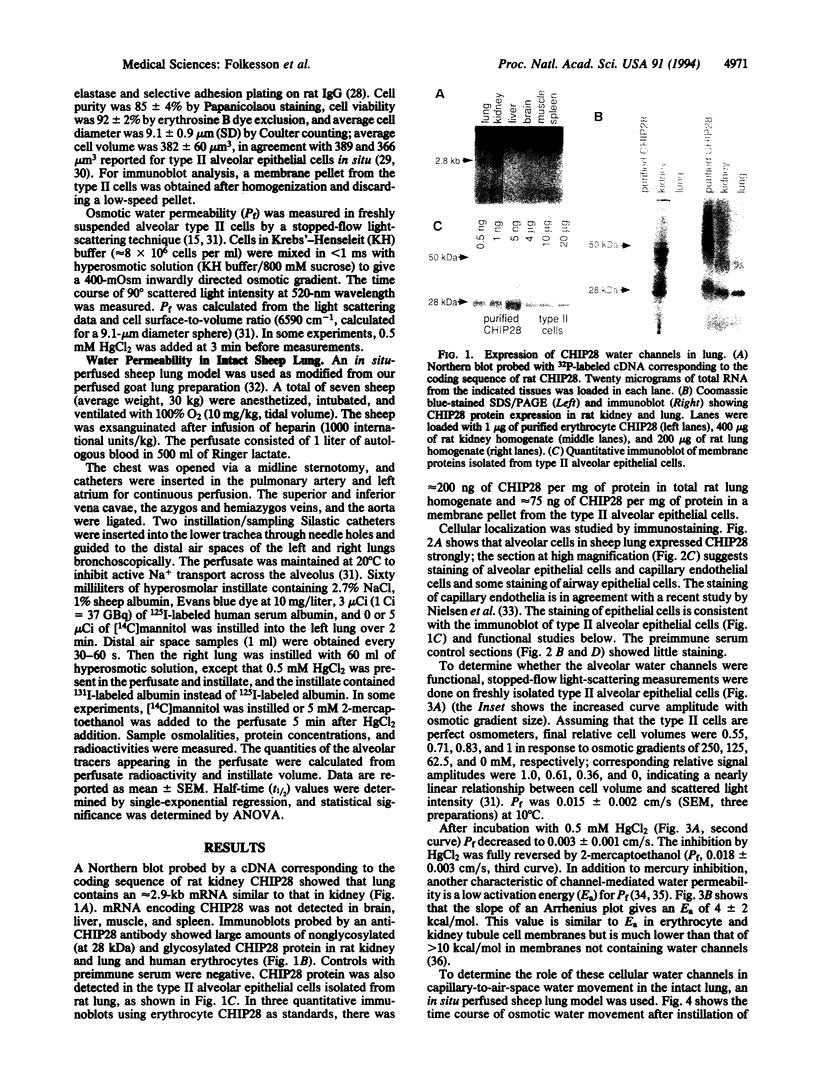
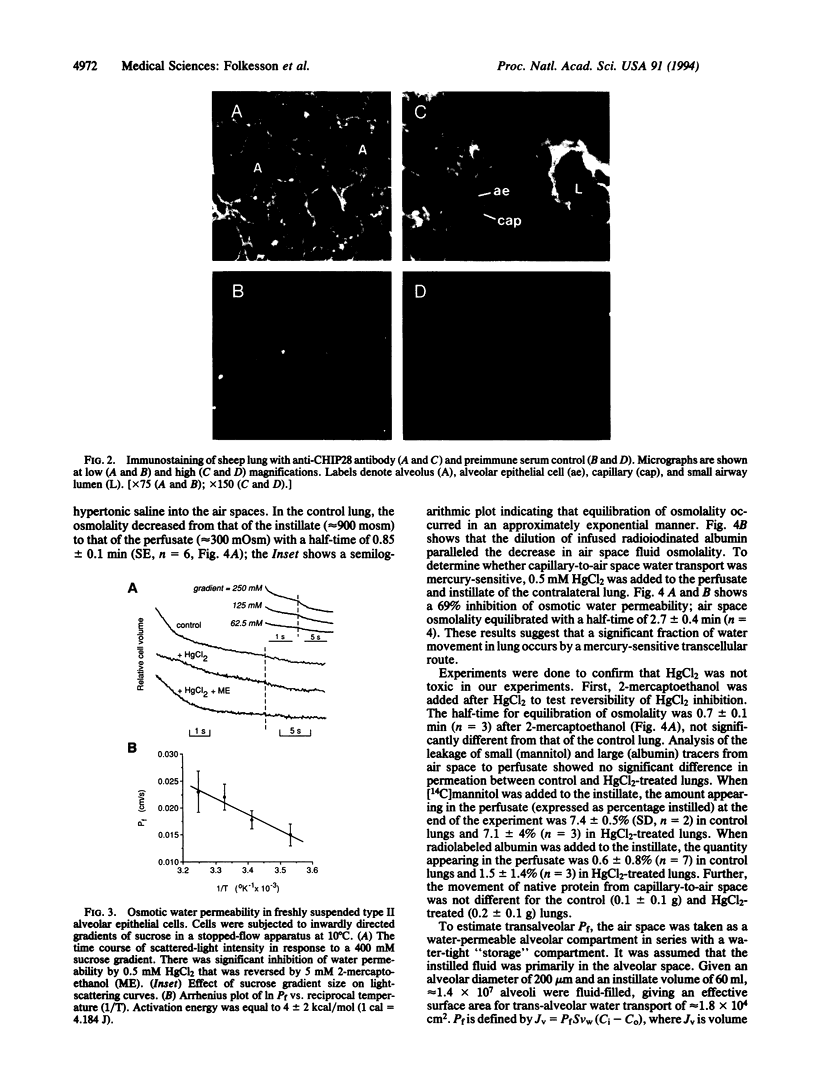
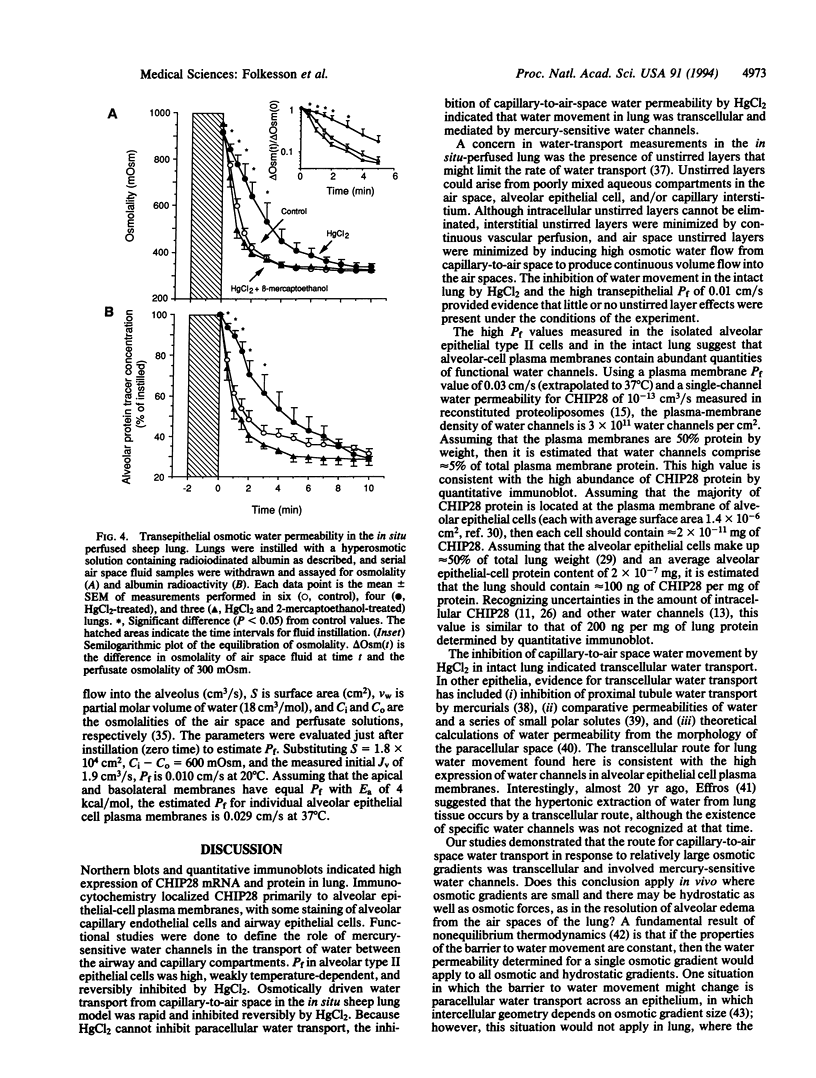

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry P. H., Diamond J. M. Effects of unstirred layers on membrane phenomena. Physiol Rev. 1984 Jul;64(3):763–872. doi: 10.1152/physrev.1984.64.3.763. [DOI] [PubMed] [Google Scholar]
- Basset G., Crone C., Saumon G. Fluid absorption by rat lung in situ: pathways for sodium entry in the luminal membrane of alveolar epithelium. J Physiol. 1987 Mar;384:325–345. doi: 10.1113/jphysiol.1987.sp016457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C. A., Verkman A. S. Osmotic gradient dependence of osmotic water permeability in rabbit proximal convoluted tubule. J Membr Biol. 1988 Oct;105(1):33–43. doi: 10.1007/BF01871104. [DOI] [PubMed] [Google Scholar]
- Berthiaume Y., Staub N. C., Matthay M. A. Beta-adrenergic agonists increase lung liquid clearance in anesthetized sheep. J Clin Invest. 1987 Feb;79(2):335–343. doi: 10.1172/JCI112817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy C., Chin E., Smith B. L., Preston G. M., Agre P. Developmental gene expression and tissue distribution of the CHIP28 water-channel protein. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4500–4504. doi: 10.1073/pnas.90.10.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapo J. D., Peters-Golden M., Marsh-Salin J., Shelburne J. S. Pathologic changes in the lungs of oxygen-adapted rats: a morphometric analysis. Lab Invest. 1978 Dec;39(6):640–653. [PubMed] [Google Scholar]
- Dobbs L. G., Gonzalez R., Williams M. C. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis. 1986 Jul;134(1):141–145. doi: 10.1164/arrd.1986.134.1.141. [DOI] [PubMed] [Google Scholar]
- Effros R. M., Mason G. R., Hukkanen J., Silverman P. New evidence for active sodium transport from fluid-filled rat lungs. J Appl Physiol (1985) 1989 Feb;66(2):906–919. doi: 10.1152/jappl.1989.66.2.906. [DOI] [PubMed] [Google Scholar]
- Effros R. M. Osmotic extraction of hypotonic fluid from the lungs. J Clin Invest. 1974 Oct;54(4):935–947. doi: 10.1172/JCI107834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettiplace R., Haydon D. A. Water permeability of lipid membranes. Physiol Rev. 1980 Apr;60(2):510–550. doi: 10.1152/physrev.1980.60.2.510. [DOI] [PubMed] [Google Scholar]
- Fushimi K., Uchida S., Hara Y., Hirata Y., Marumo F., Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature. 1993 Feb 11;361(6412):549–552. doi: 10.1038/361549a0. [DOI] [PubMed] [Google Scholar]
- Goodman B. E., Brown S. E., Crandall E. D. Regulation of transport across pulmonary alveolar epithelial cell monolayers. J Appl Physiol Respir Environ Exerc Physiol. 1984 Sep;57(3):703–710. doi: 10.1152/jappl.1984.57.3.703. [DOI] [PubMed] [Google Scholar]
- Goodman B. E., Crandall E. D. Dome formation in primary cultured monolayers of alveolar epithelial cells. Am J Physiol. 1982 Jul;243(1):C96–100. doi: 10.1152/ajpcell.1982.243.1.C96. [DOI] [PubMed] [Google Scholar]
- Haies D. M., Gil J., Weibel E. R. Morphometric study of rat lung cells. I. Numerical and dimensional characteristics of parenchymal cell population. Am Rev Respir Dis. 1981 May;123(5):533–541. doi: 10.1164/arrd.1981.123.5.533. [DOI] [PubMed] [Google Scholar]
- Hasegawa H., Ma T., Skach W., Matthay M. A., Verkman A. S. Molecular cloning of a mercurial-insensitive water channel expressed in selected water-transporting tissues. J Biol Chem. 1994 Feb 25;269(8):5497–5500. [PubMed] [Google Scholar]
- Hasegawa H., Zhang R., Dohrman A., Verkman A. S. Tissue-specific expression of mRNA encoding rat kidney water channel CHIP28k by in situ hybridization. Am J Physiol. 1993 Jan;264(1 Pt 1):C237–C245. doi: 10.1152/ajpcell.1993.264.1.C237. [DOI] [PubMed] [Google Scholar]
- Imai M., Yoshitomi K. Heterogeneity of the descending thin limb of Henle's loop. Kidney Int. 1990 Oct;38(4):687–694. doi: 10.1038/ki.1990.260. [DOI] [PubMed] [Google Scholar]
- Ma T., Frigeri A., Tsai S. T., Verbavatz J. M., Verkman A. S. Localization and functional analysis of CHIP28k water channels in stably transfected Chinese hamster ovary cells. J Biol Chem. 1993 Oct 25;268(30):22756–22764. [PubMed] [Google Scholar]
- Ma T., Hasegawa H., Skach W. R., Frigeri A., Verkman A. S. Expression, functional analysis, and in situ hybridization of a cloned rat kidney collecting duct water channel. Am J Physiol. 1994 Jan;266(1 Pt 1):C189–C197. doi: 10.1152/ajpcell.1994.266.1.C189. [DOI] [PubMed] [Google Scholar]
- Matalon S. Mechanisms and regulation of ion transport in adult mammalian alveolar type II pneumocytes. Am J Physiol. 1991 Nov;261(5 Pt 1):C727–C738. doi: 10.1152/ajpcell.1991.261.5.C727. [DOI] [PubMed] [Google Scholar]
- Matthay M. A., Landolt C. C., Staub N. C. Differential liquid and protein clearance from the alveoli of anesthetized sheep. J Appl Physiol Respir Environ Exerc Physiol. 1982 Jul;53(1):96–104. doi: 10.1152/jappl.1982.53.1.96. [DOI] [PubMed] [Google Scholar]
- Meyer M. M., Verkman A. S. Evidence for water channels in renal proximal tubule cell membranes. J Membr Biol. 1987;96(2):107–119. doi: 10.1007/BF01869237. [DOI] [PubMed] [Google Scholar]
- Nielsen S., Smith B. L., Christensen E. I., Agre P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7275–7279. doi: 10.1073/pnas.90.15.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S., Smith B. L., Christensen E. I., Knepper M. A., Agre P. CHIP28 water channels are localized in constitutively water-permeable segments of the nephron. J Cell Biol. 1993 Jan;120(2):371–383. doi: 10.1083/jcb.120.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olver R. E., Strang L. B. Ion fluxes across the pulmonary epithelium and the secretion of lung liquid in the foetal lamb. J Physiol. 1974 Sep;241(2):327–357. doi: 10.1113/jphysiol.1974.sp010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson B. E., Spring K. R. Gallbladder epithelial cell hydraulic water permeability and volume regulation. J Gen Physiol. 1982 Mar;79(3):481–505. doi: 10.1085/jgp.79.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisig P. A., Berry C. A. Evidence for transcellular osmotic water flow in rat proximal tubules. Am J Physiol. 1985 Jul;249(1 Pt 2):F124–F131. doi: 10.1152/ajprenal.1985.249.1.F124. [DOI] [PubMed] [Google Scholar]
- Preston G. M., Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11110–11114. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston G. M., Carroll T. P., Guggino W. B., Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992 Apr 17;256(5055):385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Preston G. M., Jung J. S., Guggino W. B., Agre P. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J Biol Chem. 1993 Jan 5;268(1):17–20. [PubMed] [Google Scholar]
- Sabolić I., Valenti G., Verbavatz J. M., Van Hoek A. N., Verkman A. S., Ausiello D. A., Brown D. Localization of the CHIP28 water channel in rat kidney. Am J Physiol. 1992 Dec;263(6 Pt 1):C1225–C1233. doi: 10.1152/ajpcell.1992.263.6.C1225. [DOI] [PubMed] [Google Scholar]
- Schafer J. A., Troutman S. L., Andreoli T. E. Osmosis in cortical collecting tubules. ADH-independent osmotic flow rectification. J Gen Physiol. 1974 Aug;64(2):228–240. [PMC free article] [PubMed] [Google Scholar]
- Schneeberger E. E., Lynch R. D. Structure, function, and regulation of cellular tight junctions. Am J Physiol. 1992 Jun;262(6 Pt 1):L647–L661. doi: 10.1152/ajplung.1992.262.6.L647. [DOI] [PubMed] [Google Scholar]
- Serikov V. B., Grady M., Matthay M. A. Effect of temperature on alveolar liquid and protein clearance in an in situ perfused goat lung. J Appl Physiol (1985) 1993 Aug;75(2):940–947. doi: 10.1152/jappl.1993.75.2.940. [DOI] [PubMed] [Google Scholar]
- Shi L. B., Skach W. R., Verkman A. S. Functional independence of monomeric CHIP28 water channels revealed by expression of wild-type mutant heterodimers. J Biol Chem. 1994 Apr 8;269(14):10417–10422. [PubMed] [Google Scholar]
- Smith B. L., Agre P. Erythrocyte Mr 28,000 transmembrane protein exists as a multisubunit oligomer similar to channel proteins. J Biol Chem. 1991 Apr 5;266(10):6407–6415. [PubMed] [Google Scholar]
- Van Hoek A. N., Wiener M., Bicknese S., Miercke L., Biwersi J., Verkman A. S. Secondary structure analysis of purified functional CHIP28 water channels by CD and FTIR spectroscopy. Biochemistry. 1993 Nov 9;32(44):11847–11856. doi: 10.1021/bi00095a013. [DOI] [PubMed] [Google Scholar]
- Verbavatz J. M., Brown D., Sabolić I., Valenti G., Ausiello D. A., Van Hoek A. N., Ma T., Verkman A. S. Tetrameric assembly of CHIP28 water channels in liposomes and cell membranes: a freeze-fracture study. J Cell Biol. 1993 Nov;123(3):605–618. doi: 10.1083/jcb.123.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidel M. L., Ambudkar S. V., Smith B. L., Agre P. Reconstitution of functional water channels in liposomes containing purified red cell CHIP28 protein. Biochemistry. 1992 Aug 25;31(33):7436–7440. doi: 10.1021/bi00148a002. [DOI] [PubMed] [Google Scholar]
- Zhang R., Skach W., Hasegawa H., van Hoek A. N., Verkman A. S. Cloning, functional analysis and cell localization of a kidney proximal tubule water transporter homologous to CHIP28. J Cell Biol. 1993 Jan;120(2):359–369. doi: 10.1083/jcb.120.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoek A. N., Verkman A. S. Functional reconstitution of the isolated erythrocyte water channel CHIP28. J Biol Chem. 1992 Sep 15;267(26):18267–18269. [PubMed] [Google Scholar]