Abstract
Background
Some studies have suggested that helminth infections increase the risk of malaria infection and are associated with increased number of malaria attacks and anaemia. Thus interventions to control helminth infections may have an impact on incidence of clinical malaria and anaemia. The current study assessed the impact of two anthelmintic treatment approaches on malaria infection and on anaemia in school and pre-school children in Magu district, Tanzania.
Methods
A total of 765 children were enrolled into a prospective randomized anthelmintic intervention trial following a baseline study of 1546 children. Enrolled children were randomized to receive either repeated treatment with praziquantel and albendazole four times a year (intervention group, 394 children) or single dose treatment with praziquantel and albendazole once a year (control group, 371 children). Follow up examinations were conducted at 12 and 24 months after baseline to assess the impact of the intervention. Stool and urine samples were collected and examined for schistosome and soil transmitted helminth infections. Blood samples were also collected and examined for malaria parasites and haemoglobin concentrations. Monitoring of clinical malaria attacks was performed at each school during the two years of the intervention.
Results
Out of 1546 children screened for P. falciparum, S. mansoni, S. haematobium, hookworm and T. Trichiura at baseline, 1079 (69.8%) were infected with at least one of the four parasites. There was no significant difference in malaria infection (prevalence, parasite density and frequency of malaria attacks) and in the prevalence of anaemia between the repeated and single dose anthelmintic treatment groups at 12 and 24 months follow up (p > 0.05). However, overall, there was significant improvement in mean haemoglobin concentrations (p < 0.001) from baseline levels of 122.0g/L and 123.0g/L to 136.0g/L and 136.8g/L for the repeated and single dose treatment groups, respectively, at 24 months follow-up which resulted in significant reduction in prevalence of anaemia.
Conclusions
These results suggest that repeated anthelmintic treatment did not have an impact on malaria infection compared to single dose treatment. However, both treatment approaches had overall impact in terms of improvements of haemoglobin levels and hence reductions in prevalence of anaemia.
Keywords: Anthelmintic intervention, Malaria infection, Anaemia, School children, Magu district
Background
Malaria, schistosomiasis and soil transmitted helminth infections (STH) are considered the most important parasitic infections in Sub-Saharan Africa, contributing to a major part of disease burden [1,2]. The diseases share the same geographical distribution and occur as co-infections in humans and thus interact with regards to susceptibility, infection level, and pathology [3-5]. In Tanzania, these infections occur throughout the country and are a major public health problem particularly in school and pre-school age children [5-8]. In school children, the diseases are associated with impaired physical and mental development, impaired learning capabilities, undernutrition and anaemia [2,9,10].
Globally, major control interventions are currently being undertaken to control malaria and helminth infections. These include use of insecticide impregnated bed nets, indoor residual spraying (IRS) and early and effective detection and management of cases using artemisinin based combination therapy for malaria [11-14]. For helminth infections, control interventions include mass drug administration using praziquantel and albendazole, provision of safe water, improved sanitation and health education [2,15].
Some previous studies have demonstrated that helminth infections may increase the risk of infections with P. falciparum [16-20] and other infections such as HIV and TB [21,22]. However other studies have not found this relationship [23]. Another important aspect of malaria and helminth co-infections in humans is their joint contribution to anaemia [24]. A study in Nepal [24] observed that P. vivax malaria and hookworm co-infections in pregnant women were associated with more frequent malaria attacks and severe anaemia than seen in women who harbour only one parasite infection. In the Philippines, it was demonstrated that even at low infection intensities, multiple parasite infections enhance the risk of anaemia [25,26]. Thus interventions to control helminth infections in areas where helminth infections are co-endemic with malaria could be expected to have not only an impact on prevalence and intensity of helminth infections but also an impact on disease burden due to malaria [27]. However there have been few longitudinal randomized studies to test these hypotheses [28-30]. Most studies have been cross sectional in nature and hence more longitudinal randomized studies are needed.
The objective of this study was therefore to assess the impact of an anthelmintic intervention delivered through two different approaches on malaria infection and on anaemia. The study was designed to test the hypothesis that helminth infections modulate immune responses against P. falciparum infection in an infection intensity dependent manner and thus increase susceptibility to malaria infection and related morbidity. Thus control of helminth infections would enhance immune response against P. falciparum malaria and reduce incidence of clinical malaria attacks and anaemia.
Methods
Study area and population
The study was implemented in Magu district, Mwanza region, North-western Tanzania, and involved six primary schools namely Mwamayombo, Nyashimo, Bulima, Milambi, Ihale and Ijitu. From each school, school children aged 6–13 years (grades I-V) were randomly selected and included in the study. The aim was to get at least 100 children per school. Where the required number of 100 school children was not reached, pre-school children aged 3–5 years were also enrolled into the study. Selection of pre-school children was made conveniently whereby parents and guardians living in the community surrounding each school were requested to bring their children to the examination centre.
Study design, randomization and treatments
The study was a longitudinal open label intervention trial which was preceded by cross-sectional baseline study that enrolled 1546 children in the catchment areas of the 6 primary schools. During the baseline study, the prevalence and infection intensity of P. falciparum, S. mansoni, S. haematobium, hookworm and T. trichiura was determined using standard methods as described in Kinung’hi et al. [5]. Haemoglobin concentrations were also assessed using the Haemocue method. Quality control was performed by re-examining 10% randomly selected blood slides, urine filters and Kato smears by an experienced independent technician.
Following the baseline study, 765 children were selected and included in the longitudinal study. The inclusion criteria were: Being pre-school children (age 3 to 5 years) or school children (age 6 to 13 years) living in the selected villages for at least one year. In addition, selected children were those infected with either S. mansoni or S. haematobium or both. Exclusion criteria were: Children who had stayed in the village for less than one year, children whose parents or legal guardians did not provide informed consent and children with severe malaria and anaemia. Severe malaria was defined as a seriously sick child presenting with fever (axillary temperature ≥ 37.5) plus one or more signs of seizures, respiratory distress, prostration, impaired consciousness or coma combined with the presence of P. falciparum parasitemia but in the absence of other causes of febrile illness [31,32]. Severe anaemia was defined as a child with haemoglobin concentrations below 80g/L. Children who had received any anthelminthic treatment within one year before baseline were also excluded from the study.
Haemoglobin concentrations were taken as the reference variable for calculation of sample size. A minimum sample size of 620 children (310 children for the repeated anthelmintic treatment group and 310 children for the single dose treatment group) was considered sufficient to give a power of 90% to be able to detect a significant difference of at least 5g/L between the two groups at 95% confidence interval and 5% level of precision. However, after baseline survey and assessment for inclusion criteria, 765 children were selected and randomized into either the repeated anthelmintic treatment group (intervention group, 394 children) or the single dose anthelmintic treatment group (control group, 371 children). Generation of random allocation sequence and assignment of participating children to intervention and control groups was performed by an experienced statistician.
Children in the intervention group were treated with repeated doses of praziquantel 40mg/kg and albendazole 400mg four times a year at three months interval while children in the control group were treated with a single dose of praziquantel 40mg/kg and albendazole 400mg once a year. The two treatment approaches for the intervention and control groups were based on the assumption that the intensive repeated treatment (four times a year) of the intervention group will make this group helminth free while for the control group, the standard single dose treatment (once per year) will allow for re-infection to take place in 50% or more of children in this group [33-35]. Both praziquantel and albendazole were supplied by the medical stores department of the ministry of health and social welfare (MoHSW), Tanzania. Treatments took place at the school premises and were administered by a qualified medical doctor assisted by a nurse and trained school teachers. Before taking the drugs, children were given a piece of bread and a soft drink (fruit juices) to minimise side effects of praziquantel. While albendazole was given as a single dose at a dosage rate of 400mg, praziquantel was administered according to weight. All drugs were taken orally together with clean safe drinking water under direct observation. After taking the drugs, children were allowed to rest for a period of 30–60 minutes during which side effects related to praziquantel treatment were monitored. Overall praziquantel was well tolerated except for mild abdominal discomforts that lasted for about 30 minutes.
The cohort of 765 children was followed up at 12 and 24 months after baseline. During these two follow up time points, the prevalence and infection intensity of P. falciparum, S. mansoni, S. haematobium and hookworm as well as haemoglobin concentrations were assessed. The 12 months follow up assessment took place between October and November 2007 and included 655 children. The 24 months follow up assessment took place between October and November 2008 and involved 592 children. Of the 592 children who completed both the 12 months and the 24 months surveys, 297 were from the intervention group while 292 were from the control group. This amounted to a compliance rate of 75.4% and 78.8%, respectively. This study is registered on ClinicalTrials.gov, registration number NCT00347113; registered June 30, 2006 and reported according to CONSORT guidelines for randomized trials [36,37]. The study flow chart is shown in Figure 1.
Figure 1.
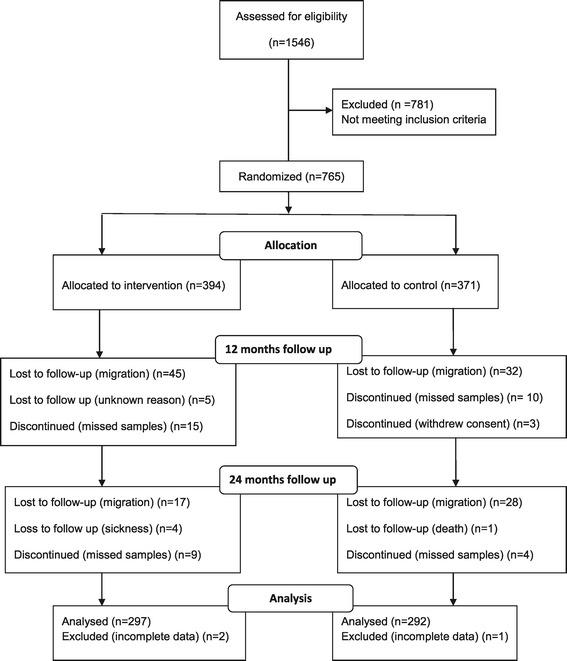
Study flow diagram.
Major reasons for loss to follow-up included migration from the study area, missing a second stool or urine sample, withdrawal of consent, sickness during follow-up survey days and death.
Monitoring of clinical malaria attacks
During the 24 months follow-up period, the incidence of clinical malaria attacks was monitored at each primary school assisted by trained school teachers. Monitoring of incidence of clinical malaria attacks was done for all children included in the longitudinal study by passive case detection whereby children were asked to report to teachers when they had febrile illness. The parents and guardians of all children enrolled in this study were also informed about the study and requested to report to teachers when their children had febrile illness. The teachers were trained on how to make presumptive diagnosis and treatment of malaria cases. They were also trained to collect finger prick blood, prepare and stain thick blood smears using the Giemsa stain method and keep records. A register of malaria cases was established at each primary school. A laboratory technician visited each school on a weekly basis to collect the Giemsa stained blood smears and all information recorded. Blood smear examination was undertaken at the parasitology laboratory of the National Institute for Medical Research (NIMR), Mwanza Centre. School children found with febrile illness were treated with Artemether-Lumefantrine which was the first line antimalarial drug at the time of the study or were referred to nearby health facilities according to national guidelines on malaria treatment. A malaria episode (case) was defined as a child presenting with febrile illness with axillary body temperature ≥37.5°C plus a positive thick blood smear for malaria parasites in the absence of signs for other infectious diseases.
Study outcomes
The following study outcome measures for malaria infection and anaemia were assessed during the study:
Prevalence of malaria parasitaemia defined as the proportion of children with a positive blood smear for malaria parasites by the Giemsa stain method.
Malaria parasite density defined as the number of malaria parasites per microlitre (μL) of blood calculated assuming 8000 white blood cells/μL of blood and estimated by counting the number of parasites per 200 leucocytes in Giemsa stained thick films at 100× magnification. Malaria parasite density was classified as low (<5000 parasites/μL of blood) and high (≥5000 parasites/μL of blood).
Incidence of clinical malaria attacks defined as number of new cases of children presenting with febrile illness with axillary body temperature ≥37.5°C plus a positive thick blood smear for malaria parasites by Giemsa stain method in the absence of signs for other infectious diseases. The incidence of clinical malaria attacks was observed for the entire duration of the intervention (two years).
Prevalence of anaemia and severe anaemia defined as the proportion of children with haemoglobin (Hb) concentrations less that 120 g/L and 80 g/L, respectively, measured by the Haemocue method.
Ethical statement
The study was approved by the Medical Research Coordination Committee (MRCC) of the National Institute for Medical Research (NIMR), Tanzania (Reference No. NIMR/HQ/R.8a/Vol. IX/355). Before commencement of the study, the research team conducted meetings with village leaders, teachers and community members of all selected villages. During these meetings, the objectives and procedures of the study were explained including study benefits and potential risks and discomforts. Written informed consent for children who participated in the study was sought from parents or legal guardians after they had been informed about the study. Children were also requested to give written assent and were informed of their right to refuse to participate in the study and to withdraw at any time during the study without jeopardizing their right of access to other health services. Invasive procedures such as collection of blood samples were explained to parents and children and were carried out using sterile disposable materials. All children found infected with any of the parasites S. mansoni, S. haematobium, soil-transmitted helminthiasis and P. falciparum and those found with ailments not targeted by the project were treated free of charge according to national guidelines. Study identification numbers were used instead of children names and information collected was kept confidential. Feedback to the study population in the form of dissemination workshops was conducted during the course of the study.
Data management and statistical analysis
Data were double entered into Dbase V software (Borland International, Scotts Valley, California, USA) and analyzed using STATA Version 10 (STATA Corp., Texas, USA). Infection intensities (of positive samples only) were calculated as geometric mean of parasites per microlitre of blood for P. falciparum and geometric mean of eggs per gram of faeces for S. mansoni and hookworms. For S. haematobium, infection intensity was calculated as geometric mean of eggs per 10ml of urine. For each survey point (baseline, 12 months follow up and 24 months follow up), the Chi-square test was used to compare proportions (prevalence) of malaria infection and prevalence of anaemia between the intervention and control groups. Likewise, for each survey point, the student’s t-test was used to compare geometric mean parasite counts and mean haemoglobin concentrations between the intervention and control groups where two groups were compared. The repeated measures analysis of variance (rmANOVA) was used to compare geometric mean parasite counts and mean haemoglobin concentrations where repeated measurements for all three survey points (baseline, 12 months follow up and 24 months follow up) were compared longitudinally. Comparison of the incidence (or risk) of clinical malaria attack among children in the intervention group and those in the control group was performed by calculating the relative risk (risk ratio) using 2×2 contingency tables. Tests were considered statistically significant at p < 0.05.
Results
Baseline characteristics
Table 1 shows baseline characteristics of children included in the longitudinal study. There were no significant differences in baseline characteristics of children in the two groups.
Table 1.
Baseline characteristics of children included in the trial by randomization group (n = 765)
| Variable | Intervention group (n = 394) | Control group (n = 371) |
|---|---|---|
| Sex | ||
| Boys (n = 392) | 193 (49.0%) | 180 (48.5%) |
| Girls (n = 373) | 201 (51.0%) | 191 (49.0%) |
| Age distribution (years) | ||
| 3 – 5 (n = 93) | 40 (10.1%) | 53 (14.3%) |
| 6 – 8 (n = 517) | 278 (70.6%) | 239 (64.4%) |
| 9 – 13 (n = 155) | 76 (19.3%) | 79 (21.3%) |
| School | ||
| Mwamayombo | 52 (51.5) | 49 (48.5) |
| Nyashimo | 85 (50.6) | 83 (49.4) |
| Bulima | 68 (50.7) | 66 (49.3) |
| Milambi | 56 (53.8) | 48 (46.2) |
| Ihale | 75 (51.4) | 71 (48.6) |
| Ijitu | 58 (51.8) | 54 (48.2) |
| All schools | 394 (51.5) | 371(48.5) |
| S. mansoni infection | ||
| Prevalence (%) | 238 (60.4) | 220 (59.3) |
| Intensity (Epg)* | 49 (42–59) | 50 (42–59) |
| S. haematobium infection | ||
| Prevalence (%) | 90 (22.8) | 85 (22.9) |
| Intensity (Eggs/10 ml)* | 15 (11–20) | 17 (13–23) |
| S. mansoni/S. haematobium co-infection | ||
| Prevalence (%) | 65 (16.6) | 61 (16.4) |
| S. mansoni intensity (Epg)* | 31 (23–42) | 41 (29–58) |
| S. haematobium intensity (egg/10 ml)* | 19 (11–28) | 16 (10–26) |
| Malaria infection | ||
| Prevalence (%) | 123 (31.2) | 123 (33.2) |
| Parasite density (mps/μL)* | 530 (414–676) | 510 (403–644) |
| Hookworm infection | ||
| Prevalence (%) | 80 (20.3) | 68 (18.3) |
| Intensity (Epg)* | 54 (39–76) | 53 (39–73) |
| Prevalence of anaemia (%) | 142 (36.7) | 134 (35.5) |
| Prevalece of severe anaemia (%) | 5 (1.3) | 4 (1.1) |
| Mean haemoglobin level (g/L)** | 122 (121–123) | 123 (122–124) |
| Multiple infections (%) | ||
| Single parasite | 199 (50.5) | 186 (50.1) |
| Two parasites | 148 (37.6) | 136 (36.7) |
| Thee/four parasites | 47 (12.0) | 49 (13.2) |
*Infection intensity expressed as geometric mean parasite counts of positive samples only with 95% confidence interval shown in brackets.
**Mean haemoglobin concentrations with 95% confidence interval shown in brackets.
Out of the 765 children included in the longitudinal study, 589 (77.0%) completed the two year follow up period and were included in the analysis of whom 291 (49.4%) were boys. Mean age was 9 years. Out of the 589 children who completed the 24 months follow up, 302 (51.4%) were infected with at least one of the parasites P. falciparum, S. mansoni, S. haematobium, Hookworm and T. trichiura. Children who were infected with P. falciparum were 144 (24.5%), out of whom 55 (38.2%) had helminth co-infections. Only 3 children (0.5%) had T. Trichiura infection and hence T. Trichiura infection was excluded from further analysis.
Effect of the intervention on malaria infection, haemoglobin concentrations and anaemia
There was no significant difference in malaria infection (prevalence and malaria parasite density) and in the prevalence of anaemia between the intervention and control groups at 12 and 24 months follow up period (p > 0.05) (Table 2). However, overall, there was a significant improvement in mean haemoglobin concentrations (F = 96.47, p < 0.001) from baseline levels of 122.0g/L (95% CI 120.5-123.4) and 123.0g/L (95% CI 121.6-124.5), for the intervention and control groups, respectively, to 136.0g/L (95% CI 134.2-137.8) and 136.8g/L (95% CI 135.0-137.7) for the intervention and control groups, respectively, at the 24 months follow-up period which resulted in a significant reduction in the prevalence of anaemia over the two years period but without significant differences between intervention and control groups (Table 2).
Table 2.
Comparison of overall prevalence and geometric mean parasite density of malaria infection, mean Hb levels and anaemia after one and two years of anthelmintic treatment by randomization groups
| Variable/randomization group | Baseline | 12 months follow up | 24 months follow up |
|---|---|---|---|
| Prevalence of P. falciparum infection (n,%) | |||
| Intervention | 123 (33.2) | 145 (44.9) | 74 (24.9) |
| Control | 123 (31.2) | 137 (42.4) | 70 (24.0) |
| p-value | 0.567 | 0.526 | 0.790 |
| Intensity of P. falciparum infection (95% CI) | |||
| Intervention | 530 (414-678) | 833 (664-1048) | 326 (279-382) |
| Control | 510 (403-645) | 906 (724-1133) | 343 (294-400) |
| p-value | 0.825 | 0.608 | 0.658 |
| Prevalence of anaemia (n,%) | |||
| Intervention | 142 (36.0) | 120 (37.2) | 43 (14.5) |
| Control | 134 (36.1) | 112 (34.7) | 38 (13.0) |
| p-value | 0.971 | 0.806 | 0.634 |
| Prevalence of severe anaemia (n,%) | |||
| Intervention | 5 (1.3) | 1 (0.3) | - |
| Control | 4 (1.1) | 1 (0.3) | - |
| p-value | NA | NA | NA |
| Mean haemoglobin level in g/L (95% CI) | |||
| Intervention | 122.0 (120.5-123.4) | 123.5 (121.9-125.0) | 136.0 (134.2-137.8) |
| Control | 123.0 (121.6-124.5) | 123.2 (121.7-124.6) | 136.8 (135.0-137.7) |
| p-value | 0.270 | 0.749 | 0.494 |
Baseline prevalence of heavy P. falciparum infection (≥5000 mps/μL) was 2.1% and 2.2% for the intervention and control group, respectively and was reduced to 0% at the end of the intervention. This represented a significant reduction over the two years follow up period but without significant differences between groups (χ2 = 0.07, p = 0.790). Likewise, there was an overall significant reduction in malaria parasite density (F = 32.94, p < 0.001) from baseline levels of 530 (95% CI 414–678) and 510 (95% CI 403–645), for the intervention and control groups, respectively, to 326 (95% CI 279–382) and 343 (95% CI 294–400) for the intervention and control groups, respectively, at the 24 months follow-up period but without significant differences between groups (t = 0.44, p = 0.658) (Table 2). However, for all groups, there was an increase in malaria prevalence and malaria parasite density from baseline to 12 months follow up (Table 2) which could be a result of other factors such as climate variability and seasonality of malaria transmission.
Incidence of clinical malaria cases
Out of the 765 children originally included in the longitudinal follow up, 589 (77.0%) children completed the two year follow up period for malaria attacks (297 were in the intervention group while 292 were in the control group). A total of 221 children presented with febrile illness suggestive of malaria of whom 114 were from the intervention group and 107 were from the control group. However only 85 children (38.5%) met the criteria of confirmed malaria cases (axillary temp ≥37.5% plus a blood smear positive for malaria parasites). Forty confirmed malaria cases were reported from the intervention group while 45 confirmed malaria cases were reported from the control group. Eighteen children (21.2%) reported more than one episode of clinical malaria attacks (2–4) per year during the first year of the intervention. However, this occurred less frequently during the second year of the intervention whereby only 7 children (8.2%) reported more than one episode of clinical malaria attacks with the highest frequency of 2 attacks per child per year. There was clustering of overall clinical malaria attacks by school which corresponded with baseline malaria prevalence at respective schools (data not shown).
Overall, there was no significant differences in the incidence of clinical malaria attacks (RR = 1.144, 95% CI 0.759-1.725, p = 0.519) or malaria parasite density (t = −0.76, p = 451) between children in the intervention group and those in the control group (Table 3).
Table 3.
Malaria case incidence and malaria parasite density by treatment group over 24 months follow up period
| Variable | Intervention group (n = 297) | Control group (n = 292) | P-value |
|---|---|---|---|
| Malaria case incidence* | 6.7 (4.8-8.9) | 7.7 (5.7-10.1) | 0.519 |
| Malaria parasite density** | 2658 (1647-4291) | 3100 (1831-5249) | 0.451 |
*Malaria case incidence defined as the number of new clinical malaria attacks observed in a given treatment group per 100 person years with 95% confidence intervals shown in brackets.
**Malaria parasite density expressed as geometric mean malaria parasites per microlitre of blood (mps/μL) (positive samples only) with 95% confidence intervals shown in brackets.
Discussion
Results of the current study suggest that repeated anthelmintic treatment (treatment with praziquantel and albendazole four times a year) did not have any impact on malaria infection (prevalence, malaria parasite density and frequency of malaria attacks) compared to the single dose annual treatment as no differences were observed between children in the repeated anthelmintic treatment (intervention) group compared to children in single dose annual treatment (control) group. This is contrary to what was expected. Previous studies had observed that helminth infections increases the risk of malaria infection [17-19,38,39] which could imply that an anthelmintic treatment intervention would result in reduced frequency of malaria attacks and/or malaria parasite densities in areas where both malaria and helminth infections are co-endemic [40]. One explanation of this finding could be that the impact of helminth infections on malaria infection is intensity dependent such that heavy helminth infections are required to induce a shift in Th1/Th2 balance which in turn would be reflected in differences in the observed malaria infection between children in the intervention and control groups. This view is supported by findings of Sokhna et al. [18], Fenton [41] and Wiria et al. [42]. Sokhna et al. [18] observed that the incidence rate of malaria attacks was significantly higher in Senegalese children infected with S. mansoni particularly those carrying the highest egg loads as compared to uninfected children. Fenton [41] observed that the outcomes of coinfection are highly dependent on helminth burden among other factors. Another recent study by Waknine-Grinber et al. [43] demonstrated that concomitant S. mansoni infection reduced the incidence of cerebral malaria in P. bergheii infected mice but the effect was dependent on S. mansoni parasite load. As most of the helminth infections in this study were light, their impact on malaraia infections might have been weak. The results are however in agreement with findings of Beasley et al. [44] who worked with school children aged 7–12 years in Tanga Region in Northeast Tanzania where malaria, S. haematobium and STHs are co-endemic. He observed that 15 to 16 weeks following anthelmintic treatment using praziquantel and albendazole, children in the treatment group had significant reductions in prevalence and infection intensity of S. haematobium and STHs and in the prevalence of anaemia compared to children in the control (placebo) group but did not differ in terms of prevalence and infection intensity of P. falciparum malaria. Another important observation was the relatively few confirmed malaria attacks relative to the number of cases who reported with febrile illness. Out of the 221 reported cases of febrile illness, only 85 (38.5%) were confirmed to be due to P. falciparum infection. This observation suggests that there are other causes of febrile illness in this study area. Similar observations have been made in other parts of Tanzania [45-47] and hence there is a need for further longitudinal studies.
As observed by previous studies investigating the association between malaria and helminth infections [23,48], one weakness of the design of the current study is the lack of a proper untreated control group due to ethical reasons. While children in the intervention group were treated with praziquantel 40mg/kg and albendazole 400mg four times a year, children in the comparison group had to be treated using the minimum recommended single dose of praziquantel 40mg/kg and albendazole 400mg annually according to the national schistosomiasis and soil-transmitted helminthiasis treatment guidelines in Tanzania. Although it was assumed that the single dose annual anthelminthic treatment of children in the control group would allow for re-infection of 50% or more of children in this group and that this level of helminth re-infection would still induce the same effect on malaria infection, the treatment might have contributed to lack of differences in malaria infection between children in the intervention group compared to those in the control group. Despite the lack of significant differences between the study groups, there was an overall reduction in prevalence of malaria parasitaemia, intensity of infection and number of malaria attacks from baseline through to the 12 months and 24 months follow up surveys. However, this reduction was not consistent over time since the prevalence and infection intensity of malaria infection was higher at the first (12 months) follow up survey. The fact that this reduction was not consistent over time suggests that other factors such as seasonal variation in malaria transmission resulting from malaria vector dynamics and climatic/environmental factors might have acted as confounding factors and contributed to the overall reduction observed.
Earlier studies to investigate the association between malaria and helminth infections using a randomized design include the study of Murray et al. [49] which suggested that treatment of children with helminth infections (A. lumbricoides) resulted into an increase of malaria cases. Two further studies with similar design were conducted in Madagascar [50,51] and showed that children aged more than 5 years treated for helminths (A. lumbricoides) had a significant increase in malaria parasite densities compared to untreated controls suggesting a negative interaction between A. lumbricoides infection and malaria parasite density. It might be difficult to compare findings of the current study with the studies of Murray and Brutus. While the study of Murray et al. [49] included a very small sample size (only 35 children were studied), all the three studies [49-51] investigated the association between P. falciparum and A. lumbricoides infections which involved a different helminth specie (A. lumbricoides) from the helminth species investigated in the current study (S. mansoni, S. haematobium and hookworms). Further, the studies of Murray and Brutus were conducted in a different epidemiological setting (and hence different disease transmission patterns) and used different treatment regimen against helminth infections. While Murray et al. [49] used piperazine to treat STH infections, Brutus et al. [50,51] used levamisole to treat STH infections. No treatment was given against schistosome infections.
Likewise, there were no significant differences in prevalence of anaemia or in haemoglobin levels between children in the intervention group compared to children in the control group over the two years assessment periods. However, for both groups, there was significant improvement in mean haemoglobin levels and hence a reduction in prevalence of anaemia. The observed changes in haemoglobin levels and the prevalence of anaemia followed a similar trend as changes in the prevalence and infection intensity of malaria suggesting that malaria infection was the most important contributor to anaemia in the studied population in line with findings of other studies [52,53]. Further, the anthelmintic intervention might have contributed to the improvement in haemoglobin levels and hence reduction in the prevalence of anaemia by acting through reductions in prevalence and infection intensity of helminth infections (S. mansoni, S. haematobium and hookworms) and the prevalence and infection intensity of P. falciparum infection in line with reports by other investigators [27,44,54,55]. This argument is supported by baseline findings [5] whereby multivariate analysis showed that P. falciparum and S. haematobium infections were the most important predictors of anaemia in the studied population. Other studies which have provided evidence on the importance of malaria infections on anaemia include studies on interventions directed against malaria such as Insecticide Treated Nets (ITNs) and chemoprophylaxis. Results of these studies demonstrated increases in haemoglobin levels and a strong impact on anaemia [56,57]. The most striking impact of the anthelmintic intervention was on severe anaemia which was reduced by 100% for both the intervention and control groups.
Conclusions
In conclusion, findings of the current study show that repeated anthelmintic treatment did not have a direct impact on malaria infection (prevalence, malaria parasite density and frequency of malaria attacks) as compared to single dose annual treatment. However, both treatments had an overall impact in terms of improvements of haemoglobin levels and hence reductions in prevalence of anaemia.
Acknowledgements
The authors extend their sincere thanks to the Management of NIMR-Mwanza Centre for logistic support during fieldwork. The support rendered to the project by the office of the district medical officer (DMO) and the office of the district education officer (DEO) for Magu district is also highly acknowledged. An equally important aspect to the successful completion of the study was the friendly cooperation of village leaders, teachers, parents and children of all villages and schools which participated in this study. Financial support for this study was provided by the Danish Development Assistance (DANIDA) through the DBL-Centre for Heath Research and Development, Faculty of Life Sciences, University of Copenhagen, Denmark, and formed part of PhD training for SMK.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SMK, PM and BJV contributed to the design of the study. SMK supervised enrolment of children into the study, field data collection and data entry. SMK performed data analysis and drafted the manuscript. CK contributed to randomization of school children, supervision of data entry and data analysis. JT critically reviewed the manuscript and contributed to statistical analysis, PM, BJV and CK critically reviewed the manuscript. All authors read and approved the final version of the manuscript.
Contributor Information
Safari M Kinung’hi, Email: kinunghi_csm@hotmail.com.
Pascal Magnussen, Email: pma@sund.ku.dk.
Coleman Kishamawe, Email: kishamawe@yahoo.com.
Jim Todd, Email: jim.todd@lshtm.ac.uk.
Birgitte J Vennervald, Email: bjv@sund.ku.dk.
References
- 1.Chitsulo L, Engels D, Montressor A, Savioli L. The global status of schistosmiasis and its control. Acta Trop. 2000;77(1):41–51. doi: 10.1016/S0001-706X(00)00122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . Report of WHO Expert Committee. Geneva: WHO; 2002. Prevention and control of schistosomiasis and soil-transmitted helminthiasis; pp. 1–24. [PubMed] [Google Scholar]
- 3.De Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19:547–51. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Utzinger J, Keiser J. Schistosomiasis and soil transmitted helminthiasis: common drugs for treatment and control. Expert Opin Pharmacotger. 2004;5:263–85. doi: 10.1517/14656566.5.2.263. [DOI] [PubMed] [Google Scholar]
- 5.Kinung’hi SM, Magnussen P, Kaatano GM, Kishamawe C, Vennervald BJ. Malaria and helminth Co-infections in school and preschool children: a cross-sectional study in Magu District, North-Western Tanzania. PLoS One. 2014;9(1):e86510. doi: 10.1371/journal.pone.0086510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lwambo NJS, Siza JE, Brooker S, Bundy DAP, Guyatt H. Patterns of concurrent hookworm infections and schistosomiasis in school children in Tanzania. Trans Roy Soc Trop Med Hyg. 1999;93:497–502. doi: 10.1016/S0035-9203(99)90349-8. [DOI] [PubMed] [Google Scholar]
- 7.Magnussen P, Ndawi B, Sheshe AK, Byskov J, Mbwana K. The Impact of a school health programme on the prevalence and morbidity of urinary schistosomiasis in Mwera Division, Pangani District, Tanzania. Trans R Soc Trop Med Hyg. 2001;95(1):58–64. doi: 10.1016/S0035-9203(01)90333-5. [DOI] [PubMed] [Google Scholar]
- 8.Magnussen P, Ndawi B, Sheshe AK, Byskov J, Mbwana K. Prevalence and morbidity of urinary schistosomiasis among school children in the Mwera Division of Pangani district, Tanazania. Ann Trop Med Parasitol. 2002;96(8):843–8. doi: 10.1179/000349802125002329. [DOI] [PubMed] [Google Scholar]
- 9.Stephenson LS, Latham MC, Ottesen EA. Malnutrition and parasitic helminth infections. Parasitology. 2000;121(Suppl):S23–38. doi: 10.1017/S0031182000006491. [DOI] [PubMed] [Google Scholar]
- 10.Hotez PJ, Brooker S, Bethony JM. Current concepts: hookworm infection. N Engl J Med. 2004;351(8):799–808. doi: 10.1056/NEJMra032492. [DOI] [PubMed] [Google Scholar]
- 11.Menendez C, Kahigwa E, Hirt R, Vounatsou P, Aponte JJ, Font F, et al. Randomized placebo-controlled trial of iron supplementation and malaria chemoprophylaxis for prevention of severe anaemia and malaria in Tanzanian infants. Lancet. 1997;350:844–50. doi: 10.1016/S0140-6736(97)04229-3. [DOI] [PubMed] [Google Scholar]
- 12.Lengeler C. Insecticide-treated nets for malaria control: real gains. Bull World Health Organ. 2004;82(2):85–91. [PMC free article] [PubMed] [Google Scholar]
- 13.Otten M, Aregawi M, Were W, Karema K, Medin A, Bekele W, et al. Initial evidence of reduction of malaria cases and dealhs in Rwanda and Ethiopia due to rapid scale- up of malaria prevention and treatment. Malar J. 2009;8:14. doi: 10.1186/1475-2875-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mashauri FM, Kinung’hi SM, Kaatano GM, Magesa SM, Kishamawe C, Mwanga JR, et al. Impact of indoor residual spraying of lambda-cyhalothrin on malaria prevalence and anemia in an Epidemic-Prone District of Muleba, North-Western Tanzania. Am J Trop Med Hyg. 2013;88(5):841–9. doi: 10.4269/ajtmh.12-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO . Preventive chemotherapy in human helminthiasis. Coordinated use of antihelmintic drugs in control interventions. A manual for health professionals and programme managers. Geneva: World Health Organization; 2006. pp. 1–62. [Google Scholar]
- 16.Tschikuka JG, Scott ME, Gray-Donald K, Kalumba ON. Multiple infection with plasmodium and helminths in communities of low and relatively high socio-economic status. Ann Trop Med Parasitol. 1996;90(3):277–93. doi: 10.1080/00034983.1996.11813053. [DOI] [PubMed] [Google Scholar]
- 17.Spiegel A, Tall A, Raphenon G, Trape JF, Druilhe P. Increased frequency of malaria attacks in subjects co-infected by intestinal worms and Plasmodium falciparum malaria. Trans Roy Soc Trop Med Hyg. 2003;97:198–9. doi: 10.1016/S0035-9203(03)90117-9. [DOI] [PubMed] [Google Scholar]
- 18.Sokhna C, Le Hesran JY, Mbaye PA, Akiana J, Camara P, Diop M, et al. Increased malaria attacks among children presenting concomitant infections by S. mamsoni in Senegal. Malar J. 2004;3:43. doi: 10.1186/1475-2875-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Hesran JY, Akiana J, Ndiaye EHM, Dia M, Senghor P, Konate L. Severe malaria attach is associeted with high prevalence of ascaris lumbricoides infection among children in rural Senegal. Trans Roy Soc Trop Med Hyg. 2004;98:397–9. doi: 10.1016/j.trstmh.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites–masters of regulation. Immunol Rev. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 21.Bentwich Z, Kalinkovich A, Weisman Z, Borkow G, Beyers N. Can eradication of helminthic infections change the face of AIDS and tuberculosis? Immunol Today. 1999;20:485–7. doi: 10.1016/S0167-5699(99)01499-1. [DOI] [PubMed] [Google Scholar]
- 22.Gallagher M, Malhotra I, Mungai PL, Wamachi AN, Kioko JM, Ouma JH, et al. The effects of maternal helminth and malaria infections on mother-to-child HIV transmission. AIDS. 2005;19(16):1849–955. doi: 10.1097/01.aids.0000189846.90946.5d. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro AE, Tukahebwa EM, Kasten J, Clerk S, Magnussen P. Epidemiology of helminth infections and their relationship to clinical malaria in Southwest Uganda. Trans Roy Soc Trop Med Hyg. 2005;99:18–24. doi: 10.1016/j.trstmh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Dreyfuss ML, Stoltzfus RJ, Shrestha JB, Pradhan EK, Leclerg SC, Khatri SK, et al. Hookworms, malaria and vitamin A deficiency contributes to anaemia and iron deficiency among pregnant women of Nepal. J Nutr. 2000;130:2527–36. doi: 10.1093/jn/130.10.2527. [DOI] [PubMed] [Google Scholar]
- 25.Ezeamama AE, Friedman JF, Acosta LP, Bellinger DC, Langdon GC, Manalo DL, et al. Helminth infection and cognitive impairment among Filipino children. Am J Trop Med Hyg. 2005;72(5):540–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Ezeamama AE, McGarvey ST, Acosta LP, Zierler S, Manalo DL, Wu HW, et al. The synergistic effect of concomitant schistosomiasis, hookworm, and trichuris infections on children’s anemia burden. PLoS Negl Trop Dis. 2008;2(6):e245. doi: 10.1371/journal.pntd.0000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tohon ZB, Mainassara HB, Garba A, Mahamane AE, Bosque-Oliva E, Ibrahim M, et al. Controlling schistosomiasis: significant decrease of anaemia in Nigerian schoolchildren. PLoS Negl Trop Dis. 2008;2(5):e241. doi: 10.1371/journal.pntd.0000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirwan P, Asaolu SO, Molloy SF, Abiona TC, Jackson AL, Holland CV. Patterns of soil-transmitted helminth infection and impact of four-monthly albendazole treatments in preschool children from semi-urban communities in Nigeria: a double-blind placebo-controlled randomised trial. BMC Infect Dis. 2009;9:20. doi: 10.1186/1471-2334-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirwan P, Jackson AL, Asaolu SO, Molloy SF, Abiona TC, Bruce MC, et al. Impact of repeated four-monthly anthelmintic treatment on Plasmodium infection in preschool children: a double-blind placebo-controlled randomized trial. BMC Infect Dis. 2010;10:277. doi: 10.1186/1471-2334-10-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nacher M. Interactions between worms and malaria: good worms or bad worms? Malar J. 2011;10:259. doi: 10.1186/1475-2875-10-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vekemans J, Mash K, Greenwood B, Leach A, Kabore W, Soulanoudjingar S. Assessment of severe malaria in a multicenter, phase III, RTS, S/AS01 malaria candidate vaccine trial: case definition, standardization of data collection and patient care. Malar J. 2011;10:221. doi: 10.1186/1475-2875-10-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anstey NM, Price RN. Improving case definitions for severe malaria. PLoS Med. 2007;4(8):e267. doi: 10.1371/journal.pmed.0040267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tukahebwa EM, Vennervald BJ, Nuwaha F, Kabatereine NB, Magnussen P. Comparative efficacy of one versus two doses of praziquantel on cure rate of Schistosoma mansoni infection and re-infection in Mayuge District, Uganda. Trans R Soc Trop Med Hyg. 2013;107(6):397–404. doi: 10.1093/trstmh/trt024. [DOI] [PubMed] [Google Scholar]
- 34.Webster BL, Diaw OT, Seye MM, Faye DS, Stothard JR, Sousa-Figueiredo JC, et al. Praziquantel treatment of school children from single and mixed infection foci of intestinal and urogenital schistosomiasis along the Senegal River Basin: monitoring treatment success and re-infection patterns. Acta Trop. 2013;128(2):292–302. doi: 10.1016/j.actatropica.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Garba A, Lamine MS, Barkiré N, Djibo A, Sofo B, Gouvras AN, et al. Efficacy and safety of two closely spaced doses of praziquantel against Schistosoma haematobium and S. mansoni and re-infection patterns in school-aged children in Niger. Acta Trop. 2013;128(2):334–44. doi: 10.1016/j.actatropica.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomized trials. BMC Med Res Methodol. 2001;1(2):1471–2288. doi: 10.1186/1471-2288-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulz KF, Altman DG, Moher D, The CONSORT Group CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11:32. doi: 10.1186/1745-6215-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Helmby H, Kullberg M, Troye-Blomberg M. Altered immune responses in mice with concomitant Schistosoma mansoni and Plasmodium chabaudi infections. Infect Immun. 1998;66(11):5167–74. doi: 10.1128/iai.66.11.5167-5174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nacher M, Singhasivanon P, Yimsamran S, Manibunyong W, Thanyavanich N, Wuthisen P, et al. Intestinal helminth infections are associated with increased incidence of Plasmodium falciparum malaria in Thailand. J Parasitol. 2002;88(1):55–8. doi: 10.1645/0022-3395(2002)088[0055:IHIAAW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 40.Druilhe P, Tall A, Sokhna C. Worms can worsen malaria. Towards a new means to roll back malaria? Trends Parasitol. 2005;21:359–62. doi: 10.1016/j.pt.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 41.Fenton A. Dances with worms: the ecological and evolutionary impacts of deworming on coinfecting pathogens. Parasitology. 2013;140:1119–32. doi: 10.1017/S0031182013000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiria AE, Hamid F, Wammes LJ, Kaisar MMM, May L. The effect of three-monthly albendazole treatment on malarial parasitemia and allergy: a household-based cluster-randomized, double-blind, placebo-controlled trial. PLoS One. 2013;8(3):e57899. doi: 10.1371/journal.pone.0057899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waknine-Grinberg JH, Gold D, Ohayon A, Flescher E, Heyfets A, Doenhoff MJ, et al. Schistosoma mansoni infection reduces the incidence of murine cerebral Malaria. Malar J. 2010;9:1186. doi: 10.1186/1475-2875-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beasley NM, Tomkins AM, Hall A, Kihamia CM, Lorri W. The impact of population level deworming on the haemoglobin levels of school children in Tanga, Tanzania. Trop Med Intl Health. 1999;4:744–50. doi: 10.1046/j.1365-3156.1999.00486.x. [DOI] [PubMed] [Google Scholar]
- 45.Crump JA, Morrissey AB, Nicholson WL, Massung RF, Stoddard RA. Etiology of severe Non-malaria febrile illness in Northern Tanzania: a prospective cohort study. PLoS Negl Trop Dis. 2013;7(7):e2324. doi: 10.1371/journal.pntd.0002324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crump JA, Ramadhani HO, Morrissey AB, Saganda W, Mwako MS. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected adults and adolescents in Northern Tanzania. Clin Infect Dis. 2011;52:341–8. doi: 10.1093/cid/ciq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crump JA, Ramadhani HO, Morrissey AB, Msuya LJ, Yang LY. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected children and infants in northern Tanzania. Trop Med Int Health. 2011;16:830–7. doi: 10.1111/j.1365-3156.2011.02774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mwangi TW, Bethony JM, Brooker S. Malaria and helminth interactions in humans: an epidemiological viewpoint. Ann Trop Med Parasitol. 2006;100(7):551–70. doi: 10.1179/136485906X118468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murray J, Murray A, Murray M, Murray C. The biological suppression of malaria: an ecological and nutritional interrelationship of the host and two parasites. Am J Clin Nutr. 1978;31:1363–6. doi: 10.1093/ajcn/31.8.1363. [DOI] [PubMed] [Google Scholar]
- 50.Brutus L, Watier L, Briand V, Hanitrasoamampionona V, Razanatsoarilala H, Cot M. Parasitic co-infections: does Ascaris lumbricoides protect against Plasmodium falciparum infection? Am J Trop Med Hyg. 2006;75(2):194–8. [PubMed] [Google Scholar]
- 51.Brutus L, Watier L, Briand V, Hanitrasoamampionona V, Razanatsoarilala H, Cot M. Confirmation of the protective effect of Ascaris lumbricoides on Plasmodium falciparum infection: results of a randomized trial in Madagascar. Am J Trop Med Hyg. 2007;77(6):1091–5. [PubMed] [Google Scholar]
- 52.Carneiro IA, Smith T, Lusingu JP, Malima R, Utzinger J, Drakeley CJ. Modeling the relationship between the population prevalence of Plasmodium falciparum malaria and anemia. Am J Trop Med Hyg. 2006;75(2 Suppl):82–9. doi: 10.4269/ajtmh.2006.75.82. [DOI] [PubMed] [Google Scholar]
- 53.Enhardt S, Burchard GD, Mantel C, Cramer JP, Kaiser S. Malaria, anaemia and malnutrition in African school children. Defining intervention strategies. J Inf Dis. 2006;194:108–14. doi: 10.1086/504688. [DOI] [PubMed] [Google Scholar]
- 54.Koukounari A, Fenwick A, Whawell S, Kabatereine NB, Kazibwe F, Tukahebwa EM, et al. Morbidity indicators of schistosomma mansoni: relationship between infection and anaemia in Ugandan school children before and after praziquantel and albedazole chemotherapy. Am J Trop Med Hyg. 2006;75(2):278–86. [PubMed] [Google Scholar]
- 55.Deribew K, Zinaye T, Petro B. Urinary schistosomiasis and malaria associated anemia in Ethiopia. Asian Pac J Trop Biomed. 2013;3(4):307–10. doi: 10.1016/S2221-1691(13)60068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schellenberg D, Menendez C, Kahigwa E, Aponte J, Vidal J, Tanner M, et al. Intermittent treatment for malaria and anaemia control at time of routine vaccination in Tanzanian infants: a randomized placebo controlled trial. Lancet. 2001;357:1471–7. doi: 10.1016/S0140-6736(00)04643-2. [DOI] [PubMed] [Google Scholar]
- 57.Shulman CE, Dorman EK, Kutts F, Kawuondo K, Bulmer JN, Peshu N, et al. Intermittent sulphadoxine-pyrimethamine to prevent severe anaemia secondary to malaria in pregnancy: a randomised placebo controllled trial. Lancet. 1999;353:632–6. doi: 10.1016/S0140-6736(98)07318-8. [DOI] [PubMed] [Google Scholar]


