Abstract
PPARγ (peroxisome proliferator-activated receptor gamma) is a nuclear receptor whose activation is dependent on a ligand. PPARγ activation by exogenous ligands, such as thiazolidinediones (TZDs), is a strategy in the treatment of type 2 diabetes for the improvement of insulin sensitivity. In addition to a ligand, PPARγ function is also regulated by posttranslational modifications, such as phosphorylation, sumoylation, and ubiquitination. Here, we report that PPARγ protein is modified by acetylation, which induces the PPARγ function in the absence of an external ligand. We observed that histone deacetylase 3 (HDAC3) interacted with PPARγ to deacetylate the protein. In immunoprecipitation, the HDAC3 protein was associated with the PPARγ protein. Inhibition of HDAC3 using RNAi-mediated knockdown or HDAC3 inhibitor increased acetylation of the PPARγ protein. Furthermore, inhibition of HDAC3 enhanced expression of PPARγ target genes such as adiponectin and aP2. The expression was associated with an increase in glucose uptake and insulin signaling in adipocytes. HDAC3 inhibition enhanced lipid accumulation during differentiation of adipocytes. PPARγ acetylation was also induced by pioglitazone and acetylation is required for PPARγ activation. In the absence of TZDs, the acetylation from HDAC3 inhibition was sufficient to induce the transcriptional activity of PPARγ. Treating the Dio mice with HDAC3 inhibitor or pioglitazone for 2 weeks significantly improved high fat diet induced–insulin resistance. Our data suggest that acetylation of PPARγ is a ligand-independent mechanism of PPARγ activation. HDAC3 inhibitor is a potential PPARγ activator for improvement of insulin sensitivity.
Keywords: type 2 diabetes, insulin sensitivity, metabolic syndrome, adipocytes, adipogenesis, PPARγ, posttranslational modifications, histone deacetylase, HDAC inhibitors, acetylation
1. Introduction
PPARγ is a well-documented transcription factor that plays an important role in the control of glucose and fatty acid metabolism. In the mechanism, PPARγ induces expression of adipocyte-specific genes and promotes differentiation of preadipocytes through transcriptional activation of target genes (Rosen and Spiegelman 2000). PPARγ is also required in the maintenance of physiological function of mature adipocytes. Insufficient PPARγ activity is associated with adipose tissue dysfunction and glucose disorders in metabolic syndrome (Fujiki, et al. 2009). At the molecular level, PPARγ forms heterodimers with the retinoid X receptor (RXR) when it binds to the gene promoter DNA of target genes. The transcriptional activity of PPARγ is regulated by ligands that determine PPARγ interaction with coactivators and corepressors (Berger and Moller 2002). TZD is a synthetic PPARγ ligand that has been widely used in clinical practice to improve insulin sensitivity in type 2 diabetes. In the absence of ligands, PPARγ binds the corepressor that is formed by HDAC3 and SMRT/NCoR. Ligand binding leads to disassociation of the corepressor complex and induces recruitment of coactivators. Although TZDs are outstanding PPARγ ligands with strong therapeutic activities in the treatment of type 2 diabetes, their side effects for the heart and bladder have caused alarm in clinical applications. It is urgent to identify a new PPARγ activator to replace TZDs in the treatment of type 2 diabetes (Ye 2011). For this reason, we explored a new strategy of PPARγ activation with a focus on HDAC3 inhibition.
Regulation of PPARγ protein by direct acetylation is a new topic in the study of PPARγ function. The PPARγ function is regulated by posttranslational modifications such as phosphorylation (Hu, et al. 1996), sumoylation (Pascual, et al. 2005), ubiquitination (Anbalagan, et al. 2012; Christianson, et al. 2008; Floyd and Stephens 2002; Hauser, et al. 2000), and histone acetylation (Qiang, et al. 2012; Sugii and Evans 2011). Phosphorylation of PPARγ at Serine 112 and 273 inhibits the PPARγ transcriptional activity. Sumoylation of PPARγ at lysine 107 in the AF1 region and at lysine 395 in the AF2 region (lysine 77 and 365 in PPARγ1, respectively) activates PPARγ by blocking the interaction between the nuclear receptor corepressor of HDAC3 and PPARγ. Ubiquitination of PPARγ leads to protein degradation following PPARγ activation by TZDs (Anbalagan et al. 2012; Christianson et al. 2008; Floyd and Stephens 2002; Hauser et al. 2000). It is largely unknown whether PPARγ protein is acetylated and, if so, how the PPARγ function is regulated by acetylation. In this study, we addressed this issue by analysis of PPARγ protein acetylation.
HDAC3 belongs to the class I HDAC proteins, which play important roles in the regulation of histone protein acetylation in the process of chromatin remodeling and gene transcription. HDACs have three classes, class I (HDAC1, 2, 3, 8, 11), class II (HDAC4, 5, 6, 7, 9, 10) (Huang, et al. 2000), and class III (SIRT1-7) (Blander and Guarente 2004). TSA is a pan-HDAC inhibitor for class I and class II HDACs. In our previous studies, we reported that HDAC inhibitors such as sodium butyrate and TSA promoted ligand-induced PPARγ function in adipocytes in vitro (Gao, et al. 2006) and prevented high fat diet–induced obesity in mice (Gao, et al. 2009). HDAC3, a member of class I HDACs, has been reported by our and other laboratories to regulate PPARγ function in adipocytes (Fajas, et al. 2002; Gao et al. 2006; Guan, et al. 2005; Miard and Fajas 2005). However, it is unknown whether HDAC3 inhibition is sufficient to activate PPARγ in the absence of classical ligands.
In this study, we found that PPARγ protein is acetylated. The acetylation was induced by a ligand and decreased by HDAC3. HDAC3 inhibition induced PPARγ acetylation and activation in the absence of exogenous ligands. The current study suggests that HDAC3 inhibition is a new approach to activate PPARγ in the absence of exogenous ligands.
2. Materials and methods
2.1. Mouse models and treatment
Diet-induced obesity (Dio) male C57BL/6J mice were purchased from the Jackson laboratory (Bar Harbor, ME) at 16-week-old, which had been fed a high fat diet (HFD, 60% calories as fat, Research Diets D12492) for 10 weeks. The mice were group-housed two to four mice per cage in the animal facility of the Pennington Biomedical Research Center with 12:12-h light-dark cycle and temperature of 22–24°C. The mice had free access to water and diet. The mice were treated with HDAC3 inhibitor by intraperitoneal injection at 10 µg/kg body weight per day for 2 weeks. Pioglitazone at the dose of 10 mg/kg body weight per day was used as a positive control. The pioglitazone was administered into the diet, and this group of mice was injected with the same amount of DMSO in PBS by intraperitoneal injection every day. All animal experiments were approved by the Institutional Animal Care and Use Committee at the Pennington Biomedical Research Center.
2.2. Cell culture and reagents
The cell lines 3T3-L1 (CL-173) and HEK293 (CRL-1573) were purchased from the American Type Culture Collection and maintained in 10% and 5% fetal bovine serum, Dulbecco’s modified Eagle’s medium in a 5% CO2 incubator. The cells were starved in Dulbecco’s modified Eagle’s medium containing 0.25% fatty acid-free bovine serum albumin overnight before treatment with 150 nM of HDAC3 inhibitor. HDAC3 inhibitor (cat. #EB1003) was purchased from KeraFAST (Boston, MA). Pioglitazone (cat. #E6910) was purchased from Sigma.
2.3. Adipogenesis
3T3-L1 preadipocytes were grown into confluence in a six-well or 100-mm plate. Then they were differentiated into adipocytes using a standard protocol. The 3T3-L1 cells were incubated in the adipogenic cocktail (5 µg/ml insulin, 0.5 mM isobutylmethylxanthine, and 10 µm dexamethasone) for 2 days. This was followed by incubation in an insulin-supplemented medium for an additional 4 days. The normal medium was used at day 7 to maintain the adipocytes. Adipogenesis was quantified with oil red O staining, as described previously (Gao et al. 2006).
2.4. Glucose uptake
3T3-L1 preadipocytes (5 × 105/well) were differentiated into adipocytes in a 12-well plate. After serum starvation in 0.25% BSA DMEM overnight, the cells were treated with HDAC inhibitors, and glucose uptake was measured as described elsewhere (Gao, et al. 2004).
2.5. Immunoblot
Whole cell lysates were prepared by sonication in lysis buffer and used in Western blots, as described elsewhere (Gao, et al. 2002). Antibodies to acetyl-lysine (ab21623), β-Actin (ab6276), HDAC3 (ab2379), and GFP (ab290) were purchased from Abcam (Cambridge, MA). Monoclonal PPARγ (E-8, sc-7273x) and HA (sc-7392) antibodies were from Santa Cruz.
2.6. Immunoprecipitation (IP) and HDAC assay
IP was carried out using whole-cell lysates (400 µg of total protein), 2–4 µg of antibody, and 40 µl of protein G-Sepharose beads (Amersham Biosciences), as described elsewhere (Gao et al. 2002). Histone deacetylase assay was conducted using a histone deacetylase assay kit (17–320; Upstate). Briefly, PPARγ was immunoprecipitated and then was used as a substrate in HDAC assay. Recombinant HDAC3 protein (cat. #H00008841, ABNOVA) was added into the reactions as an enzyme in the assay.
2.7. HDAC3 inhibitor specificity test
HDAC3 inhibitor specificity was measured using a Fluor-de-Lys® HDAC3/NCOR1 fluorometric drug discovery kit (BML-AK531, Enzo Life Science) and a Fluor-de-Lys® HDAC1 fluorometric drug discovery assay kit (BML-AK511, Enzo Life Science) as described in the manufacturer's instructions.
2.8. Intraperitoneal insulin tolerance test (ITT)
Fourteen-week-old Dio mice, which had already been given a high fat diet (D12492) for 8 weeks, were purchased from JAX Lab (stock #000664). After quarantine, the mice were divided into three groups. Each group had 8 mice. For 2 weeks, 10 µg/kg body weight/day of HDAC3 inhibitor was administrated by intraperitoneal injection. Control groups were given PBS with 0.1% DMSO (solvent). Pioglitazone was applied in the diet at the dose of 10 mg/kg body weight/day. ITT was conducted by intraperitoneal injection of insulin (I9278, Sigma) at 0.75 unit/kg of body weight in mice after a 4-h fast, as described elsewhere (Gao et al. 2009). Blood glucose was monitored in the tail vein blood using the FreeStyle blood glucose monitoring system (TheraSense, Phoenix, AZ).
2.9. Transfection and Luciferase Assay
Transient transfection was conducted in triplicate in 12-well plates. HEK293 cells (1.5 × 105/well) were plated for 16 h and transfected with plasmid DNA utilizing Lipofectamine. The PPARγ reporter system was constituted utilizing 0.2 µg each of PPRE (3×)-luciferase, PPARγ2, and RXRα in each well (Gao, et al. 2006). The cells were treated with 1µM pioglitazone or 150nM HDAC3 inhibitor for 16 h to activate PPARγ2 after transfection for 24 h. The luciferase assay was conducted using the luciferase substrate system (Promega) with a 96-well luminometer (Gao, et al. 2006). Each experiment was repeated at least three times.
2.10. Statistical analysis
All experiments were repeated independently at least three times with consistent results. For most figures, a representative bar graph showed the mean ±S.E. of multiple independent experiments normalized to appropriate controls. Student’s t-test or one-way analysis of variance was used as appropriate in statistical analyses of the data. P<0.05 was considered to indicate statistical significance.
3. Results
3.1. PPARγ is acetylated in adipocytes
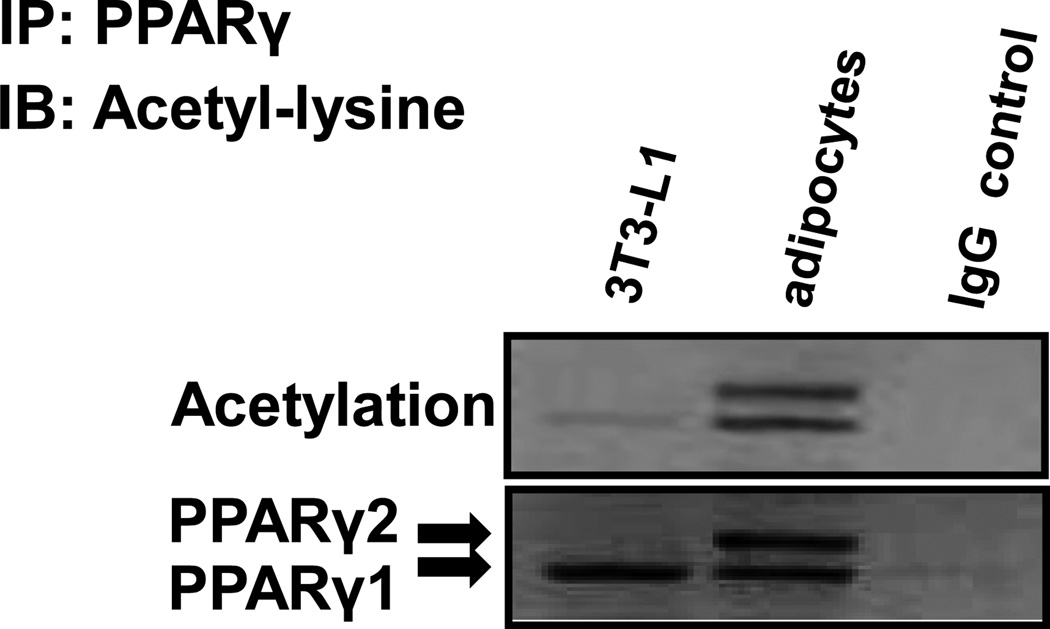
PPARγ function is regulated by posttranslational modifications, such as phosphorylation (Hu et al. 1996), sumoylation (Geiss-Friedlander and Melchior 2007; Yang and Gregoire 2006), and ubiquitination (Anbalagan et al. 2012; Christianson et al. 2008; Floyd and Stephens 2002; Hauser et al. 2000). However, whether PPARγ can be regulated by acetylation is not well known. We examined PPARγ acetylation in 3T3-L1 adipocytes before and after differentiation. The acetylation was examined in an immunoblot with the acetylation-specific antibody after PPARγ isolation by immunoprecipitation (IP) (Fig. 1). In the undifferentiated cells, the purified PPARγ protein exhibited no significant signal in the acetylation assay (Fig. 1). In the differentiated cells, there are two isoforms of PPARγ, PPARγ1 and PPARγ2, with different sizes of molecular weight. Both isoforms of PPARγ proteins expressed a strong signal of acetylation (Fig. 1). The levels of acetylation were identical between PPARγ1 and PPARγ2. The data suggest that PPARγ protein is acetylated in the adipocytes.
Figure 1.
PPARγ is an acetylated protein. PPARγ was precipitated with a monoclonal PPARγ antibody (E-8, sc-7273x, Santa Cruz) using 500 µg of protein from 3T3-L1 preadipocytes and fully differentiated 3T3-L1 adipocyte lysates. Mouse IgG was used as a negative control with the adipocyte lysates. The acetylation was detected by using rabbit acetyl-lysine antibody.
3.2. HDAC3 regulates PPARγ acetylation
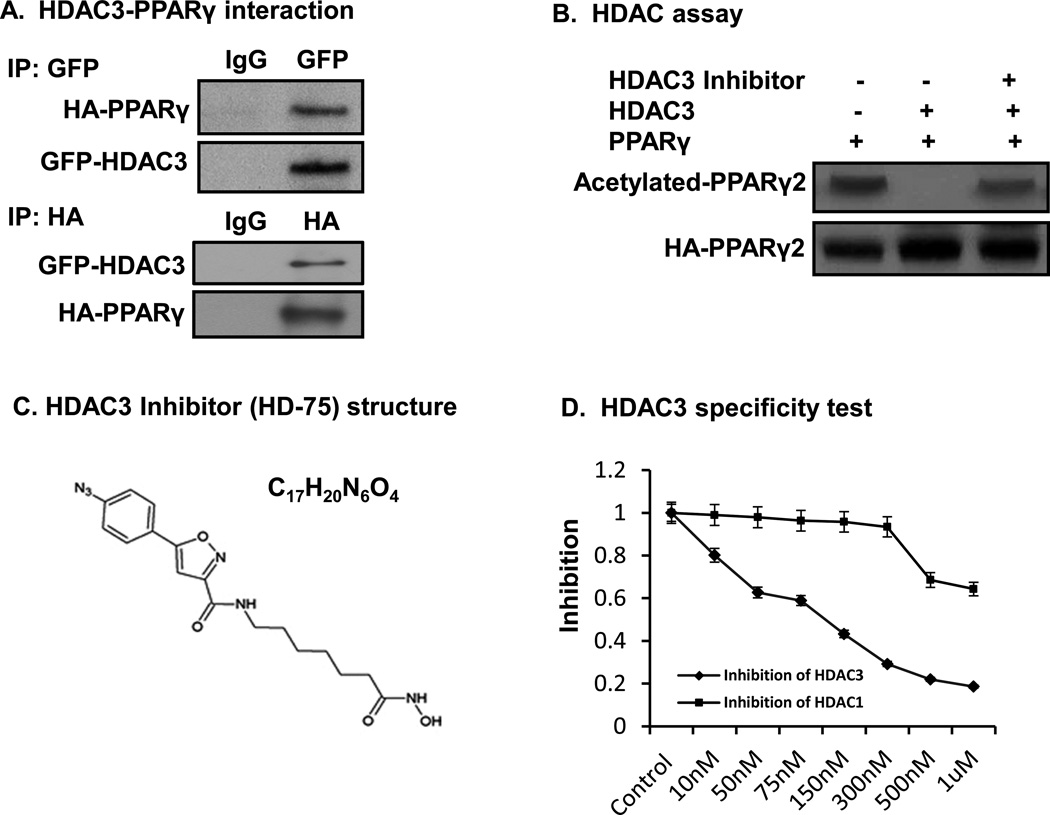
It is generally believed that PPARγ is associated with the nuclear receptor corepressor in the absence of a ligand. Disassociation of the corepressor complex is induced by the interaction of ligand PPARγ, which is required for recruitment of coactivators and acetylation of histone proteins in the initiation of gene transcription. The corepressor contains HDAC3 and SMRT or NCoR. HDAC3 inhibits the transcription by deacetylating histone proteins (Gao, et al. 2005; Hu, et al. 2001; Krogsdam, et al. 2002; Treuter, et al. 1998; Zamir, et al. 1997). However, it is unknown whether HDAC3 regulates acetylation of PPARγ protein. To address this issue, we examined HDAC3–PPARγ interaction in cells through immunoprecipitation. In the study, GFP-tagged HDAC3 was expressed, together with HA-tagged PPARγ, in a transient cotransfection of HEK293 cells. Antibodies to GFP and HA were used to isolate HDAC3 and PPARγ in IP, respectively. We observed that the HDAC3 product contained PPARγ and that PPARγ product contained HDAC3 (Fig. 2A). The data suggest that HDAC3 physically interacts with PPARγ. To test HDAC3 in deacetylation PPARγ, we determined their enzyme and substrate relationship. In the assay, a recombinant HDAC3 protein (cat. #H00008841, ABNOVA) was used as the deacetylase enzyme. Acetylation was induced in HA-tagged PPARγ2 in HEK 293 cells with HDAC inhibitor TSA (200nM for 30 minutes). When HDAC3 was inhibited with a chemical inhibitor (HD-75) in a deacetylation assay in test tube, PPARγ acetylation was preserved by the inhibitor (Fig. 2B). The acetylation was reduced in the absence of the inhibitor (Fig. 2B). The data suggest that as a protein deacetylase, HDAC3 directly deacetylates PPARγ protein.
Figure 2.
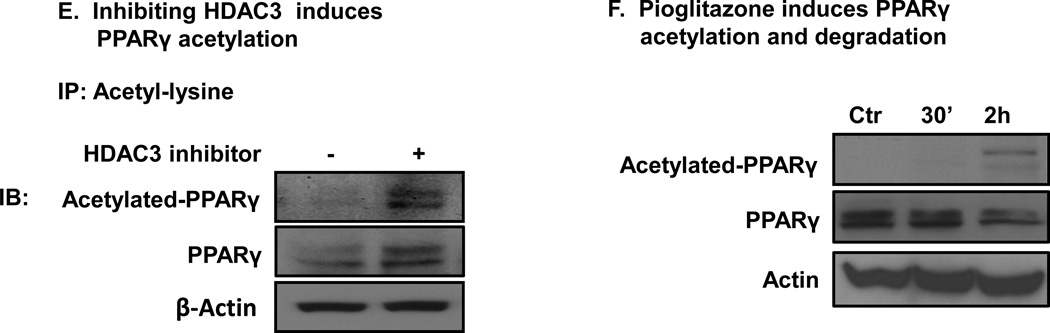
HDAC3 regulates PPARγ acetylation. A. Physical interaction of PPARγ with HDAC3. Co-IP of HA-tagged PPARγ or GFP-tagged HDAC3 in HEK293 cells. B. HDAC3 deacetylates PPARγ in HDAC assay. HA-PPARγ2 plasmids were transfected into HEK293 cells. The cells were treated with 200 nM TSA for 30 minutes to induce protein acetylation. PPARγ was immunoprecipitated on beads, and the IP product on beads was served as a substrate in HDAC assay. C. HDAC3 inhibitor structure. D. HDAC3 specificity test. 10ng of HDAC3 protein was used in the HDAC3 assay. E. Inhibiting HDAC3 induces PPARγ acetylation. 3T3-L1 adipocytes were treated with 150 nM of HDAC3 inhibitor for 2 h before being harvested. 500 µg of protein was used in IP with anti-acetyl-lysine antibody. E. Pioglitazone induces PPARγ acetylation in adipocytes.
We tested the specificity of HDAC3 inhibitor HD-75 (Fig. 2C) using a Fluor-de-Lys® HDAC3/NCOR1 fluorometric drug discovery kit and a Fluor-de-Lys® HDAC1 fluorometric drug discovery assay kit. HDAC1 assay kit was used as a control for the specificity of HDAC3 inhibitor. These kits are ideal for chemical library screening for candidate inhibitors. In the HDAC3 assay, the deacetylase activity of HDAC3 was inhibited by the HDAC3 inhibitor at IC50 of 150nM. In this dosage, it only slightly inhibited HDAC1 activity (Fig. 2D). This suggested that HD-75 has specificity for HDAC3.
PPARγ acetylation was examined by the inhibition of HDAC3 using HDAC3 inhibition in 3T3-L1 adipocytes. We tested that HD-75 has a strong induction of PPARγ acetylation at the dose of 150nM in 3T3-L1 adipocytes. In the study, 3T3-L1 adipocytes were treated with 150nM of HDAC3 inhibitor for two hours. The cells were harvested and 500ug of the whole cell lysates protein was used in IP with anti-acetyl-lysine antibody. We detected a stronger PPARγ signal in the IP product in the sample treated by the HDAC3 inhibitor. PPARγ acetylation was significantly enhanced by HDAC3 inhibitor also in the blot (Fig. 2E). 40ug of protein from 500ug/500ul IP samples was used for the loading control indicated by the β-Actin in the immonoblot (Fig. 2E, lane 3). The data suggest that PPARγ acetylation was regulated by the HDAC3 inhibitor in cells.
PPARγ activation by the ligands was reported to induce degradation of the PPARγ protein (Floyd and Stephens 2002; Hauser et al. 2000). It is unknown whether the ligand induces PPARγ acetylation. We addressed this question by examining PPARγ acetylation in pioglitazone-treated 3T3-L1 adipocytes. In cells, pioglitazone enhanced PPARγ acetylation (Fig. 2F). The data suggest that pioglitazone induces PPARγ acetylation. Acetylation of PPARγ by inhibition of HDAC3 did not cause PPARγ degradation. This suggests that acetylation of PPARγ by inhibition of HDAC3 may be different from pioglitazone-induced acetylation of PPARγ.
3.3. HDAC3 inhibitor induces PPARγ Acetylation at multiple lysine sites
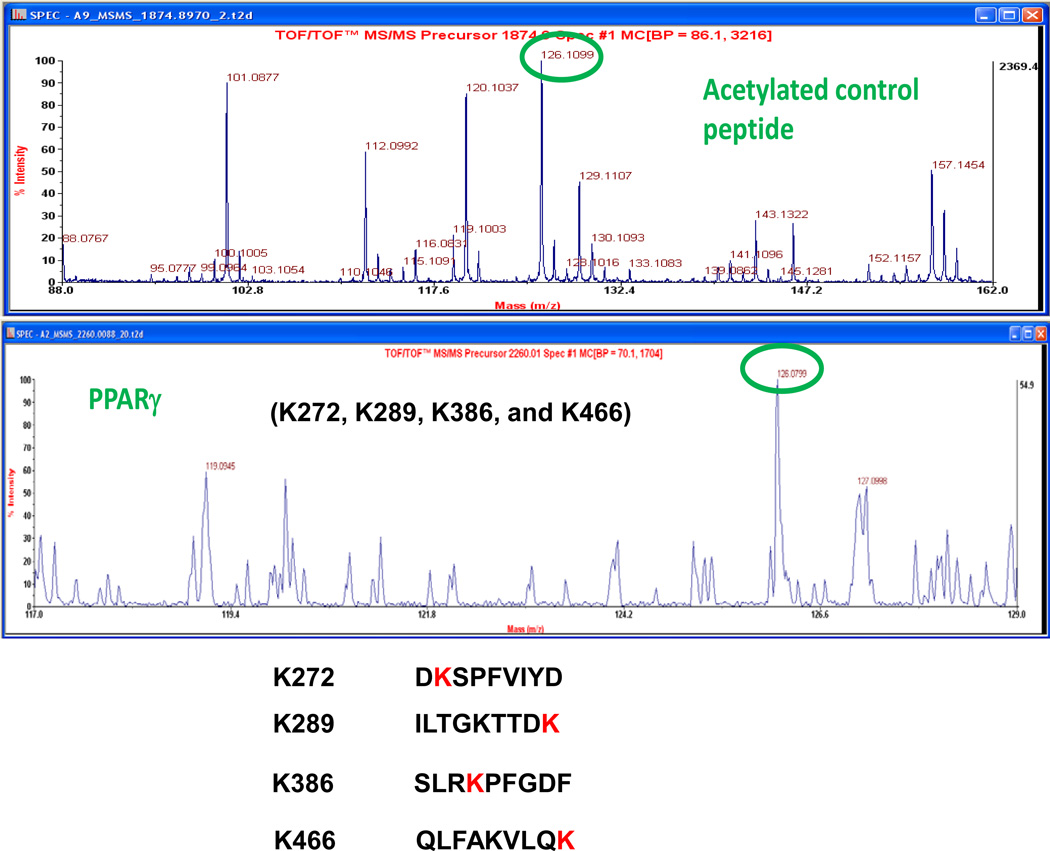
PPARγ acetylation sites were analyzed by Mass Spectrometry. In the study, PPARγ was expressed in a transient transfection of HEK293 cells. Antibody to PPARγ was used to isolate PPARγ in IP, respectively. PPARγ in the IP product was purified by SDS-Page. These analyses were performed by the Proteomics Core facility at Applied Biomics, Inc. (23785 Cabot Blvd. Suite 311 Hayward, CA 94545). We used their service and detected four acetylated lysine residues in PPARγ (Figure 3). Four acetylated lysine sites (K289, K386, K462 and K466) were detected by Mass Spectrometry (lower panel). The data confirmed that PPARγ acetylation was induced by HDAC3 inhibitor. Further study is needed for the function of acetylated lysine sites in PPARγ.
Figure 3.
Acetylation sites analysis by Mass Spectrometry. Acetylated control peptide by Mass Spectrometry was generated a 126.1 peak (top panel). Four acetylated lysine sites (K289, K386, K462 and K466) were detected by MS (lower panel). The MS figure shows one of the four acetylated lysine sites.
3.4. Acetylation induces PPARγ function
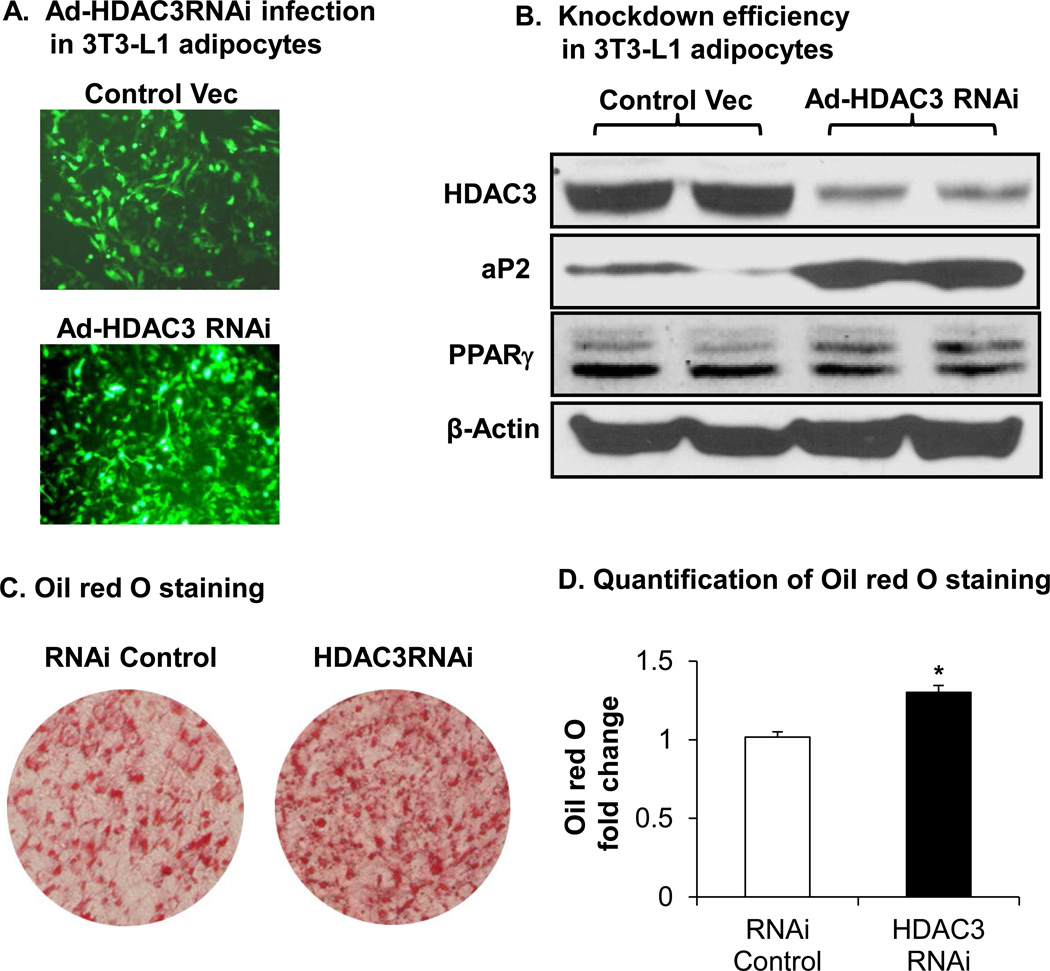
To test acetylation modification in the regulation of PPARγ function, we determined the transcriptional activity of PPARγ by quantifying expression of the target genes. PPARγ acetylation was induced in 3T3-L1 adipocytes through inhibition of HDAC3 activity with gene knockdown. A vector-based GFP-positive RNAi to HDAC3 was delivered by adenovirus, which infected 3T3-L1 adipocytes with 90% efficiency (Fig. 4A). The efficiency was quantified for GFP-positive cells under the fluorescence microscope. Expression of PPARγ target genes was determined in an immunoblot. In the control cells that were infected with the control virus, HDAC3 protein was observed with an abundant protein band. In the cells infected by RNAi virus, HDAC3 was reduced by 90% according to the decreased signal of HDAC3 protein band (Fig. 4B). Knockdown of HDAC3 significantly enhanced expression of PPARγ responsive genes, including aP2 (FABP4) (Fig. 4B). The results suggest that HDAC3 inhibition promotes the transcriptional activity of PPARγ in adipocytes.
Figure 4.
Acetylation induces PPARγ activation. A. 3T3-L1 adipocytes were infected with adeno-GFP-HDAC3 RNAi virus. B. Knockdown efficiency of HDAC3 in 3T3-L1 adipocytes with a 24-h infection and PPARγ target gene aP2 were measured in the immunoblot. C. Knockdown HDAC3 promotes adipogenesis. 3T3-L1 preadipocytes were infected with 50 µl of adenovirus in a 10-cm cell dish for 24 h, and then the cells were induced for adipogenesis with a standard protocol. After 8 days of induction, the cells were stained with oil red O. Adeno-RNAi empty vector virus was used as a negative control. D. Adipogenesis was quantified with a microscope and color absorbance. Data presented with triplicates by student’s t-test, *, p<0.05.
HDAC3 knockdown promotes adipogenesis. PPARγ induces expression of a variety of genes in the pathways for lipid biosynthesis and storage, which is required for differentiation of preadipocytes. Adipogenesis is often used to determine PPARγ function. We conducted an adipogenesis to test the approach of HDAC3 inhibition in the regulation of PPARγ function. HDAC3 was inhibited in 3T3-L1 preadipocytes by gene knockdown using the adenovirus RNAi delivery system (Fig. 4C).
Adipogenesis was induced at 48 h after virus infection. We observed the green fluorescence in cells from day 1 to day 8 in adipogenesis (data not shown). Lipid accumulation was quantified by oil red O staining in the differentiated cells 8 days later. HDAC3 knockdown increased the lipid content by 30% in this adipogenesis system (Fig. 4D). The data suggest that inhibition of HDAC3 promotes lipid accumulation in adipocytes. The result further supports that inhibition of HDAC3 is an approach to promote PPARγ function.
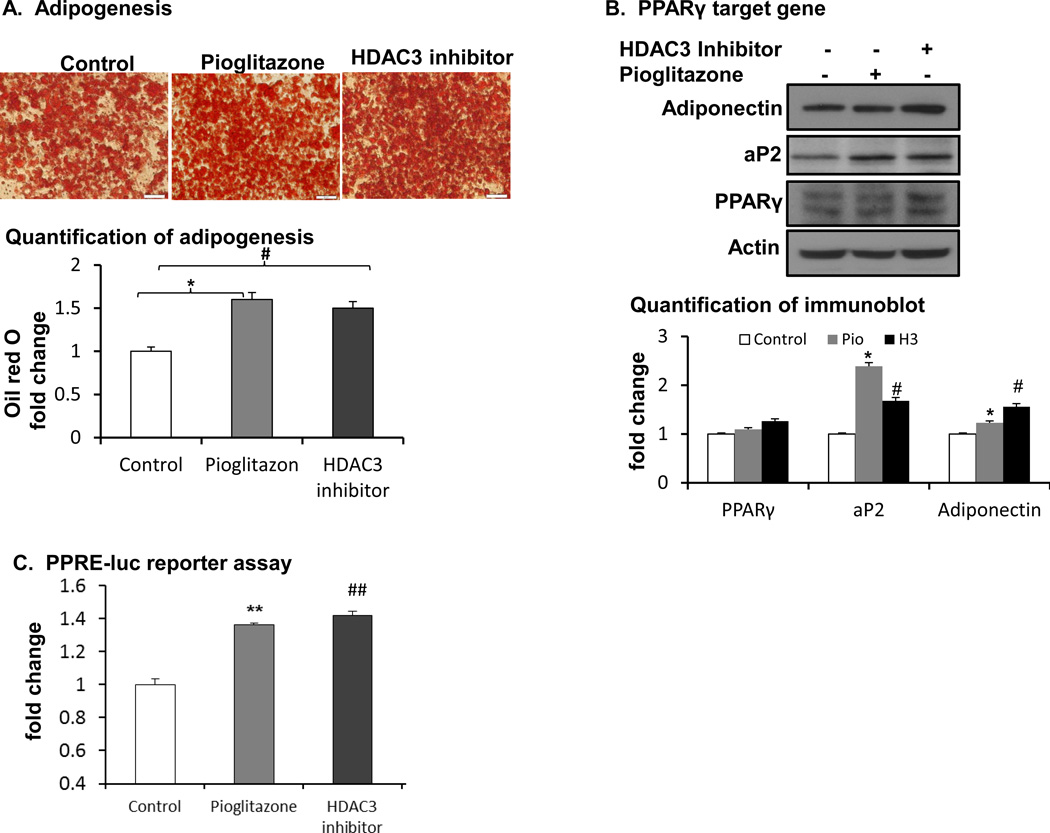
3.5. HDAC3 inhibitor promotes adipogenesis
A chemical inhibitor of HDAC3 was tested in the regulation of adipogenesis in an effort to identify a new PPARγ activator independent of TZDs. In the study, activation of PPARγ by the HDAC3 inhibitor was tested in the adipocyte differentiation model. 3T3-L1 adipocytes were differentiated in the standard adipogenic cocktail. The HDAC3 inhibitor was added to the culture medium at 150 nM during adipogenesis. Pioglitazone was used as a positive control. At the end of differentiation, the degree of differentiation was determined by oil red O staining of intracellular lipids. The HDAC3 inhibitor enhanced lipid accumulation in the cells, as indicated by the results (Fig. 5A). Oil red O staining was enhanced by 50% in the inhibitor- and pioglitazone-treated groups (Fig. 5A lower panel). HDAC3 inhibitor has the same effect with pioglitazone in inducing adipogenesis. PPARγ target genes including adiponectin and aP2 were enhanced by the inhibitor and pioglitazone in western blot (Fig. 5B). Comparing the HDAC3 inhibitor with pioglitazone, HDAC3 inhibitor enhanced more adiponectin expression. Adiponectin is known to promote insulin sensitivity. In reporter assay, HDAC3 inhibitor has the same effect with pioglitazone in inducing PPARγ transcriptional activity (Fig. 5C). The data suggest that the HDAC3 inhibitor activates PPARγ function in the absence of exogenous ligands and the potency of HDAC3 inhibitor is similar to that of pioglitazone.
Figure 5.
Inhibiting HDAC3 promoted PPARγ function. A. Adipogenesis. HDAC3 inhibitor (150nM) was added into a standard inducing cocktail, and adipogenesis was induced in a 12-well plate. Adipogenesis was evaluated using oil red O staining methods. The experiments were conducted at least 3 times, and each trial had constant results. B. PPARγ target gene expression by western blot and quantification. C. PPRE-Luc reporter assay. #, HDAC3 inhibitor vs. control, p<0.05, ##, p<0.001; *, Pioglitazone vs. control, p<0.05, **, p<0.001 by student’s t-test. Pio: pioglitazone; H3: HDAC3 inhibitor.
3.6. Inhibition of HDAC3 enhances insulin sensitivity
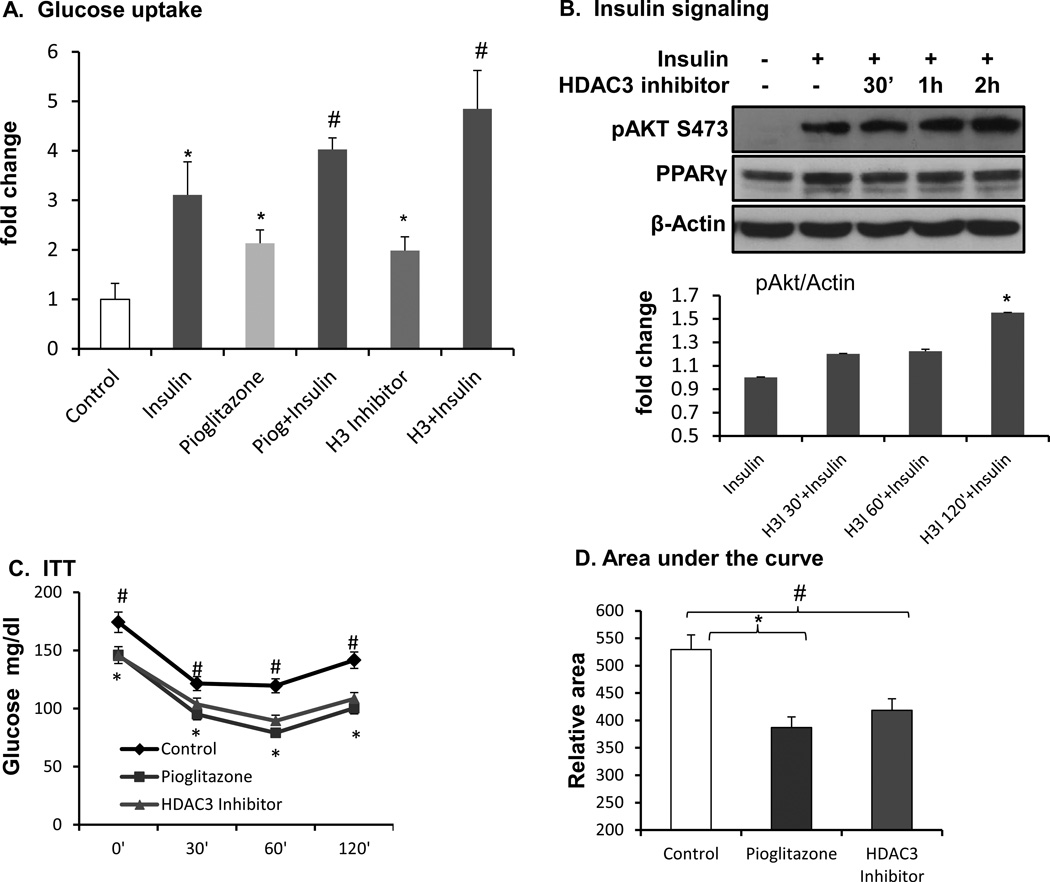
To further investigate the effect of HDAC3 inhibitor in the activation of PPARγ, first, we used glucose uptake to determine PPARγ function, which may enhance glucose uptake by induction of IRS-2 and Glut4 in the insulin-signaling pathway. Pioglitazone was used as a positive control. The results indicated that PPARγ ligand increased insulin-induced glucose uptake in 3T3-L1 adipocytes (Fig. 6A). The HDAC3 inhibitor exhibited the same activity in the promotion of glucose uptake (Fig. 6A). Insulin signaling activity was examined by Akt Serine 473 phosphorylation thereafter. Insulin-induced phosphorylation of Akt Serine 473 was enhanced by the HDAC3 inhibitor (Fig. 6B). To test that HDAC3 inhibitor enhances insulin sensitivity in vivo, we treated the Dio mice with HDAC3 inhibitor by intraperitoneal injection at 10 µg/kg body weight per day for 2 weeks. The treatment was given to 16-week-old Dio mice, which had been fed a high fat diet for 10 weeks. In our previous studies, feeding the B6 mice for 12 weeks with a high fat diet induced insulin resistance. Pioglitazone at the dose of 10 mg/kg body weight per day was used as a positive control. The pioglitazone was administered into the diet, and this group of mice was injected with the same amount of DMSO in PBS by intraperitoneal injection every day. After 2 weeks of treatment, insulin sensitivity was evaluated by an insulin tolerance test after 4 h of fasting. HDAC3 inhibitor and pioglitazone both significantly reduced glucose levels and enhanced insulin sensitivity (Fig. 6C). Body weight and food intake were not changed in the mice by the 2 week treatment. The results suggest that the HDAC3 inhibitor enhanced PPARγ function to serve as an insulin sensitizer in adipocytes.
Figure 6.
Inhibiting HDAC3 increased glucose uptake and insulin sensitivity. A. Glucose uptake. Fully differentiated 3T3-L1 adipocytes were treated with HDAC3 inhibitor overnight, and then glucose uptake was measured. *, drug vs. control; #, drug+insulin vs. insulin; p<0.05 by student’s t-test. H3I: HDAC3 inhibitor. B. Insulin signaling. C. Insulin tolerance test. D. Area under the curve of ITT. #, HDAC3 inhibitor vs. control; *, Pioglitazone vs. control; *, #, p<0.05 by student’s t-test, n=8.
4. DISCUSSION
Our data suggest that acetylation of PPARγ is induced by pioglitazone. Pioglitazone induces PPARγ activation through recruitment of coactivators and disassociation of corepressors. The coactivators contribute to the transcriptional activation by inducing histone acetylation, which is required for chromatin structure change. There has been little information about PPARγ acetylation, though PPARγ activity is regulated by protein modification such as phosphorylation (Hu et al. 1996), sumoylation (Pascual et al. 2005), and ubiquitination (Anbalagan et al. 2012; Christianson et al. 2008; Floyd and Stephens 2002; Hauser et al. 2000). In a recent study, it was reported that PPARγ acetylation (Lysine 268 and 293) was induced by a ligand and decreased by SIRT1 in HEK293 cells (Qiang et al. 2012). Although the study suggests a role of acetylation in the regulation of PPARγ function, it is not known whether the acetylation happens in the absence of a ligand. In that study, PPARγ acetylation was regulated by SIRT1 and the effect was investigated in “browning” of white adipose tissue. The acetylation inhibits brown adipocyte differentiation in the white adipose tissue (inguinal fat) by blocking PPARγ interaction with the coactivator Prdm16. They reported that inhibition of the acetylation promotes preadipocyte differentiation into brown adipocytes in response to cold challenge at 4°C cold room. Their study suggests that PPARγ acetylation favors lipid accumulation and preadipocyte differentiation into white adipocytes, which un-favors brown adipocyte differentiation. HDAC1/HDAC3 was reported to modulate PPARγ transcription through the sumoylated CEBPD in hepatic lipogenesis (Lai, et al. 2008). However, there is no report that PPARγ was acetylated by HDAC1/HDAC3. Our data suggest that HDAC3 regulates PPARγ acetylation and function directly. In this current study, we observed that PPARγ acetylation was induced by pioglitazone (Fig. 2F). PPARγ acetylation was induced by inhibition of HDAC3 in the absence of an exogenous ligand. The ligand-independent acetylation enhanced the transcriptional activity of PPARγ, as indicated by PPARγ target gene expression, lipid accumulation in adipogenesis, and insulin-induced glucose uptake. Our results suggest that PPARγ acetylation is a new approach to increase PPARγ activity and this acetylation may occur in the absence of exogenous ligands. It is not known whether acetylation correlates with phosphorylation, sumoylation, and ubiquitination in PPARγ protein.
In terms of the mechanism by which PPARγ acetylation leads to PPARγ activation, we would like to propose a model here. In this model, we suggest that there is a basal level of acetylation in the PPARγ protein in the absence of a ligand. The corepressor removes the acetylation constantly to prevent PPARγ activation without a ligand. The corepressor activity is abolished when it is disassociated from the PPARγ protein in response to a ligand. When the corepressor activity is inhibited, the basal acetylation is accumulated in the PPARγ protein in the absence of deacetylation activity. Acetylated PPARγ induces recruitment of acetyltransferases (HATs), such as p300/CBP, to induce gene transcription, which in turn induces histone acetylation in PPARγ responsive genes (Freedman 1999; Graves, et al. 1992; Rosen and Spiegelman 2000; Rosen, et al. 2000). This model explains the role of PPARγ acetylation in PPARγ activation in the absence of a ligand. Our data suggest that PPARγ acetylation is coupled with histone acetylation. Histone acetylation is required for gene transcription, but histone acetylation is likely a consequence of PPARγ acetylation. This possibility remains to be tested.
Our data suggest that HDAC3 inhibitor is a new PPARγ activator that exhibits potency comparable to pioglitazone. TZDs are the most powerful insulin sensitizer in the treatment of T2DM (Tontonoz and Spiegelman 2008). TZD-based medicines include pioglitazone (Actos by Takeda Pharmaceuticals) and rosiglitazone (Avandia by GlaxoSmithKline). Although TZD-based medicines have outstanding therapeutic activities, the adverse effects, such as heart attacks and bladder cancer, have significantly reduced their applications in the treatment of T2DM (Cariou, et al. 2012; Ferrara, et al. 2011; Rosen 2010; Tseng 2012). We believe that PPARγ activation remains as an excellent approach in the treatment of type 2 diabetes. All of the TZD-based medicines activate PPARγ. However, their side effects are different for the heart and bladder, suggesting that the side effects are not due to PPARγ activation. It is likely that the side effects are the off-target activities of the medicine. Our data suggest that HDAC3 inhibitor is a potential new generation of PPARγ activator and an insulin sensitizer. Inhibition of HDAC3 is beneficial in preventing neuronal death (Bardai, et al. 2012), improving beta-cell function (Chou, et al. 2012), and having anticancer effects (Bhaskara, et al. 2008; Escaffit, et al. 2007). Moreover, inhibition of HDAC3 promotes the transcriptional activities of myocyte enhancer factor 2 (MEF2) (Gregoire, et al. 2007; Naya and Olson 1999), implying that inhibition of HDAC3 may protect heart function. In our previous study, pan-inhibitors of HDACs prevented HFD-induced obesity and insulin resistance in mice (Gao et al. 2009). These findings suggest that HDAC3 inhibitor may be able to avoid the side effects of synthetic PPARγ ligands in vivo.
In conclusion, we report that the transcription factor PPARγ is modulated by acetylation in response to ligands. The acetylation is sufficient to induce PPARγ function in the absence of exogenous ligands. HDAC3 inhibitor is able to activate PPARγ in a ligand-independent manner. These observations indicate that HDAC3 inhibitor may be a ligand-independent PPARγ activator. Inhibition of HDAC3 may present a new approach to improve insulin sensitivity in the treatment of type 2 diabetes.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health COBRE grant (2P20RR021945), American Diabetes Association Junior Faculty Award 1-09-JF-17 and the National Natural Science Foundation of China (Grant no. 81370915).
The abbreviations used are
- PPARγ
peroxisome proliferator- activated receptor, gamma
- aP2
fatty acid binding protein 4
- TZDs
thiazolidinediones
- HDAC3
histone deacetylase 3
- RXR
retinoid X receptor
- SMRT
silencing mediator for retinoid and thyroid hormone receptors
- NCoR
nuclear receptor corepressor
- ITT
intraperitoneal insulin tolerance test
- IP
immunoprecipitation
- T2DM
type 2 diabetes mellitus
Footnotes
Declaration of interest
The authors declare that they have no conflicts of interest concerning this article.
REFERENCES
- Anbalagan M, Huderson B, Murphy L, Rowan BG. Post-translational modifications of nuclear receptors and human disease. Nucl Recept Signal. 2012;10:e001. doi: 10.1621/nrs.10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardai FH, Price V, Zaayman M, Wang L, D'Mello SR. Histone deacetylase (HDAC1) is a molecular switch between neuronal survival and death. J Biol Chem. 2012 doi: 10.1074/jbc.M112.394544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- Bhaskara S, Chyla BJ, Amann JM, Knutson SK, Cortez D, Sun ZW, Hiebert SW. Deletion of histone deacetylase 3 reveals critical roles in S phase progression and DNA damage control. Mol Cell. 2008;30:61–72. doi: 10.1016/j.molcel.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- Cariou B, Charbonnel B, Staels B. Thiazolidinediones and PPARgamma agonists: time for a reassessment. Trends Endocrinol Metab. 2012;23:205–215. doi: 10.1016/j.tem.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Chou DH, Holson EB, Wagner FF, Tang AJ, Maglathlin RL, Lewis TA, Schreiber SL, Wagner BK. Inhibition of histone deacetylase 3 protects beta cells from cytokine-induced apoptosis. Chem Biol. 2012;19:669–673. doi: 10.1016/j.chembiol.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JL, Nicoloro S, Straubhaar J, Czech MP. Stearoyl-CoA desaturase 2 is required for peroxisome proliferator-activated receptor gamma expression and adipogenesis in cultured 3T3-L1 cells. J Biol Chem. 2008;283:2906–2916. doi: 10.1074/jbc.M705656200. [DOI] [PubMed] [Google Scholar]
- Escaffit F, Vaute O, Chevillard-Briet M, Segui B, Takami Y, Nakayama T, Trouche D. Cleavage and cytoplasmic relocalization of histone deacetylase 3 are important for apoptosis progression. Mol Cell Biol. 2007;27:554–567. doi: 10.1128/MCB.00869-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajas L, Egler V, Reiter R, Hansen J, Kristiansen K, Debril MB, Miard S, Auwerx J. The retinoblastoma-histone deacetylase 3 complex inhibits PPARgamma and adipocyte differentiation. Dev Cell. 2002;3:903–910. doi: 10.1016/s1534-5807(02)00360-x. [DOI] [PubMed] [Google Scholar]
- Ferrara A, Lewis JD, Quesenberry CP, Jr, Peng T, Strom BL, Van Den Eeden SK, Ehrlich SF, Habel LA. Cohort study of pioglitazone and cancer incidence in patients with diabetes. Diabetes Care. 2011;34:923–929. doi: 10.2337/dc10-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd ZE, Stephens JM. Interferon-gamma-mediated activation and ubiquitin-proteasome-dependent degradation of PPARgamma in adipocytes. J Biol Chem. 2002;277:4062–4068. doi: 10.1074/jbc.M108473200. [DOI] [PubMed] [Google Scholar]
- Freedman LP. Increasing the complexity of coactivation in nuclear receptor signaling. Cell. 1999;97:5–8. doi: 10.1016/s0092-8674(00)80708-4. [DOI] [PubMed] [Google Scholar]
- Fujiki K, Kano F, Shiota K, Murata M. Expression of the peroxisome proliferator activated receptor gamma gene is repressed by DNA methylation in visceral adipose tissue of mouse models of diabetes. BMC Biol. 2009;7:38. doi: 10.1186/1741-7007-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Chiao P, Zhang X, Lazar MA, Seto E, Young HA, Ye J. Coactivators and corepressors of NF-kappaB in IkappaB alpha gene promoter. J Biol Chem. 2005;280:21091–21098. doi: 10.1074/jbc.M500754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, He Q, Peng B, Chiao PJ, Ye J. Regulation of nuclear translocation of HDAC3 by IkappaBalpha is required for tumor necrosis factor inhibition of peroxisome proliferator-activated receptor gamma function. J Biol Chem. 2006;281:4540–4547. doi: 10.1074/jbc.M507784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem. 2002;277:48115–48121. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Zhang X, Zuberi A, Hwang D, Quon MJ, Lefevre M, Ye J. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Mol Endocrinol. 2004;18:2024–2034. doi: 10.1210/me.2003-0383. [DOI] [PubMed] [Google Scholar]
- Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- Graves RA, Tontonoz P, Spiegelman BM. Analysis of a tissue-specific enhancer: ARF6 regulates adipogenic gene expression. Mol Cell Biol. 1992;12:1202–1208. doi: 10.1128/mcb.12.3.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire S, Xiao L, Nie J, Zhang X, Xu M, Li J, Wong J, Seto E, Yang XJ. Histone deacetylase 3 interacts with and deacetylates myocyte enhancer factor 2. Mol Cell Biol. 2007;27:1280–1295. doi: 10.1128/MCB.00882-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan HP, Ishizuka T, Chui PC, Lehrke M, Lazar MA. Corepressors selectively control the transcriptional activity of PPARgamma in adipocytes. Genes Dev. 2005;19:453–461. doi: 10.1101/gad.1263305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser S, Adelmant G, Sarraf P, Wright HM, Mueller E, Spiegelman BM. Degradation of the peroxisome proliferator-activated receptor gamma is linked to ligand-dependent activation. J Biol Chem. 2000;275:18527–18533. doi: 10.1074/jbc.M001297200. [DOI] [PubMed] [Google Scholar]
- Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- Hu X, Li Y, Lazar MA. Determinants of CoRNR-dependent repression complex assembly on nuclear hormone receptors. Mol Cell Biol. 2001;21:1747–1758. doi: 10.1128/MCB.21.5.1747-1758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EY, Zhang J, Miska EA, Guenther MG, Kouzarides T, Lazar MA. Nuclear receptor corepressors partner with class II histone deacetylases in a Sin3-independent repression pathway. Genes Dev. 2000;14:45–54. [PMC free article] [PubMed] [Google Scholar]
- Krogsdam AM, Nielsen CA, Neve S, Holst D, Helledie T, Thomsen B, Bendixen C, Mandrup S, Kristiansen K. Nuclear receptor corepressor-dependent repression of peroxisome-proliferator-activated receptor delta-mediated transactivation. Biochem J. 2002;363:157–165. doi: 10.1042/0264-6021:3630157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai PH, Wang WL, Ko CY, Lee YC, Yang WM, Shen TW, Chang WC, Wang JM. HDAC1/HDAC3 modulates PPARG2 transcription through the sumoylated CEBPD in hepatic lipogenesis. Biochim Biophys Acta. 2008;1783:1803–1814. doi: 10.1016/j.bbamcr.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Miard S, Fajas L. Atypical transcriptional regulators and cofactors of PPARgamma. Int J Obes (Lond) 2005;29(Suppl 1):S10–S12. doi: 10.1038/sj.ijo.0802906. [DOI] [PubMed] [Google Scholar]
- Naya FJ, Olson E. MEF2: a transcriptional target for signaling pathways controlling skeletal muscle growth and differentiation. Curr Opin Cell Biol. 1999;11:683–688. doi: 10.1016/s0955-0674(99)00036-8. [DOI] [PubMed] [Google Scholar]
- Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR, et al. Brown Remodeling of White Adipose Tissue by SirT1-Dependent Deacetylation of Ppargamma. Cell. 2012;150:620–632. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen CJ. Revisiting the rosiglitazone story--lessons learned. N Engl J Med. 2010;363:803–806. doi: 10.1056/NEJMp1008233. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000;16:145–171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- Sugii S, Evans RM. Epigenetic codes of PPARgamma in metabolic disease. FEBS Lett. 2011;585:2121–2128. doi: 10.1016/j.febslet.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- Treuter E, Albrektsen T, Johansson L, Leers J, Gustafsson JA. A regulatory role for RIP140 in nuclear receptor activation. Mol Endocrinol. 1998;12:864–881. doi: 10.1210/mend.12.6.0123. [DOI] [PubMed] [Google Scholar]
- Tseng CH. Pioglitazone and bladder cancer in human studies: is it diabetes itself, diabetes drugs, flawed analyses or different ethnicities? J Formos Med Assoc. 2012;111:123–131. doi: 10.1016/j.jfma.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Yang XJ, Gregoire S. A recurrent phospho-sumoyl switch in transcriptional repression and beyond. Mol Cell. 2006;23:779–786. doi: 10.1016/j.molcel.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Ye J. Challenges in Drug Discovery for Thiazolidinedione Substitute. Yao Xue Xue Bao. 2011;1:137–142. doi: 10.1016/j.apsb.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir I, Zhang J, Lazar MA. Stoichiometric and steric principles governing repression by nuclear hormone receptors. Genes Dev. 1997;11:835–846. doi: 10.1101/gad.11.7.835. [DOI] [PubMed] [Google Scholar]