Abstract
Objectives
Some perinatally infected children do not regain normal CD4 T cell counts despite suppression of HIV-1 plasma viremia by antiretroviral therapy (ART), The frequency, severity, and significance of these discordant treatment responses remain unclear.
Design
We examined the persistence of CD4 lymphocytopenia despite virologic suppression (VS) in 933 children (>5 years of age) in the US, Latin America, and the Caribbean.
Methods
CD4 T-cell trajectories were examined and Kaplan Meier methods used to estimate median time to CD4 T cell count ≥ 500 cells/μL.
Results
After 1 year of VS, most (99%) children achieved a CD4 T cell count of ≥200 cells/μl, but CD4 T cell counts remained below 500 cells/μL after 1 and 2 years of VS in 14% and 8%. Median times to first CD4 T cell count ≥ 500 cells/μl were 1.29, 0.78, and 0.46 years for children with <200, 200–349, and 350–499 cells/μL at the start of VS. New AIDS-defining events occurred in 9 children, including 4 in the first 6 months of VS. Other infectious and HIV-related diagnoses occurred more frequently and across a wide range of CD4 counts.
Conclusions
ART improved CD4 counts in most children, but the time to CD4 count of ≥ 500 cells was highly dependent upon baseline immunological status. Some children did not reach a CD4 T cell count of 500 cells/μl despite 2 years of VS. AIDS defining events occurred in 1% of the population, including children in whom VS and improved CD4 T cell counts were achieved.
Keywords: immune reconstitution, pediatrics, HIV, antiretroviral therapy, opportunistic infections, AIDS
INTRODUCTION
In most HIV-infected infants, children, and adults, combination antiretroviral therapy (cART) results in suppression of plasma viral load and an increase in peripheral CD4 T lymphocyte cell counts [1–2]. In the United States (US) and Western Europe, the availability of cART has been associated with a marked reduction in HIV-related mortality attributable to perinatal HIV infection [3–5]. These successes are being recapitulated in resource limited settings [3, 6–9].
Unfortunately, a discordant treatment response is seen in some pediatric patients in whom immunologic reconstitution does not occur despite virologic suppression (VS)[10–18]. This immunological failure (IF) phenotype has not been rigidly defined, but in a child age 5 years of age or older at baseline, it may be defined as a failure to achieve or maintain a CD4 T cell count above the level associated with severe immune suppression (CD4 <200 cells/mm3) [2].
A variety of explanations could account for this discordant IF-VS phenotype, including the antiretroviral agents being used, depletion of bone marrow precursor cells that must undergo thymic differentiation into T cells, presence of active co-infections, malnutrition, failure of HIV RNA assays to detect the genetic subtype of HIV-1 with which the child is infected, or laboratory error [2]. Previous reports suggest that IF despite VS is more common in children with a lower nadir CD4+ T cell count and older age, but conflicting data have been reported [6, 19]. In all of these reports, the number of children with the IF-VS phenotype appears to be small, and, consequently, the frequency and clinical significance of IF among children with prolonged VS has remained unclear. In one recent study of adults with persistently low CD4 T cell counts during virologically successful therapy [20], the incidence rate of new AIDS events was higher in the first six months after VS was achieved than in later periods of follow-up. After 2 years of successful suppression, no new AIDS-defining illnesses were seen, despite persistent severe CD4+ lymphocytopenia (<200 cells/μL3). No comparable data are available to inform the management of children and adolescents whose CD4 T cells remain abnormal despite successful suppression of HIV plasma viremia by antiretroviral therapy.
We examined the frequency and clinical significance of the IF-VS phenotype in perinatally HIV-infected patients to enhance our understanding of immune reconstitution in HIV-infected children and the risks of continuing a cART regimen that has failed to achieve substantial improvement in CD4 T cell counts. We hypothesized that children and adolescents with incomplete immune reconstitution in the setting of sustained virologic suppression are at greater risk of new HIV/AIDS related clinical events than individuals whose CD4 T cell counts improve or remain above levels indicative of immune suppression.
MATERIALS AND METHODS
Study Population
The source populations for this study were the Adolescent Master Protocol (AMP) of the Pediatric HIV/AIDS Cohort Study (PHACS), the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Protocol 219C (219C), and the NICHD International Site Development Initiative (NISDI) [3, 21–22]. These prospective cohort studies were designed to evaluate the impact of HIV-infection and antiretroviral therapy (ART) on HIV-infected children and adolescents with perinatal infection and enrolled over 3700 perinatally HIV-1 infected children and adolescents in the US, Latin America, and the Caribbean. The protocols were approved by human subjects review boards at each participating institution and written informed consent was obtained from each child’s parent or legal guardian. The final study population for our analysis was restricted to perinatally HIV-1 infected children only and included those with a documented period of VS lasting at least 1 year in duration beginning at or after age 5 years with available CD4 T cell counts at: 1) the start of the ART that led to VS, 2) the start of VS, and 3) at 1 year into the period of VS. Periods of VS were restricted to those starting after age 5 years to discount the variability in CD4 T cell count prior to 5 years of age [23]. For children with multiple periods of VS meeting the above eligibility criteria, their first period of VS was chosen for analyses.
Study definitions
Periods of VS were defined as beginning with two consecutive HIV viral load measures <400 copies/ml of plasma and maintaining subsequent measurements below 400 copies/ml, but allowing for isolated (single) measurements of ≥400 copies/ml (i.e. intermittent viremia). The period of VS was said to have ended at either the final <400 reading that preceded two consecutive ≥400 readings or the final viral load <400 copies/mL during follow-up if virologic rebound did not occur. If the last measurement during follow-up was ≥400 copies/mL and was preceded by a qualifying suppression period, the suppression period was defined to have ended at the time of the preceding viral load measurement of <400 copies/mL. A cART regimen was defined as at least three antiretroviral drugs from at least two different drug classes. Diagnoses with onset during the first three years of VS were reviewed and classified as meeting the definition of an AIDS-defining condition based on the age-appropriate Centers for Disease Control and Prevention (CDC) classification system (i.e. meeting the definition of a CDC class C event) [24–25]. Other clinical outcomes of interest during the first three years of VS included bacterial meningitis, sepsis, cardiomyopathy, cervical dysplasia, mucocutaneous herpes simplex virus (HSV) infections, herpes zoster, lymphoid interstitial pneumonia, nephropathy, nocardiosis, oropharyngeal candidiasis, pneumonia, and disseminated varicella.
Statistical Methods
CD4 T cell count trajectories during VS were examined by plotting mean CD4 T cell counts during the study period of VS by CD4 count at the start of VS and fitting piece-wise linear regression models to estimate the observed slopes. CD4 T cell counts at 6 months, 1 year, and 2 years after VS were also compared relative to counts at the start of VS. Kaplan-Meier survival curves were used to estimate the median time to achieve CD4 T cell counts ≥500 cells/μl, relative to CD4 T cell count categories at the beginning of VS. Kaplan-Meier survival curves were also used to estimate time to first sustained measures of CD4 <200, <350, and <500 cells/μl among those with CD4 ≥500 cells/μl at baseline to inform CD4 monitoring strategies. To evaluate the clinical significance of incomplete immune reconstitution during VS, incidence rates for first AIDS-defining event during the first three years of follow-up were calculated by CD4 T cell count at the time of the event and CD4 T cell count at baseline. Events were also plotted by time since VS and CD4 count at the time of the event. Similar analyses were conducted for the other clinical outcomes of interest. Sensitivity analyses excluding those with intermittent (single) viral loads ≥10,000 copies/ml were also conducted for the evaluation of incidence of all clinical events. All analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
Study population
Records were available for 3,784 children with perinatal HIV enrolled in the AMP and 219C cohorts in the US and Puerto Rico and NISDI sites in Central and South America (Brazil, Mexico, Argentina, Puerto Rico, and Peru). Among these, CD4 T cell counts and plasma HIV viral load data needed for analysis were available for 933 children who experienced at least 1 year of VS at 5 years of age or more after starting a cART regimen (Supplemental Table 1). Most were seen at centers in the US (87%) (Table 1), with the majority of the AMP/219C cohort born prior to 1996 (83%). By contrast, the majority of the NISDI cohort (72%) was born after 1996. Prior to the initiation of a suppressive cART regimen, many had only been previously treated with a non-cART regimen (32% of children from the United States and Puerto Rico, 15% from NISDI sites). At the time of first suppressive cART, participants had a median age of 8.8 years. VS was achieved in most cases with cART that employed an HIV protease inhibitor plus nucleoside/nucleotide reverse transcriptase inhibitors (nRTIs) (52%), a non-nucleoside reverse transcriptase inhibitor plus nRTIs (12%), or both protease inhibitor and non-nucleoside reverse transcriptase inhibitor in combination with nRTIs (21%) (Table 1).
Table 1.
Characteristics of Study Population
| Characteristics | Total (N=933) |
Study cohort | |
|---|---|---|---|
| AMP/219C* (N=825) |
NISDI (N=108) |
||
| Female Gender | 492 (53%) | 436 (53%) | 56 (52%) |
| Age (years) at start of ART resulting in VS** | 8.8 (6.2, 11.6) | 8.8 (6.2, 11.8) | 8.5 (5.6, 10.6) |
| Birth cohort | |||
| Born prior to 1996 | 718 (77%) | 688 (83%) | 30 (28%) |
| Country of origin | |||
| Argentina | 8 (1%) | 0 (0%) | 8 (7%) |
| Brazil | 79 (8%) | 0 (0%) | 79 (73%) |
| Mexico | 19 (2%) | 0 (0%) | 19 (18%) |
| Peru | 2 (0%) | 0 (0%) | 2 (2%) |
| Puerto Rico | 13 (1%) | 13 (2%) | 0 (0%) |
| US | 812 (87%) | 812 (98%) | 0 (0%) |
| Race/ethnicity | |||
| Black/Mulato (Non-Hispanic) | 464 (50%) | 458 (56%) | 6 (6%) |
| Hispanic(Regardless of Race) & Native American | 334 (36%) | 246 (30%) | 88 (81%) |
| White/Asian/Other (Non-Hispanic) | 132 (14%) | 118 (14%) | 14 (13%) |
| Missing | 3 (0%) | 3 (0%) | 0 (0%) |
| CDC C diagnosis prior to VS | 300 (32%) | 299 (36%) | 1 (1%) |
| Ever on cART prior to suppressing regimen | |||
| Yes | 585 (63%) | 508 (62%) | 77 (71%) |
| No, previous non-cART | 279 (30%) | 263 (32%) | 16 (15%) |
| No, no prior regimen | 69 (7%) | 54 (7%) | 15 (14%) |
| Type of regimen immediately preceding suppressing regimen | |||
| cART | 411 (44%) | 351 (43%) | 60 (56%) |
| Non-cART regimen | 310 (33%) | 291 (35%) | 19 (18%) |
| Off Antiretroviral therapy | 143 (15%) | 129 (16%) | 14 (13%) |
| No prior regimen | 69 (7%) | 54 (7%) | 15 (14%) |
| Type of regimen at start of VS† | |||
| cART (PI + nRTIs) | 483 (52%) | 421 (51%) | 62 (57%) |
| cART (PI + NNRTI ± nRTIs) | 200 (21%) | 195 (24%) | 5 (5%) |
| cART (NNRTI + nRTIs) | 115 (12%) | 84 (10%) | 31 (29%) |
| Other cART regimen# | 36 (4%) | 28 (3%) | 5 (5%) |
| Non-cART regimen | 95 (10%) | 90 (11%) | 5 (5%) |
| Antiretroviral therapy data incomplete/missing | 4 (<1%) | 4 (<1%) | 0 (0%) |
| Highest known viral load (log10 copies/mL) at start of VS*** | 4.8 (4.0, 5.4) | 4.7 (3.9, 5.4) | 5.2 (4.7, 5.8) |
| Lowest known CD4 T cells at start of VS | |||
| Absolute number*** | 368 (196, 583) | 353 (187, 576) | 458 (276, 664) |
| CD4% | 17 (11, 25) | 17 (10,25) | 19 (13,25) |
| Time from start of suppressing ART to VS | |||
| Median | 5.6 (2.3, 20.4) | 6.4 (2.3, 22.5) | 3.1 (1.8, 6.1) |
| <9 months | 541 (58%) | 453 (55%) | 88 (81%) |
| ≥9 to ≤15 months | 84 (9%) | 76 (9%) | 8 (7%) |
| >15 months | 308 (33%) | 296 (36%) | 12 (11%) |
Data are presented as number (%) or median (IQR).
Includes subjects enrolled in AMP (n=76), 219C (n =592) or both (n=157).
VS = virologic suppression
p <0.001 for comparison between AMP/219C and NISDI cohorts
PI = HIV protease inhibitor, NNRTI = non-nucleoside analogue reverse transcriptase inhibitor, nRTI = nucleoside/nucleotide reverse transcriptase inhibitor
cART with fusion inhibitor or integrase inhibitor (with or without PI or NNRTI).
Immunological Changes in Response to cART
At the time of initiation of cART that resulted in VS, 92 children (10%) had CD4 T cell counts below 200 cells/μL, while 118 (13%) had 200 to 349 CD4 T cells/μL and 138 (15%) had 350 to 499 CD4 T cells/μL (Table 2). Early improvements in CD4 T cell counts were commonly seen, and by the time VS was achieved, the number of children with CD4 T cell counts under 500/μL had decreased from 348 to 206 (37% to 22% of the total cohort) and only 29 (3%) children still had a CD4 T cell count of <200 cells/μL (Table 2). In children whose CD4 T cell counts were initially <500 /μL, a biphasic response was seen with CD4 T cell numbers rising more rapidly during the first six months after cART initiation than in subsequent months (Figure 1, panel A). The rate of initial rise was twice as great in children with baseline CD4 T cell counts <200 /μL than in children with 350 to 499 CD4 T cells (556 versus 258 cells/year). (Supplemental Table 2). The time until the first CD4 T cell count exceeded 500/μL was strongly and inversely related to the baseline CD4 values (Figure 1, Panel B): the median time to reach this value was 1.29, 0.78 and 0.46 years for children with CD4 T cells at baseline of <200, 200–<350, and 350 to 499 cells/μL, respectively. The proportion of children with CD4 T cell counts <500 cells/μL progressively declined during VS, but CD4 counts below this threshold were observed in 14% after one year and 8% at two years (Supplemental Table 3).
Table 2.
Frequencies of subjects by CD4 count at time of initiation of virologic suppressing cART and at start of suppression period
| CD4 count (cells/μL) at start of suppressing ART* | |||||
|---|---|---|---|---|---|
| CD4 count (cells/μL) at start of virologic suppression** | Total (N=933) |
<200 (N=92) |
200 to 349 (N=118) |
350 to 499 (N=138) |
≥500 (N=585) |
| <200 | 29 (3%) | 24 (26%) | 3 (3%) | 1 (1%) | 1 (0%) |
| 200 to 349 | 58 (6%) | 20 (22%) | 23 (19%) | 11 (8%) | 4 (1%) |
| 350 to 499 | 119 (13%) | 19 (21%) | 45 (38%) | 30 (22%) | 25 (4%) |
| ≥500 | 727 (78%) | 29 (32%) | 47 (40%) | 96 (70%) | 555 (95%) |
CD4 reading nearest to start of suppressing ART (up to 6 months prior to ART start date allowed).
Nearest CD4 reading to the start of virologic suppression (readings up to 3 months after start allowed).
Figure 1.
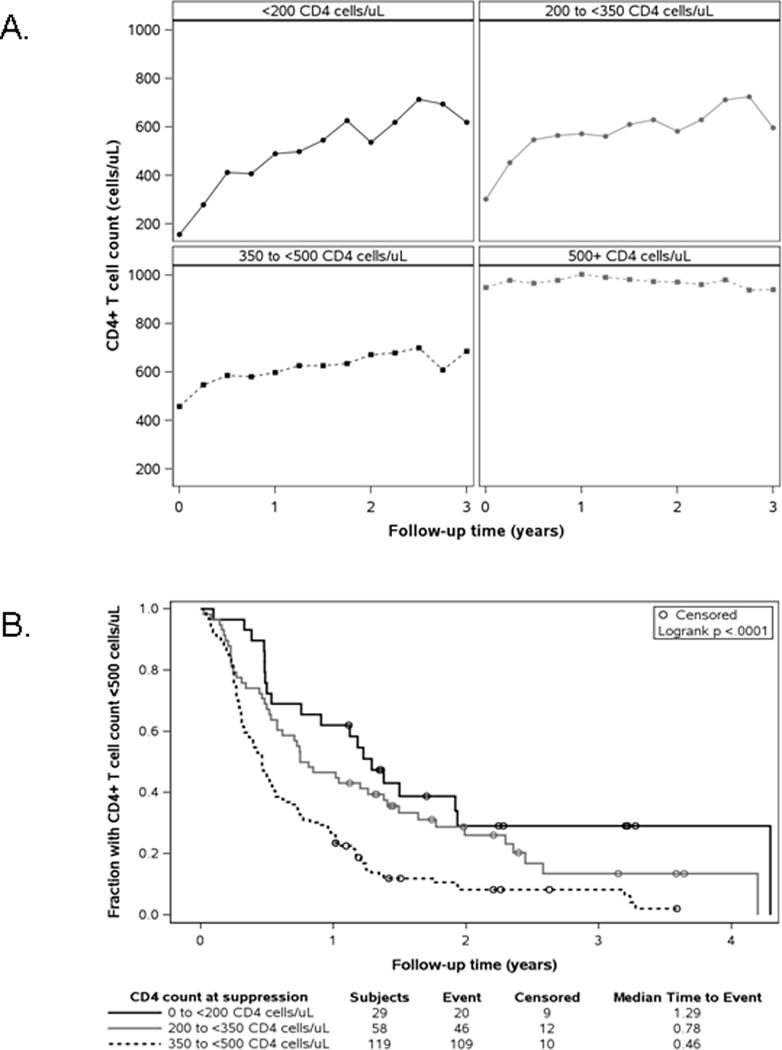
Tempo of immune reconstitution during periods of virological suppression.
A. CD4 count (cells/μL) means over follow-up, by CD4 count at the start of the virologic suppression periods.
B. Kaplan-Meier survival curves for time to first CD4 count ≥500 cells/μL, by CD4 count at the start of the virologic suppression period.
CD4 T cell counts were very stable among the 727 children (78% of the total study population) whose counts were 500 cells/μL or higher at the time that VS was confirmed: fewer than 10% experienced sustained periods in which CD4 T counts dropped below thresholds of either 350 or 200 cells/μL (Figure 2, Panel A). This was also true for the NISDI cohort when examined separately from children in the United States in Puerto Rico (Supplemental Figure 1).
Figure 2.
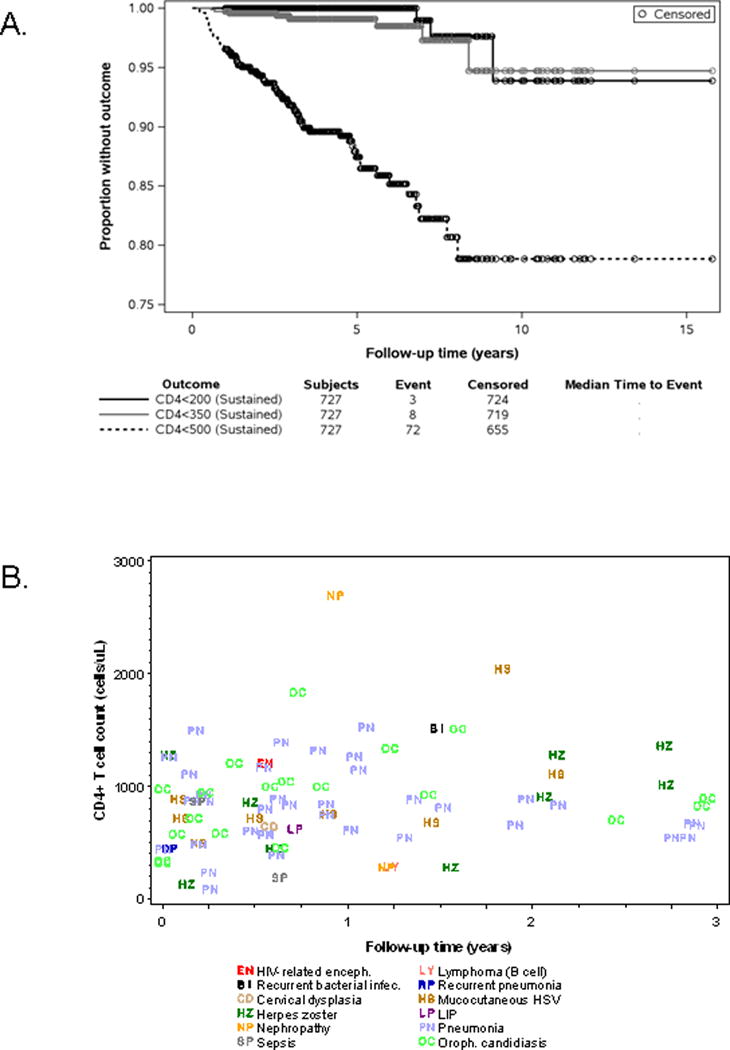
Development of low CD4 counts and serious clinical events during virologic suppression.
A. Kaplan-Meier survival curves for time to first sustained low CD4 count outcome, among subjects with CD4 ≥ 500 at the start of the virologic suppression period.
B. New Clinical Events (CDC C and non-AIDS defining) during the first three years of study follow-up, by CD4 count at event. Only first clinical event for an individual is shown
These data indicate that durable increases in CD4 T cell counts occur in children with perinatal HIV infection, although maximal improvement in the T cell count may not occur for years after VS has occurred. However, prolonged CD4 lymphocytopenia is seen in some individuals even after prolonged virologic suppression. (Supplemental Table 3).
Clinical events occurring during virologic suppression
To assess the clinical impact of low CD4 T cell counts at the beginning of and during virologic suppression, we examined the incidence of new clinical events indicative of immune suppression. As outlined in Table 3, 9 children experienced one or more new CDC C events during the first three years following VS, including 4 in the first 6 months following VS and 5 at a time when CD4 T cell counts were 500 cells/μl or more. Incidence rates for the first CDC C event during this follow-up period did not statistically differ by person-time spent with CD4 T cell counts <500 cells/μL or by CD4 T cell counts at start of virologic suppression (data not shown).
Table 3.
Details of the first CDC C classification event by subjects during the first three years of study follow-up
| Days from start of period of virologic suppression | Age at Time of Event | CD4 T cell count at time of event | Event |
|---|---|---|---|
| 0 | 6 | 152 | Esophageal candidiasis |
| 13 | 15 | 454 | Recurrent pneumonia* |
| 60 | 15 | 889 | Mucocutaneous HSV (chronic) |
| 168 | 11 | 182 | Mucocutaneous HSV (chronic) |
| 201 | 12 | 1209 | HIV-related encephalopathy |
| 405 | 14 | 1409 | Recurrent pneumonia* |
| 450 | 7 | 286 | Lymphoma (B cell) |
| 544 | 11 | 1516 | Recurrent bacterial infections |
| 624 | 11 | 2070 | Recurrent pneumonia* |
Culture-confirmed bacterial pneumonia occurring within two years of a previous episode.
We also examined reports of non-AIDS defining clinical events of interest. Ninety individuals experienced a CDC C event and/or a non-AIDS defining clinical event of interest throughout the first three years of virologic suppression; these events largely occurred at a time when CD4 counts were ≥ 500 cells/μl. (Figure 2, Panel B and Supplemental Table 4). Similar to the CDC C events, incidence rates for these events did not statistically differ by person-time spent with CD4 T cell counts <500 cells/μL or by CD4 T cell counts at start of virologic suppression. We performed a similar analysis with data related to the CDC-C and the other clinical events combined, with similar findings: the incidence rates of these events were not influenced by these CD4 T cell parameters. Twenty-three subjects experienced recurrent events (CDC C and/or non-AIDS defining) during the first three years of follow-up; 14 of these individuals experienced one or more episodes of pneumonia after an earlier significant clinical event. (Supplemental Table 5).
DISCUSSION
In adults, discordant virologic and immunological responses to antiretroviral therapy have been associated with a higher risk of death from both AIDS-defining and non-AIDS-defining causes among those with persistently low CD4+ lymphocyte counts. [20, 26–28]. A failure of cART to increase CD4 T cell counts, despite suppression of HIV-1 plasma viremia, also occurs in infants, children and adolescents, but the frequency and clinical significance of this phenomenon have been unclear. In this multinational, large pediatric observational study, we found that a failure to achieve or maintain a CD4 T cell count above 200 cells/μL in the setting of VS appears to be rare: at 2 years into VS, only 4 children had CD4 counts recorded below this threshold. Subsequent sustained periods of severe CD4 lymphocytopenia (<200 cells/μL) also appeared to be very uncommon during prolonged follow-up, occurring in only 3 of 727 (0.4%) individuals with data extending to 15 years of follow up (Figure 2, Panel A). These data contrast with studies of adults, in whom failure to achieve full CD4+ T cell count reconstitution appears to be more common; this likely reflects residual thymic function generally seen in children and adolescents, including those with histories of AIDS defining conditions [27, 29].
However, immune reconstitution was protracted in some children. In agreement with smaller pediatric studies [15, 30–35], the time to achieve a maximal CD4 T cell count was highly dependent on the CD4 count at the beginning of the cART regimen that resulted in VS. We found this to be true when data from the smaller NISDI and the larger AMP/219c cohorts were examined separately, or when the data were examined together. Children with the lowest CD4 T cell counts showed the greatest relative and absolute initial improvements in CD4 T cell concentration, but took the longest to achieve a normal CD4 count threshold. These data are consistent with adult cohort studies [26–27]. Data from this study also suggest that clinicians should anticipate that the maximal improvement in CD4 T cell count may not occur for several years after VS has occurred.
The clinical significance of having low CD4 T cell counts during VS has been evaluated in a limited number of studies of adults. Zoufaly et al examined clinical outcomes of HIV-infected adults with discordant virologic and immunological responses to antiretroviral therapy [20]. New AIDS-defining events occurred most frequently during the initial 6 months of complete VS and decreased significantly thereafter. We attempted to replicate this analysis but found that AIDS defining illnesses were uncommon in the pediatric cohorts studied: only 9 study subjects experienced one or more new CDC C events during the first 2 years of VS. This likely reflects the fact that only 10% of subjects’ studied had CD4 T cell counts below 200 cells/μL at the beginning of cART. It may also reflect the fact that majority of children were receiving some form of antiretroviral therapy when the regimens resulting in VS were begun, as immunologic improvements may occur even if HIV viremia is not fully suppressed. However, we also noted a high frequency of serious, albeit non-AIDS defining, illnesses among children in this cohort (Supplemental Table 5). The occurrence of these events throughout the follow-up period was independent of CD4 T cell count and is indicative of ongoing immunologic deficits. Nonetheless, incidence rates for CDC C defining and non-AIDS defining clinical events did not statistically differ by person-time spent with CD4 T cell counts <500 cells/μL (compared to time above this threshold) or by CD4 T cell counts at start of virologic suppression, serving as a reminder that CD4 T cell counts are an imperfect indicator of immune function [36].
The strengths of this study include its multinational composition, prolonged data collection period, and study population that reflects the racial and ethnic diversity of communities most affected by pediatric HIV in the western hemisphere. However, this analysis has several limitations. First, it focused on children 5 years of age or more. In contrast to this older population, disease progression is more rapid among infants and younger children, in whom AIDS defining illnesses often occur at CD4 T cell counts that are well above normal range for adolescents and adults. In addition, the spacing of viral load measurements from some individual children may have been broad enough to miss periods of HIV viremia that had deleterious effects on immune reconstitution. We used <400 copies/ml as the threshold for defining VS, as most data in the cohorts studied were accumulated prior to widespread use of HIV-1 RNA monitoring with greater levels of sensitivity. It is possible that lower level viremia, or transient increases in HIV viral load, may have interfered with or disrupted immune reconstitution in some children. In an effort to determine if subjects with high transient increases in viral load might have significantly influenced our results, we performed an additional analysis restricted to subjects who did not experience a viral load at or above 10,000 copies/mL during the first three years of follow-up. Removing these 34 subjects from the original set of 933 did not alter our estimates of the rates of CDC C or other clinical events or the impact of baseline CD4 T cell counts on CD4 cell reconstitution (data not shown).
Although not the primary focus of this study, we also examined the stability of CD4 T cell counts in children who had counts of 500 cells/μL or more at the time that virologic suppression was confirmed. It was very uncommon for these children to have subsequent reductions in CD4 T cell counts to below 500 cells/μL, lending support to the recent suggestion that infrequent CD4 count monitoring may be reasonable during long periods of VS. [2]
Overall, the vast majority of children with perinatal HIV infection in this study who began a cART regimen associated with VS experienced significant improvements in CD4 T cell counts. This immunological improvement was not maximized in some individuals for several years. Additionally, a small, but significant proportion of children (8%) did not reach a CD4 T cell count of 500 cells/μl or more during VS during the periods of observation. The continued occurrence of clinically important diagnoses despite CD4 cell reconstitution in most subjects highlights the need for improved measures of immune function in children.
Supplementary Material
Supplemental Figure 1. Kaplan-Meier survival curves for time to first sustained low CD4 count outcome among NISDI subjects with CD4 ≥ 500 at the start of the virologic suppression period.
Acknowledgments
We thank the children and families for their participation in PHACS, and the individuals and institutions involved in the conduct of PHACS. The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard University School of Public Health (HD052102) (Principal Investigator: George Seage; Project Director: Julie Alperen) and the Tulane University School of Medicine (HD052104) (Principal Investigator: Russell Van Dyke; Co-Principal Investigator: Kenneth Rich; Project Director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc (PI: Julie Davidson).
The following institutions participated in conducting PHACS AMP in 2013, in alphabetical order: Ann & Robert H. Lurie Children’s Hospital of Chicago, Baylor College of Medicine, Bronx Lebanon Hospital Center, Children’s Diagnostic & Treatment Center, Children’s Hospital, Boston, Jacobi Medical Center, Rutgers – New Jersey Medical School, St. Christopher’s Hospital for Children, St. Jude Children’s Research Hospital, San Juan Hospital/Department of Pediatrics, Tulane University Health Sciences Center, University of California, San Diego, and the University of Colorado Denver Health Sciences Center.
The following sites participated in PACTG 219/219C: University of New Jersey Medical and Dental School – Department of Pediatrics, Division of Allergy, Immunology & Infectious Diseases, Boston Medical Center, Division of Pediatric Infectious Diseases, Med, Children’s Hospital LA – Department of Pediatrics, Division of Clinical Immunology & Allergy, Long Beach Memorial Medical Center, Miller Children’s Hospital, Harbor – UCLA Medical Center – Department of Pediatrics, Division of Infectious Diseases, Johns Hopkins Hospital & Health System – Department of Pediatrics, Division of Infectious Diseases, University of Maryland Medical Center, Division of Pediatric Immunology & Rheumatology, Texas Children’s Hospital, Allergy & Immunology Clinic, Cook County Hospital, Children’s Hospital of Columbus, Ohio, University of Miami Miller School of Medicine, Division of Pediatric Immunology & Infectious Disease, University of California San Francisco School of Medicine, Department of Pediatrics, Children’s Hospital & Research Center Oakland, Pediatric Clinical Research Center & Research Lab, University of California San Diego Mother, Child & Adolescent HIV Program, Duke University School of Medicine – Department of Pediatrics, Children’s Health Center, University of North Carolina at Chapel Hill School of Medicine – Department of Pediatrics, Division of Immunology and Infectious Diseases, Schneider Children’s Hospital, Harlem Hospital Center, New York University School of Medicine, Division of Pediatric Infectious Diseases, Children’s National Medical Center, ACT, University of Washington School of Medicine – Children’s Hospital and Regional Medical Center, University of Illinois College of Medicine at Chicago, Department of Pediatrics, Yale University School of Medicine – Department of Pediatrics, Division of Infectious Disease, SUNY at Stony Brook School of Medicine, Division of Pediatric Infectious Diseases, Howard University Hospital, Department of Pediatrics & Child Health, LA County/University of Southern California Medical Center, University of Florida Health Science Center Jacksonville, Division of Pediatric Infectious Disease & Immunology, North Broward Hospital District, Children’s Diagnostic & Treatment Center, University of Rochester Medical Center, Golisano Children’s Hospital, Medical College of Virginia, St. Jude Children’s Research Hospital, Department of Infectious Diseases, University of Puerto Rico, U. Children’s Hospital AIDS, Children’s Hospital of Philadelphia, Center for Pediatric & Adolescent AIDS, St. Christopher’s Hospital for Children/Drexel University College of Medicine, Bronx-Lebanon Hospital Center, Infectious Diseases, New York Medical College/Metropolitan Hospital Center, University of Massachusetts Memorial Children’s Medical School, Department of Pediatrics, Baystate Health, Baystate Medical Center, Connecticut Children’s Medical Center, Medical College of Georgia School of Medicine, Department of Pediatrics, Division of Infectious Disease, University of South Alabama College of Medicine, Southeast Pediatric ACTU, LSU Health Sciences Center, Tulane University Health Sciences Center, St. Josephs Hospital and Medical Center, Cooper University Hospital – Children’s Hospital Boston, Division of Infectious Diseases, David Geffen School of Medicine at UCLA – Department of Pediatrics, Division of Infectious Diseases, Children’s Hospital of Orange County, Children’s Memorial Hospital – Department of Pediatrics, Division of Infectious Disease, University of Chicago – Department of Pediatrics, Division of Infectious Disease, Mt. Sinai Hospital Medical Center – Chicago, Women’s & Children’s HIV Program, Columbia University Medical Center, Pediatric ACTU, Incarnation Children’s Center, Cornell University, Division of Pediatric Infectious Diseases & Immunology, University of Miami Miller School of Medicine – Jackson Memorial Hospital, Bellevue Hospital (Pediatric), San Francisco General (Pediatric), Phoenix Children’s Hospital, Metropolitan Hospital Center (N.Y.), University of Cincinnati, SUNY Downstate Medical Center, Children’s Hospital at Downstate, North Shore University Hospital, Jacobi Medical Center, University of South Florida – Department of Pediatrics, Division of Infectious Diseases, Cornell University, Oregon Health & Science University – Department of Pediatrics, Division of Infectious Diseases, Children’s Hospital of the King’s Daughters, Infectious Disease, Lincoln Medical & Mental Health Center, Mt. Sinai School of Medicine, Division of Pediatric Infectious Diseases, Emory University Hospital, San Juan City Hospital, UMDNJ – Robert Wood Johnson, Ramon Ruiz Arnau University Hospital, Medical University of South Carolina, SUNY Upstate Medical University, Department of Pediatrics, Wayne State University School of Medicine, Children’s Hospital of Michigan, Children’s Hospital at Albany Medical Center, Children’s Medical Center of Dallas, Children’s Hospital – University of Colorado at Denver and Health Sciences, Center, Pediatric Infectious Diseases, Columbus Children’s Hospital, University of Florida College of Medicine – Department of Pediatrics, Division of Immunology, Infectious Diseases & Allergy, University of Mississippi Medical Center, Palm Beach County Health Department, Children’s Hospital LA – Department of Pediatrics, Division of Adolescent Medicine, Vanderbilt University Medical Center, Division of Pediatric Infectious Diseases, Washington University School of Medicine at St. Louis, St. Louis Children’s Hospital, Children’s Hospital & Medical Center, Seattle ACTU, Oregon Health Sciences University, St. Luke’s-Roosevelt Hospital Center, Montefiore Medical Center – Albert Einstein College of Medicine, Children’s Hospital, Washington, D.C., Children’s Hospital of the King’s Daughters, University of Alabama at Birmingham, Department of Pediatrics, Division of Infectious Diseases, Columbus Regional HealthCare System, The Medical Center, Sacred Heart Children’s Hospital/CMS of Florida, Bronx Municipal Hospital Center/Jacobi Medical Center.
Note: The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or U.S. Department of Health and Human Services.
Funding:
The Pediatric HIV/AIDS Cohort Study (PHACS) was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard University School of Public Health (HD052102) and the Tulane University School of Medicine (HD052104). This work was also supported by the National Institutes of Health under cooperative agreements to the IMPAACT Network [U01 AI068616] NICHD International Site Development Initiative (NISDI) [HD-3-3345, N01-HD-8-0001].
Footnotes
Paul Krogstad (PK), Russell B. Van Dyke (RVD), Kunjal Patel, Rohan Hazra (RH), Mark J. Abzug, James Oleske, George R. Seage III, Paige L Williams, William Borkowsky, Andrew Wiznia, were involved in study design. Kunjal Patel and Brad Karalius performed the data acquisition and analysis, and participated in drafting and revision of manuscript with PK and RVD, with input from all authors. RVD, RH, and Jorge Pinto were responsible for oversight of the study for the contributing PHACS and NISDI networks. All authors played a role in review in review of the final manuscript.
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2014 Available at aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines.
- 2.Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. 2014 Available at aidsinfo.nih.gov/guidelines/html/2/pediatric-arv-guidelines.
- 3.Brady MT, Oleske JM, Williams PL, et al. Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era. J Acquir Immune Defic Syndr. 2010;53:86–94. doi: 10.1097/QAI.0b013e3181b9869f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Judd A, Doerholt K, Tookey PA, et al. Morbidity, mortality, and response to treatment by children in the United Kingdom and Ireland with perinatally acquired HIV infection during 1996–2006: planning for teenage and adult care. Clin Infect Dis. 2007;45:918–24. doi: 10.1086/521167. [DOI] [PubMed] [Google Scholar]
- 5.Kapogiannis BG, Soe MM, Nesheim SR, et al. Mortality trends in the US Perinatal AIDS Collaborative Transmission Study (1986–2004) Clin Infect Dis. 2011;53:1024–34. doi: 10.1093/cid/cir641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puthanakit T, Aurpibul L, Oberdorfer P, et al. Sustained immunologic and virologic efficacy after four years of highly active antiretroviral therapy in human immunodeficiency virus infected children in Thailand. Pediatr Infect Dis J. 2007;26:953–6. doi: 10.1097/INF.0b013e318125720a. [DOI] [PubMed] [Google Scholar]
- 7.Puthanakit T, Aurpibul L, Oberdorfer P, et al. Hospitalization and mortality among HIV-infected children after receiving highly active antiretroviral therapy. Clin Infect Dis. 2007;44:599–604. doi: 10.1086/510489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins IJ, Jourdain G, Hansudewechakul R, et al. Long-term survival of HIV-infected children receiving antiretroviral therapy in Thailand: a 5-year observational cohort study. Clin Infect Dis. 2010;51:1449–57. doi: 10.1086/657401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciaranello AL, Chang Y, Margulis AV, et al. Effectiveness of pediatric antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. Clin Infect Dis. 2009;49:1915–27. doi: 10.1086/648079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiappini E, Galli L, Gabiano C, Tovo PA, de Martino M. Early triple therapy vs mono or dual therapy for children with perinatal HIV infection. JAMA. 2006;295:626–8. doi: 10.1001/jama.295.6.626-b. [DOI] [PubMed] [Google Scholar]
- 11.Chiappini E, Galli L, Tovo PA, et al. Virologic, immunologic, and clinical benefits from early combined antiretroviral therapy in infants with perinatal HIV-1 infection. Aids. 2006;20:207–15. doi: 10.1097/01.aids.0000200529.64113.3e. [DOI] [PubMed] [Google Scholar]
- 12.de Martino M, Galli L, Moriondo M, et al. Dissociation of responses to highly active antiretroviral therapy: Notwithstanding virologic failure and virus drug resistance, both CD4+ and CD8+ T lymphocytes recover in HIV-1 perinatally infected children. J Acquir Immune Defic Syndr. 2001;26:196–7. doi: 10.1097/00042560-200102010-00018. [DOI] [PubMed] [Google Scholar]
- 13.Flynn PM, Rudy BJ, Douglas SD, et al. Virologic and immunologic outcomes after 24 weeks in HIV type 1-infected adolescents receiving highly active antiretroviral therapy. J Infect Dis. 2004;190:271–9. doi: 10.1086/421521. [DOI] [PubMed] [Google Scholar]
- 14.Kovacs A, Montepiedra G, Carey V, et al. Immune reconstitution after receipt of highly active antiretroviral therapy in children with advanced or progressive HIV disease and complete or partial viral load response. J Infect Dis. 2005;192:296–302. doi: 10.1086/430922. [DOI] [PubMed] [Google Scholar]
- 15.Nikolic-Djokic D, Essajee S, Rigaud M, et al. Immunoreconstitution in children receiving highly active antiretroviral therapy depends on the CD4 cell percentage at baseline. J Infect Dis. 2002;185:290–8. doi: 10.1086/338567. [DOI] [PubMed] [Google Scholar]
- 16.Piketty C, Weiss L, Thomas F, Mohamed AS, Belec L, Kazatchkine MD. Long-term clinical outcome of human immunodeficiency virus-infected patients with discordant immunologic and virologic responses to a protease inhibitor-containing regimen. J Infect Dis. 2001;183:1328–35. doi: 10.1086/319861. [DOI] [PubMed] [Google Scholar]
- 17.Rutstein RM, Gebo KA, Flynn PM, et al. Immunologic function and virologic suppression among children with perinatally acquired HIV Infection on highly active antiretroviral therapy. Med Care. 2005;43:III15–22. doi: 10.1097/01.mlr.0000175636.34524.b9. [DOI] [PubMed] [Google Scholar]
- 18.Sufka SA, Ferrari G, Gryszowka VE, et al. Prolonged CD4+ cell/virus load discordance during treatment with protease inhibitor-based highly active antiretroviral therapy: immune response and viral control. J Infect Dis. 2003;187:1027–37. doi: 10.1086/368359. [DOI] [PubMed] [Google Scholar]
- 19.van Rossum AM, Scherpbier HJ, van Lochem EG, et al. Therapeutic immune reconstitution in HIV-1-infected children is independent of their age and pretreatment immune status. Aids. 2001;15:2267–75. doi: 10.1097/00002030-200111230-00008. [DOI] [PubMed] [Google Scholar]
- 20.Zoufaly A, an der Heiden M, Kollan C, et al. Clinical outcome of HIV-infected patients with discordant virological and immunological response to antiretroviral therapy. J Infect Dis. 2011;203:364–71. doi: 10.1093/jinfdis/jiq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hazra R, Stoszek SK, Freimanis Hance L, et al. Cohort Profile: NICHD International Site Development Initiative (NISDI): a prospective, observational study of HIV-exposed and HIV-infected children at clinical sites in Latin American and Caribbean countries. Int J Epidemiol. 2009;38:1207–14. doi: 10.1093/ije/dyn239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Dyke RB, Patel K, Siberry GK, et al. Antiretroviral treatment of US children with perinatally acquired HIV infection: temporal changes in therapy between 1991 and 2009 and predictors of immunologic and virologic outcomes. J Acquir Immune Defic Syndr. 2011;57:165–73. doi: 10.1097/QAI.0b013e318215c7b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raszka WV, Jr, Meyer GA, Waecker NJ, et al. Variability of serial absolute and percent CD4+ lymphocyte counts in healthy children born to human immunodeficiency virus 1-infected parents. Military Pediatric HIV Consortium. Pediatr Infect Dis J. 1994;13:70–2. [PubMed] [Google Scholar]
- 24.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 25.1994 Revised Classification System for Human Immunodeficiency Virus Infection in Children Less Than 13 Years of Age. MMWR Recomm Rep. 1994;43:1–10. [Google Scholar]
- 26.Moore DM, Hogg RS, Yip B, et al. Discordant immunologic and virologic responses to highly active antiretroviral therapy are associated with increased mortality and poor adherence to therapy. J Acquir Immune Defic Syndr. 2005;40:288–93. doi: 10.1097/01.qai.0000182847.38098.d1. [DOI] [PubMed] [Google Scholar]
- 27.Engsig FN, Zangerle R, Katsarou O, et al. Long-term Mortality in HIV-Positive Individuals Virally Suppressed for >3 Years With Incomplete CD4 Recovery. Clin Infect Dis. 2014;58:1312–21. doi: 10.1093/cid/ciu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zoufaly A, Cozzi-Lepri A, Reekie J, et al. Immuno-virological discordance and the risk of non-AIDS and AIDS events in a large observational cohort of HIV-patients in Europe. PLoS One. 2014;9:e87160. doi: 10.1371/journal.pone.0087160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JC, Boechat MI, Belzer M, et al. Thymic volume, T-cell populations, and parameters of thymopoiesis in adolescent and adult survivors of HIV infection acquired in infancy. Aids. 2006;20:667–74. doi: 10.1097/01.aids.0000216366.46195.81. [DOI] [PubMed] [Google Scholar]
- 30.Essajee SM, Kim M, Gonzalez C, et al. Immunologic and virologic responses to HAART in severely immunocompromised HIV-1-infected children. Aids. 1999;13:2523–32. doi: 10.1097/00002030-199912240-00005. [DOI] [PubMed] [Google Scholar]
- 31.Lewis J, Walker AS, Castro H, et al. Age and CD4 count at initiation of antiretroviral therapy in HIV-infected children: effects on long-term T-cell reconstitution. J Infect Dis. 2012;205:548–56. doi: 10.1093/infdis/jir787. [DOI] [PubMed] [Google Scholar]
- 32.Resino S, Bellon JM, Gurbindo D, Leon JA, Munoz-Fernandez MA. Recovery of T-cell subsets after antiretroviral therapy in HIV-infected children. Eur J Clin Invest. 2003;33:619–27. doi: 10.1046/j.1365-2362.2003.01168.x. [DOI] [PubMed] [Google Scholar]
- 33.Resino S, Seoane E, Perez A, Ruiz-Mateos E, Leal M, Munoz-Fernandez MA. Different profiles of immune reconstitution in children and adults with HIV-infection after highly active antiretroviral therapy. BMC Infect Dis. 2006;6:112. doi: 10.1186/1471-2334-6-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez CA, Koch S, Goodenow M, Sleasman JW. Clinical implications of discordant viral and immune outcomes following protease inhibitor containing antiretroviral therapy for HIV-infected children. Immunol Res. 2008;40:271–86. doi: 10.1007/s12026-007-0031-1. [DOI] [PubMed] [Google Scholar]
- 35.Diniz L, MMM M, Camargos L, Amaral L, Goulart E, Pinto J. Evaluation of long-term immunological and virological response to highly active antiretroviral therapy in a cohort of HIV infected children. HIV&AIDS Review. 2011;10:70–5. [Google Scholar]
- 36.Mocroft A, Furrer HJ, Miro JM, et al. The incidence of AIDS-defining illnesses at a current CD4 count >/= 200 cells/muL in the post-combination antiretroviral therapy era. Clin Infect Dis. 2013;57:1038–47. doi: 10.1093/cid/cit423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Kaplan-Meier survival curves for time to first sustained low CD4 count outcome among NISDI subjects with CD4 ≥ 500 at the start of the virologic suppression period.


