Abstract
The leaves of the native North American plant, Eriodictyon californicum were once used to mask the bitter taste of pharmaceuticals, an application currently of importance. Ten flavonoids (1–10) were isolated from the leaves of E. californicum, of which the structure and absolute configuration of 6-methoxyhesperetin (8) were assigned for the first time. In addition, the absolute configurations at C-2 were established for 4′-isobutyrylhomoeriodictyol (3) and 6-methoxyhomoeriodictyol (7). Using a cell-based assay, it was determined that the 7-methoxylated flavanones, sakuranetin (2), and 6-methoxysakuranetin (9), and the flavone, jaceosidin (10), are antagonists of hTAS2R31.
Keywords: Eriodictyon californicum, leaves, bitterness-masking, flavonoids, 6-methoxyhesperetin, 6-methoxysakuranetin, jaceosidin, sakuranetin
INTRODUCTION
The adverse reaction to bitter tastants most likely evolved as a means of detecting toxins in order to eliminate them from the diet (1,2). Indeed, through agricultural or food processing techniques, the bitter principles of many foods have been either eliminated or substantially reduced (1–3). However, the bitter components sought to be eliminated from foodstuffs tend to be the active constituents of “functional foods” (2). Although uncooked vegetables with comparatively high concentrations of bitter constituents may be a healthy choice, consumers tend to favor taste when selecting foods, a process that has indeed developed on an evolutionary timescale (2). Another possible benefit of vegetables with increased amounts of secondary metabolites is a lower dependence on pesticides in agriculture (2). Aside from the realm of functional foods, the bitter off-taste of some artificial sweeteners causes avoidance of reduced calorie products, which could otherwise be beneficial for health both by prevention of dental caries, as well as in the regulation caloric intake (4,5). The bitterness of certain medicines can also cause human health problems due to reduced patient compliance, especially among the young (6). The most common means of masking a bitter taste is by creating a physical barrier to the tastant, but this is not practical for use in food applications, or in some liquid medicines (7). Other bitterness-masking techniques run counter to maintaining good health, such as masking taste with salt, sugar, or fats (2,7).
In the present work, the dried leaves of the North American species Eriodictyon californicum Decne. (Hydrophyllaceae) were selected for an investigation of its bitterness-masking constituents, due to the documented flavor profile of this plant as having an initial bitter taste that turns sweet (8), as well as the more recent literature on this plant (9). Also, the range of taste sensations caused by flavonoids from sweet to tasteless to bitter demonstrates that interesting interactions between flavonoids and the taste receptors do occur (10). Previous work on E. californicum by Ley et al. identified the sodium salt of homoeriodictyol and eriodictyol as the principal bitterness-masking agents from this species (9). Homoeriodictyol was then used as a lead compound for a series of analogues with bitterness-masking properties (11,12). Further evidence of the intriguing flavor properties of E. californicum are the identification of hesperetin as a sweetness-enhancer (13), and the isolation of bitter benzoic acid derivatives from this plant (14).
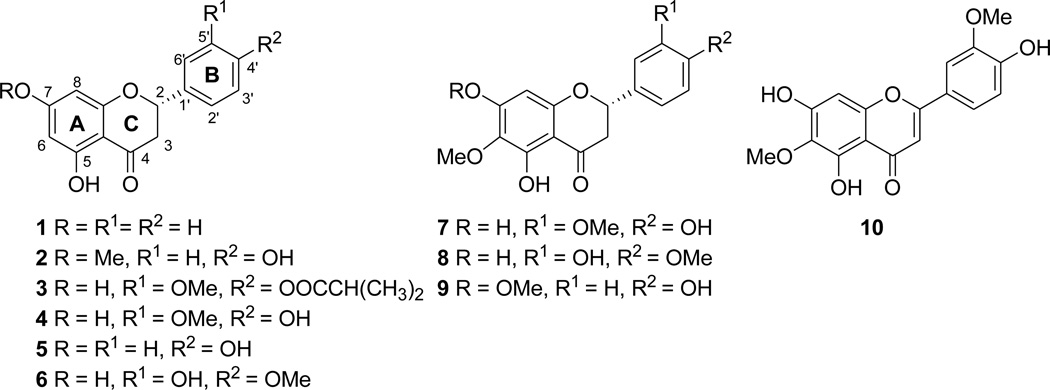
Ten flavonoids (1–10) (Figure 1) were isolated from the leaves of E. californicum and evaluated in a cell-based assay to determine their potential bitterness masking activities, with three of these compounds [sakuranetin (2), 6-methoxysakuranetin (9), and jaceosidin (10)] found to reduce the response to saccharin in hTAS2R31 (formerly known as hTAS2R44) transfected cells selectively. Sensory evaluation of sakuranetin (2) was also attempted to verify these results. 6-Methoxyhesperetin (8) has been reported as a constituent of Populus balsamifera L. (Salicaceae) with unresolved stereochemistry, but no spectroscopic or physical data were provided to support the structure proposed (15). Along with the structure confirmation and absolute configuration assignment of 8, this is also the first report of the absolute configuration assignments of compounds 3 and 7.
Figure 1.
Structures of Compounds Tested in the Bitterness Receptor Assay.
MATERIALS AND METHODS
Plant Material
Leaves of Eriodictyon californicum were collected by Dr. Richard Spjut at Whiskeytown Lake, California, in September, 2002, and a voucher specimen was deposited at the World Botanical Associates Herbarium, Laurel, Maryland accession number WBA-4400-33.
General Experimental Procedures
Melting points were obtained using a Fisher-Johns melting point apparatus. Optical rotations were measured with a Perkin-Elmer model 343 polarimeter. Circular dichroism (CD) spectra were recorded on a JASCO J-810 spectropolarimeter. IR spectra were recorded on a Nicolet 6700 FT-IR spectrometer. NMR spectra were recorded at room temperature on Bruker Avance DRX-300 MHz and DRX-400 MHz NMR spectrometers. High-resolution mass spectra were recorded on a LCT-TOF mass spectrometer. HPLC was performed using a semi-preparative reversed-phase phenol column (YMC pack-Ph, 150 mm × 20 mm i.d.), with Hitachi Prep-36 pumps, a Hitachi L-2200 Elite LaChrom autosampler, and a Hitachi L-2400 Elite LaChrom UV detector.
Extraction and Isolation
The dried leaves of E. californicum (2.0 kg) were ground and extracted exhaustively with methanol (30 separate extractions with 4 L of methanol), yielding 525 g of extract. This dried methanol extract was then partitioned between 375 mL of 9:1 methanol-water and 375 of mL hexane, in aliquots of approximately 60 g at a time. The methanol/water layer was evaporated to a thick tar and partitioned with 375 mL of chloroform and 375 mL of water. The aqueous layer afforded 200 g in total of extract, and, at the interface between the solvents a viscous syrup (170 g) formed that was insoluble in a wide variety of solvent, and only partially soluble in methanol. The resulting chloroform layer was partially detannified with 1% NaCl in water to give a final chloroform-soluble extract of 110 g (16). This extract was then separated into five fractions using silica gel vacuum-liquid chromatography (VLC). Fraction 2 (73.8 g), eluted with 20%–60% ethyl acetate in hexane was selected for further study.
Diaion® HP-20 column chromatography was utilized to remove chlorophylls yielding 48.5 g of a flavonoid-rich fraction. This fraction was then subjected to open column chromatography with coarse silica gel as the stationary phase and a step-gradient of hexane to ethyl acetate as the mobile phase, yielding ten sub-fractions.
Pinocembrin (1) (4.8 mg) was isolated via HPLC from subfraction 4 (tR 46.1 min) using a water to methanol gradient (30% from 0–20 min, 50% MeOH 40 min, 70% MeOH 70 min). Sakuranetin (2) (300 mg) was isolated via HPLC from subfraction 6 (tR 51.6 min) using a water to methanol gradient (30%–40% MeOH 25 min, 40%–45% MeOH 80 min), and 4′-isobutrylhomoeriodictyol (3) (16.0 mg) was also obtained from this subfraction via the same HPLC method (tR 78.3 min). Subfraction 7 afforded a precipitate and a syrup-like mother liquor. The precipitate yielded homoeriodictyol (4) (10 g), which was obtained by washing with dichloromethane, while naringenin (5) (22.5 mg) and hesperetin (6) (6.9 mg) were also isolated from this precipitate using HPLC with (0%–5% MeOH 5 min, 5%–30% MeOH 15 min, 30%–40% MeOH 40 min, 40%–100% MeOH 120 min), with tR values of 43.5 and 49.9 min, respectively. The supernatant of subfraction 7 yielded 6-methoxyhomoeriodictyol (7) (6.0 mg), 6-methoxyhesperetin (8) (8.0 mg), and 6-methoxysakuranetin (9) (6.0 mg), which were separated via HPLC with a water to methanol gradient (10%–100% MeOH over 90 min) eluting at 49.3 min, 51.1 min, and 55.8 min, respectively. Jaceosidin (10) (7.7 mg) was isolated via silica gel chromatography from subfraction 9 using dichloromethane as the mobile phase.
Pinocembrin (1)
White needles, mp 189–190 °C (lit. 189–192 °C) (18); [α]25D −45 (MeOH, c 1) (lit. −50) (19); HRESIMS m/z 279.0633 [M+Na]+ (calcd for C15H12O4Na+ 279.0643.) CD, IR, and NMR data were consistent with literature values (18–20).
Sakuranetin (2)
White needles mp 140–141 °C (lit. 151–153 °C) (21); [α]25D −18 (CHCl3, c 1.0) (lit. −21) (19); CD (MeOH, Δε) 326.6 nm (1.9 degree•L•mol−1•m−1) 288.0 nm (−7.3 degree•L•mol−1•m−1); HRESIMS m/z 309.0731 [M+Na]+ (calcd for C16H14O5Na+ 309.0739). IR and NMR data were consistent with literature values (21–23).
4′-Isobutyrylhomoeriodictyol (3)
White needles, mp 149 −150 °C (lit. 149–150 °C) (24); [α]25D −87 (MeOH, c 1.0); CD (MeOH, Δε) 326.8 nm (4.4 degree•L•mol−1•m−1) 288.0 nm (−20.5 degree•L•mol−1•m−1); IR (NaCl) υmax 3382, 2973, 2934, 1758, 1738, 1642, 1609, 1512, 1467, 1423, 1344, 1313, 1272, 1182, 1160, 1088 cm−1; HRESIMS m/z 395.1090 [M+Na]+ (calcd for C20H20O7Na+ 395.1107). NMR data were consistent with literature values (24).
Homoeriodictyol (4)
Light yellow needles, mp 224–226 °C (lit. 227–229 °C) (24); [α]25D +17 (CHCl3, c 1.0) (lit. +8) (25); IR (NaCl) υmax 3359, 1610, 1641, 1519, 1464, 1435, 1342, 1272, 1184, 1160, 1087, 1067 cm−1; HRESIMS m/z 309.0688 [M+Na]+ (calcd for C16H14O6Na+ 309.0688). CD and NMR data were consistent with literature values (24,26,27).
Naringenin (5)
White needles, mp 256–257 °C (227–229 °C); (28) [α]25D −12 (MeOH, c 1.0) (lit. −17.5) (28); CD (MeOH, Δε) 326.4 nm (2.0 degree•L•mol−1•m−1) 288.4 nm (−7.6 degree•L•mol−1•m−1); HRESIMS m/z 295.0582 [M+Na]+ (calcd for C15H12O3Na+ 295.0582). IR and NMR data were consistent with literature values (28–30).
Hesperetin (6)
White needles, mp 226–227 °C (224–225 °C) (31); [α]25D −29 (MeOH c 1.0) (lit. −10.6 to −18.8) (32); CD (MeOH, Δε) 327.6 nm (2.9 degree•L•mol−1•m−1) 290.2 nm (−11.4 degree•L•mol−1•m−1); HRESIMS m/z 309.0686 [M+Na]+ (calcd for C16H14O6Na+ 309.0688). IR and NMR data were consistent with literature values (21,33,34).
6-Methoxyhomoeriodictyol (7)
White needles, mp 161–162 °C (230–232 °C) (24); [α]25D −14 (MeOH, c 1.0); CD (MeOH, Δε) 326.6 nm (2.9 degree•L•mol−1•m−1) 284.2 nm (−10.5 degree•L•mol−1•m−1); IR (NaCl) υmax 3383, 2925, 2851, 1644, 1506, 1462, 1436, 1342, 1296, 1275, 1159, 1090, cm−1; HRESIMS m/z 355.0797 [M+Na]+ (calcd for C17H16O7Na + 355.0794). NMR data were consistent with literature values (24,35).
6-Methoxyhesperetin (8)
White needles, mp 234–235 °C; [α]25D −11 (MeOH, c 1.0); CD (MeOH, Δε) 334.6 nm (2.5 degree•L•mol−1•m−1) 293.0 nm (−9.7 degree•L•mol−1•m−1); IR (NaCl) υmax 3374, 2926, 2843, 1643, 1586, 1503, 1461, 1342, 1296, 1276, 1160, 1090, cm−1; 1H NMR (300 MHz, acetone-d6) δH 2.76 (1H, dd, J = 3.0, 17.2 Hz, H-3α), 3.16 (1H dd, J = 12.6, 17.2 Hz, H-3β), 3.78 (3H, s, OMe-4′), 3.86 (3H, s, OMe-6), 5.42 (1H, dd, J = 12.6, 3.0 Hz, H-2), 6.02 (1H, s, H-8), 6.98 (2H, m, H-5′, 6′), 7.04 (1H, s, H-2′), 7.75 (1H, s, OH), 9.17 (1H, s, OH), 12.31 (1H, s, OH-5); 13C NMR δC 198.0 (C-4), 159.9 (C-9), 159.5 (C-7), 156.3 (C-5), 148.7 (C-4′), 147.6 (C-3′), 132.9 (C-1′), 129.9 (C-6), 118.8 (C-6′), 114.4 (C-2′), 112.3 (C-5′), 103.4 (C-10), 95.7 (C-8), 79.9 (C-2), 60.8 (OMe-6), 56.4 (OMe-4′), 43.6 (C-3); HRESIMS m/z 355.0794 [M+Na]+ (calcd for C17H16O7Na + 355.0794).
6-Methoxysakuranetin (9)
White needles, mp 155–156 °C (lit. 173–174 °C);(36) [α]25D −8 (MeOH, c 1) (lit. −3.2);(36) CD (MeOH, Δε) 335.8 nm (2.1 degree•L•mol−1•m−1) 289.8 nm (−8.0 degree•L•mol−1•m−1); HRESIMS m/z 339.0825 [M+Na]+ (calcd for C17H16O6Na+ 339.0845). IR and NMR data were consistent with literature values (25,36,37).
Jaceosidin (10)
Dark yellow needles, mp 229–230 °C (lit. 229–230 °C);(38) HRESIMS m/z 353.0653 [M+Na]+ (calcd for C17H14O7Na+ 353.0638). IR and NMR data were consistent with literature values (39,40).
Cell-Based Bioassay
Putative antagonist activities for these compounds were evaluated in vitro essentially as previously described (17). Briefly, HEK-293T cells expressing a chimeric G-protein α-subunit, and stably transfected with the human bitter receptor, hTAS2R31, were used to determine the bitterness-masking potential of compounds 1–10 isolated against 1 mM sodium saccharin. These compounds were tested initially at 25 µM to determine whether they might inhibit activation of hTAS2R31 by saccharin. Test compounds were also evaluated for inhibition of a non-taste GPCR pathway to ensure that any apparent inhibitory activity was bitter receptor-dependent and not simply due to non-specific effects on intracellular calcium handling, as previously described (17). The half-maximal inhibitory concentration (IC50) was then determined for selectively active components (greater than 50% inhibition at 25 µM) in order to assess the potency of the receptor antagonists.
Sensory Testing on Sakuranetin (2)
Sakuranetin (2) was dissolved in 95% ethanol at a concentration of 1%. This was then submitted to a bitterness-masking taste test with both an acesulfame K-aspartame mixture and rebaudioside A in a panel of four tasters. These flavorists then made an evaluation of the effects of sakuranetin the negative control for the acesulfame K-aspartame mixture and rebaudioside A.
RESULTS AND DISCUSSION
The known compounds pinocembrin (1) (18–20,26), sakuranetin (2) (20–23), 4′-isobutyrylhomoeriodictyol (3) (24), homoeriodictyol (4) (24–27), naringenin (5) (28–30), hesperetin (6) (21,30–34), 6-methoxyhomoeriodictyol (7) (24,35), 6-methoxysakuranetin (9) (24,36,37), and jaceosidin (10) (38–40) were identified by comparing their physical and spectroscopic data with literature values. Those data not previously available in the literature are given in the Materials and Methods section. The absolute configurations of the isolated flavanones (1–7,9) were determined by CD spectroscopy, with these 2S flavanones showing a positive Cotton effect due to the n→π* transition near 330 nm and a negative Cotton effect from the π→π* near 290 nm (26).
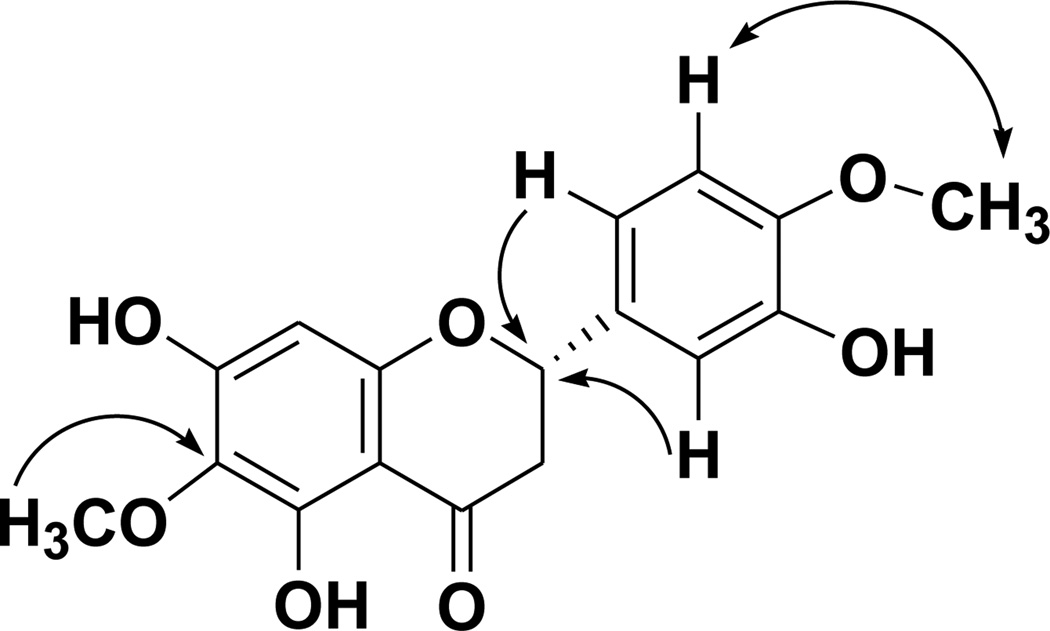
The mass spectrum of 6-methoxyhesperetin (8) showed the parent ion peak at m/z 355.0794 [M+Na]+, consistent with the molecular formula, C17H16O7. 6-Methoxyhesperetin (8) exhibited the characteristic peaks for a flavanone in its 1H NMR spectrum with a coupled doublet of doublets system, representing the protons at positions C-2 and C-3, observed at δH 2.76 (1H, dd, J = 3.0, 17.2 Hz, H-3α), 3.16 (1H dd, J = 12.6, 17.2 Hz, H-3β), and 5.42 (1H, dd, J = 12.6, 3.0 Hz, H-2). The 1H NMR spectroscopic data also revealed the presence of two methoxy substituents at δH 3.78 (3H, s, OMe-4′) and 3.86 (3H, s, OMe-6). The signal at δH 6.02 (1H, s, H-8) was indicative of a proton on the A-ring, while the signals at δH 6.98 (2H, m, H-5′,-6′) and 7.04 (1H, s, H-2′) revealed three unsubstituted positions on the B-ring. Three hydroxy group signals were also seen in the 1H NMR spectrum [δH 7.75 (1H,), 9.17 (1H), 12.31 (1H, s, OH-5)] with a sharp downfield singlet indicative of a hydroxy substituent at the C-5 position. A B-ring singlet in the 1H NMR spectrum was assigned to the H-2′ proton, while the multiplet at δH 6.98 could be attributed to splitting between the nearly overlapping peaks of H-5′ and H-6′. The 13C NMR spectrum of compound 8 confirmed the presence of a flavanone carbon skeleton for this molecule, with characteristic C-ring resonances at δC 198.0 (C-4), 79.9 (C-2), and 43.6 (C-3). HMBC correlations were used to place one of the methoxy groups at C-6 (δC 60.8) and the other on the B-ring (δC 56.4, C-4). The position of the B-ring methoxy group was determined by a NOE correlation. Further evidence for the B-ring substitution pattern of compound 8 was obtained by comparison with the 1H NMR spectrum of the closely related compound, hesperetin (6). Key 2D-NMR correlations for compound 8 are shown in Figure 2. CD spectroscopy was used to determine the absolute configuration at the C-2 stereocenter of 8. The absolute configuration of S at the C-2 stereocenter was assigned due to the observed positive Cotton effect at 334.6 nm and negative Cotton effect at 293.0 nm (26).
Figure 2.
Selected HMBC (single-headed arrows) and NOESY (double-headed arrow) Correlations for Compound 8
Of the ten flavonoids from the leaves of E. californicum tested for antagonistic activity against hTAS2R31, three (2, 9, 10) were found to inhibit the response to saccharin by more than 50% (Table 1). It would appear that flavanones methoxylated at the C-7 position are good candidates for masking the bitterness due to hTAS2R31 activity, in light of the activity of 2 and 9. It can also be established that methoxylation of the C-6 position does not greatly affect activity at this receptor, as evidenced by the data obtained for the previously mentioned compounds 2 and 9, which were both found to be receptor antagonists.. This insight regarding the C-6 position is supported also by comparison of the activities of compounds 4 and 6 and their 6-methoxy analogues, 7 and 8, which were not found to significantly suppress the response of hTAS2R31 to saccharin at a concentration level of 25 µM. The related compound 1, was slightly active. In light of this slight activity, the C-7 methoxlyated derivative of this compound may be an intriguing lead for future bitterness-masking tests.
Table 1.
Evaluation of Compounds 1–10 Using a Bitterness Receptor Assay.
| compound number | percent inhibition vs. controla |
IC50 (µM) |
|---|---|---|
| 1 | 25.5±24.3 | - |
| 2 | 82.4±23.4 | 5.5±2.5 |
| 3 | −55.0±31.7 | - |
| 4 | 8.2±13.8 | - |
| 5 | −5.7±7.1 | - |
| 6 | −8.1±12.2 | - |
| 7 | 12.5±14.0 | - |
| 8 | 11.0±11.9 | - |
| 9 | 69.5±33.1 | 10.2±5.4 |
| 10 | 59.9±33.6 | 11.7±6.0 |
Compounds producing greater than 50% inhibition of the response of hTAS2R31 to saccharin at 25 µM were considered active. The data are presented as the mean + s.d.m of n=3–4 replicate experiments. Compounds 2, 9, and 10 were selected as antagonists of hTAS2R32 and the half-maximal inhibitory concentrations (IC50) were determined as an indicator of antagonist potency. For IC50 determinations, at least three separate experiments were performed in duplicate and the data were fitted in GraphPad Prism using a 4-parameter logistic fit equation.
Homoeriodicyol (4), although previously identified as a bitterness masker, at 25 µM was not found to inhibit the saccharin response of hTAS2R31, which was the threshold level for determining EC50 values in this work (9). The apparent lack of an effect for 4 could be due to the fact that the concentrations used for in vitro screening (25 µM) were much lower than those used for tasting (331 µM), calculated from Ley et al. (9). Alternatively, compound 4 may be eliciting its reported bitter masking effects through other bitter receptors that were not tested as part of this study.
Another interesting inference is that flavones may be more active than flavanones, as evidenced by the antagonistic activity of compound 10 compared to that of compound 7. Although only one flavone was tested in the present investigation, it is possible that its functional group substitution pattern plays a different role in flavones when compared to flavanones in regard to potential bitterness-masking applications. It is known, however, that flavanones and flavones that possess the same substitution pattern can display quite different taste properties (7).
In order to verify these results with sensory data, sakuranetin (2) was submitted to a bitterness-masking taste test against both an acesulfame K-aspartame mixture and rebaudioside A in a panel of four trained tasters. Acesulfame K is a known activator of hTAS2R31 (4). As an aqueous solution could not be obtained, sakuranetin (2) was tested at 1% in ethanol. While the test results were inconsistent, two flavorists reported sakuranetin (2) to have an effect on the bitterness of acesulfame K-aspartame (“cuts backend”, “delayed bitterness”). The limited aqueous solubility of sakuranetin (2), however precluded a full evaluation of its bitterness-masking capabilities.
Eriodictyon californicum appears to be an excellent source of potential leads for bitterness-masking applications, with nearly one third of the molecules isolated from the plant in the present investigation showing selective inhibition of the bitterness receptor hTAS2R31. This percentage of active components is particularly striking when the fact is considered that these molecules were not the product of a bioactivity-guided fractionation process. These simple flavonoids could also serve as a source for either semi-synthetic analogues or completely artificial compounds, particularly those retaining a methoxy group substitution at the C-7 position.
Finally, the type of bioassay technique employed in this investigation to evaluate the compounds isolated is worthy of note (17). The ability to obtain data for limited amounts of water-insoluble compounds is particularly germane to phytochemical research. This is evidenced by the presented attempt to obtain taste data for sakuranetin (2). Bioactivity-guided fractionation techniques are often incongruent with taste testing since the amount of total sample used for tasting increases with each round of fractionation. This approach has further benefits due to the omission of human volunteer tasters, avoiding problems with reproducibility, especially by removing taster bias, and bypassing safety concerns involved with taste testing until after compounds are established as potential leads. The method employed also affords the benefits of high-throughput screening approaches to the arena of flavors research, and better allows development of targeted bitterness-masking agents via the investigation of discrete bitterness receptors.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Dr. J. M. Cassady for donating the plant material for this project. We would also like to acknowledge Mr. J. W. Fowble and Dr. K. B. Green-Church for maintenance of the NMR and MS facilities, respectively.
Footnotes
Supporting Information Available.
400 MHz H 1H NMR and 13C NMR, and 300 MHz H NOESY and HMBC spectra for compound 8 are available in the supporting information, as well as the CD data for compounds 3, 7, and 8.
REFERENCES
- 1.Roy G. The applications and future implications of bitterness reduction and inhibition in food products. Crit. Rev. Food Sci. Nutr. 1990;29:59–71. doi: 10.1080/10408399009527516. [DOI] [PubMed] [Google Scholar]
- 2.Drewnowski A, Gomez-Carneros C. Bitter taste, phytonutrients, and the consumer: a review. Am. J. Clin. Nutr. 2000;72:1424–1435. doi: 10.1093/ajcn/72.6.1424. [DOI] [PubMed] [Google Scholar]
- 3.Wink M. Plant breeding: importance of plant secondary metabolites for protection against pathogens and herbivores. Theor. Appl. Genet. 1988;75:225–233. [Google Scholar]
- 4.Kuhn C, Bufe B, Winnig M, Hofmann T, Frank O, Behrens M, Lewtschenko T, Slack JP, Ward CD, Meyerhof W. Bitter taste receptors for saccharin and acesulfame K. J. Neurosci. 2004;24:10260–10265. doi: 10.1523/JNEUROSCI.1225-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ly KA, Milgrom P, Rothen M. Xylitol, sweeteners, and dental caries. Pediatr. Dent. 2006;28:154–163. 192–198. [PubMed] [Google Scholar]
- 6.Binello A, Cravotto G, Nano GM, Spagliardi P. Synthesis of chitosan–cyclodextrin adducts and evaluation of their bitter-masking properties. Flavour Frag. J. 2004;19:394–400. [Google Scholar]
- 7.Ley JP. Masking bitter taste by molecules. Chemosens. Percept. 2008;1:58–77. [Google Scholar]
- 8.The Pharmacopeia of the United States of America. Philadelphia, PA: P. Blakiston's Son & Co.; 1916. Anonymous; pp. 148–149. 9th Decennial Revision; [Google Scholar]
- 9.Ley JP, Krammer G, Reinders G, Gatfield IL, Bertram H-J. Evaluation of bitter masking flavanones from Herba Santa (Eriodictyon californicum (H. and A.) Torr., Hydrophyllaceae) J. Agric. Food Chem. 2005;53:6061–6066. doi: 10.1021/jf0505170. [DOI] [PubMed] [Google Scholar]
- 10.Horowitz RM, Gentili B. Taste and structure in phenolic glycosides. J. Agric. Food Chem. 1969;17:696–700. [Google Scholar]
- 11.Ley JP, Blings M, Paetz S, Krammer GE, Bertram H-J. New bitter- masking compounds: hydroxylated benzoic acid amides of aromatic amines as structural analogues of homoeriodictyol. J. Agric. Food Chem. 2006;54:8574–8579. doi: 10.1021/jf0617061. [DOI] [PubMed] [Google Scholar]
- 12.Ley JP, Paetz S, Blings M, Hoffmann-Lücke P, Bertram H-J, Krammer GE. Structural analogues of homoeriodictyol as flavor modifiers. Part III: short chain gingerdione derivatives. J. Agric. Food Chem. 2008;56:6656–6664. doi: 10.1021/jf8006536. [DOI] [PubMed] [Google Scholar]
- 13.Ley J, Kindel G, Paetz S, Riess T, Haug M, Schmidtmann R, Krammer G. Use of hesperetin for enhancing the sweet taste. 20080305052. U.S. Patent. 2008 Dec.
- 14.Reichelt KV, Hartmann B, Weber B, Ley JP, Krammer GE, Engel K-H. Identification of bisprenylated benzoic acid derivatives from Yerba Santa (Eriodictyon spp.) using sensory-guided fractionation. J. Agric. Food Chem. 2010;58:1850–1859. doi: 10.1021/jf903286s. [DOI] [PubMed] [Google Scholar]
- 15.Seitembetova A. Zh. Relationship between antioxidant activity and molecular structure of some flavonoids. Izvestiya Ministerstva Obrazovaniya i Nauki Respubliki Kazakhstan, Natsional'noi Akademii Nauk Respubliki Kazakhstan, Seriya Khimicheskaya. 2000;2:107–111. [Google Scholar]
- 16.Wall ME, Wani MC, Brown DM, Fullas F, Oswald JB, Josephson FF, Thornton NM, Pezzuto JM, Beecher CWW, Farnsworth NR, Cordell GA, Kinghorn AD. Effect of tannins on screening of plant extracts for enzyme inhibitory activity and techniques for their removal. Phytomedicine. 1996;3:281–286. doi: 10.1016/S0944-7113(96)80067-5. [DOI] [PubMed] [Google Scholar]
- 17.Slack JP, Brockhoff A, Batram C, Menzel S, Sonnabend C, Born S, Galindo MM, Kohl S, Thalmann S, Ostopovici-Halip L, Simons CT, Ungureanu I, Duineveld K, Bologa CG, Behrens M, Furrer S, Oprea TI, Meyerhof W. Modulation of bitter taste perception by a small molecule hTAS2R antagonist. Curr. Biol. 2010;20:1104–1109. doi: 10.1016/j.cub.2010.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung JH, Pummangura S, Chaichantipyuth C, Patarapanich C, McLaughlin JL. Bioactive constituents of Melodorum fruticosum. Phytochemistry. 1990;29:1667–1670. [Google Scholar]
- 19.Melliou E, Chinou I. Chemical analysis and antimicrobial activity of Greek propolis. Planta Med. 2004;70:515–519. doi: 10.1055/s-2004-827150. [DOI] [PubMed] [Google Scholar]
- 20.Ichino K, Tanaka H, Ito K. Two novel flavonoids from the leaves of Lindera umbellata var. lancea and L. umbellata. Tetrahedron. 1998;44:3251–3260. [Google Scholar]
- 21.Vasconcelos JM, Silva AMS, Cavaleiro JAS. Chromones and flavanones from Artemisia campestris subsp. maritima. Phytochemistry. 1998;49:1421–1424. [Google Scholar]
- 22.Agrawal PK. Carbon-13 NMR of Flavonoids. Amsterdam: Elsevier Science Publishers B.V.; 1989. [Google Scholar]
- 23.Jerz G, Waibel R, Achenbach H. Cyclohexanoid protoflavanones from the stem-bark and roots of Ongokea gore. Phytochemistry. 2005;66:1698–1706. doi: 10.1016/j.phytochem.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y-K, Ho DK, Cassady JM. Isolation of potential cancer chemopreventive agents from Eriodictyon californicum. J. Nat. Prod. 1992;55:357–363. doi: 10.1021/np50081a012. [DOI] [PubMed] [Google Scholar]
- 25.Tatuta H. Asymmetric synthesis. II. Synthesis of optically active hydroxyflavanones from hydroxychalcones. Nippon Kagaku Kaishi (1921–47) 1940;61:1048–1050. [Google Scholar]
- 26.Gaffield W. Circular dichroism, optical rotatory dispersion and absolute configuration of flavanones, 3-hydroxyflavanones and their glycosides. Tetrahedron. 1970;26:4093–4108. [Google Scholar]
- 27.Ibrahim A-RS, Galal AM, Ahmed MS, Mossa GS. O-Demethylation and sulfation of 7-methoxylated flavanones by Cunninghamella elegans. Chem. Pharm. Bull. 2003;51:203–206. doi: 10.1248/cpb.51.203. [DOI] [PubMed] [Google Scholar]
- 28.Jeon SH, Chun W, Choi YJ, Kwon YS. Cytotoxic constituents from the bark of Salix hulteni. Arch. Pharm. Res. 2008;31:978–982. doi: 10.1007/s12272-001-1255-9. [DOI] [PubMed] [Google Scholar]
- 29.Selenski C, Pettus TRR. (±)-Diinsininone: made Nature’s way. Tetrahedron. 2006;62:5298–5307. doi: 10.1016/j.tet.2006.01.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giorgioa E, Parrinellob N, Caccamese S, Rosini C. Non-empirical assignment of the absolute configuration of (−)-naringenin, by coupling the exciton analysis of the circular dichroism spectrum and the ab initio calculation of the optical rotatory power. Org. Biomol. Chem. 2004;2:3602–3607. doi: 10.1039/b411110a. [DOI] [PubMed] [Google Scholar]
- 31.Chaliha BP, Sastry GP, Rao PR. Citrus jambhiri peel. Isolation of a new flavone. Tetrahedron. 1965;21:1441–1443. [Google Scholar]
- 32.Arakawa H, Nakazaki M. Absolute configuration of the optically active flavanones. J. Liebigs Ann. Chem. 1960;636:111–117. [Google Scholar]
- 33.Heneczkowski M, Kopacz M, Nowak D, Kuzniar A. Infrared spectrum analysis of some flavonoids. Acta Pol. Pharm. 2001;58:415–420. [PubMed] [Google Scholar]
- 34.Lee SYH, Munerol B, Pollard S, Youdim KA, Pannala AS, Kuhnle GGC, Debnam ES, Rice-Evans C, Spencer JPE. The reaction of flavanols with nitrous acid protects against N-nitrosamine formation and leads to the formation of nitroso derivatives which inhibit cancer cell growth. Free Rad. Biol. Med. 2006;40:323–334. doi: 10.1016/j.freeradbiomed.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 35.Li R, Fan N, Mabry TJ. Flavonoids and a coumarin from Gutierrezia sphaerocephala. Phytochemistry. 1988;27:1556–1559. [Google Scholar]
- 36.Uchida M, Rüedi P, Eugster CH. Drüsenfarbstoffe aus Labiaten: Ecklonochinone A und B zwei neuartige Dibenzo-p-dioxin-o-chinone aus Plectranthus ecklonii BENTH. Helv. Chim. Acta. 1980;63:225–231. [Google Scholar]
- 37.Fernandez C, Fraga BM, Hernandez MG, Arteaga JM. Flavonoid aglycones from some Canary Islands species of Sideritis. J. Nat. Prod. 1988;51:591–593. doi: 10.1021/np50057a027. [DOI] [PubMed] [Google Scholar]
- 38.Wollenweber E, Mann K. Exudate flavonoids in three essential oil plants from the Ciskei (South Africa) Fitoterapia. 1989;60:249–251. [Google Scholar]
- 39.Min S-W, Kim N-J, Baek N-I, Kim D-H. Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps on carrageenan-induced inflammation in mice. J Ethnopharmacol. 2009;125:497–500. doi: 10.1016/j.jep.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Nakasugi T, Nakashima M, Komai K. Antimutagens in gaiyou (Artemisia argyi Levl. et Vant.) J. Agric. Food Chem. 2000;48:3256–3266. doi: 10.1021/jf9906679. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.