Abstract
Background
Angiogenesis is the formation of new blood vessels from pre-existing vasculature. Many factors and substances may stimulate angiogenesis and exhibit proliferative effect. In this study, we aimed to investigate the angiogenic and proliferative effects of sodium nitrite.
Material/Methods
The angiogenic activity of sodium nitrite was examined in vivo in the chick chorioallantoic membrane (CAM) model and in vitro in tube formation assay of human umbilical vein endothelial cells (HUVECs). The proliferative activity of sodium nitrite was also determined through MTT assay on HUVECs.
Results
In CAM assay, sodium nitrite had an angiogenic effect especially at high concentrations compared with the control group and this was statistically significant. There was a proliferative effect on HUVECs in the presence of sodium nitrite for 24 and 48 h, and this was statistically significant (p<0.05). Comparing the tube length/area ratio values, there was statistically significant increase in the sodium nitrite group compared to the control group (p<0.05).
Conclusions
The results provide evidence that sodium nitrite induces angiogenesis in vitro and in vivo.
Keywords: Angiogenesis Inducing Agents, Chorioallantoic Membrane, Human Umbilical Vein Endothelial Cells, Sodium Nitrite
Background
Every organ and tissue is penetrated by blood and lymphatic vascular systems in order to distribute nutrients and oxygen. Angiogenesis depends on many stimulating or inhibiting factors. Vascular endothelium growth factors (VEGF) and their receptors are the main regulators for proliferation and migration of endothelial cells, forming the basis of any vessel. Angiogenesis is new blood vessel formation from the preexisting vasculature; it is a strictly controlled process, which is activated only under certain conditions such as ischemia, wound healing, organ regeneration, and formation of placenta [1–3].
Jia et al. [4] wrote that “Therapeutic angiogenesis has been recently proposed to induce new blood vessel growth for the treatment or prevention of critical limb ischemia by pharmacological and molecular targeting with VEGF, fibroblastic growth factor, granulocyte colony-stimulating factors, granulocyte-macrophage colony-stimulating factors, angiogenic gene therapy and endothelial progenitor cells.”
Doganci et al. [5] stated that “One of the pharmacological strategies is to increase nitric oxide (NO) in order to stimulate angiogenesis in conditions such as ischemia-reperfusion injury, cerebral ischemia, kidney injury, coronary artery disease, and peripheral artery disease. In several studies it was shown that administration of NO or NO donors prior to ischemia attenuates the negative consequences of ischemia-reperfusion injury. During physiological hypoxia and pathological hypoxia nitrite is reduced to NO, regulating hypoxic vasodilatation, cellular respiration, mitochondrial reactive oxygen species generation, angiogenesis, and cellular death programs. Nitrite in human plasma exist at concentration of 100 to 300 nmol/l and may be reduced NO by iron-containing enzymes, including hemoglobin, myoglobin, neuroglobin, xanthine oxidoreductase, endothelial nitric oxide synthase, mitochondrial electron transport chain proteins, and the hepatic cytochrome P450 system. The rate and extent of nitrite reduction are coupled to deoxygenation and proton generation. Thus NO generation is coupled to oxygen and pH gradients and maximized in ischemic tissues”.
Although cytoprotective effects of nitrite have been studied in ischemic conditions, its angiogenic, proliferative and migratory effects are not thoroughly defined. Induction of angiogenesis may be important in ischemic disease such as critical limb ischemia, which is a serious condition with limited therapeutic options. As a nitric oxide, donor sodium nitrite may be a good alternative in ischemic conditions for inducing neoangiogenesis in patients with no surgical and interventional therapy options. In this study we aimed to show the angiogenic, proliferative, and migratory effects of sodium nitrite as a NO donor in normo-oxygenic conditions in vivo and in vitro.
Material and Methods
This study was performed in Gulhane Military Academy of Medicine in the Departments of Biochemistry, and Cancer, and Stem Cell Research Laboratory between July and September 2014.
Sodium Nitrite (NaNO2) solution
Sodium Nitrite (NaNO2) solution was prepared as stock solution; 11 mg of sodium nitrite was dissolved in 2 ml of distilled water to prepare a stock solution of 80 mM. Required concentrations of sodium nitrite solutions were prepared with serial dilutions from this stock solution.
Cell culture
Human umbilical vein endothelial cells (HUVEC) were provided as ATCC (LGC standards GmbH, Germany). The cell line cultures were maintained in F-12 K medium (ATCC 30-2004, Germany) supplemented with 10% heat-inactivated FBS (Biochrome KG, Berlin, Germany), antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin), and 2 mM L-glutamine under an atmosphere of 95% air and 5% CO2 at 37°C.
Cell viability assay
To evaluate the cell viability assay, we used the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method with the same procedures detailed to our previously published study (6). Briefly, the cells (10 000–20 000/well) were incubated in a 96-well plate in the presence of various concentrations of sodium nitrite solution (0.25, 0.5, 1, and 10 μM) in a final volume of 0.2 ml for 24–48 hours to determine the effect on endothelial cell proliferation.
In vitro endothelial cell tube formation assay
The In vitro endothelial cell tube formation assay was used to determine whether sodium nitrite had an effect on tube formation of endothelial cells. This was performed with 1 μM of sodium nitrite solution as described in our previously published study [6].
Chick Chorioallantoic Membrane (CAM) assay
The chick chorioallantoic membrane (CAM) assay was used for in vivo evaluation of angiogenesis. Sodium nitrite solutions with concentrations of 50, 100, and 150 μM were used to evaluate their effects on angiogenesis in vivo. For this purpose, fertilized eggs were incubated for 6 days, and then the above-mentioned concentrations of sodium nitrite were used to determine whether sodium nitrite had an angiogenic effect and whether this effect was dose-dependent. A standardized procedure in our published study was used to perform CAM assay, and the effect on CAM area was scored [6]. The workflow is summarized in Figure 1.
Figure 1.
Workflow for CAM assay.
Statistics
We used the chi-square test for the non-parametric tests. In addition, Yates correction analysis was performed for statistically significant difference. Statistical analysis was carried out using MedCalc for Windows, version 12.5 (MedCalc Software, Ostend, Belgium) and we used it in the same data situations as a Pearson’s correlation. The statistical analysis was carried out using the Statistical Package for the Social Sciences software for Windows, Version 15.00 (SPSS Inc., Chicago, Illinois, USA).
The value of p≤0.05 was considered statistically significant.
Results
Cell viability assay of sodium nitrite
Sodium nitrite showed a proliferative effect on MTT assay after an incubation period of 24 and 48 h. The proliferation in sodium nitrite added wells was significantly higher than in the control wells (p<0.05) (Figure 2).
Figure 2.
Cell proliferation of HUVECs under sodium nitrite incubation (cell viability was indicated as percentage of control). HUVECs: human umbilical vein endothelial cells.
Effect of sodium nitrite on tube formation
Tube length/area ratio was significantly higher in the sodium nitrite group compared to the control group at the 18th hour of incubation (p<0.05). These results provided evidence that sodium nitrite showed a proangiogenic effect (Figures 3 and 4).
Figure 3.
Ratio of tube length to area under sodium nitrite incubation and control.
Figure 4.
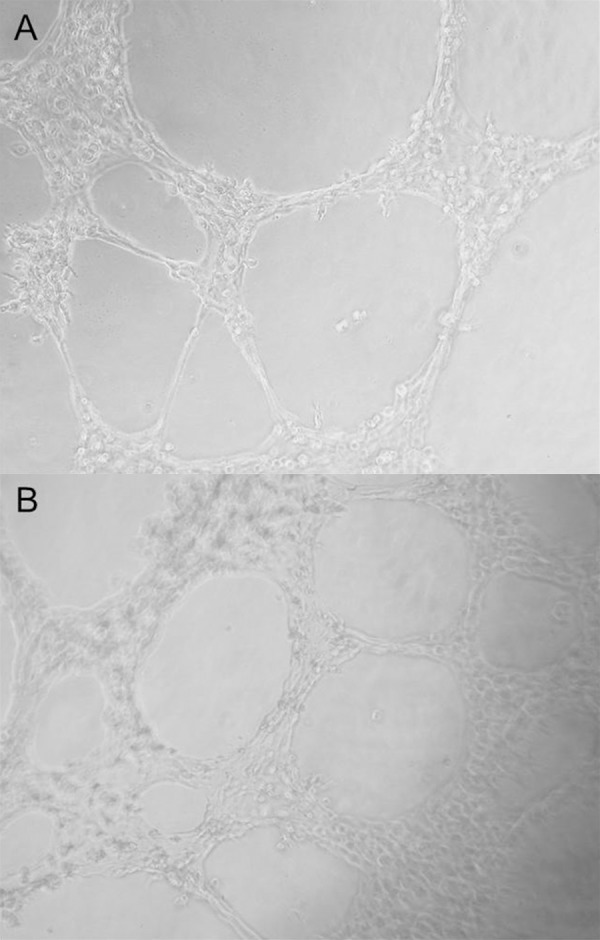
Tube formations of control group (A) and under sodium nitrite incubation (B). A and B were photographed using ×40 magnification.
Sodium nitrite induces angiogenesis on CAM
The vessel formation on each CAM area was macroscopically scored similarly to the previous studies and the results were presented in Table 1 [6]. Treatment with sodium nitrite solutions caused a significant increase (budding, sprouting, and extravasation) on CAM vessel growth. Affected vessels were thicker and had increased branching. Sprouting of new vessels from existing vessels can be clearly seen in Figure 5. Significant growth was seen for 100 μM sodium nitrite solution. The efficacies of different concentrations of sodium nitrite solutions were compared using the χ2 test. The difference was statistically significant (Yates correction χ2=14.3, p<0.05) and there was a strong correlation between the efficacies and doses according to Spearman’s correlation test (r=0.783, p<0.001). These results showed that sodium nitrite had an angiogenic effect in vivo.
Table 1.
Macroscopic evaluation of the effect of sodium nitrite treatment on CAM.
| Group | Efficacy | ||||
|---|---|---|---|---|---|
| Ineffective | +1 | +2 | Total | ||
| Control | n | 6 | 0 | 0 | 6 |
| % | 100.0 | 0 | 0 | 100.0 | |
| 50 μM | n | 4 | 2 | 0 | 6 |
| % | 66.67 | 33.33 | 0 | 100.0 | |
| 100 μM | n | 1 | 2 | 3 | 6 |
| % | 16.67 | 33.33 | 50.0 | 100.0 | |
| 150 μM | n | 0 | 3 | 4 | 7 |
| % | 0 | 42.86 | 57.14 | 100.0 | |
| Total | n | 11 | 7 | 7 | 25 |
| % | 44.0 | 28.0 | 28.0 | 100.0 | |
CAM – chorioallantoic membrane.
Figure 5.

Effect of sodium nitrite solution on CAM before (A) and after 24 h (B, C). A and B were photographed using ×1.0, C was photographed using ×4.0 magnification. White circle shows the area where sodium nitrite was placed first. White arrows show extravasation, budding, and sprouting of new vessels from existing vessels. CAM: chorioallantoic membrane.
Discussion
Pharmacological treatment with nitrite may offer an alternative therapeutic approach. The intrinsic biological activity of nitrite is limited at physiological pH ranges and oxygen tension. Reduction of nitrite to NO is optimal in conditions like chronic tissue ischemia or ischemia reperfusion. Therefore, this reduction process may serve as an important mechanism to maintain NO reservoirs during pathophysiological states [4]. However, we do not have enough information about the behavior of sodium nitrite under physiological conditions.
In this study, we aimed to clearly show the relation between sodium nitrite and angiogenesis in both in vivo and in vitro models. Because we have limited treatment options in conditions such as critical limb ischemia, any treatment strategy that increases angiogenesis has great importance for preserving limb and/or patient life expectancy.
As a new treatment modality in critical limb ischemia, therapeutic angiogenesis is a promising therapy for this group of patients [4]. Bir et al. [7] demonstrated that administration of nitrite resulted in increased tissue nitrite bioavailability, as well as increased levels of S-nitrosothiol and S-nitrosheme in the ischemic hindlimb of diabetic mice. They also showed that the proangiogenic effects of sodium nitrite were related to the activity of xanthine oxidoreductase. In another study, Bir et al. [8] reported that “delayed sodium nitrite therapy rapidly increased ischemic limb arterial vessel diameter and branching in a NO-dependent manner. Spy imaging angiography over time showed that nitrite therapy enhanced ischemic gracilis collateral vessel formation from the profunda femoris to the saphenous artery. Immunofluorescent staining of smooth muscle cell actin also confirmed that sodium nitrite therapy increased arteriogenesis.”
Kumar et al. [9] reported that “sodium nitrite therapy exerts cytoprotective effects against acute ischemia/reperfusion injury in both hearth and liver, consistent with the model of bioactive NO formation from nitrite during ischemic stress. We tested the hypothesis that chronic sodium nitrite therapy can selectively augment angiogenic activity and tissue perfusion in the murine hind-limb ischemia model. Nitrite therapy significantly increased ischemic limb vascular density and stimulated endothelial cell proliferation. Sodium nitrite therapy also increased ischemic tissue nitrite and NO metabolites compared to nonischemic limbs. Use of the scavenger carboxy PTIO completely abolished sodium nitrite-dependent ischemic tissue blood flow and angiogenic activity consistent with nitrite reduction to NO being the proangiogenic mechanism.”
Doganci et al. [5] reported a cardioprotective role of sodium nitrite against myocardial ischemia-reperfusion injury when it is given in the pre-ischemic period.
In all of the above studies, nitrite was reduced to NO only under special conditions such as ischemia, hypoxia, and low pH, and these studies did not compare the difference of NO, VEGF, EC, and angiogenesis in normal and ischemic regions. We performed our work in normal physiological conditions.
According to the in vitro results of the present study, there was a proliferative effect on HUVECs in the presence of sodium nitrite for 24 and 48 h, and this effect was statistically significant (p<0.05). CAM assay is a widely used in vivo method to analyze the effects of many different molecules on angiogenesis at varied concentrations. In the present study, we examined the effects of sodium nitrite at different concentrations on CAM assay. We found that sodium nitrite had an angiogenic effect especially at high concentrations compared with the control group and this was statistically significant. Comparing the tube length/area ratio values, there was a statistically significant increase in sodium nitrite group compared to the control group (p<0.05). We found that sodium nitrite had a proangiogenic effect.
Conclusions
The results of this study, which aimed to demonstrate the relation between sodium nitrite and angiogenesis, showed that sodium nitrite has an inducing effect on angiogenesis in in vitro and in vivo models. With its potential angiogenic effect, sodium nitrite may have a role in therapeutic angiogenesis in diseases such as peripheral arterial disease and critical limb ischemia.
Footnotes
Source of support: Departmental sources
References
- 1.Karamysheva AF. Mechanisms of angiogenesis. Biochemistry (Mosc) 2008;73:751–62. doi: 10.1134/s0006297908070031. [DOI] [PubMed] [Google Scholar]
- 2.Katrancioglu N, Karahan O, Kilic AT, et al. The antiangiogenic effects of levosimendan in a CAM assay. Microvasc Res. 2012;83:263–66. doi: 10.1016/j.mvr.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Jia W, Gao XJ, Zhang ZD, et al. S100A4 silencing suppresses proliferation, angiogenesis and invasion of thyroid cancer cells through downregulation of MMP-9 and VEGF. Eur Rev Med Pharmacol Sci. 2013;17:1495–508. [PubMed] [Google Scholar]
- 4.Jia G, Sowers JR. New thoughts in an old player: role of nitrite in the treatment of ischemic revascularization. Diabetes. 2014;63:39–41. doi: 10.2337/db13-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doganci S, Yildirim V, Bolcal C, et al. Sodium nitrite and cardioprotective effect in pig regional myocardial ischemia-reperfusion injury model. Adv Clin Exp Med. 2012;21:713–26. [PubMed] [Google Scholar]
- 6.Yesildal F, Aydin F, Deveci S, et al. Aspartame induces angiogenesis in vitro and in vivo models. Hum Exp Toxicol. 2015;34:260–65. doi: 10.1177/0960327114537535. [DOI] [PubMed] [Google Scholar]
- 7.Bir SC, Pattillo CB, Pardue S, et al. Nitrite anion therapy protects against chronic ischemic tissue injury in db/db diabetic mice in a NO/VEGF-dependent manner. Diabetes. 2014;63:270–81. doi: 10.2337/db13-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bir SC, Pattillo CB, Pardue S, et al. Nitrite anion stimulates ischemic arteriogenesis involving NO metabolism. Am J Physiol Heart Circ Physiol. 2012;303:H178–88. doi: 10.1152/ajpheart.01086.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar D, Branch BG, Pattillo CB, et al. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. Proc Natl Acad Sci USA. 2008;105:7540–45. doi: 10.1073/pnas.0711480105. [DOI] [PMC free article] [PubMed] [Google Scholar]





