Abstract
Variation within and around the leucine-rich repeat kinase 2 (LRRK2) gene is associated with familial and sporadic Parkinson’s disease (PD). Here, we discuss the prevalence of LRRK2 substitutions in different populations and their association with PD, as well as molecular and cellular mechanisms of pathologically relevant LRRK2 mutations. Kinase activation was proposed as a universal molecular mechanism for all pathogenic LRRK2 mutations, but later reports revealed heterogeneity in the effect of mutations on different activities of LRRK2. One mutation (G2019S) increases kinase activity, whereas mutations in the Ras of complex proteins (ROC)–C-terminus of ROC (COR) bidomain impair the GTPase function of LRRK2. Some risk factor variants, including G2385R in the WD40 domain, actually decrease the kinase activity of LRRK2. We suggest a model where LRRK2 mutations exert different molecular mechanisms but interfere with normal cellular function of LRRK2 at different levels of the same downstream pathway. Finally, we discuss the current state of therapeutic approaches for LRRK2-related PD.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-014-0284-z) contains supplementary material, which is available to authorized users.
Key Words: Parkinson’s disease, kinase inhibitors, GTPase, vesicular trafficking, cytoskeleton, treatment.
Introduction
Parkinson’s disease (PD) is a devastating neurodegenerative disorder affecting about 1.5 % of the population older then 65 years [1]. The typical clinical presentation of PD includes resting tremor, rigidity, bradykinesia, and postural instability [2]. Initially, PD was considered as a purely idiopathic disorder, but a growing number of genetic factors are now recognized to contribute to lifetime risk of disease [3].
Among the known autosomal-dominant genes, mutations in the leucine-rich repeat kinase 2 (LRRK2) gene are a relatively common cause of PD [4, 5]. Clinically, LRRK2-associated PD closely resembles idiopathic disease [6, 7], although pathological phenotypes in patients with the same mutation are variable [8]. The gene consists of 51 exons [9, 10] and is located on chromosome 12, in the locus originally designated as PARK8 [11]. The same locus has been identified by genome-wide association studies as conferring lifetime risk for sporadic PD.
LRRK2 encodes a large (286-kDa) complex protein composed of a central catalytic Ras of complex proteins (ROC)–C-terminus of ROC (COR)–kinase tridomain surrounded by several protein–protein interacting domains (Fig. 1). LRRK2 is an active kinase [12–15], and, as a member of the ROCO family of proteins [16, 17], can both bind and hydrolyse GTP [18–20]. It was initially proposed that GTP binding could stimulate the kinase activity of LRRK2, although subsequent results suggested that kinase activity of LRRK2 depends on the ability to bind guanosine nucleotides rather than the GTP-bound state [21–23], an important mechanistic distinction. Reciprocal regulation of GTP binding by the kinase domain may also occur, as LRRK2 autophosphorylates several residues within the ROC domain. Phosphorylation of T1491 and T1503 residues apparently diminishes GTP binding [24–29], suggesting that LRRK2 GTPase may be functionally downstream of the kinase activity [30], although more complex situations may occur. These suggestions show that there is intramolecular communication between different LRRK2 domains, pointing out how complex the molecule is.
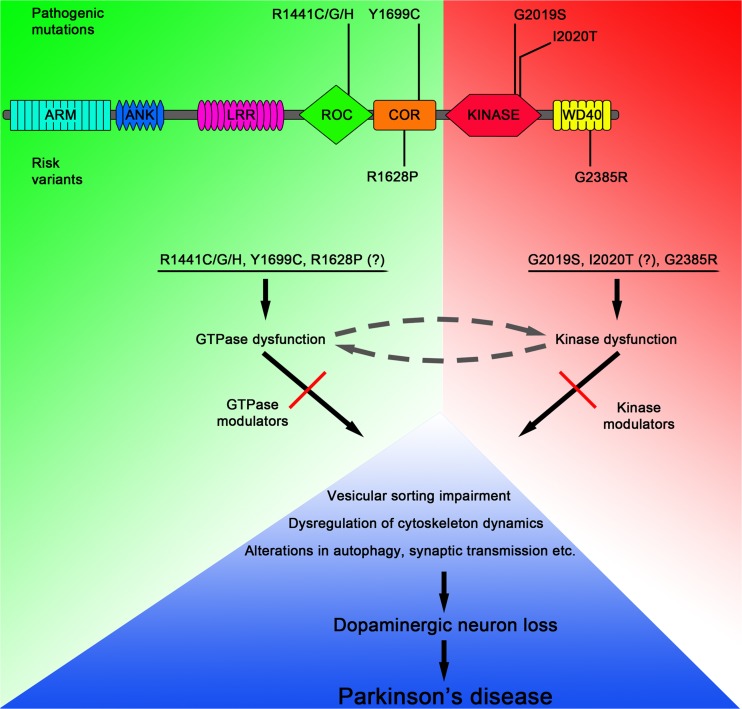
Fig. 1.
LRRK2 domain structure, localization of mutations, and proposed pathogenic mechanisms. ARM = armadillo repeats; ANK = ankyrin repeats; ROC = Ras of complex proteins; COR = C-terminus of ROC, kinase domain, and WD40 domain; GTP
Furthermore, these activities are probably regulated by cellular signaling. Similar to many other GTPases, GTP hydrolysis of LRRK2 might be regulated by guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs) [30, 31]. GEFs activate GTPases by promoting the exchange of GDP for GTP, while GAPs stimulate GTP hydrolysis and inactivation of enzymes. ARHGEF7 and ArfGAP1 were recently suggested to play these respective roles for LRRK2 [32–34]. The weak intrinsic GTPase activity of LRRK2 in vitro may be owing to the absence of the regulatory factors in assays of purified protein [35]. Dimerization is another potential mechanism of regulation of GTP binding and hydrolysis by LRRK2 [36]. In this model, GTP binding promotes dimerization and activation of the protein. Some studies have suggested that a dimer is the catalytically active form of LRRK2 [24, 37–39], although this has not yet been confirmed using structural analyses. The dimeric form of LRRK2 may also be essential for kinase activity of the protein [37, 38]. LRRK2 is also phosphorylated by other, as yet unknown, kinases.
LRRK2 also contains several domains that might be important for protein–protein interactions outside the catalytic core such as armadillo, ankyrin, leucine-rich, and WD40 repeats (Fig. 1). LRRK2 physically interacts with many proteins, such as microtubules [40–43], 14-3-3 proteins [44, 45], mitogen-activated protein kinase 6 [46], and Wnt signaling pathway proteins [47]. Therefore, LRRK2 may also function as a scaffold for many signaling pathways and might therefore be implicated in the number of cellular functions [48].
The physiological function of LRRK2 is unknown, but LRRK2 is involved in autophagy [49, 50], vesicle sorting and trafficking [51, 52], dopamine homeostasis [53, 54], dopamine receptor activation, synaptogenesis [55], and cytoskeleton dynamics (Fig. 2) [56, 57]. Many of these functions depend on LRRK2 activity and could, therefore, be affected by different mutations. In this review, we discuss the diversity of mechanisms of different pathogenic mutations and risk factor variants of LRRK2. We also discuss whether we can predict useful therapeutic strategies based on what we know about LRRK2 biology and pathogenesis.
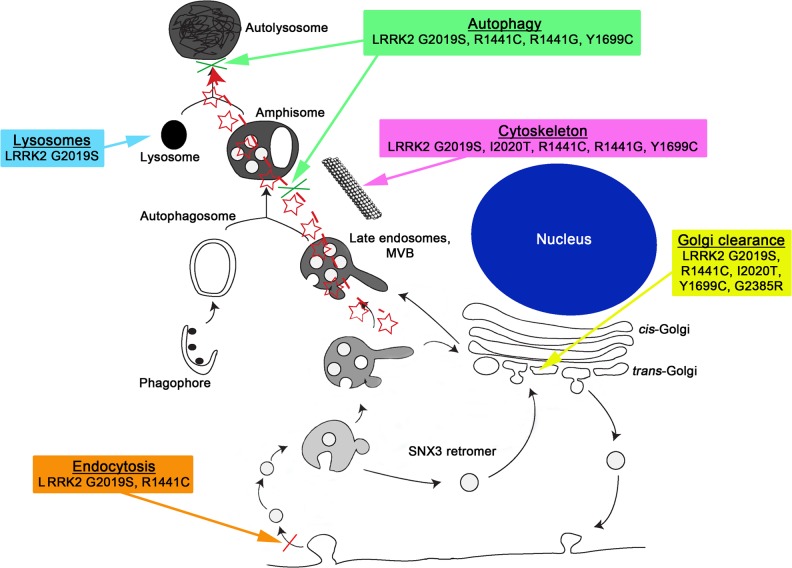
Fig. 2.
Pathogenic mechanisms of LRRK2 mutations (modified from [151]). Many pathogenic mechanisms have been reported for LRRK2 mutations. Among those there is a growing number of evidence of involvement of LRRK2 in different vesicle trafficking events, including endocytosis [152, 171], Golgi clearance [43, 51, 126, 150, 151], cytoskeleton dynamics [40–43, 53, 115, 126, 138, 139, 150, 172–174], lysosomal dynamics [143], and autophagy [124, 140, 142, 146, 175]. Different pathogenic LRRK2 mutations can cause alterations of at least some of these events. Please note that lack of indication that a specific mutation has a given effect does not necessarily mean that a negative result has been reported; in most cases not all mutations have been tested
Diversity of LRRK2 Mutations
Many mutations have been reported in LRRK2 in patients with PD, but only 6 of these (R1441C/G/H, Y1699C, G2019S, and I2020T) found in the central catalytic ROC–COR–kinase triple domain clearly segregate with the disease (Fig. 1; Table 1) [48, 58, 59]. Some additional LRRK2 variants represent risk factors for the disease (G2385R, R1628P), while the role of other reported substitutions remains unclear. We will discuss these different mutations below based on where they are in the protein as this leads most easily into a discussion about variable effects on function.
Table 1.
Pathogenic mutations and risk factor variants of LRRK2
| Substitution | LRRK2 domain | Molecular mechanism | Cellular effects | Invertebrate and rodent models | Therapeutic strategy | |
|---|---|---|---|---|---|---|
| Pathogenic mutations | G2019S | Kinase | Kinase activation | Acute toxicity, neurite shortening, endocytosis impairment, lysosomal positioning alteration, autophagy impairment, Golgi clearance, cytoskeleton dynamics imbalance | DA neurodegeneration, reduction of dopamine levels in vivo, locomotor dysfunction in Caenorhabditis elegans; modest DA neuron loss, diminished dopamine transmission and motor deficits in mouse | Inhibition of kinase activity |
| I2020T | Kinase | Unclear. May be kinase activation | Acute toxicity, neurite shortening, Golgi clearance, cytoskeleton dynamics imbalance | DA neuron loss in Drosophila | Inhibition of kinase activity (?) | |
| R1441C/G/H | ROC | GTP hydrolysis disruption, destabilization of ROC domain | Acute toxicity, neurite shortening, endocytosis impairment, autophagy impairment, Golgi clearance, cytoskeleton dynamics imbalance | DA neurodegeneration, reduction of dopamine levels in vivo, locomotor dysfunction in C. elegans; diminished dopamine release, bradykinesia and coordination deficiency in mouse | Modulation of GTPase activity | |
| Y1699C | COR | GTP hydrolysis disruption, strengthening of ROC–COR interactions | Acute toxicity, neurite shortening, autophagy impairment, Golgi clearance, cytoskeleton dynamics imbalance | DA neuron loss and locomotor deficits in Drosophila | Modulation of GTPase activity | |
| Risk variants | R1628P | COR | Unknown. Presumably may affect GTPase activity (?) | - | - | Modulation of GTPase activity (?) |
| G2385R | WD40 | Partial loss of kinase activity | Reversal of some effects of G2019S mutant, Golgi clearance | DA neuron loss and locomotor deficits in Drosophila | Restoration of kinase activity |
ROC Ras of complex proteins; COR C-terminus of ROC; DA dopamine
Mutations in the Kinase Domain
The most common LRRK2 mutation is G2019S, which was first identified in PD patients of European ancestry [60–63]. The highest incidence of this variant is in people of Ashkenazi Jewish descent (almost 20 %) [64] and in North African patients (about 40 %) [65]. The G2019S substitution is located in the activation loop of the kinase domain of LRRK2, and causes stabilization of the enzyme in the active form, leading to enhanced phosphorylation activity (Fig. 1) [31, 66]. A 2–3-fold increase in kinase activity of G2019S LRRK2 compared with wild-type protein has been consistently seen by many groups [12, 13, 15, 20, 56, 67–73].
The I2020T substitution is also located within the activation loop of the kinase domain (Fig. 1). This mutation is causative in the first Japanese family reported for the PARK8 locus [11, 74]. In contrast to the adjacent G2019S mutation, how I2020T affects protein function is controversial. Some reports suggest an increased kinase activity for I2020T mutant [14, 75, 76] presumably via stabilization of the active state confirmation of the kinase [77], while others revealed unchanged [44] or even decreased kinase activity [67]. Although methodological differences between assays may contribute to the apparent differences between studies [78], a more likely interpretation of these data is that the effect of this mutation is not more than a 40 % increase over the wild type. In most assays, this is within measurement error. Overall, this suggests that the effects of G2019S and I2020T on kinase activity of LRRK2 are quite different from each other. The differential effects of these two mutations is surprising given that these are apparently similar substitutions at adjacent residues and a convincing structural explanation has not yet been proposed.
ROC–COR Bidomain Mutations
The second most common LRRK2 mutation, R1441C, has been found in several different populations [79]. Interestingly, two other variations of the same codon (R1441G and R1441H) have been reported. The R1441G variant is frequent in some Basque families, but rare elsewhere [80]. The R1441H mutation was reported in several PD families [81–85], but is generally infrequent worldwide [86].
The R1441 residue is located at the interface of the ROC and COR domains, and likely stabilizes the interactions between these regions [87]. The R1441 mutations cause a decrease in GTPase activity of LRRK2, although this activity is low and has been difficult to measure reliably, leading to some uncertainty about relevance of this observation [18–20, 88]. The mechanism of diminished activity is likely via disruption of hydrogen bonding and stacking interactions normally provided by the arginine side chain [18–20, 87], which may cause an enhancement in the affinity of LRRK2 for GTP with a subsequent decrease in GTP hydrolysis [89]. Substitution of the positively charged arginine to neutral amino acids (cytosine or glycine) is expected to cause misfolding of ROC domain [90]. R1441 substitutions may also alter the interaction between the ROC and COR domains [91]. Data on kinase activity of R1441C mutant of LRRK2 have been inconsistent. Some groups have reported increased kinase activity of R1441C LRRK2 [15, 20], while others did not detect any difference with the wild-type protein [13, 44, 67, 68]. As discussed for I2020T, the apparent differences between studies likely result from the fact that the effects on kinase activity are modest at best and often within measurement error.
The Y1699C mutation was originally discovered in a large family from Germany, Denmark, and Canada, and another family from the UK [9, 10], although it is relatively rare in most populations [4, 3]. The Y1699 residue is located on the outer surface of the COR domain and substitution with cytosine at this likely alters interactions between the ROC and COR domains, leading to a reduction in GTPase activity [88, 91]. As for R1441 mutations, the kinase function of Y1699C LRRK2 is similar to wild-type protein [13, 44, 67, 68].
Overall, it therefore seems most likely that the major effect of both ROC and COR mutations is on GTPase rather than kinase activity. In general, the GTP-bound form of proteins is the active version and it logically follows that that ROC and COR mutations increase the activation state of the protein. Therefore, despite these mutations having a loss of GTPase function, they likely result in a persistent function compared with wild-type protein.
The ROC–COR bidomain mutations have another shared effect in that they all decrease S910, S935 phosphorylation-dependent 14-3-3 binding [44]. I2020T is also dephosphorylated but G2019S is not. Decreased phosphorylation of Ser935 is a result of an enhanced affinity of R1441C and Y1699C mutants for protein phosphatase 1α [92]. Additionally, loss of 14-3-3 binding correlates with an increased number of inclusion bodies formed in the cytoplasm of cells [44]. Collectively, the effects of ROC–COR domain mutations, and perhaps I2020T, are likely driven by altered protein folding.
Genetic Risk Factors Associated with Parkinson’s Disease
Two variants in LRRK2 that are predominantly found in Asian populations, G2385R [93–97] and R1628P [98–101], increase the lifetime risk of PD about 2-fold [102]. Molecular modeling predicts that the G2385 residue is located on the outer surface of the WD40 domain towards the C-terminus of LRRK2 [31]. The substitution of a neutral and flexible glycine for a positively charged arginine at this position could interfere with interdomain interactions within LRRK2. The WD40 and extreme C-terminus of LRRK2 are also required for kinase activity of LRRK2 as C-terminally truncated constructs have a loss of kinase activity [67, 73, 103]. In our hands, using a range of different constructs and assays, the G2385R substitution causes a 40–50 % loss of LRRK2 kinase function. Interestingly, a double G2019S/G2385R LRRK2 mutant had kinase activity indistinguishable from wild-type protein, suggesting an opposite effect of G2019S and G2385R substitutions on kinase function of LRRK2 within the same molecule [73]. These results reinforce that there are multiple intramolecular interactions within LRRK2 and that some disease-associated variants affect such communications.
LRRK2 Polymorphisms that may Affect Protein Function
A number of LRRK2 polymorphisms have been reported, but only few are likely to be pathogenically relevant. The N1437H substitution was found in 2 Norwegian families and 1 patient from Sweden [58, 104]. Owing to the limited genetic data, whether this mutation is pathogenic is uncertain. Direct evaluation of GTPase and kinase activity of N1437H LRRK2 has not yet been reported, although this variant does cause increased GTP binding [58]. When overexpressed in HEK293 cells, N1437H LRRK2 has an increased phosphorylation at Ser1292 [105] and diminished phosphorylation of Ser935 and, hence, 14-3-3 binding [92]. The measured effects are similar to other ROC–COR domain mutations, supporting pathogenicity.
An I2012T substitution was initially suggested to be pathogenic [10]. However, owing to the limited data about familial segregation it is difficult to be certain that this is a causative mutation. The I2012T variant lies within the predicted Mg2+ binding region of the kinase domain and thus could influence LRRK2 kinase activity [31]. Supporting this, Jaleel et al. [67] demonstrated the I2012T mutant has decreased kinase activity, but subsequent studies did not find any difference in phosphorylation activity compared with wild-type LRRK2 [44]. Therefore, further genetic and biochemical evaluation of the I2012T substitution is still needed.
The R1398H LRRK2 polymorphism is quite common (>5 %) in several populations and apparently plays a protective role against development of PD [102, 106–109]. The R1398 residue is located in the switch II motif of the ROC domain and could be important for the interaction with the γ-phosphate of GTP [87]. Intriguingly, the hypothesis-testing R1398L substitution has increased GTPase activity in vitro [33, 110, 111]. However, R1398Q does not change GTPase activity of LRRK2, in contrast to expectations [110]. It would be interesting to check if R1398H LRRK2 has enhanced GTPase activity, as having the opposite effect of R1441C or Y1699C mutations might explain why this is a protective variant.
Finally, it should be noted that there are many more variants in LRRK2, many of which are very rare and/or only found in controls and not PD cases. It is likely that the LRRK2 protein tolerates a fairly extensive series of amino acid substitutions and many of these are not associated with altered risk of PD and are instead benign polymorphisms.
Biological Effects of LRRK2 Mutations
Strikingly, despite how the different mutations in LRRK2 have varied effects on activities of the molecule, some aspects of their effects in cells and animals are similar. Revealing the specific and detailed cellular pathogenic mechanisms for each LRRK2 mutation that result from the primary effects of mutations may help not only to understand physiological functions of the protein better, but also allow the development of pharmacological interventions to halt the progression of PD.
Direct Toxic Effects of LRRK2 Expression in Neurons
An obvious question about LRRK2 mutations is whether they directly cause neuronal cell death. Overexpression of G2019S LRRK2 in cultured primary neurons and neuroblastoma cell lines mediates cellular toxicity and triggers apoptotic cell death [13, 15, 70, 103]. Similarly, overexpression of R1441C or Y1699C also triggers cell death [13]. In some cases, the acute toxic effects could be attenuated by mutation in kinase domain to render it inactive [13, 15, 112] or by pharmacological inhibition of LRRK2 kinase [113]. However, whether this is strictly owing to loss of kinase activity of LRRK2 itself is not clear as the hybrid mutants with artificial kinase dead mutations are less stable than kinase active LRRK2 [112]. Similarly, kinase inhibition can destabilize LRRK2 [50].
Transgenic expression of human G2019S LRRK2 in Drosophila causes dendrite degeneration and dopaminergic (DA) neurons loss followed by locomotor dysfunction [114, 115]. Importantly, this effect was diminished by pharmacological inhibition of kinase activity of G2019S mutant or by overexpression of parkin, a protein implicated in autosomal-recessive Parkinsonism [116, 117]. It was also suggested that DA neuron loss in Drosophila triggered by overexpression of G2019S hLRRK2 is caused by an increase in bulk protein synthesis and be prevented by overexpression of the phosphodeficient T136A ribosomal protein s15 [118]. Transgenic overexpression of human I2020T LRRK2 in Drosophila also induces DA neuron degeneration by a mechanism that is proposed to involve phosphorylation of 4E-BP [75], although this mechanism is controversial [72, 119]. Overexpression of human LRRK2 in Caenorhabditis elegans also mediate age-dependent DA neurodegeneration, reduction of dopamine levels in vivo, behavioral deficits, and locomotor dysfunction that is more profound for G2019S than wild-type protein [120, 121]. Transgenic expression of human R1441C mutant LRRK2 in C. elegans induces age-dependent DA neurodegeneration and locomotor deficit accompanied by a reduction in dopamine levels [120]. In Drosophila, a dLRRK Y1383C mutant (analogous to human Y1699C) caused prominent vesicular aggregation of the protein and DA neuron loss [75]. Transgenic overexpression of human Y1699C or G2385R LRRK2 in Drosophila is also associated with DA neuron loss accompanied by locomotor deficits [117, 122].
Gene transfer models have been used to further support the idea that mutant LRRK2 can be directly toxic to neurons in vivo. Injection of adenoviruses expressing human G2019S but not wild-type LRRK2 to the striatum of adult rats induces hyperphosphorylation of tau and progressive DA neuron loss [123]. In mice, herpes simplex virus amplicon vectors expressing human G2019S LRRK2 caused degeneration of DA neurons, which was attenuated by pharmacological inhibition of LRRK2 kinase activity [113].
In contrast to cell culture or acute experiments, dopamine cell loss is not generally seen using transgenic rodent models. Two transgenic mouse models overexpressing human G2019S LRRK2 exhibit modest age-dependent DA neuron loss associated with accumulation of autophagosomes and mitochondrial alterations [124, 125], but other G2019S LRRK2 transgenic mouse models had diminished dopamine transmission and motor deficits without detectable neuron loss [53, 54]. The only I2020T LRRK2 transgenic mouse model reported to date exhibits no obvious loss of DA neurons [126]. R1441G bacterial artificial chromosome transgenic mice do not demonstrate detectable nigral neuron loss but develop age-dependent bradykinesia and coordination deficiency presumably due to diminished dopamine release, which could be partially reversed upon levodopa administration [127, 128]. Similar results were received for R1441C knock-in mouse model, where the point mutation of LRRK2 caused deterioration in stimulated DA neurotransmission and D2 receptor function but, again, no cell loss [129].
Collectively, these results show that while LRRK2 may be directly toxic to DA neurons, whether this holds true in systems with more chronic lower level expression is unclear. As this situation may more closely mirror the situation in patients with physiological levels of expression over lifetime, it might be argued that the transgenic results are more relevant and predict that LRRK2 is not directly toxic to neurons in vivo. Further complicating the picture, endogenous LRRK2 is not actually very highly expressed in presynaptic dopamine neurons but is expressed in postsynaptic striatal neurons and in microglia [130–135]. Therefore, an open question is whether the effects of LRRK2 mutations are really neuronal cell-autonomous. Because of these uncertainties, it is valid to consider other effects of LRRK2 mutations that may be relevant to the development of pathophysiological states.
Effects of LRRK2 on Cellular Phenotypes and Physiology
In neurons, G2019S LRRK2 induces shortening of neurite length and, perhaps, alteration of their branching [53, 56, 57]. I2020T also induces the shortening and debranching of neurites [56, 126]. Acute overexpression of R1441C LRRK2 induces neurite shortening [13, 56, 70, 103]. Primary neurons derived from bacterial artificial chromosome transgenic (Y1699C) mice have reduced neurite outgrowth and branching, which was partially rescued with staurosporine, a nonspecific kinase inhibitor [136]. It should be noted that wild-type LRRK2 may share the essential ability of mutants to shorten neurites as neurons from LRRK2 knockout mice have longer neurites [57]. This might support the idea that LRRK2 mutations can induce a persistent functional state.
Neurite shortening might therefore be a way to assay pathogenic mutations in LRRK2. However, the G2385R LRRK2 variant does not induce neurite shortening (at least in M17 neuroblastoma cells), and even alleviated the effect of G2019S mutant when present on the same construct [73]. Furthermore, the effects of mutant LRRK2 on neurite length have a limited developmental window [55]. It might be that altered development of neurons changes their later sensitivity to the PD process, but that hypothesis is difficult to assess without an in vivo model that shows both neurite length changes and neurodegeneration, both of which are lacking in current models. Therefore, neurite shortening is a helpful assay for LRRK2 but the relevance either to the effects of all mutations or disease pathogenesis is unclear.
What the neurite shortening effects have done is to provide a platform to explore mechanisms related to mutant LRRK2. The effects of the G2019S can be overcome by overexpression of Rac1 [137], a Rho GTPase involved in actin cytoskeleton remodeling. I2020T-mediated neurite shortening may involve changes in tubulin-associated tau [138]. Abnormally phosphorylated tau was found in the brainstem of LRRK2 I2020T mutation carriers [139]. Alterations in autophagy may also contribute to neurite shortening caused by G2019S LRRK2 [140]. These results highlight 2 functional themes for LRRK2 that may be important in understanding pathogenesis; altered cytoskeletal dynamics and changes in vesicular trafficking, particularly related to the autophagy–lysosome system. This latter idea is particularly intriguing as endogenous LRRK2 is associated with vesicular structures in cells and brain [141], and is particularly associated with vesicles related to late endosomes and autophagosomes [142]. Several groups have shown that LRRK2 mutations induce the formation of autophagy-related intracellular inclusions [35].
The Drosophila LRRK2 homolog (lrrk) interacts with Rab7, a small GTPase involved in late endosome processing, and introduction of point mutation analogous to human G2019S substitution promotes formation of Rab7-positive inclusions in Drosophila follicle cells [143]. Overexpression of another GTPase implicated in vesicle sorting and trafficking, the Drosophila Rab7L1 ortholog, rescued DA neuron loss mediated by G2019S mutant [51]. This effect may be related to retromer function [51]; mutations in a retromer protein, VPS35, are a genetic cause of PD in humans [144, 145]. The control of vesicular sorting and recycling is critical to most cells as a major way of regulating organelle distribution and turnover, again often by the autophagy–lysosome system. Interestingly, all mutations tested in LRRK2 cause alterations in markers of autophagy function [146], suggesting that this is a common effect of mutations.
In C. elegans, LRK-1 plays a pivotal role in polarized sorting of synaptic vesicle proteins to the Golgi [147]. A LRRK2 interactor, ArfGAP1, associates with the Golgi apparatus as a part of the coat protein I complex [148, 149]. Golgi apparatus fragmentation is a prominent pathological feature of DA neurons in G2019S LRRK2 transgenic mice [150]. The fragmentation could result in or be a consequence of altered vesicle sorting and trafficking, both of which are essential steps in the autophagy pathway. We have recently suggested that the effects of LRRK2 on autophagy and on Golgi turnover are, in fact, related. By looking for direct protein interactors of LRRK2, we recovered both Rab7L1 (also identified by Macleod et al. [51]) and cyclin-G associated kinase (GAK) [151]. Both Rab7L1 and GAK are associated with the Golgi apparatus and, specifically, overlap in their distribution at the trans-Golgi network. We were able to show both in cells and in an acute expression model in vivo that LRRK2 promotes turnover of trans-Golgi network-derived vesicles. Previous results in mice showed that trans- rather than cis- Golgi markers are better measures of LRRK2-induced Golgi fragmentation [150]. Importantly, all mutations in LRRK2 exaggerated this phenomenon, including the risk factor G2385R, which had an effect in between wild-type LRRK2 and the Mendelian mutants. Although these results need to be confirmed and extended, they suggest that the pathogenic effects of LRRK2 are shared by all mutations, despite their different biochemical effects. Our interpretation is that all mutations cause, to some extent, persistent or poorly regulated function and could cause alterations at different levels of the same pathophysiological pathway.
An open question is whether this general cell biological event has particular relevance to neurons. LRRK2 plays a role in the regulation of synaptic vesicle endocytosis and recycling, which involves vesicle sorting and trafficking [52, 152]. Surprisingly, both knockdown and overexpression of wild-type or mutant LRRK2 impairs synaptic vesicle endocytosis, meaning that the relationship between mutation and normal function is more complex than for Golgi turnover. The precise mechanism of these phenomena is unresolved but it may be mediated via interaction of LRRK2 with the number of proteins involved in orchestration of synaptic vesicle endocytosis and trafficking including synataxin 1A and Rab5 GTPase [52, 152]. Considering that the WD40 domain of LRRK2 interacts with many proteins involved in orchestration of synaptic vesicle dynamics [52], it is possible that G2385R mutant LRRK2 may have similar effects. However, because of the limited expression of LRRK2 in presynaptic dopamine neurons it remains to be determined how changes in presynaptic sorting cause disease.
If vesicular sorting in general, or synaptic recycling specifically, is a direct effect of LRRK2 mutations, then this may have secondary effects that are measurable in cells and could contribute to pathogenesis. Dopamine neurons derived from pluripotent stem cells from R1441C mutation carriers revealed mitochondrial pathology presumably due to mitochondrial DNA damage, which could be rescued pharmacologically or by gene correction [153, 154]. Similar effects on mitochondrial function have been seen in G2019S fibroblasts [155]. Because the Golgi is a major source of lipids for many organelles, it would be of interest to see if the effects of LRRK2 on mitochondria are a consequence of Golgi dysfunction or represent an independent pathway for pathogenesis.
Overall, both vesicle sorting impairment and cytoskeleton dynamic dysregulation could potentially lead to disruption of vesicle trafficking and turnover in DA neurons followed by neurodegeneration and development of frank PD symptoms.
Therapeutic Strategies for LRRK2-associated PD
All current therapeutic strategies of PD are symptomatic and do not halt or slow down progression of the disease. The identification of LRRK2 mutations has led to several proposals for mechanism-based therapies [3]. As the most common LRRK2 mutation (G2019S) augments kinase activity, development of LRRK2 kinase inhibitors has been proposed as a promising approach [156]. Many kinase inhibitors will block LRRK2 activity, with CZC-25146 and LRRK2-IN1 having high specificity for LRRK2 [157–159]. Neither of these kinase inhibitors would be taken into clinical trials owing to their poor blood–brain barrier permeability [159], although more recent inhibitors may be have better distribution in the brain [160–162].
However, even if selective potent and bioavailable LRRK2 kinase inhibitors were developed, there are some concerns about likely side effects. Because LRRK2 is heavily expressed in kidneys and lungs [163], and deletion of LRRK2 in mice causes primarily accumulation of cytoplasmic inclusions in those tissues [49, 50], LRRK2 inhibitors could cause significant impairment of kidney and lung functions. Furthermore, apart from G2019S none of other LRRK2 mutations consistently augment kinase activity of the protein [78]. Therefore, application of kinase inhibitors in case of these substitutions may or may not be sensible, and alternative therapeutic approaches might be considered in these cases [164, 165]. Moreover, some variants, like G2385R, have diminished kinase activity and are still associated with disease [73]. Given these data, it is unclear if kinase inhibitors are a viable option for LRRK2-based therapeutics.
However, there are still other possible regions within LRRK2 that might be targeted therapeutically. Because pathogenic mutations in ROC–COR have impaired GTP hydrolysis, it might be that the GTP-bound form of LRRK2 is pathogenic [18–20]. It has been suggested that GTP binding is essential for LRRK2-mediated toxicity [15, 111]. The proposed facilitation of GTP hydrolysis by LRRK2 via activation of its GAP (ArfGAP1) might also be a target [165], although this would require targeting protein–protein interactions. Along the same lines, our recent results showing enhancement of LRRK2 activity in cells by interactions with Rab7L1 and GAK may suggest additional targets. Dimerization of LRRK2 itself is also thought to contribute to its activity and might be targetable if the structural basis of this phenomenon were elucidated [164].
Kinases usually participate in intracellular signaling cascades. Therefore, modulation of up- and downstream effectors of LRRK2 could compensate for the pathogenic effects of mutations [164]. For example, activation of 4E-BP by rapamycin or transgenic overexpression of 4E-BP prevents dopamine neuron loss in Drosophila models [75, 166]. 4E-BP binds and negatively regulates eukaryotic initiation factor 4E (eIF4E). Strikingly, mutations in EIF4G1, another interacting partner of eIF4E, are also implicated in autosomal-dominant PD [167]. Therefore, it would be of interest to study functional interactions of LRRK2, 4E-BP, and EIF4G1 in mammalian models.
Because the death of DA neurons starts several years before the onset of PD symptoms [168], the most effective time to intervene in disease would be before motor symptoms occur. However, whether this is ethically appropriate is uncertain, as LRRK2 mutations are not fully penetrant, even by the age of 80 [7, 169, 170]. Therefore, identification of the very earliest symptoms of LRRK2 patients is likely to be an important step in deciding when to apply therapeutics. However, the limiting factor right now is having therapeutics on hand, including potentially different approaches for different mutations.
Conclusions
Substantial progress has been made in understanding the molecular mechanisms of pathogenic LRRK2 mutations. However, the intracellular pathophysiologic effects of mutations remains unclear. Therefore, a major challenge is to identify the normal cellular function of LRRK2 and to understand how LRRK2 mutations consistently affect this function. To do so would, in our opinion, help facilitate development of new LRRK2-targeted etiological medications for the treatment of PD. There is, as there has been for many years now, still a need to develop better in vivo models that have robust degeneration such that any proposed events can be tied into one of the major events in the disease process. Eventually, such efforts may help to prevent or halt neuronal damage in PD and establish etiological treatment of the disorder.
Electronic supplementary material
(PDF 1224 kb)
Acknowledgments
We thank Dr. Aleksandra Beilina for fruitful discussions.
Funding
This work was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Competing Interests
The authors have no competing interests.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 2.Halliday G, Lees A, Stern M. Milestones in Parkinson's disease—clinical and pathologic features. Mov Disord. 2011;26:1015–1021. doi: 10.1002/mds.23669. [DOI] [PubMed] [Google Scholar]
- 3.Singleton AB, Farrer MJ, Bonifati V. The genetics of Parkinson's disease: progress and therapeutic implications. Mov Disord. 2013;28:14–23. doi: 10.1002/mds.25249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simón-Sánchez J, Schulte C, Bras JM, et al. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satake W, Nakabayashi Y, Mizuta I, et al. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nat Genet. 2009;41:1303–1307. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- 6.Haugarvoll K, Wszolek ZK. Clinical features of LRRK2 parkinsonism. Parkinsonism Relat Disord. 2009;15(Suppl. 3):S205–S208. doi: 10.1016/S1353-8020(09)70815-6. [DOI] [PubMed] [Google Scholar]
- 7.Healy DG, Falchi M, O'Sullivan SS, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case-control study. Lancet Neurol. 2008;7:583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cookson MR, Hardy J, Lewis PA. Genetic neuropathology of Parkinson’s disease. Int J Clin Exp Pathol. 2008;1:217–231. [PMC free article] [PubMed] [Google Scholar]
- 9.Paisan-Ruiz C, Jain S, Evans EW, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 10.Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Funayama M, Hasegawa K, Kowa H, Saito M, Tsuji S, Obata F. A new locus for Parkinson's disease (PARK8) maps to chromosome 12p11.2-q13.1. Ann Neurol. 2002;51:296–301. doi: 10.1002/ana.10113. [DOI] [PubMed] [Google Scholar]
- 12.West AB, Moore DJ, Biskup S, et al. Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greggio E, Jain S, Kingsbury A, et al. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis. 2006;23:329–341. doi: 10.1016/j.nbd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Gloeckner CJ, Kinkl N, Schumacher A, et al. The Parkinson disease causing LRRK2 mutation I2020T is associated with increased kinase activity. Hum Mol Genet. 2006;15:223–232. doi: 10.1093/hmg/ddi439. [DOI] [PubMed] [Google Scholar]
- 15.Smith WW, Pei Z, Jiang H, Dawson VL, Dawson TM, Ross CA. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci. 2006;9:1231–1233. doi: 10.1038/nn1776. [DOI] [PubMed] [Google Scholar]
- 16.Lesage S, Leutenegger AL, Brice A. LRRK2: a gene belonging to the ROCO family is implicated in the Parkinson's disease. Med Sci (Paris) 2005;21:1015–1017. doi: 10.1051/medsci/200521121015. [DOI] [PubMed] [Google Scholar]
- 17.Marin I. The Parkinson disease gene LRRK2: evolutionary and structural insights. Mol Biol Evol. 2006;23:2423–2433. doi: 10.1093/molbev/msl114. [DOI] [PubMed] [Google Scholar]
- 18.Lewis PA, Greggio E, Beilina A, Jain S, Baker A, Cookson MR. The R1441C mutation of LRRK2 disrupts GTP hydrolysis. Biochem Biophys Res Commun. 2007;357:668–671. doi: 10.1016/j.bbrc.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Tan YC, Poulose S, Olanow CW, Huang XY, Yue Z. Leucine-rich repeat kinase 2 (LRRK2)/PARK8 possesses GTPase activity that is altered in familial Parkinson's disease R1441C/G mutants. J Neurochem. 2007;103:238–247. doi: 10.1111/j.1471-4159.2007.04743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo L, Gandhi PN, Wang W, Petersen RB, Wilson-Delfosse AL, Chen SG. The Parkinson's disease-associated protein, leucine-rich repeat kinase 2 (LRRK2), is an authentic GTPase that stimulates kinase activity. Exp Cell Res. 2007;313:3658–3670. doi: 10.1016/j.yexcr.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taymans JM, Vancraenenbroeck R, Ollikainen P, et al. LRRK2 kinase activity is dependent on LRRK2 GTP binding capacity but independent of LRRK2 GTP binding. PLoS One. 2011;6:e23207. doi: 10.1371/journal.pone.0023207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu M, Dobson B, Glicksman MA, Yue Z, Stein RL. Kinetic mechanistic studies of wild-type leucine-rich repeat kinase 2: characterization of the kinase and GTPase activities. Biochemistry. 2010;49:2008–2017. doi: 10.1021/bi901851y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito G, Okai T, Fujino G, et al. GTP binding is essential to the protein kinase activity of LRRK2, a causative gene product for familial Parkinson's disease. Biochemistry. 2007;46:1380–1388. doi: 10.1021/bi061960m. [DOI] [PubMed] [Google Scholar]
- 24.Greggio E, Zambrano I, Kaganovich A, et al. The Parkinson disease-associated leucine-rich repeat kinase 2 (LRRK2) is a dimer that undergoes intramolecular autophosphorylation. J Biol Chem. 2008;283:16906–16914. doi: 10.1074/jbc.M708718200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greggio E, Taymans JM, Zhen EY, et al. The Parkinson's disease kinase LRRK2 autophosphorylates its GTPase domain at multiple sites. Biochem Biophys Res Commun. 2009;389:449–454. doi: 10.1016/j.bbrc.2009.08.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamikawaji S, Ito G, Iwatsubo T. Identification of the autophosphorylation sites of LRRK2. Biochemistry. 2009;48:10963–10975. doi: 10.1021/bi9011379. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Moore DJ, Xiong Y, Dawson TM, Dawson VL. Reevaluation of phosphorylation sites in the Parkinson disease-associated leucine-rich repeat kinase 2. J Biol Chem. 2010;285:29569–29576. doi: 10.1074/jbc.M110.127639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gloeckner CJ, Boldt K, von Zweydorf F, et al. Phosphopeptide analysis reveals two discrete clusters of phosphorylation in the N-terminus and the Roc domain of the Parkinson-disease associated protein kinase LRRK2. J Proteome Res. 2010;9:1738–1745. doi: 10.1021/pr9008578. [DOI] [PubMed] [Google Scholar]
- 29.Webber PJ, Smith AD, Sen S, Renfrow MB, Mobley JA, West AB. Autophosphorylation in the leucine-rich repeat kinase 2 (LRRK2) GTPase domain modifies kinase and GTP-binding activities. J Mol Biol. 2011;412:94–110. doi: 10.1016/j.jmb.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taymans JM. The GTPase function of LRRK2. Biochem Soc Trans. 2012;40:1063–1069. doi: 10.1042/BST20120133. [DOI] [PubMed] [Google Scholar]
- 31.Mata IF, Wedemeyer WJ, Farrer MJ, Taylor JP, Gallo KA. LRRK2 in Parkinson's disease: protein domains and functional insights. Trends Neurosci. 2006;29:286–293. doi: 10.1016/j.tins.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Haebig K, Gloeckner CJ, Miralles MG, et al. ARHGEF7 (Beta-PIX) acts as guanine nucleotide exchange factor for leucine-rich repeat kinase 2. PLoS One. 2010;5:e13762. doi: 10.1371/journal.pone.0013762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stafa K, Trancikova A, Webber PJ, Glauser L, West AB, Moore DJ. GTPase activity and neuronal toxicity of Parkinson's disease-associated LRRK2 is regulated by ArfGAP1. PLoS Genet. 2012;8:e1002526. doi: 10.1371/journal.pgen.1002526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong Y, Yuan C, Chen R, Dawson TM, Dawson VL. ArfGAP1 is a GTPase activating protein for LRRK2: reciprocal regulation of ArfGAP1 by LRRK2. J Neurosci. 2012;32:3877–3886. doi: 10.1523/JNEUROSCI.4566-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsika E, Moore DJ. Mechanisms of LRRK2-mediated neurodegeneration. Curr Neurol Neurosci Rep. 2012;12:251–260. doi: 10.1007/s11910-012-0265-8. [DOI] [PubMed] [Google Scholar]
- 36.Gasper R, Meyer S, Gotthardt K, Sirajuddin M, Wittinghofer A. It takes two to tango - regulation of G proteins by dimerization. Nat Rev Mol Cell Biol. 2009;10:423–429. doi: 10.1038/nrm2689. [DOI] [PubMed] [Google Scholar]
- 37.Berger Z, Smith KA, Lavoie MJ. Membrane localization of LRRK2 is associated with increased formation of the highly active LRRK2 dimer and changes in its phosphorylation. Biochemistry. 2010;49:5511–5523. doi: 10.1021/bi100157u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sen S, Webber PJ, West AB. Dependence of leucine-rich repeat kinase 2 (LRRK2) kinase activity on dimerization. J Biol Chem. 2009;284:36346–36356. doi: 10.1074/jbc.M109.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Civiero L, Vancraenenbroeck R, Belluzzi E, et al. Biochemical characterization of highly purified leucine-rich repeat kinases 1 and 2 demonstrates formation of homodimers. PLoS One. 2012;7:e43472. doi: 10.1371/journal.pone.0043472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gandhi PN, Wang X, Zhu X, Chen SG, Wilson-Delfosse AL. The Roc domain of leucine-rich repeat kinase 2 is sufficient for interaction with microtubules. J Neurosci Res. 2008;86:1711–1720. doi: 10.1002/jnr.21622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kett LR, Boassa D, Ho CC, et al. LRRK2 Parkinson disease mutations enhance its microtubule association. Hum Mol Genet. 2012;21:890–899. doi: 10.1093/hmg/ddr526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caesar M, Zach S, Carlson CB, Brockmann K, Gasser T, Gillardon F. Leucine-rich repeat kinase 2 functionally interacts with microtubules and kinase-dependently modulates cell migration. Neurobiol Dis. 2013;54:280–288. doi: 10.1016/j.nbd.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 43.Gillardon F. Leucine-rich repeat kinase 2 phosphorylates brain tubulin-beta isoforms and modulates microtubule stability--a point of convergence in parkinsonian neurodegeneration? J Neurochem 2009;110:1514-1522. [DOI] [PubMed]
- 44.Nichols RJ, Dzamko N, Morrice NA, et al. 14-3-3 binding to LRRK2 is disrupted by multiple Parkinson's disease-associated mutations and regulates cytoplasmic localization. Biochem J. 2010;430:393–404. doi: 10.1042/BJ20100483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dzamko N, Deak M, Hentati F, et al. Inhibition of LRRK2 kinase activity leads to dephosphorylation of Ser(910)/Ser(935), disruption of 14-3-3 binding and altered cytoplasmic localization. Biochem J. 2010;430:405–413. doi: 10.1042/BJ20100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu CH, Chan D, Greggio E, et al. MKK6 binds and regulates expression of Parkinson's disease-related protein LRRK2. J Neurochem. 2010;112:1593–1604. doi: 10.1111/j.1471-4159.2010.06568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sancho RM, Law BM, Harvey K. Mutations in the LRRK2 Roc-COR tandem domain link Parkinson's disease to Wnt signalling pathways. Hum Mol Genet. 2009;18:3955–3968. doi: 10.1093/hmg/ddp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cookson MR. The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson's disease. Nat Rev Neurosci. 2010;11:791–797. doi: 10.1038/nrn2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tong Y, Yamaguchi H, Giaime E, et al. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein, and apoptotic cell death in aged mice. Proc Natl Acad Sci U S A. 2010;107:9879–9884. doi: 10.1073/pnas.1004676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herzig MC, Kolly C, Persohn E, et al. LRRK2 protein levels are determined by kinase function and are crucial for kidney and lung homeostasis in mice. Hum Mol Genet. 2011;20:4209–4223. doi: 10.1093/hmg/ddr348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.MacLeod DA, Rhinn H, Kuwahara T, et al. RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson's disease risk. Neuron. 2013;77:425–439. doi: 10.1016/j.neuron.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piccoli G, Condliffe SB, Bauer M, et al. LRRK2 controls synaptic vesicle storage and mobilization within the recycling pool. J Neurosci. 2011;31:2225–2237. doi: 10.1523/JNEUROSCI.3730-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Melrose HL, Dachsel JC, Behrouz B, et al. Impaired dopaminergic neurotransmission and microtubule-associated protein tau alterations in human LRRK2 transgenic mice. Neurobiol Dis. 2010;40:503–517. doi: 10.1016/j.nbd.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li X, Patel JC, Wang J, et al. Enhanced striatal dopamine transmission and motor performance with LRRK2 overexpression in mice is eliminated by familial Parkinson's disease mutation G2019S. J Neurosci. 2010;30:1788–1797. doi: 10.1523/JNEUROSCI.5604-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parisiadou L, Yu J, Sgobio C, et al. LRRK2 regulates synaptogenesis and dopamine receptor activation through modulation of PKA activity. Nat Neurosci. 2014;17:367–376. doi: 10.1038/nn.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MacLeod D, Dowman J, Hammond R, Leete T, Inoue K, Abeliovich A. The familial Parkinsonism gene LRRK2 regulates neurite process morphology. Neuron. 2006;52:587–593. doi: 10.1016/j.neuron.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 57.Parisiadou L, Xie C, Cho HJ, et al. Phosphorylation of ezrin/radixin/moesin proteins by LRRK2 promotes the rearrangement of actin cytoskeleton in neuronal morphogenesis. J Neurosci. 2009;29:13971–13980. doi: 10.1523/JNEUROSCI.3799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aasly JO, Vilarino-Guell C, Dachsel JC, et al. Novel pathogenic LRRK2 p.Asn1437His substitution in familial Parkinson's disease. Mov Disord. 2010;25:2156–2163. doi: 10.1002/mds.23265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gasser T. Molecular pathogenesis of Parkinson disease: insights from genetic studies. Expert Rev Mol Med. 2009;11:e22. doi: 10.1017/S1462399409001148. [DOI] [PubMed] [Google Scholar]
- 60.Goldwurm S, Di Fonzo A, Simons EJ, et al. The G6055A (G2019S) mutation in LRRK2 is frequent in both early and late onset Parkinson's disease and originates from a common ancestor. J Med Genet. 2005;42:e65. doi: 10.1136/jmg.2005.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gilks WP, Abou-Sleiman PM, Gandhi S, et al. A common LRRK2 mutation in idiopathic Parkinson's disease. Lancet. 2005;365:415–416. doi: 10.1016/S0140-6736(05)17830-1. [DOI] [PubMed] [Google Scholar]
- 62.Infante J, Rodriguez E, Combarros O, et al. LRRK2 G2019S is a common mutation in Spanish patients with late-onset Parkinson's disease. Neurosci Lett. 2006;395:224–226. doi: 10.1016/j.neulet.2005.10.083. [DOI] [PubMed] [Google Scholar]
- 63.Bras JM, Guerreiro RJ, Ribeiro MH, et al. G2019S dardarin substitution is a common cause of Parkinson's disease in a Portuguese cohort. Mov Disord. 2005;20:1653–1655. doi: 10.1002/mds.20682. [DOI] [PubMed] [Google Scholar]
- 64.Ozelius LJ, Senthil G, Saunders-Pullman R, et al. LRRK2 G2019S as a cause of Parkinson's disease in Ashkenazi Jews. N Engl J Med. 2006;354:424–425. doi: 10.1056/NEJMc055509. [DOI] [PubMed] [Google Scholar]
- 65.Lesage S, Dürr A, Tazir M, et al. LRRK2 G2019S as a cause of Parkinson's disease in North African Arabs. N Engl J Med. 2006;354:422–423. doi: 10.1056/NEJMc055540. [DOI] [PubMed] [Google Scholar]
- 66.Liu M, Bender SA, Cuny GD, Sherman W, Glicksman M, Ray SS. Type II Kinase inhibitors show an unexpected inhibition mode against Parkinson's disease-linked LRRK2 mutant G2019S. Biochemistry. 2013;12:1725–1736. doi: 10.1021/bi3012077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jaleel M, Nichols RJ, Deak M, et al. LRRK2 phosphorylates moesin at threonine-558: characterization of how Parkinson's disease mutants affect kinase activity. Biochem J. 2007;405:307–317. doi: 10.1042/BJ20070209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anand VS, Reichling LJ, Lipinski K, et al. Investigation of leucine-rich repeat kinase 2 : enzymological properties and novel assays. FEBS J 2009;276:466-478. [DOI] [PubMed]
- 69.Luzon-Toro B. Rubio de la Torre E, Delgado A, Perez-Tur J, Hilfiker S. Mechanistic insight into the dominant mode of the Parkinson's disease-associated G2019S LRRK2 mutation. Hum Mol Genet. 2007;16:2031–2039. doi: 10.1093/hmg/ddm151. [DOI] [PubMed] [Google Scholar]
- 70.Iaccarino C, Crosio C, Vitale C, Sanna G, Carri MT, Barone P. Apoptotic mechanisms in mutant LRRK2-mediated cell death. Hum Mol Genet. 2007;16:1319–1326. doi: 10.1093/hmg/ddm080. [DOI] [PubMed] [Google Scholar]
- 71.Covy JP, Giasson BI. Identification of compounds that inhibit the kinase activity of leucine-rich repeat kinase 2. Biochem Biophys Res Commun. 2009;378:473–477. doi: 10.1016/j.bbrc.2008.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumar A, Greggio E, Beilina A, et al. The Parkinson's disease associated LRRK2 exhibits weaker in vitro phosphorylation of 4E-BP compared to autophosphorylation. PLoS One. 2010;5:e8730. doi: 10.1371/journal.pone.0008730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rudenko IN, Kaganovich A, Hauser DN, et al. The G2385R variant of leucine-rich repeat kinase 2 associated with Parkinson's disease is a partial loss-of-function mutation. Biochem J. 2012;446:99–111. doi: 10.1042/BJ20120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Funayama M, Hasegawa K, Ohta E, et al. An LRRK2 mutation as a cause for the parkinsonism in the original PARK8 family. Ann Neurol. 2005;57:918–921. doi: 10.1002/ana.20484. [DOI] [PubMed] [Google Scholar]
- 75.Imai Y, Gehrke S, Wang HQ, et al. Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO J. 2008;17:2432–2443. doi: 10.1038/emboj.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kamikawaji S, Ito G, Sano T, Iwatsubo T. Differential effects of familial Parkinson mutations in LRRK2 revealed by a systematic analysis of autophosphorylation. Biochemistry. 2013;52:6052–6062. doi: 10.1021/bi400596m. [DOI] [PubMed] [Google Scholar]
- 77.Ray S, Bender S, Kang S, Lin R, Glicksman MA, Liu M. The Parkinson disease-linked LRRK2 protein mutation I2020T stabilizes an active state conformation leading to increased kinase activity. J Biol Chem. 2014;289:13042–13053. doi: 10.1074/jbc.M113.537811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Greggio E, Cookson MR. Leucine-rich repeat kinase 2 mutations and Parkinson's disease: three questions. ASN Neuro 2009;1. [DOI] [PMC free article] [PubMed]
- 79.Haugarvoll K, Rademakers R, Kachergus JM, et al. Lrrk2 R1441C parkinsonism is clinically similar to sporadic Parkinson disease. Neurology. 2008;70:1456–1460. doi: 10.1212/01.wnl.0000304044.22253.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simon-Sanchez J, Marti-Masso JF, Sanchez-Mut JV, et al. Parkinson's disease due to the R1441G mutation in Dardarin: a founder effect in the Basques. Mov Disord. 2006;21:1954–1959. doi: 10.1002/mds.21114. [DOI] [PubMed] [Google Scholar]
- 81.Mata IF, Kachergus JM, Taylor JP, et al. Lrrk2 pathogenic substitutions in Parkinson's disease. Neurogenetics. 2005;6:171–177. doi: 10.1007/s10048-005-0005-1. [DOI] [PubMed] [Google Scholar]
- 82.Zabetian CP, Samii A, Mosley AD, et al. A clinic-based study of the LRRK2 gene in Parkinson disease yields new mutations. Neurology. 2005;65:741–744. doi: 10.1212/01.wnl.0000172630.22804.73. [DOI] [PubMed] [Google Scholar]
- 83.Spanaki C, Latsoudis H, Plaitakis A. LRRK2 mutations on Crete: R1441H associated with PD evolving to PSP. Neurology. 2006;67:1518–1519. doi: 10.1212/01.wnl.0000239829.33936.73. [DOI] [PubMed] [Google Scholar]
- 84.Ross OA, Spanaki C, Griffith A, et al. Haplotype analysis of Lrrk2 R1441H carriers with parkinsonism. Parkinsonism Relat Disord. 2009;15:466–467. doi: 10.1016/j.parkreldis.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang L, Quadri M, Guedes LC, et al. Comprehensive LRRK2 and GBA screening in Portuguese patients with Parkinson's disease: identification of a new family with the LRRK2 p.Arg1441His mutation and novel missense variants. Parkinsonism Relat Disord. 2013;19:897–900. doi: 10.1016/j.parkreldis.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 86.Puschmann A. Monogenic Parkinson's disease and parkinsonism: clinical phenotypes and frequencies of known mutations. Parkinsonism Relat Disord. 2013;19:407–415. doi: 10.1016/j.parkreldis.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 87.Deng J, Lewis PA, Greggio E, Sluch E, Beilina A, Cookson MR. Structure of the ROC domain from the Parkinson's disease-associated leucine-rich repeat kinase 2 reveals a dimeric GTPase. Proc Natl Acad Sci U S A. 2008;105:1499–1504. doi: 10.1073/pnas.0709098105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Daniels V, Vancraenenbroeck R, Law BM, et al. Insight into the mode of action of the LRRK2 Y1699C pathogenic mutant. J Neurochem. 2011;116:304–315. doi: 10.1111/j.1471-4159.2010.07105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liao J, Wu CX, Burlak C, et al. Parkinson disease-associated mutation R1441H in LRRK2 prolongs the "active state" of its GTPase domain. Proc Natl Acad Sci U S A. 2014;111:4055–4060. doi: 10.1073/pnas.1323285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Y, Dunn L, Greggio E, et al. The R1441C mutation alters the folding properties of the ROC domain of LRRK2. Biochim Biophys Acta. 2009;1792:1194–1197. doi: 10.1016/j.bbadis.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gotthardt K, Weyand M, Kortholt A, Van Haastert PJ, Wittinghofer A. Structure of the Roc-COR domain tandem of C. tepidum, a prokaryotic homologue of the human LRRK2 Parkinson kinase. EMBO J. 2008;27:2239–2249. doi: 10.1038/emboj.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lobbestael E, Zhao J, Rudenko IN, et al. Identification of protein phosphatase 1 as a regulator of the LRRK2 phosphorylation cycle. Biochem J. 2013;456:119–128. doi: 10.1042/BJ20121772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim JM, Lee JY, Kim HJ, et al. The LRRK2 G2385R variant is a risk factor for sporadic Parkinson's disease in the Korean population. Parkinsonism Relat Disord. 2010;16:85–88. doi: 10.1016/j.parkreldis.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 94.Tan EK, Zhao Y, Skipper L, et al. The LRRK2 Gly2385Arg variant is associated with Parkinson's disease: genetic and functional evidence. Hum Genet. 2007;120:857–863. doi: 10.1007/s00439-006-0268-0. [DOI] [PubMed] [Google Scholar]
- 95.Fu X, Zheng Y, Hong H, et al. LRRK2 G2385R and LRRK2 R1628P increase risk of Parkinson's disease in a Han Chinese population from Southern Mainland China. Parkinsonism Relat Disord. 2013;19:397–398. doi: 10.1016/j.parkreldis.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 96.Fung HC, Chen CM, Hardy J, Singleton AB, Wu YR. A common genetic factor for Parkinson disease in ethnic Chinese population in Taiwan. BMC Neurol. 2006;6:47. doi: 10.1186/1471-2377-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Funayama M, Li Y, Tomiyama H, et al. Leucine-rich repeat kinase 2 G2385R variant is a risk factor for Parkinson disease in Asian population. Neuroreport. 2007;18:273–275. doi: 10.1097/WNR.0b013e32801254b6. [DOI] [PubMed] [Google Scholar]
- 98.Ross OA, Wu YR, Lee MC, et al. Analysis of Lrrk2 R1628P as a risk factor for Parkinson's disease. Ann Neurol. 2008;64:88–92. doi: 10.1002/ana.21405. [DOI] [PubMed] [Google Scholar]
- 99.Tan EK, Tan LC, Lim HQ, et al. LRRK2 R1628P increases risk of Parkinson's disease: replication evidence. Hum Genet. 2008;124:287–288. doi: 10.1007/s00439-008-0544-2. [DOI] [PubMed] [Google Scholar]
- 100.Yu L, Hu F, Zou X, et al. LRRK2 R1628P contributes to Parkinson's disease susceptibility in Chinese Han populations from mainland China. Brain Res. 2009;1296:113–116. doi: 10.1016/j.brainres.2009.08.047. [DOI] [PubMed] [Google Scholar]
- 101.Zhang Z, Burgunder JM, An X, et al. LRRK2 R1628P variant is a risk factor of Parkinson's disease among Han-Chinese from mainland China. Mov Disord. 2009;24:1902–1905. doi: 10.1002/mds.22371. [DOI] [PubMed] [Google Scholar]
- 102.Ross OA, Soto-Ortolaza AI, Heckman MG, et al. Association of LRRK2 exonic variants with susceptibility to Parkinson’s disease: a case–control study. Lancet Neurol. 2011;10:898–908. doi: 10.1016/S1474-4422(11)70175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jorgensen ND, Peng Y, Ho CC, et al. The WD40 domain is required for LRRK2 neurotoxicity. PLoS One. 2009;4:e8463. doi: 10.1371/journal.pone.0008463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Puschmann A, Englund E, Ross OA, et al. First neuropathological description of a patient with Parkinson's disease and LRRK2 p.N1437H mutation. Parkinsonism Relat Disord. 2012;18:332–338. doi: 10.1016/j.parkreldis.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sheng Z, Zhang S, Bustos D, et al. Ser1292 autophosphorylation is an indicator of LRRK2 kinase activity and contributes to the cellular effects of PD mutations. Sci Transl Med 2012;4:164ra1. [DOI] [PubMed]
- 106.Chen L, Zhang S, Liu Y, et al. LRRK2 R1398H polymorphism is associated with decreased risk of Parkinson's disease in a Han Chinese population. Parkinsonism Relat Disord. 2011;17:291–292. doi: 10.1016/j.parkreldis.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 107.Tan EK, Peng R, Teo YY, et al. Multiple LRRK2 variants modulate risk of Parkinson disease: a Chinese multicenter study. Hum Mutat. 2010;31:561–568. doi: 10.1002/humu.21225. [DOI] [PubMed] [Google Scholar]
- 108.Rubio JP, Topp S, Warren L, et al. Deep sequencing of the LRRK2 gene in 14,002 individuals reveals evidence of purifying selection and independent origin of the p.Arg1628Pro mutation in Europe. Hum Mutat. 2012;33:1087–1098. doi: 10.1002/humu.22075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Heckman MG, Elbaz A, Soto-Ortolaza AI, et al. The protective effect of LRRK2 p.R1398H on risk of Parkinson's disease is independent of MAPT and SNCA variants. Neurobiol Aging 2014;35:266 e5- e14. [DOI] [PMC free article] [PubMed]
- 110.Biosa A, Trancikova A, Civiero L, et al. GTPase activity regulates kinase activity and cellular phenotypes of Parkinson's disease-associated LRRK2. Hum Mol Genet. 2013;22:1140–1156. doi: 10.1093/hmg/dds522. [DOI] [PubMed] [Google Scholar]
- 111.Xiong Y, Coombes CE, Kilaru A, et al. GTPase activity plays a key role in the pathobiology of LRRK2. PLoS Genet. 2010;6:e1000902. doi: 10.1371/journal.pgen.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Skibinski G, Nakamura K, Cookson MR, Finkbeiner S. Mutant LRRK2 toxicity in neurons depends on LRRK2 levels and synuclein but not kinase activity or inclusion bodies. J Neurosci. 2014;34:418–433. doi: 10.1523/JNEUROSCI.2712-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee BD, Shin JH, VanKampen J, et al. Inhibitors of leucine-rich repeat kinase-2 protect against models of Parkinson's disease. Nat Med. 2010;16:998–1000. doi: 10.1038/nm.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu Z, Wang X, Yu Y, et al. A Drosophila model for LRRK2-linked parkinsonism. Proc Natl Acad Sci U S A. 2008;105:2693–2698. doi: 10.1073/pnas.0708452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lin CH, Tsai PI, Wu RM, Chien CT. LRRK2 G2019S mutation induces dendrite degeneration through mislocalization and phosphorylation of tau by recruiting autoactivated GSK3ss. J Neurosci. 2010;30:13138–13149. doi: 10.1523/JNEUROSCI.1737-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu Z, Hamamichi S, Lee BD, et al. Inhibitors of LRRK2 kinase attenuate neurodegeneration and Parkinson-like phenotypes in Caenorhabditis elegans and Drosophila Parkinson's disease models. Hum Mol Genet. 2011;20:3933–3942. doi: 10.1093/hmg/ddr312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ng CH, Mok SZ, Koh C, et al. Parkin protects against LRRK2 G2019S mutant-induced dopaminergic neurodegeneration in Drosophila. J Neurosci. 2009;29:11257–11262. doi: 10.1523/JNEUROSCI.2375-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Martin I, Kim JW, Lee BD, et al. Ribosomal protein s15 phosphorylation mediates lrrk2 neurodegeneration in Parkinson's disease. Cell. 2014;157:472–485. doi: 10.1016/j.cell.2014.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Trancikova A, Mamais A, Webber PJ, et al. Phosphorylation of 4E-BP1 in the mammalian brain is not altered by LRRK2 expression or pathogenic mutations. PLoS One. 2012;7:e47784. doi: 10.1371/journal.pone.0047784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yao C, El Khoury R, Wang W, et al. LRRK2-mediated neurodegeneration and dysfunction of dopaminergic neurons in a Caenorhabditis elegans model of Parkinson's disease. Neurobiol Dis. 2010;40:73–81. doi: 10.1016/j.nbd.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saha S, Guillily MD, Ferree A, et al. LRRK2 modulates vulnerability to mitochondrial dysfunction in Caenorhabditis elegans. J Neurosci. 2009;29:9210–9218. doi: 10.1523/JNEUROSCI.2281-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Venderova K, Kabbach G, Abdel-Messih E, et al. Leucine-rich repeat kinase 2 interacts with Parkin, DJ-1 and PINK-1 in a Drosophila melanogaster model of Parkinson's disease. Hum Mol Genet. 2009;18:4390–4404. doi: 10.1093/hmg/ddp394. [DOI] [PubMed] [Google Scholar]
- 123.Dusonchet J, Kochubey O, Stafa K, et al. A rat model of progressive nigral neurodegeneration induced by the Parkinson's disease-associated G2019S mutation in LRRK2. J Neurosci. 2011;31:907–912. doi: 10.1523/JNEUROSCI.5092-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ramonet D, Daher JP, Lin BM, et al. Dopaminergic neuronal loss, reduced neurite complexity and autophagic abnormalities in transgenic mice expressing G2019S mutant LRRK2. PLoS One. 2011;6:e18568. doi: 10.1371/journal.pone.0018568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen CY, Weng YH, Chien KY, et al. (G2019S) LRRK2 activates MKK4-JNK pathway and causes degeneration of SN dopaminergic neurons in a transgenic mouse model of PD. Cell Death Differ. 2012;19:1623–1633. doi: 10.1038/cdd.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Maekawa T, Mori S, Sasaki Y, et al. The I2020T Leucine-rich repeat kinase 2 transgenic mouse exhibits impaired locomotive ability accompanied by dopaminergic neuron abnormalities. Mol Neurodegener. 2012;7:15. doi: 10.1186/1750-1326-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li Y, Liu W, Oo TF, et al. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson's disease. Nat Neurosci. 2009;12:826–828. doi: 10.1038/nn.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dranka BP, Gifford A, Ghosh A, et al. Diapocynin prevents early Parkinson's disease symptoms in the leucine-rich repeat kinase 2 (LRRK2R(1)(4)(4)(1)G) transgenic mouse. Neurosci Lett. 2013;549:57–62. doi: 10.1016/j.neulet.2013.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tong Y, Pisani A, Martella G, et al. R1441C mutation in LRRK2 impairs dopaminergic neurotransmission in mice. Proc Natl Acad Sci U S A. 2009;106:14622–14627. doi: 10.1073/pnas.0906334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Higashi S, Biskup S, West AB, et al. Localization of Parkinson's disease-associated LRRK2 in normal and pathological human brain. Brain Res. 2007;1155:208–219. doi: 10.1016/j.brainres.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 131.Higashi S, Moore DJ, Colebrooke RE, et al. Expression and localization of Parkinson's disease-associated leucine-rich repeat kinase 2 in the mouse brain. J Neurochem. 2007;100:368–381. doi: 10.1111/j.1471-4159.2006.04246.x. [DOI] [PubMed] [Google Scholar]
- 132.Galter D, Westerlund M, Carmine A, Lindqvist E, Sydow O, Olson L. LRRK2 expression linked to dopamine-innervated areas. Ann Neurol. 2006;59:714–719. doi: 10.1002/ana.20808. [DOI] [PubMed] [Google Scholar]
- 133.Melrose H, Lincoln S, Tyndall G, Dickson D, Farrer M. Anatomical localization of leucine-rich repeat kinase 2 in mouse brain. Neuroscience. 2006;139:791–794. doi: 10.1016/j.neuroscience.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 134.Taymans JM, Van den Haute C, Baekelandt V. Distribution of PINK1 and LRRK2 in rat and mouse brain. J Neurochem. 2006;98:951–961. doi: 10.1111/j.1471-4159.2006.03919.x. [DOI] [PubMed] [Google Scholar]
- 135.Miklossy J, Arai T, Guo JP, et al. LRRK2 expression in normal and pathologic human brain and in human cell lines. J Neuropathol Exp Neurol. 2006;65:953–963. doi: 10.1097/01.jnen.0000235121.98052.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dachsel JC, Behrouz B, Yue M, Beevers JE, Melrose HL, Farrer MJ. A comparative study of Lrrk2 function in primary neuronal cultures. Parkinsonism Relat Disord. 2010;16:650–655. doi: 10.1016/j.parkreldis.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chan D, Citro A, Cordy JM, Shen GC, Wolozin B. Rac1 protein rescues neurite retraction caused by G2019S leucine-rich repeat kinase 2 (LRRK2) J Biol Chem. 2011;286:16140–16149. doi: 10.1074/jbc.M111.234005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kawakami F, Yabata T, Ohta E, et al. LRRK2 phosphorylates tubulin-associated tau but not the free molecule: LRRK2-mediated regulation of the tau-tubulin association and neurite outgrowth. PLoS One. 2012;7:e30834. doi: 10.1371/journal.pone.0030834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ujiie S, Hatano T, Kubo S, et al. LRRK2 I2020T mutation is associated with tau pathology. Parkinsonism Relat Disord. 2012;18:819–823. doi: 10.1016/j.parkreldis.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 140.Plowey ED, Cherra SJ, 3rd, Liu YJ, Chu CT. Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells. J Neurochem. 2008;105:1048–1056. doi: 10.1111/j.1471-4159.2008.05217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Biskup S, Moore DJ, Celsi F, et al. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60:557–569. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- 142.Alegre-Abarrategui J, Christian H, Lufino MM, et al. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum Mol Genet. 2009;18:4022–4034. doi: 10.1093/hmg/ddp346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dodson MW, Zhang T, Jiang C, Chen S, Guo M. Roles of the Drosophila LRRK2 homolog in Rab7-dependent lysosomal positioning. Hum Mol Genet. 2012;21:1350–1363. doi: 10.1093/hmg/ddr573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zimprich A, Benet-Pages A, Struhal W, et al. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet. 2011;89:168–175. doi: 10.1016/j.ajhg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vilarino-Guell C, Wider C, Ross OA, et al. VPS35 mutations in Parkinson disease. Am J Hum Genet. 2011;89:162–167. doi: 10.1016/j.ajhg.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Manzoni C, Mamais A, Dihanich S, et al. Pathogenic Parkinson's disease mutations across the functional domains of LRRK2 alter the autophagic/lysosomal response to starvation. Biochem Biophys Res Commun. 2013;441:862–866. doi: 10.1016/j.bbrc.2013.10.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sakaguchi-Nakashima A, Meir JY, Jin Y, Matsumoto K, Hisamoto N. LRK-1, a C. elegans PARK8-related kinase, regulates axonal-dendritic polarity of SV proteins. Curr Biol. 2007;17:592–598. doi: 10.1016/j.cub.2007.01.074. [DOI] [PubMed] [Google Scholar]
- 148.Cukierman E, Huber I, Rotman M, Cassel D. The ARF1 GTPase-activating protein: zinc finger motif and Golgi complex localization. Science. 1995;270:1999–2002. doi: 10.1126/science.270.5244.1999. [DOI] [PubMed] [Google Scholar]
- 149.McMahon HT, Mills IG. COP and clathrin-coated vesicle budding: different pathways, common approaches. Curr Opin Cell Biol. 2004;16:379–391. doi: 10.1016/j.ceb.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 150.Lin X, Parisiadou L, Gu XL, et al. Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson's-disease-related mutant alpha-synuclein. Neuron. 2009;64:807–827. doi: 10.1016/j.neuron.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Beilina A, Rudenko IN, Kaganovich A, et al. Unbiased screen for interactors of leucine-rich repeat kinase 2 supports a common pathway for sporadic and familial Parkinson disease. Proc Natl Acad Sci U S A. 2014;111:2626–2631. doi: 10.1073/pnas.1318306111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shin N, Jeong H, Kwon J, et al. LRRK2 regulates synaptic vesicle endocytosis. Exp Cell Res. 2008;314:2055–2065. doi: 10.1016/j.yexcr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 153.Cooper O, Seo H, Andrabi S, et al. Pharmacological rescue of mitochondrial deficits in iPSC-derived neural cells from patients with familial Parkinson's disease. Sci Transl Med 2012;4:141ra90. [DOI] [PMC free article] [PubMed]
- 154.Sanders LH, Laganiere J, Cooper O, et al. LRRK2 mutations cause mitochondrial DNA damage in iPSC-derived neural cells from Parkinson's disease patients: Reversal by gene correction. Neurobiol Dis. 2014;62:381–386. doi: 10.1016/j.nbd.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mortiboys H, Johansen KK, Aasly JO, Bandmann O. Mitochondrial impairment in patients with Parkinson disease with the G2019S mutation in LRRK2. Neurology. 2010;75:2017–2020. doi: 10.1212/WNL.0b013e3181ff9685. [DOI] [PubMed] [Google Scholar]
- 156.Vancraenenbroeck R, Lobbestael E, Maeyer MD, Baekelandt V, Taymans JM. Kinases as targets for Parkinson's disease: from genetics to therapy. CNS Neurol Disord Drug Targets. 2011;10:724–740. doi: 10.2174/187152711797247858. [DOI] [PubMed] [Google Scholar]
- 157.Ramsden N, Perrin J, Ren Z, et al. Chemoproteomics-based design of potent LRRK2-selective lead compounds that attenuate Parkinson's disease-related toxicity in human neurons. ACS Chem Biol. 2011;6:1021–1028. doi: 10.1021/cb2002413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Deng X, Dzamko N, Prescott A, et al. Characterization of a selective inhibitor of the Parkinson's disease kinase LRRK2. Nat Chem Biol. 2011;7:203–205. doi: 10.1038/nchembio.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kramer T, Lo Monte F, Goring S, Okala Amombo GM, Schmidt B. Small molecule kinase inhibitors for LRRK2 and their application to Parkinson's disease models. ACS Chem Neurosci. 2012;3:151–160. doi: 10.1021/cn200117j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Choi HG, Zhang J, Deng X, et al. Brain penetrant LRRK2 inhibitor. ACS Med Chem Lett. 2012;3:658–662. doi: 10.1021/ml300123a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Troxler T, Greenidge P, Zimmermann K, et al. Discovery of novel indolinone-based, potent, selective and brain penetrant inhibitors of LRRK2. Bioorg Med Chem Lett. 2013;23:4085–90. doi: 10.1016/j.bmcl.2013.05.054. [DOI] [PubMed] [Google Scholar]
- 162.Estrada AA, Liu X, Baker-Glenn C, et al. Discovery of highly potent, selective, and brain-penetrable leucine-rich repeat kinase 2 (LRRK2) small molecule inhibitors. J Med Chem. 2012;55:9416–9433. doi: 10.1021/jm301020q. [DOI] [PubMed] [Google Scholar]
- 163.Westerlund M, Belin AC, Anvret A, Bickford P, Olson L, Galter D. Developmental regulation of leucine-rich repeat kinase 1 and 2 expression in the brain and other rodent and human organs: Implications for Parkinson's disease. Neuroscience. 2008;152:429–436. doi: 10.1016/j.neuroscience.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 164.Rudenko IN, Chia R, Cookson MR. Is inhibition of kinase activity the only therapeutic strategy for LRRK2-associated Parkinson's disease? BMC Med. 2012;10:20. doi: 10.1186/1741-7015-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lee BD, Dawson VL, Dawson TM. Leucine-rich repeat kinase 2 (LRRK2) as a potential therapeutic target in Parkinson's disease. Trends Pharmacol Sci. 2012;33:365–373. doi: 10.1016/j.tips.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tain LS, Mortiboys H, Tao RN, Ziviani E, Bandmann O, Whitworth AJ. Rapamycin activation of 4E-BP prevents parkinsonian dopaminergic neuron loss. Nat Neurosci. 2009;12:1129–1135. doi: 10.1038/nn.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chartier-Harlin MC, Dachsel JC, Vilarino-Guell C, et al. Translation initiator EIF4G1 mutations in familial Parkinson disease. Am J Hum Genet. 2011;89:398–406. doi: 10.1016/j.ajhg.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Halliday GM, McCann H. The progression of pathology in Parkinson's disease. Ann N Y Acad Sci. 2010;1184:188–195. doi: 10.1111/j.1749-6632.2009.05118.x. [DOI] [PubMed] [Google Scholar]
- 169.Latourelle JC, Sun M, Lew MF, et al. The Gly2019Ser mutation in LRRK2 is not fully penetrant in familial Parkinson's disease: the GenePD study. BMC Med. 2008;6:32. doi: 10.1186/1741-7015-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Goldwurm S, Zini M, Mariani L, et al. Evaluation of LRRK2 G2019S penetrance: relevance for genetic counseling in Parkinson disease. Neurology. 2007;68:1141–1143. doi: 10.1212/01.wnl.0000254483.19854.ef. [DOI] [PubMed] [Google Scholar]
- 171.Matta S, Van Kolen K, da Cunha R, et al. LRRK2 controls an EndoA phosphorylation cycle in synaptic endocytosis. Neuron. 2012;75:1008–1021. doi: 10.1016/j.neuron.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 172.Niu J, Yu M, Wang C, Xu Z. Leucine-rich repeat kinase 2 disturbs mitochondrial dynamics via Dynamin-like protein. J Neurochem. 2012;122:650–658. doi: 10.1111/j.1471-4159.2012.07809.x. [DOI] [PubMed] [Google Scholar]
- 173.Wang X, Yan MH, Fujioka H, et al. LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum Mol Genet. 2012;21:1931–1944. doi: 10.1093/hmg/dds003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Law BM, Spain VA, Leinster VH, et al. A direct interaction between leucine-rich repeat kinase 2 and specific beta-tubulin isoforms regulates tubulin acetylation. J Biol Chem. 2014;289:895–908. doi: 10.1074/jbc.M113.507913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bravo-San Pedro JM, Niso-Santano M, Gomez-Sanchez R, et al. The LRRK2 G2019S mutant exacerbates basal autophagy through activation of the MEK/ERK pathway. Cell Mol Life Sci. 2013;70:121–136. doi: 10.1007/s00018-012-1061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)