Abstract
The triad is a skeletal muscle substructure responsible for the regulation of excitation–contraction coupling. It is formed by the close apposition of the T-tubule and the terminal sarcoplasmic reticulum. A rapidly growing list of skeletal myopathies, here referred to as triadopathies, are caused by gene mutations in components of the triad. These disorders, at their root, are caused by defects in excitation contraction coupling and intracellular calcium homeostasis. Secondary abnormalities in triad structure and/or function are also reported in several muscle diseases, most notably certain muscular dystrophies. This review highlights the current understanding of both primary and secondary triadopathies, and identifies important concepts yet to be fully addressed in the field. The emphasis of the review is both on the pathogenesis of triadopathies and their potential treatment.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-014-0300-3) contains supplementary material, which is available to authorized users.
Key Words: Excitation–contraction coupling, RYR1, triad, congenital myopathies, malignant hyperthermia
Introduction
The primary function of skeletal muscle is to generate force to initiate and control movement. To accomplish this, muscle utilizes several unique substructures dedicated to force production and regulation [1]. These substructures include the neuromuscular junction (NMJ), the triad, and the sarcomere. As knowledge of the genetic basis of human skeletal myopathies has grown, it has become apparent that many muscle diseases fall into categories based on the fact that they primarily involve alterations of one of these structures. Consideration of muscle diseases by substructural categorization is useful in terms of understanding disease pathogenesis and for therapy development. This review focuses on the triad and disorders that affect triad structure and function—the so-called “triadopathies.”
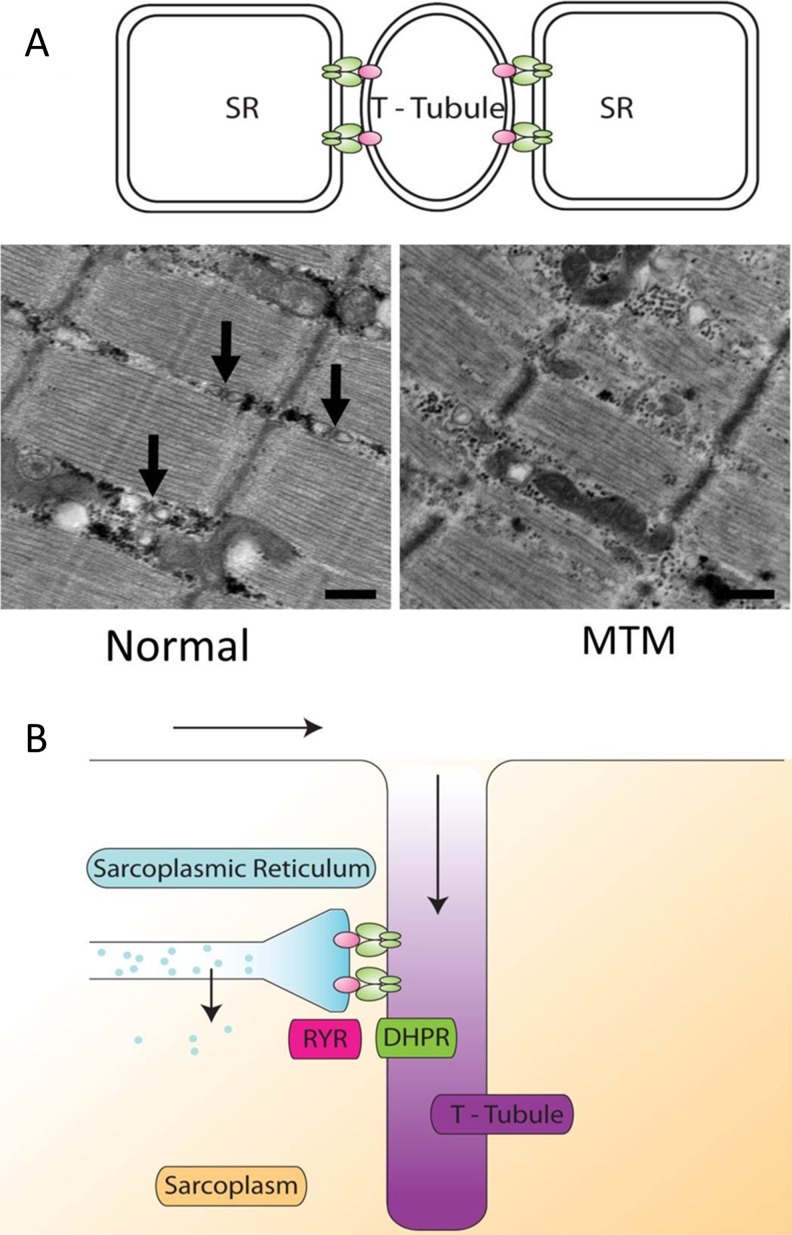
The triad is an essential skeletal muscle substructure. It represents the close apposition of the transverse tubule (T-tubule) membrane with 2 flanking terminal cisternae of the sarcoplasmic reticulum (SR) (Fig. 1A). The primary role of the triad is to coordinate excitation–contraction coupling (EC coupling). EC coupling is the process by which neuronal input to skeletal muscle [through the release of acetylcholine (ACh) at the NMJ] is transduced into muscle contraction (Fig. 1B). At the triad, membrane depolarization (stimulated by an action potential initiated through ACh binding to ACh receptors at the NMJ) activates dihydropyridine receptors (DHPRs; located in the T-tubule), which, in turn, triggers the opening of the skeletal muscle ryanodine receptor (RyR1) calcium release channels in the terminal SR [2]. Activation of RyR1 results in the release of calcium ions stored in the SR, which then bind to troponin C at the thin filament to initiate actin–myosin interactions during the cross bridge cycle. As a result of cross bridge cycling, actin filaments are pulled toward the center of the sarcomere, the sarcomere shortens, and the muscle contracts and generates force. The process is finally reversed upon cessation of calcium release following membrane repolarization and the subsequent reuptake of calcium into the SR through the activity of SR calcium ATPases (SERCAs) [3]. In addition to EC coupling, the triad also participates in the regulation of calcium homeostasis, which, in turn, regulates key cellular process such as oxidative stress, autophagy, and apoptosis.
Fig. 1.
Structure and function of the triad. (A) The triad is a structure formed by the interface between the T-tubule and 2 portions of the sarcoplasmic reticulum (SR). It is normally seen on electron microscopy of longitudinal sections as a triplet of structures (arrows) between myofibrils and slightly offset from the Z-line. In triadopathies such as myotubular myopathy (MTM), the triad may be absent or disorganized. (B) The triad is a critical structure in the process of excitation–contractions coupling whereby electrical impulses (arrows) travel down in the membrane and into the T-tubules. Interaction between the dihydropyridine receptor (DHPR) in the T-tubule and the ryanodine receptor (RYR) in the SR produces the release of calcium from the SR into the sarcoplasm. This calcium then participates in a variety of cellular processes, including, especially, muscle contraction
Recently, defects in triad structure and function have emerged as important underlying causes and/or contributors to a wide range of human muscle diseases [4]. Broadly speaking, alterations in the triad can be grouped into primary “triadopathes”—genetic myopathies where the primary pathogenic mechanism involves an alteration in triad function—and diseases with secondary triad defects. Here we review key features of both the primary triadopathies (Table 1) and disorders in whcih triad dysfunction plays a key secondary role in the disease process (Table 2). The discussion highlights the critical role of the triad in muscle disease, as well as the development of several potential therapeutic targets and interventions based on triad pathology.
Table 1.
Primary triadopathies
| Disorder | Gene | Relationship to triad |
|---|---|---|
| Malignant hyperthermia susceptibility |
RYR1
CACNA1S |
Triad hypersensitivity to triggering agents; excessive calcium release from RyR1 |
| RYR1-related myopathies | RYR1 | Aberrant EC coupling due to reduced RyR1 function |
| Centronuclear myopathies |
MTM1
DNM2 BIN1 RYR1 TTN |
Abnormal triad structure and impaired EC coupling. Exact mechanism(s) unclear. May involve aberrant tubulogenesis and/or abnormal membrane recycling |
| Native American myopathy | STAC3 | Abnormal EC coupling. Malignant hyperthermia susceptibility. STAC3 may serve as a linker between DHPR and RyR1 |
| Tubular aggregate myopathy |
ORAI1
STIM1 |
Abnormal SOCE. Tubular aggregates likely SR-derived |
EC = excitation–contraction; RyR1 = ryanodine receptor; DHPR = dihydropyridine receptor; SOCE = store-operated calcium entry; SR = sarcoplasmic reticulum
Table 2.
Secondary triadopathies
| Disorder | Gene(s) | Relationship to Triad |
|---|---|---|
| Myotonic dystrophy type I | DMPK (CTG repeat expansion in 3’UTR) | Abnormal splicing of key triad gene products (RyR1, DHPR, and BIN1), leading to abnormal EC coupling and altered muscle histopathology |
| Dystrophinopathies | DMD | Hypernitrosylated RyR1 leading to chronic channel “leakiness” |
| Sarcoglycanopathies | SGCA, SGCB, SGCD, and SGCG | Hypernitrosylated RyR1 leading to chronic channel “leakiness” |
| Dysferlinopathies | DYSF | Dysferlin is a T-tubule component. May stabilize T-tubule and/or chaperone triad proteins |
| Calpainopathies | CAPN3 | Partial localization to the triad. Loss of CAPN3 associated with reduced RyR1 expression |
| SEPN1-related myopathies | SEPN1 | Histopathology similar to RYR1-related myopathies. Direct interaction with RyR1 |
| CCDC78-associated myopathy | CCDC78 | Partial localization to the triad. Mutation associated with RyR1 accumulation and abnormal triad structure |
UTR = untranslated region; RyR1 = ryanodine receptor; DHPR = dihydropyridine receptor; BIN1 = bridging integrator 1; EC = excitation–contraction; CAPN3 = calpain-3
Primary Triadopathies
Malignant Hyperthermia Susceptibility
What Is It?
Malignant hyperthermia (MH) is a pharmacogenetic disorder in which susceptible individuals develop a catastrophic hypermetabolic response to a subset of anesthetics [5, 6]. The classic triggering agents are the inhaled volatile gases (halothane, isoflurane, sevoflurane, etc.), either alone or in combination with a depolarizing muscle relaxing agent like succinylcholine [7, 8]. The presenting signs and symptoms occur either during or just after general anesthesia, and include muscle rigidity (particularly masseter muscle rigidity), rapidly increasing body temperature, respiratory and metabolic acidosis, and cardiac rhythm changes. Muscle breakdown is also often detected through observation of elevated creatine kinase levels and/or myoglobinuria. The consequences of MH are dire, and death is the uniform outcome if untreated. Even with treatment, significant complications commonly occur, affecting 25% of MH survivors [9]. These include renal and/or cardiac failure, coma, pulmonary edema, and disseminated intravascular coagulation. The estimated incidence of MH is between 1:3000 and 1:16,000 anesthetics [10].
MH susceptibility (MHS) is the condition wherein a person has an underlying risk of developing MH. Those with previous MH reactions are considered to have MHS for future anesthesias. However, this term is most usefully applied to individuals who have not had an MH reaction but who either 1) carry a mutation in either RYR1 or CACNA1S that is associated with the development of MH (see below); and/or 2) have a positive diagnostic contracture test (caffeine–halothane contracture test in North America or in vitro contracture test in Europe). These contracture tests have high sensitivity (false negative rate approaching 0%) with very good specificity (false positive rate between 10% and 20%). Interestingly, not all individuals who have MHS will develop MH, even after exposure to an established triggering anesthetic.
What Causes It?
MH is a pharmacogenetic disorder. The syndrome involves a proper inciting stimulus (i.e., exposure to inhaled anesthetic) occurring in an individual with a susceptible genetic background. Mutations in two genes encoding triad proteins are well established as causes of MHS. The most common genetic change, occurring in > 70% of individuals with genetically solved MH, is mutation in the skeletal muscle ryanodine receptor (RYR1) [6, 11, 12]. Mutations in RYR1 associated with MH are almost always heterozygous missense changes that alter protein function [13]. The mutations are inherited in an autosomal dominant fashion. The other characterized cause of MH (occurring in 1% of genetically solved cases) are autosomal dominant mutations of the L-type calcium channel of the T-tubule (CACNA1S) [14]. Of note, MHS is genetically heterogeneous, and additional genetic causes are suspected, both from linkage studies that exclude RYR1 and CACNA1S and from direct genetic testing where mutations in the RYR1 and CACNA1S genes are not present in individuals with confirmed MHS [12]. It is currently estimated that approximately 30% of individuals with MHS do not have a known genetic basis. Ongoing studies using next generation sequencing approaches are likely to identify new MH causative genes in the near future.
How Is The Triad Involved?
RYR1 encodes the calcium release channel located in the terminal cisternae of the SR. As mentioned in the “Introduction”, RyR1 is a key component of the EC coupling apparatus, responsible for the regulated release of calcium from the SR. MH-associated mutations in RYR1 destabilize the closed state of the channel and result in calcium release channels that are hypersensitive to activation by triggering agents. In other words, MH mutant channels release an excessive amount of calcium in the setting of inciting stimuli (particularly inhaled volatile anesthetics). In turn, the uncontrolled calcium release triggers massive muscle contraction and hypermetabolism, which leads to severe muscle breakdown and the pathologic features associated with an MH crisis (hyperthermia, cyanosis, hyperkalemia, cardiac rhythm disturbances, kidney damage, etc.). Most mutations in CACNA1S associated with MHS are thought to also enhance RyR1 sensitivity to activation [14, 15]. CACNA1S is the pore-forming subunit of the DHPR, which, under physiologic conditions, functions both as an L-type calcium channel and as a voltage sensor for activation of RyR1 during EC coupling. MH mutations in CACNA1S increase RyR1 sensitivity to activation by triggering agents, which then results in excessive calcium release from RyR1.
How Is The Disorder Treated?
The standard therapy for an MH reaction is discontinuation of the triggering agent, whole body cooling, and rapid administration of dantrolene [6, 9]. If given promptly, dantrolene is very effective in minimizing the consequences of MH. The exact mechanism of action for dantrolene is uncertain. Current theories support the concept that dantrolene reduces RyR1 calcium flux and myoplasmic calcium overload, thus limiting the uncontrolled muscle contractions associated with an MH reaction. However, the precise mechanism(s) by which dantrolene accomplishes this is the subject of continued research.
What Are Key Current Issues Related To The Disorder?
Much current effort related to MHS surrounds attempts to identify additional genetic causes for the disorder. Initial efforts using whole exome sequencing have largely resulted in validation of RYR1 mutations as the primary cause of disease in the majority of cases [11, 16]. These studies have also revealed many RYR1 variants of unknown significance that require further validation in order to be listed as pathogenic. How to establish pathogenicity for such variants is challenging and is an area of active research. Another topic of interest is the overlap between MHS and other conditions associated with either hyperthermia (e.g., heat stroke) or excessive muscle damage (rhabdomyolysis) [17]. There are several individual case reports that suggest that at least a fraction of these conditions are associated with mutations in RYR1. A comprehensive analysis of 39 families with unexplained myalgias and exertional rhabdomyolysis identified causative RYR1 mutations in 14 of the families [18, 19]. These results are suggestive of an important overlap between these conditions and MH, though additional systematic analyses are needed, and the potential clinical value of dantrolene within these settings has also not been adequately studied.
RYR1-related Myopathies
What Are They?
RYR1-related myopathies are a diverse group of conditions with varied clinical and histopathologic presentations that are caused by mutations in RYR1 [20]. While MHS (discussed above) is also caused by mutations in RYR1, the term RYR1-related myopathies generally refers to disorders associated with persistent/chronic muscle dysfunction. These include several subtypes of congenital myopathy: central core disease [21], multiminicore myopathy [22], centronuclear myopathy (CNM) [23], congenital fiber-type disproportion [24], and core-rod myopathy (Fig. 2). Patients with these disorders experience a broad range of clinical symptoms and disease severity. Common features include eye muscle weakness (ophthalmoparesis), extremity muscle weakness, and joint contractures [25]. Patients also frequently exhibit various dynamic muscle symptoms, including MHS, periodic paralysis [26], exertional rhabdomyolysis [19], exercise intolerance, exercise-related myalgias, and fatigue [27]. As with MHS, these dynamic conditions can also exist in the absence of overt muscle weakness. Lastly, there are also particular clinical syndromes that are associated, at least in part, with RYR1 mutations; these include King–Denborough syndrome (KDS) and late-onset axial myopathy [28, 29].
Fig. 2.
Pathological findings associated with triadopathies. (A–D) Representative histopathologic changes in specific ryanodine receptor (RYR1)-related myopathies. Micrographs depict (A) centronuclear myopathy (CNM), (B) multiminicore disease (MMC), and (C, D) central core disease (CCD). (E–I) Histopathologic images from cases of myotubular myopathy caused by myotubularin (MTM1) mutations. Depicted are (E) myofiber hypotrophy, (F) mitochondrial mislocalization, and (G) necklace fibers. (H) Characteristic radial strands, as typically seen in CNM due to DNM2 mutation. (I) Electron micrograph of tubular aggregates from an individual with tubular aggregate myopathy. Stains shown on light microscopy images are either hematoxylin and eosin (red/blue) or nicotinamide adenine dinucleotide (blue). Bar = 40 μm for light microscopy and 2 μm for electron microscopy images
What Causes Them?
The unifying feature of these conditions is that they are all caused by mutations in RYR1. Heterozygous mutations (either autosomal dominantly inherited or de novo) are most commonly associated with central core disease and are seen with a very broad range of clinical symptomatology (de novo mutations typically being more severe than inherited) [21, 25]. Recessive mutations are most frequently encountered in other myopathic subtypes (particularly minicore myopathy and CNM), and are more prevalent in cases with a severe clinical picture [25, 30]. Recessive mutations are also more commonly associated with external ophthalmoparesis, a finding rarely observed with dominant/de novo mutations [25, 30].
How Is The Triad Involved?
In general, most RYR1-related myopathies are thought to result from RYR1 mutations that alter SR calcium release channel function [31]. This can take the form of either reduced RyR1 expression (e.g., with a nonsense or frameshift mutation) or altered channel function (as seen with missense mutations). The ultimate consequence of these changes is impaired EC coupling and diminished muscle force generation, which ultimately leads to muscle weakness. Some mutations are in known functional domains of RyR1 and result in predictable alterations in regulated calcium release from the SR. However, many mutations are not so obviously correlated with clear reduction in calcium release, and their pathogenicity is far less clear. Of note, in many recessive cases, RyR1 expression is markedly reduced and this is thought to be a key mechanism of disease that correlates with disease severity [30].
How Are These Disorders Treated?
At present, there are no rigorously studied therapies for RYR1-related myopathies. Case reports exist for a few candidate therapies and additional pharmacologic strategies have been proposed based on preclinical studies. Salbutamol, a β-adrenergic agonist, was shown to improve both muscle strength and respiratory function in a pilot trial of 13 patients with core myopathy [32]. The specific mechanism of action for the drug in this setting is not known. Thus, additional clinical studies are needed to determine true potential efficacy of this therapy. There is a single case report of improvement in motor function with oral dantrolene in a patient with central core disease and mild myopathy [20]; however, there are also descriptions of dantrolene causing increased muscle weakness after administration. Therefore, the clinical utility of dantrolene outside of MH is unclear. Response to dantrolene may ultimately depend on the specific RYR1 mutation and that mutation’s effect on the dynamics of calcium release; this is clearly an area of much needed additional research, likely at the preclinical level.
In terms of new drug development, perhaps the most promising “lead” at present is N-acetylcysteine (NAC). In a collaborative study [33], we (Dowling) uncovered increased cellular stress and aberrant oxidative stress in a zebrafish model of RYR1-related myopathy and in myotubes from patients with RYR1 mutations. Exposure of the zebrafish mutants to NAC improved muscle structure and motor endurance. Importantly, treatment of patient myotubes with NAC decreased pro-oxidant-mediated cell death. Consideration of a clinical trial to test the efficacy of NAC in RYR1-related myopathy patients is underway. However, as oxidative stress is likely a “secondary” consequence of impaired calcium handling due to RYR1 mutation, therapy with NAC does not address the primary problem of altered EC coupling in these disorders. Therefore, other strategies are clearly needed. One such pharmacologic strategy would be chemical modulators that improve the ability of RyR1 to release calcium. An example of a potential therapeutic is the Rycal class of drugs, which stabilize RyRs to “improve” their ability to release calcium [34]. Rycals are under consideration for the treatment for heart failure, but have yet to be tested in preclinical models of RYR1-related myopathies.
Another set of therapies under consideration are gene editing strategies. RYR1 is too large for conventional gene therapy approaches, so other techniques must be utilized. In principal, de novo/dominant RYR1 mutations are candidates for targeting by exon skipping, allele-specific gene silencing or trans-splicing. Such approaches, if used to produce a loss of function allele in the setting of a heterozygous mutation, could be of potential benefit because haploinsufficiency for RYR1 does not cause overt clinical symptoms. Of course, the challenge would be targeting only the mutated allele. One example of a gene-based approach was provided by our group (Dirksen) using allele-specific silencing in 2 mouse models of central core disease [35]. We developed small interfering RNA specific to the heterozygous mutation in the mouse (Y524S in one model and I4898T in another) to preferentially knock down the mutant allele and restore EC coupling in adult mouse muscle.
What Are Key Current Issues Related To The Disease?
With the application of next generation sequencing technology to clinical neuromuscular diagnostics, the identification of patients with RYR1 mutations has expanded exponentially. It is now clear that RYR1-related myopathies are the most common nondystrophic muscle conditions of childhood and that the clinical spectrum for the disorders is vast [36, 37]. One important issue to emerge is how to interpret identified sequence variants in RYR1 that are of uncertain pathogenicity [11, 16]. AS RYR1 mutations are associated with such a broad range of disease, most novel variants identified in RYR1 have the potential of causing disease. Thus, the challenge for the clinical and research communities is to develop tools and algorithms that will aid and assist in the clinical interpretation of newly identified RYR1 variants.
Another interesting issue is the clinical and histopathologic variability associated with RYR1 mutations, both among unrelated individuals and within families with the same mutation [25]. It is not clear why different histopathologic phenotypes (e.g., core myopathy and CNM) emerge and whether there is any special significance (either at the pathogenic level or in terms of clinical presentation and outcome) with regard to the histological subtype of myopathy a given patient manifests. Also, the factors that account for the variable expressivity of clinical disease among individuals with the same mutation—a variability that can be relatively dramatic in some instances—have not been explored in depth, but may represent both external factors and gene-based modifiers. One explanation for interfamilial clinical variability, at least in some cases, is the presence of additional (second-site) RYR1 mutations segregating within the family, as has been described by Klein et al. [25].
A final important consideration is the relative lack of therapeutic candidates for RYR1-related myopathies. Other than NAC, no treatments are currently being considered for clinical testing. One barrier to treatment development is the relatively small number of preclinical models, and the difficulty presented by the phenotypes of those models. The Ryr1 knockout mouse (“dyspedic”) dies at birth, and thus is not suitable for most in vivo studies [38, 39]. The available RyR1 “knockin” mouse models, in which identified disease mutations are engineered into the mouse genome, model these conditions well and have provided a wealth of information about MHS, core myopathy, and RyR1 pathophysiology [40]. However, because of cost and low inherent throughput in working with mice, these models have not been ideal for drug discovery. In addition, no mouse model exists that recapitulates a non-MH/CCD-related RYR1 myopathy. Recently, the zebrafish model has been utilized as an excellent preclinical vertebrate model for therapy identification [39]. This is well illustrated by the fact that NAC was identified as a potential therapeutic intervention using this system [33]. Additional studies using the existing zebrafish model (a recessive loss of expression mutant in ryr1b that most closely models RYR1-related CNM) will hopefully yield additional candidates. There is also a need to develop additional zebrafish mutants designed to reflect the wider range of clinical and pathologic presentations of RYR1-related myopathies.
CNMs
What Are They?
CNMs are a subset of the broader category of muscle diseases called congenital myopathies [4]. CNMs are unified by common histopathologic features on muscle biopsy, most notably the presence of large, centrally located nuclei in at least 10% (and often in excess of 25%) of muscle fibers [41]. CNMs can be further subdivided into groups based on genetic cause [42]. The most common genetic cause is mutations in myotubularin (encoded by MTM1). Patients with MTM1 mutations are said to have myotubular myopathy [43]. MTM1 is an X-linked gene and mutations primarily affect males. In its classic form, myotubular myopathy is an extremely severe disorder characterized by weakness and respiratory failure that presents at birth [44]. A significant percentage of individuals with myotubular myopathy do not survive the first year of life, and those that do exhibit lifelong disabilities, including dependence on ventilators, wheelchairs, and gastrostomy tubes.
RYR1 mutations are likely the second most common cause of CNM [22, 30]. The clinical presentation is varied, though individuals usually present with significant symptoms in childhood. Some individuals with RYR1-related CNM present with severe symptoms that phenocopy those seen with MTM1 mutations. The next most common cause of CNM is mutations in DNM2, which present as either de novo or dominant mutations [45, 46]. There is a mild adult onset form of DNM2-related CNM, usually caused by mutations within the middle domain of DNM2. There is also a severe childhood form that is often associated with mutations in the DNM2 PH domain. Two rare causes of CNM are mutations in BIN1 (bridging integrator 1, also called amphiphysin 2) and Titin (TTN), both of which exhibit an autosomal recessive form of inheritance [47, 48]. Patients with TTN mutations also may present with cardiomyopathy. In addition, approximately 25% of cases of CNM are genetically unsolved [49].
What Causes It?
As mentioned above, there are 5 known genetic causes of CNM. Mutations in MTM1, RYR1, and BIN1 are all recessively inherited and thought to result from a loss of gene function. TTN mutations are also recessive, though the consequences of mutations in the TTN gene product remain unclear. At least a part of the TTN protein is not translated in some patients with TTN-related CNM [48]. The pathomechanism of DNM2 mutations has been somewhat perplexing [50]. All DNM2 mutations are either dominant or de novo missense mutations, suggesting a potentially toxic gain-of-function effect produced by the mutations. This hypothesis is supported by the observation that nonsense mutations in DNM2 that result in haploinsufficiency do not cause disease [51]. However, several studies have shown that loss of basic DNM2 functions (such as the regulation of endocytosis) occurs with some mutations, suggesting that loss of function may also contribute to the disease mechanism.
How Is The Triad Involved?
The identification of triad abnormalities as a key driver of CNM pathogenesis is a relatively recent discovery. Our group (Dowling) identified structural changes in the triad and altered EC coupling in a zebrafish model of myotubular myopathy [52]. In the same study, we observed similar structural alterations in the triad in biopsies from patients with MTM1 mutations. These findings were soon confirmed by the description of similar changes in the mouse model of myotubular myopathy [53]. Investigation of muscle biopsies from CNM patients with BIN1 and DNM2 mutations [54–57], as well as supporting data from animal models of these diseases, revealed that triad changes also represent an important aspect of these CNMs. Lastly, the identification of mutations in RYR1 as a cause of CNM cemented the link between triad dysfunction and this congenital muscle disease.
The role of RyR1 dysfunction in muscle disease is discussed in the previous section; the reason why certain RYR1 mutations cause the distinctive histopathologic changes associated with CNM is not clear. BIN1 was identified as a modulator of T-tubule biogenesis well before mutations in this gene were linked to CNM [58]. BIN1 is a BAR (Bin1-Amphiphysin-RVS167) domain-containing protein that regulates and modifies membrane curvature; loss of BIN1 function is hypothesized to alter T-tubule formation and maintenance. DNM2 has multiple cellular functions, many of which relate to its ability to regulate membrane trafficking. Moreover, DNM2 interacts directly with BIN1. Thus, a leading hypothesis for how DNM2 mutations affect the triad is that mutant DNM2 proteins alter/prevent normal BIN1 function(s). The reason why mutations in MTM1 cause defects in triad structure is not clear. MTM1 is a lipid phosphatase that regulates the levels of the phosphoinositides PI3P and PI3,5P2. While MTM1 is localized to the triad, evidence linking alterations in levels of PI3P and/or PI3,5P2 at the triad is less robust. Thus, the mechanism underlying the association between loss of MTM1 function and defective triad structure is still uncertain. Lastly, other than the histopathology, there is no known link to date between TTN and the triad. Given the enormous size of TTN and its numerous protein–protein interactions, one possibility is that CNM-related mutations in TTN interfere with or alter other proteins that more directly regulate the triad. One intriguing possibility is calpain-3, the expression of which is diminished in the muscle biopsies of patients with TTN-related CNM [48]. A potential association between calpain-3 and the triad is discussed in the “Secondary Triadopathies” section.
It is important to note that pathways and structures other than the triad are likely also affected in CNMs. Other than RYR1, all of the known CNM-associated proteins have well-documented functions in processes unrelated to the triad. For example, MTM1 has been implicated as a regulator of autophagy and of the desmin intermediate filament network [59–61]. In addition, loss of MTM1 in both zebrafish and mouse models is associated with alterations of the NMJ (see below) [62, 63].
How Is The Disorder Treated?
There have been several exciting therapeutic advances discovered using preclinical models of CNM. By far the most promising is related to myotubular myopathy. An international collaborative group demonstrated that gene therapy with MTM1 not only prevents, but also reverses, the phenotype of Mtm1 knockout mice [64]. This same group also demonstrated the efficacy of local adenoassociated virus–Mtm1 infusion as a treatment for dogs with Mtm1 mutations. Another parallel advance has been the use of enzyme replacement therapy with MTM1 protein, where a pilot study using locally administered MTM1 improved the disease phenotype in Mtm1 knockout mice [65]. Both MTM1 gene and protein therapies are poised for testing in patients via clinical trials.
Enhancing signaling at the NMJ is another therapeutic strategy being developed for both myotubular myopathy and DNM2-related CNM. In zebrafish models of both conditions, treatment with edrophonium (an acetylcholinesterase inhibitor) significantly improved motor function [63, 66]. Similarly, in the mouse model of myotubular myopathy, treatment with pyridostigmine (another acetylcholinesterase inhibitor) significantly improved exercise tolerance [62]. At present, there are also several case reports of individuals with MTM1, DNM2, and, very recently, RYR1 mutations exhibiting positive responses to pyridostigmine, though this has not been systematically studied [27, 63, 66]. Given that there is evidence for structural abnormalities of the NMJ in models of at least some of the CNMs [62, 66], it is likely that pyridostigmine works by addressing this aspect of disease pathology, and not through direct effects on the triad.
What Are Current Issues Related To The Disease?
One of the major unanswered questions regarding CNM is the primary cause(s) for the signature pathogenic features (i.e., central nuclei) of the disorder. Because the gene products of several CNM genes, particularly MTM1 and DNM2, regulate multiple cellular processes, it was initially assumed that the central nuclei resulted from a disrupted process independent of triad pathology. However, the fact that RYR1 mutations, the gene product of which exclusively functions within the triad, also cause the disorder suggests that triad disturbance may be sufficient to trigger central nucleation. Interestingly, work in the Mtm1 knockout mice has shown that triad changes precede the presence of central nuclei [53], lending additional evidence to the hypothesis that disruption of normal triad structure/function leads to mislocalization of nuclei. Additional studies are needed to elucidate the specific molecular changes and sequence of events that underlie this phenomenon.
Native American Myopathy
What Is It?
Native American myopathy (NAM) is a rare inherited muscle disease that, at present, has only been reported in the Lumbee Native Americans of North Carolina [67]. The cardinal clinical features of the condition are mild muscle weakness, ptosis, myopathic facies, short stature, kyphoscoliosis, and a distinctive gait. Susceptibility to MH is also associated with this myopathy.
What Causes It?
NAM is caused by a recessive missense mutation (W284S) in STAC3(68). STAC3 (SH3 and cysteine rich domain 3) encodes an approximately 50-kDa protein with SH3 and cysteine-rich domains. The precise mechanisms by which mutations in STAC3 result in myopathy are currently unclear.
How Is The Triad Involved?
STAC3 localizes to the triad where it interacts with both the DHPR and RyR1. Loss of Stac3 in either zebrafish or mouse disrupts EC coupling [68, 69]. A current hypothesis is that STAC3 serves as either an essential linker or as a chaperone for the mechanical communication between the DHPR and RyR1.
How Is It Treated?
There are currently no treatments for NAM, nor any putative therapeutic strategies. Individuals with NAM who experience an MH reaction are treated with dantrolene.
What Are Some Current Issues Related To The Disease?
It will be of interest to see whether STAC3 mutations are confined to the Lumbee population or are more widely observed. It is possible that the clinical phenotype of individuals outside the Lumbee population will be different. One potential allelic phenotype is KDS, a condition that shares many similarities to NAM including weakness, an unusual gait, and MH susceptibility. To date, only RYR1 mutations have been found as a cause KDS, though they do not account for all cases [28].
Another question related to STAC3 is why individuals with NAM are susceptible to MH. Loss of STAC3 causes an impairment in EC coupling and a reduction in regulated SR calcium release [68, 69]. STAC3 complementary DNA containing the NAM mutation does not fully rescue the stac3 knockout phenotype in zebrafish [68], suggesting that, at least in part, it may represent a hypomorphic allele. It is therefore perplexing how an apparent reduction in EC coupling caused by STAC3 mutation is associated with MH, a phenotype usually associated with hyper-responsive calcium release.
Tubular Aggregate Myopathy
What Is It?
Tubular aggregate myopathy is a mild, slowly progressive muscle condition that often begins in early childhood [70]. It features prominent myalgias and muscle cramping. It is also a component of a broader syndrome called Stormorken syndrome, which, in addition to tubular aggregates, also features hematopoietic abnormalities [71]. The condition is diagnosed by muscle biopsy where aggregates of hexagonal tubules are seen by electron microscopy. Of note, tubular aggregates can also be seen as a secondary pathologic change in other muscle diseases.
What Causes It?
Both tubular aggregate myopathy and Stormorken syndrome are caused by mutations in either STIM1 or ORAI1 [71–73]. The STIM1 and ORAI1 mutations described thus far are dominant and result in constitutive activation of the respective gene products. Of note, recessive loss of function expression mutations in both genes are associated with immunodeficiency syndromes [74].
What Is The Relationship To The Triad?
ORAI1 is the pore-forming component of the store-operated calcium release-activated calcium channel, while STIM1 is the calcium-sensing component of the channel [75]. ORA1I and STIM1 interact with each other upon store depletion, and both proteins are involved with the process of store-operated calcium entry (SOCE) [76, 77]. SOCE is a critical aspect of the regulation of calcium within the muscle fiber and it is intimately linked with EC coupling. In fact, enhanced SOCE activation has been shown to be an important part of the pathogenesis of MH [78, 79]. STIM1 and ORAI1 are found at the triad, but are distinct from the DHPR/RyR1 interface [75]. The tubular aggregates that are the pathognomonic histopathologic abnormality of the disorder are likely composed of SR-derived membranes [80].
How Is It Treated?
There are currently no treatments for the myopathic symptoms associated with myopathies due to either ORAI1 or STIM1 mutations.
What Are Some Current Issues Related To The Disease?
Tubular aggregates are the defining microscopic observation in patients with tubular aggregate myopathy. However, tubular aggregates are seen in a broader range of myopathic states. For example, experimental evidence has linked accumulation of tubular aggregates with muscle aging [81]. Therefore, it is possible that tubular aggregate myopathy may represent a premature aging or senescence syndrome. Future studies are needed to address this hypothesis. Another critical question requiring additional experimentation is the mechanism by which abnormalities in SOCE lead to the formation of tubular aggregates.
Secondary Triadopathies
Abnormalities at the triad, usually in its function and not its structure, have also been described as secondary pathomechanisms in several muscle diseases. This section will briefly review these associations and discuss the potential implications for disease treatment.
Myotonic Dystrophy Type I
Myotonic dystrophy type I (DM1) is a multisystem disorder that is caused by a CTG repeat expansion in the 3’ untranslated region of DMPK [82]. DM1 primarily results from impaired alternative splicing of selected mRNA transcripts in tissues in which dystrophia myotonica-protein kinase is expressed. The repeat expansion disrupts splicing by sequestering splicing regulatory factors such as muscleblind and other CUG binding proteins (e.g., CUGBP1) [83]. Splicing of several genes associated with triad function are disrupted in DM1. In particular, splice changes have been described for RyR1, DHPR subunits, and BIN1. The splice changes in RyR1 affect a domain involved with channel inhibition and result in altered/impaired EC coupling [84, 85]. Altered splicing of the DHPR leads to changes in L-type Ca2+ channel conductance [86]. Missplicing of BIN1 results in enrichment of an inactive form of the gene product, which, in turn, disrupts T-tubule formation and maintenance, as determined by targeted alteration of the spliced exon in the mouse [87]. The BIN1 splicing alteration is proposed to be responsible for the central nucleation pattern commonly observed in muscle from patients with DM1 and to contribute to the muscle weakness observed in this disease.
Muscular Dystrophies
Dystrophinopathies
Mutations in dystrophin (DMD) cause the allelic conditions Duchenne muscular dystrophy and Becker muscular dystrophy [88]. Dystrophin encodes a massive protein that links the sarcolemmal membrane to the actin cytoskeleton. It also interacts with numerous regulatory proteins. A primary function of dystrophin is to stabilize the muscle membrane during muscle contraction. Loss of dystrophin results in increased membrane fragility, which leads to subsequent damage to the myofiber due to stress-induced membrane microtears. There is also considerable data to suggest that loss of dystrophin alters various signaling pathways (e.g., nitric oxide synthase) [89].
Interestingly, there is evidence that, in addition to its typical plasma membrane localization, dystrophin is also found at the T-tubule [90]. While its role at the T-tubule and in the triad is not well understood, there is evidence of abnormal EC coupling in the most common preclinical model of dystrophinopathy, the mdx mouse (which harbors a nonsense mutation in Dmd) [91]. There are also data showing that this EC coupling abnormality, at least in part, is due to RyR1 dysfunction. Specifically, Bellinger et al. [92] have shown that RyR1 is hypernitrosylated in the mdx mouse, and that RyR1 hypernitrosylation leads to a “leaky” RyR1 channel. Chronic RyR1 “leakiness” is associated with reduced calcium release during EC coupling and contributes to intracellular calcium-mediated toxicity (including enhanced oxidative stress).
Two therapies related to altered RyR1 function and abnormal calcium homeostasis have been tested in the mdx mouse model. One is Rycal (mentioned earlier), which is an RyR1 sensitizer that improves stimulus-dependent RyR1 calcium release. Rycals are currently under consideration for the treatment of cardiomyopathy associated with dystrophinopathy, which is thought to be due, in part, to defective RyR2 signaling in the heart [93]. The second therapeutic approach involves the overexpression of SERCA1. SERCA1, a protein primarily localized in the longitudinal SR, is responsible for the reuptake of calcium into the SR and thus the rate of muscle relaxation. Goonasekera et al. [94] recently demonstrated that transgenic upregulation of SERCA1 in mdx mice reduced development of the dystrophic muscle pathology in this model.
Sarcoglycanopathies
Sarcoglycanopathies are muscular dystrophies caused by gene mutations in any one of 4 sarcoglycan genes [95]. The sarcoglycans form a complex at the sarcolemmal membrane that is part of the dystrophin-associated membrane complex. Recessive mutations in sarcoglycan genes cause a phenotype very similar to that seen in the dystrophinopathies. Similar to dystropin mutations, there is also evidence of impaired EC coupling and altered RyR1 nitrosylation in a mouse model of sarcoglycan deficiency [96]. In addition, in the same study that examined mdx mice, Goonasekera et al. [94] showed that transgenic SERCA upregulation improves the dystrophic phenotype in sarcoglycan-deficient animals, again via a mechanism similar to that proposed for dystrophinopathy.
Dysferlinopathies
Dysferlin is a membrane-associated protein primarily implicated in the regulation of membrane repair. Loss of dysferlin function is associated with autosomal recessive limb girdle muscular dystrophy type 2B (LGMD2B) [97]. Dysferlin is also a component of the T-tubule. Its precise function at the T-tubule is not entirely clear, though dysferlin has been shown to stabilize stress-induced calcium signaling in the T-tubule membrane and serves as a chaperone for T-tubule proteins in the triad such as the DHPR [98, 99].
Calpainopathies
Calpain-3 (CAPN3) is a nonlysosomal cysteine protease implicated in the cleavage and/or breakdown of multiple key skeletal muscle proteins. Mutations in CAPN3 cause autosomal recessive limb girdle muscular dystrophy 2A (LGMD2A), a slowly and variably progressive disorder associated with proximal muscle weakness [100]. Kramerova et al. [101] identified CAPN3 localization at the triad (amongst other places). They showed that Capn3 knockout in mice was associated with decreased expression of RyR1 in skeletal muscle, as well as with impaired SR calcium release [101]. Based on these results, the authors proposed that impaired triad function was an important aspect of the disease process in patients with LGMD2A. Further work is clearly necessary to strengthen the association and causal links between CAPN3, triad structure/function, and LGMD2A disease pathogenesis associated with CAPN3 mutations. However, the fact that the precise relationship between CAPN3 function and LGMD2A is poorly understood despite much research makes this avenue of exploration worthwhile.
SEPN1-related Myopathies
SEPN1 encodes selenoprotein-N, an endoplasmic reticulum-resident protein that is part of the selenoprotein family. Recessive mutations of SEPN1 are associated with multiminicore myopathy, rigid spine muscular dystrophy, congenital fiber-type disproportion, and Mallory Body myopathy [102]. Loss of SEPN1 function is associated with increased oxidative stress in muscle [103], accelerated muscle pathology in the setting of physiologic stressors [104], and with abnormalities in muscle stem cells [105].
SEPN1 function, and the diseases that result from SEPN1 mutations, likely include significant triad involvement. The most compelling evidence for this is the substantial clinical and histopathologic overlap between SEPN1- and RYR1-related myopathies [106]. In particular, both are associated with prominent axial weakness and are the primary causes of core myopathy. In addition, a direct interaction between RyR1 and SEPN1 has been demonstrated [107]. The study that documented this interaction also provided evidence that loss of Sepn1 in zebrafish is associated with impaired calcium handling. Moreover, as with RYR1-related myopathies, there is abundant evidence that SEPN1 myopathies are associated with enhanced oxidative stress. Furthermore, NAC improves survival in myocytes from patients with SEPN1 mutations, and thus represents a potential therapeutic intervention for this disease [108]. On the contrary, and despite this evidence, SEPN1 expression is largely confined to muscle development and the gene product is not abundantly found in adult mouse muscle [109], making a primary role in triad biology less likely. Additional studies are clearly needed to better delineate a potential link between SEPN1 and RyR1 at the triad.
CCDC78-associated Myopathy
Our group (Dowling) recently identified a dominant splice site mutation in CCDC78 in a family with an unusual congenital myopathy associated with moderate muscle weakness, exercise intolerance, and a combination of cores, protein aggregates, and central nuclei on muscle biopsy [110]. Using a morpholino-based approach, we showed that zebrafish that model the splice site alteration seen in patients exhibit abnormal triad structure and accumulate RyR1 and DHPR aggregates throughout their myofibers. Similar aggregates were also observed in patient muscle biopsies. Given that CCDC78 localizes, at least in part, to the triad, it is reasonable to speculate that 1) CCDC78 plays a role in triad structure/function and 2) CCDC78 mutations act, at least in part, by disrupting EC coupling. Little additional information is currently available regarding the role of CCDC78 in muscle. A study of CCDC78 in Xenopus identified it as a required component of the deuterosome [111]. How and to what degree this may relate to its function in muscle remains to be understood.
Summary
The triad is a key muscle substructure responsible for controlling muscle contraction. Abnormalities in the triad are an emerging primary cause of an eclectic array of distinct skeletal myopathies called triadopathies. The overall incidence of triadopathies, as well as the breadth of gene mutations associated with the triad, is likely to increase as whole exome and whole genome sequencing approaches are applied to genetically unsolved myopathies. Therapy directly targeted at improving triad structure and function, and particularly at normalizing RyR1 activity, represents a promising treatment strategy for both the primary and secondary triadopathies. New drug discovery is clearly needed, as are new preclinical models to advance mechanistic understanding of disease pathogenesis and to improve therapy identification/development. In this review, we detailed issues related to altered triad stability and function in the triadopathies, highlighting studies that lay the groundwork for future mechanistic and translational research into this interesting and clinically relevant field.
Electronic supplementary material
(PDF 1199 kb)
Acknowledgments
This work was supported by grants from CureCMD (to J.J.D.), muscular dystrophy association (MDA186999 to J.J.D.), and National Institutes of Health (AR053349 to R.T.D.; 1R03AR062810 and 1K08AR054835 to J.J.D.; and 1K08AR059750 to M.W.L.). We thank Carsten Bonnemann and Maria Rita Santi for contributing biopsy photomicrographs and Elizabeth Gibbs for assistance with Figure 1.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Engel A, Franzini-Armstrong C. Myology: basic and clinical. 3. New York: McGraw-Hill; 2004. [Google Scholar]
- 2.Melzer W, Herrmann-Frank A, Luttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim Biophys Acta. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- 3.Rossi AE, Dirksen RT. Sarcoplasmic reticulum: the dynamic calcium governor of muscle. Muscle Nerve. 2006;33:715–731. doi: 10.1002/mus.20512. [DOI] [PubMed] [Google Scholar]
- 4.Nance JR, Dowling JJ, Gibbs EM, Bonnemann CG. Congenital myopathies: an update. Curr Neurol Neurosci Rep. 2012;12:165–174. doi: 10.1007/s11910-012-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg H, Davis M, James D, Pollock N, Stowell K. Malignant hyperthermia. Orphanet J Rare Dis. 2007;2:21. doi: 10.1186/1750-1172-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg H, Sambuughin N, Riazi S, Dirksen R. Malignant hyperthermia susceptibility. In: Pagon RA, Adam MP, Bird TD, et al. (eds) GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2014; 2003 Dec 19 [updated 2013 Jan 31]. [PubMed]
- 7.Denborough M. Malignant hyperthermia. Lancet. 1998;352:1131–1136. doi: 10.1016/S0140-6736(98)03078-5. [DOI] [PubMed] [Google Scholar]
- 8.McCarthy EJ. Malignant hyperthermia: pathophysiology, clinical presentation, and treatment. AACN Clin Issues. 2004;15:231–237. doi: 10.1097/00044067-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Larach MG, Gronert GA, Allen GC, Brandom BW, Lehman EB. Clinical presentation, treatment, and complications of malignant hyperthermia in North America from 1987 to 2006. Anesth Analg. 2010;110:498–507. doi: 10.1213/ANE.0b013e3181c6b9b2. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly AJ. Malignant hyperthermia. Epidemiology, pathophysiology, treatment. AORN J. 1994;59:393–395. doi: 10.1016/s0001-2092(07)70404-0. [DOI] [PubMed] [Google Scholar]
- 11.Gonsalves SG, Ng D, Johnston JJ, et al. Using exome data to identify malignant hyperthermia susceptibility mutations. Anesthesiology. 2013;119:1043–1053. doi: 10.1097/ALN.0b013e3182a8a8e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stowell KM. DNA testing for malignant hyperthermia: the reality and the dream. Anesth Analg. 2014;118:397–406. doi: 10.1213/ANE.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 13.Robinson R, Carpenter D, Shaw MA, Halsall J, Hopkins P. Mutations in RYR1 in malignant hyperthermia and central core disease. Hum Mutat. 2006;27:977–989. doi: 10.1002/humu.20356. [DOI] [PubMed] [Google Scholar]
- 14.Carpenter D, Ringrose C, Leo V, et al. The role of CACNA1S in predisposition to malignant hyperthermia. BMC Med Genet. 2009;10:104. doi: 10.1186/1471-2350-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yarotskyy V, Dirksen RT. Cav1.1 in malignant hyperthermia. In: Weiss N, Koschak A, editors. Pathologies of calcium channels. Berlin: Springer-Verlag; 2014. [Google Scholar]
- 16.Kim JH, Jarvik GP, Browning BL, et al. Exome sequencing reveals novel rare variants in the ryanodine receptor and calcium channel genes in malignant hyperthermia families. Anesthesiology. 2013;119:1054–1065. doi: 10.1097/ALN.0b013e3182a8a998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capacchione JF, Muldoon SM. The relationship between exertional heat illness, exertional rhabdomyolysis, and malignant hyperthermia. Anesth Analg. 2009;109:1065–1069. doi: 10.1213/ane.0b013e3181a9d8d9. [DOI] [PubMed] [Google Scholar]
- 18.Tobin JR, Jason DR, Challa VR, Nelson TE, Sambuughin N. Malignant hyperthermia and apparent heat stroke. JAMA. 2001;286:168–169. doi: 10.1001/jama.286.2.168. [DOI] [PubMed] [Google Scholar]
- 19.Dlamini N, Voermans NC, Lillis S, et al. Mutations in RYR1 are a common cause of exertional myalgia and rhabdomyolysis. Neuromuscul Disord. 2013;23:540–548. doi: 10.1016/j.nmd.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Jungbluth H, Dowling JJ, Ferreiro A, Muntoni F. 182nd ENMC International Workshop: RYR1-related myopathies, 15–17th April 2011, Naarden, The Netherlands. Neuromuscul Disord. 2012;22:453–462. doi: 10.1016/j.nmd.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Jungbluth H. Central core disease. Orphanet J Rare Dis. 2007;2:25. doi: 10.1186/1750-1172-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jungbluth H. Multi-minicore disease. Orphanet J Rare Dis. 2007;2:31. doi: 10.1186/1750-1172-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilmshurst JM, Lillis S, Zhou H, et al. RYR1 mutations are a common cause of congenital myopathies with central nuclei. Ann Neurol. 2010;68:717–726. doi: 10.1002/ana.22119. [DOI] [PubMed] [Google Scholar]
- 24.Clarke NF, Waddell LB, Cooper ST, et al. Recessive mutations in RYR1 are a common cause of congenital fiber type disproportion. Hum Mutat. 2010;31:E1544–E1550. doi: 10.1002/humu.21278. [DOI] [PubMed] [Google Scholar]
- 25.Klein A, Lillis S, Munteanu I, et al. Clinical and genetic findings in a large cohort of patients with ryanodine receptor 1 gene-associated myopathies. Hum Mutat. 2012;33:981–988. doi: 10.1002/humu.22056. [DOI] [PubMed] [Google Scholar]
- 26.Zhou H, Lillis S, Loy RE, et al. Multi-minicore disease and atypical periodic paralysis associated with novel mutations in the skeletal muscle ryanodine receptor (RYR1) gene. Neuromuscul Disord. 2010;20:166–173. doi: 10.1016/j.nmd.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Illingworth MA, Main M, Pitt M, et al. RYR1-related congenital myopathy with fatigable weakness, responding to pyridostigimine. Neuromuscul Disord. 2014;24:707–712. doi: 10.1016/j.nmd.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Dowling JJ, Lillis S, Amburgey K, et al. King-Denborough syndrome with and without mutations in the skeletal muscle ryanodine receptor (RYR1) gene. Neuromuscul Disord. 2011;21:420–427. doi: 10.1016/j.nmd.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Jungbluth H, Lillis S, Zhou H, et al. Late-onset axial myopathy with cores due to a novel heterozygous dominant mutation in the skeletal muscle ryanodine receptor (RYR1) gene. Neuromuscul Disord. 2009;19:344–347. doi: 10.1016/j.nmd.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Amburgey K, Bailey A, Hwang JH, et al. Genotype-phenotype correlations in recessive RYR1-related myopathies. Orphanet J Rare Dis. 2013;8:117. doi: 10.1186/1750-1172-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Treves S, Jungbluth H, Muntoni F, Zorzato F. Congenital muscle disorders with cores: the ryanodine receptor calcium channel paradigm. Curr Opin Pharmacol. 2008;8:319–326. doi: 10.1016/j.coph.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Messina S, Hartley L, Main M, et al. Pilot trial of salbutamol in central core and multi-minicore diseases. Neuropediatrics. 2004;35:262–266. doi: 10.1055/s-2004-821173. [DOI] [PubMed] [Google Scholar]
- 33.Dowling JJ, Arbogast S, Hur J, et al. Oxidative stress and successful antioxidant treatment in models of RYR1-related myopathy. Brain. 2012;135:1115–1127. doi: 10.1093/brain/aws036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersson DC, Marks AR. Fixing ryanodine receptor Ca leak – a novel therapeutic strategy for contractile failure in heart and skeletal muscle. Drug Discov Today Dis Mechan. 2010;7:e151–e157. doi: 10.1016/j.ddmec.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loy RE, Lueck JD, Mostajo-Radji MA, Carrell EM, Dirksen RT. Allele-specific gene silencing in two mouse models of autosomal dominant skeletal myopathy. PloS One. 2012;7:e49757. doi: 10.1371/journal.pone.0049757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amburgey K, McNamara N, Bennett LR, McCormick ME, Acsadi G, Dowling JJ. Prevalence of congenital myopathies in a representative pediatric united states population. Ann Neurol. 2011;70:662–665. doi: 10.1002/ana.22510. [DOI] [PubMed] [Google Scholar]
- 37.Maggi L, Scoto M, Cirak S, et al. Congenital myopathies–clinical features and frequency of individual subtypes diagnosed over a 5-year period in the United Kingdom. Neuromuscul Disord. 2013;23:195–205. doi: 10.1016/j.nmd.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Takekura H, Nishi M, Noda T, Takeshima H, Franzini-Armstrong C. Abnormal junctions between surface membrane and sarcoplasmic reticulum in skeletal muscle with a mutation targeted to the ryanodine receptor. Proc Natl Acad Sci U S A. 1995;92:3381–3385. doi: 10.1073/pnas.92.8.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirata H, Watanabe T, Hatakeyama J, et al. Zebrafish relatively relaxed mutants have a ryanodine receptor defect, show slow swimming and provide a model of multi-minicore disease. Development. 2007;134:2771–2781. doi: 10.1242/dev.004531. [DOI] [PubMed] [Google Scholar]
- 40.Kushnir A, Betzenhauser MJ, Marks AR. Ryanodine receptor studies using genetically engineered mice. FEBS Lett. 2010;584:1956–1965. doi: 10.1016/j.febslet.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jungbluth H, Wallgren-Pettersson C, Laporte J. Centronuclear (myotubular) myopathy. Orphanet J Rare Dis. 2008;3:26. doi: 10.1186/1750-1172-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biancalana V, Beggs AH, Das S, et al. Clinical utility gene card for: Centronuclear and myotubular myopathies. Eur J Hum Genet 2012;20. [DOI] [PMC free article] [PubMed]
- 43.Laporte J, Hu LJ, Kretz C, et al. A gene mutated in X-linked myotubular myopathy defines a new putative tyrosine phosphatase family conserved in yeast. Nat Genet. 1996;13:175–182. doi: 10.1038/ng0696-175. [DOI] [PubMed] [Google Scholar]
- 44.Das S, Dowling J, Pierson CR. X-linked centronuclear myopathy. In: Pagon RA, Adam MP, Ardinger HH, et al. (eds) GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2014. 2002 [updated 2011 Oct 6].
- 45.Bitoun M, Maugenre S, Jeannet PY, et al. Mutations in dynamin 2 cause dominant centronuclear myopathy. Nat Genet. 2005;37:1207–1209. doi: 10.1038/ng1657. [DOI] [PubMed] [Google Scholar]
- 46.Bohm J, Biancalana V, Dechene ET, et al. Mutation spectrum in the large GTPase dynamin 2, and genotype-phenotype correlation in autosomal dominant centronuclear myopathy. Hum Mutat. 2012;33:949–959. doi: 10.1002/humu.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicot AS, Toussaint A, Tosch V, et al. Mutations in amphiphysin 2 (BIN1) disrupt interaction with dynamin 2 and cause autosomal recessive centronuclear myopathy. Nat Genet. 2007;39:1134–1139. doi: 10.1038/ng2086. [DOI] [PubMed] [Google Scholar]
- 48.Ceyhan-Birsoy O, Agrawal PB, Hidalgo C, et al. Recessive truncating titin gene, TTN, mutations presenting as centronuclear myopathy. Neurology. 2013;81:1205–1214. doi: 10.1212/WNL.0b013e3182a6ca62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dowling JJ. Titin and centronuclear myopathy: The tip of the iceberg for TTN-ic mutations? Neurology. 2013;81:1189–1190. doi: 10.1212/WNL.0b013e3182a6cc43. [DOI] [PubMed] [Google Scholar]
- 50.Durieux AC, Prudhon B, Guicheney P, Bitoun M. Dynamin 2 and human diseases. J Mol Med. 2010;88:339–350. doi: 10.1007/s00109-009-0587-4. [DOI] [PubMed] [Google Scholar]
- 51.Koutsopoulos OS, Kretz C, Weller CM, et al. Dynamin 2 homozygous mutation in humans with a lethal congenital syndrome. Eur J Hum Genet. 2013;21:637–642. doi: 10.1038/ejhg.2012.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dowling JJ, Vreede AP, Low SE, et al. Loss of myotubularin function results in T-tubule disorganization in zebrafish and human myotubular myopathy. PLoS Genet. 2009;5:e1000372. doi: 10.1371/journal.pgen.1000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al-Qusairi L, Weiss N, Toussaint A, et al. T-tubule disorganization and defective excitation-contraction coupling in muscle fibers lacking myotubularin lipid phosphatase. Proc Natl Acad Sci U S A. 2009;106:18763–18768. doi: 10.1073/pnas.0900705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toussaint A, Cowling BS, Hnia K, et al. Defects in amphiphysin 2 (BIN1) and triads in several forms of centronuclear myopathies. Acta Neuropathol. 2011;121:253–266. doi: 10.1007/s00401-010-0754-2. [DOI] [PubMed] [Google Scholar]
- 55.Durieux AC, Vignaud A, Prudhon B, et al. A centronuclear myopathy-dynamin 2 mutation impairs skeletal muscle structure and function in mice. Hum Mol Genet. 2010;19:4820–4836. doi: 10.1093/hmg/ddq413. [DOI] [PubMed] [Google Scholar]
- 56.Gibbs EM, Davidson AE, Telfer WR, Feldman EL, Dowling JJ. The myopathy-causing mutation DNM2-S619L leads to defective tubulation in vitro and in developing zebrafish. Dis Models Mechan. 2014;7:157–161. doi: 10.1242/dmm.012286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith LL, Gupta VA, Beggs AH. Bridging integrator 1 (Bin1) deficiency in zebrafish results in centronuclear myopathy. Hum Mol Genet. 2014;23:3566–3578. doi: 10.1093/hmg/ddu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee E, Marcucci M, Daniell L, et al. Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science. 2002;297:1193–1196. doi: 10.1126/science.1071362. [DOI] [PubMed] [Google Scholar]
- 59.Al-Qusairi L, Prokic I, Amoasii L, et al. Lack of myotubularin (MTM1) leads to muscle hypotrophy through unbalanced regulation of the autophagy and ubiquitin-proteasome pathways. FASEB J. 2013;27:3384–3394. doi: 10.1096/fj.12-220947. [DOI] [PubMed] [Google Scholar]
- 60.Fetalvero KM, Yu Y, Goetschkes M, et al. Defective autophagy and mTORC1 signaling in myotubularin null mice. Mol Cell Biol. 2013;33:98–110. doi: 10.1128/MCB.01075-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hnia K, Tronchere H, Tomczak KK, et al. Myotubularin controls desmin intermediate filament architecture and mitochondrial dynamics in human and mouse skeletal muscle. J Clin Invest. 2011;121:70–85. doi: 10.1172/JCI44021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dowling JJ, Joubert R, Low SE, et al. Myotubular myopathy and the neuromuscular junction: a novel therapeutic approach from mouse models. Dis Models Mechan. 2012;5:852–859. doi: 10.1242/dmm.009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robb SA, Sewry CA, Dowling JJ, et al. Impaired neuromuscular transmission and response to acetylcholinesterase inhibitors in centronuclear myopathies. Neuromuscul Disord. 2011;21:379–386. doi: 10.1016/j.nmd.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 64.Childers MK, Joubert R, Poulard K, et al. Gene therapy prolongs survival and restores function in murine and canine models of myotubular myopathy. Sci Transl Med. 2014;6:220–10. doi: 10.1126/scitranslmed.3007523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lawlor MW, Armstrong D, Viola MG, et al. Enzyme replacement therapy rescues weakness and improves muscle pathology in mice with X-linked myotubular myopathy. Hum Mol Genet. 2013;22:1525–1538. doi: 10.1093/hmg/ddt003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gibbs EM, Clarke NF, Rose K, et al. Neuromuscular junction abnormalities in DNM2-related centronuclear myopathy. J Mol Med. 2013;91:727–737. doi: 10.1007/s00109-013-0994-4. [DOI] [PubMed] [Google Scholar]
- 67.Stamm DS, Aylsworth AS, Stajich JM, et al. Native American myopathy: congenital myopathy with cleft palate, skeletal anomalies, and susceptibility to malignant hyperthermia. Am J Med Genet A. 2008;146A:1832–1841. doi: 10.1002/ajmg.a.32370. [DOI] [PubMed] [Google Scholar]
- 68.Horstick EJ, Linsley JW, Dowling JJ, et al. Stac3 is a component of the excitation-contraction coupling machinery and mutated in Native American myopathy. Nat Commun. 2013;4:1952. doi: 10.1038/ncomms2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nelson BR, Wu F, Liu Y, et al. Skeletal muscle-specific T-tubule protein STAC3 mediates voltage-induced Ca2+ release and contractility. Proc Natl Acad Sci U S A. 2013;110:11881–11886. doi: 10.1073/pnas.1310571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jain D, Sharma MC, Sarkar C, et al. Tubular aggregate myopathy: a rare form of myopathy. J Clin Neurosci. 2008;15:1222–1226. doi: 10.1016/j.jocn.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 71.Misceo D, Holmgren A, Louch WE, et al. A dominant STIM1 mutation causes Stormorken syndrome. Hum Mutat. 2014;35:556–564. doi: 10.1002/humu.22544. [DOI] [PubMed] [Google Scholar]
- 72.Nesin V, Wiley G, Kousi M, et al. Activating mutations in STIM1 and ORAI1 cause overlapping syndromes of tubular myopathy and congenital miosis. Proc Natl Acad Sci U S A. 2014;111:4197–4202. doi: 10.1073/pnas.1312520111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bohm J, Chevessier F, Maues De Paula A, et al. Constitutive activation of the calcium sensor STIM1 causes tubular-aggregate myopathy. Am J Hum Genet. 2013;92:271–278. doi: 10.1016/j.ajhg.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feske S. ORAI1 and STIM1 deficiency in human and mice: roles of store-operated Ca2+ entry in the immune system and beyond. Immunol Rev. 2009;231:189–209. doi: 10.1111/j.1600-065X.2009.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dirksen RT. Checking your SOCCs and feet: the molecular mechanisms of Ca2+ entry in skeletal muscle. J Physiol. 2009;587:3139–3147. doi: 10.1113/jphysiol.2009.172148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stathopulos PB, Schindl R, Fahrner M, et al. STIM1/Orai1 coiled-coil interplay in the regulation of store-operated calcium entry. Nat Commun. 2013;4:2963. doi: 10.1038/ncomms3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wei-Lapierre L, Carrell EM, Boncompagni S, Protasi F, Dirksen RT. Orai1-dependent calcium entry promotes skeletal muscle growth and limits fatigue. Nat Commun. 2013;4:2805. doi: 10.1038/ncomms3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yarotskyy V, Protasi F, Dirksen RT. Accelerated activation of SOCE current in myotubes from two mouse models of anesthetic- and heat-induced sudden death. PloS One. 2013;8:e77633. doi: 10.1371/journal.pone.0077633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duke AM, Hopkins PM, Calaghan SC, Halsall JP, Steele DS. Store-operated Ca2+ entry in malignant hyperthermia-susceptible human skeletal muscle. J Biol Chem. 2010;285:25645–25653. doi: 10.1074/jbc.M110.104976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morgan-Hughes JA. Tubular aggregates in skeletal muscle: their functional significance and mechanisms of pathogenesis. Curr Opin Neurol. 1998;11:439–442. doi: 10.1097/00019052-199810000-00005. [DOI] [PubMed] [Google Scholar]
- 81.Boncompagni S, Protasi F, Franzini-Armstrong C. Sequential stages in the age-dependent gradual formation and accumulation of tubular aggregates in fast twitch muscle fibers: SERCA and calsequestrin involvement. Age. 2012;34:27–41. doi: 10.1007/s11357-011-9211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Udd B, Krahe R. The myotonic dystrophies: molecular, clinical, and therapeutic challenges. Lancet Neurol. 2012;11:891–905. doi: 10.1016/S1474-4422(12)70204-1. [DOI] [PubMed] [Google Scholar]
- 83.Todd PK, Paulson HL. RNA-mediated neurodegeneration in repeat expansion disorders. Ann Neurol. 2010;67:291–300. doi: 10.1002/ana.21948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kimura T, Lueck JD, Harvey PJ, et al. Alternative splicing of RyR1 alters the efficacy of skeletal EC coupling. Cell Calcium. 2009;45:264–274. doi: 10.1016/j.ceca.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kimura T, Nakamori M, Lueck JD, et al. Altered mRNA splicing of the skeletal muscle ryanodine receptor and sarcoplasmic/endoplasmic reticulum Ca2 + -ATPase in myotonic dystrophy type 1. Hum Mol Genet. 2005;14:2189–2200. doi: 10.1093/hmg/ddi223. [DOI] [PubMed] [Google Scholar]
- 86.Tang ZZ, Yarotskyy V, Wei L, et al. Muscle weakness in myotonic dystrophy associated with misregulated splicing and altered gating of Ca(V)1.1 calcium channel. Hum Mol Genet. 2012;21:1312–1324. doi: 10.1093/hmg/ddr568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fugier C, Klein AF, Hammer C, et al. Misregulated alternative splicing of BIN1 is associated with T tubule alterations and muscle weakness in myotonic dystrophy. Nat Med. 2011;17:720–725. doi: 10.1038/nm.2374. [DOI] [PubMed] [Google Scholar]
- 88.Morrison LA. Dystrophinopathies. Handb Clin Neurol. 2011;101:11–39. doi: 10.1016/B978-0-08-045031-5.00002-5. [DOI] [PubMed] [Google Scholar]
- 89.Rahimov F, Kunkel LM. The cell biology of disease: cellular and molecular mechanisms underlying muscular dystrophy. J Cell Biol. 2013;201:499–510. doi: 10.1083/jcb.201212142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Watkins SC, Hoffman EP, Slayter HS, Kunkel LM. Immunoelectron microscopic localization of dystrophin in myofibres. Nature. 1988;333:863–866. doi: 10.1038/333863a0. [DOI] [PubMed] [Google Scholar]
- 91.Capote J, DiFranco M, Vergara JL. Excitation-contraction coupling alterations in mdx and utrophin/dystrophin double knockout mice: a comparative study. Am J Physiol Cell Physiol. 2010;298:C1077–C1086. doi: 10.1152/ajpcell.00428.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bellinger AM, Reiken S, Carlson C, et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med. 2009;15:325–330. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fauconnier J, Thireau J, Reiken S, et al. Leaky RyR2 trigger ventricular arrhythmias in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2010;107:1559–1564. doi: 10.1073/pnas.0908540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goonasekera SA, Lam CK, Millay DP, et al. Mitigation of muscular dystrophy in mice by SERCA overexpression in skeletal muscle. J Clin Invest. 2011;121:1044–1052. doi: 10.1172/JCI43844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kirschner J, Lochmuller H. Sarcoglycanopathies. Handb Clin Neurol. 2011;101:41–46. doi: 10.1016/B978-0-08-045031-5.00003-7. [DOI] [PubMed] [Google Scholar]
- 96.Andersson DC, Meli AC, Reiken S, et al. Leaky ryanodine receptors in beta-sarcoglycan deficient mice: a potential common defect in muscular dystrophy. Skelet Muscle. 2012;2:9. doi: 10.1186/2044-5040-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Amato AA, Brown RH., Jr Dysferlinopathies. Handb Clin Neurol. 2011;101:111–118. doi: 10.1016/B978-0-08-045031-5.00007-4. [DOI] [PubMed] [Google Scholar]
- 98.Kerr JP, Ward CW, Bloch RJ. Dysferlin at transverse tubules regulates Ca homeostasis in skeletal muscle. Front Physiol. 2014;5:89. doi: 10.3389/fphys.2014.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kerr JP, Ziman AP, Mueller AL, et al. Dysferlin stabilizes stress-induced Ca2+ signaling in the transverse tubule membrane. Proc Natl Acad Sci U S A. 2013;110:20831–20836. doi: 10.1073/pnas.1307960110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gallardo E, Saenz A, Illa I. Limb-girdle muscular dystrophy 2A. Handb Clin Neurol. 2011;101:97–110. doi: 10.1016/B978-0-08-045031-5.00006-2. [DOI] [PubMed] [Google Scholar]
- 101.Kramerova I, Kudryashova E, Wu B, Ottenheijm C, Granzier H, Spencer MJ. Novel role of calpain-3 in the triad-associated protein complex regulating calcium release in skeletal muscle. Hum Mol Genet. 2008;17:3271–3280. doi: 10.1093/hmg/ddn223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Castets P, Lescure A, Guicheney P, Allamand V. Selenoprotein N in skeletal muscle: from diseases to function. J Mol Med. 2012;90:1095–1107. doi: 10.1007/s00109-012-0896-x. [DOI] [PubMed] [Google Scholar]
- 103.Arbogast S, Ferreiro A. Selenoproteins and protection against oxidative stress: selenoprotein N as a novel player at the crossroads of redox signaling and calcium homeostasis. Antioxid Redox Signal. 2010;12:893–904. doi: 10.1089/ars.2009.2890. [DOI] [PubMed] [Google Scholar]
- 104.Rederstorff M, Castets P, Arbogast S, et al. Increased muscle stress-sensitivity induced by selenoprotein N inactivation in mouse: a mammalian model for SEPN1-related myopathy. PloS One. 2011;6:e23094. doi: 10.1371/journal.pone.0023094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Castets P, Bertrand AT, Beuvin M, et al. Satellite cell loss and impaired muscle regeneration in selenoprotein N deficiency. Hum Mol Genet. 2011;20:694–704. doi: 10.1093/hmg/ddq515. [DOI] [PubMed] [Google Scholar]
- 106.Jungbluth H, Sewry CA, Muntoni F. Core myopathies. Semin Pediatr Neurol. 2011;18:239–249. doi: 10.1016/j.spen.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 107.Jurynec MJ, Xia R, Mackrill JJ, et al. Selenoprotein N is required for ryanodine receptor calcium release channel activity in human and zebrafish muscle. Proc Natl Acad Sci U S A. 2008;105:12485–12490. doi: 10.1073/pnas.0806015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arbogast S, Beuvin M, Fraysse B, Zhou H, Muntoni F, Ferreiro A. Oxidative stress in SEPN1-related myopathy: from pathophysiology to treatment. Ann Neurol. 2009;65:677–686. doi: 10.1002/ana.21644. [DOI] [PubMed] [Google Scholar]
- 109.Castets P, Maugenre S, Gartioux C, et al. Selenoprotein N is dynamically expressed during mouse development and detected early in muscle precursors. BMC Develop Biol. 2009;9:46. doi: 10.1186/1471-213X-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Majczenko K, Davidson AE, Camelo-Piragua S, et al. Dominant mutation of CCDC78 in a unique congenital myopathy with prominent internal nuclei and atypical cores. Am J Hum Genet. 2012;91:365–371. doi: 10.1016/j.ajhg.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Klos Dehring DA, Vladar EK, Werner ME, Mitchell JW, Hwang P, Mitchell BJ. Deuterosome-mediated centriole biogenesis. Develop Cell. 2013;27:103–112. doi: 10.1016/j.devcel.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1199 kb)