Abstract
Purpose
We sought to quantify the proportion of uterine cancer survivors who self-report poor physical function. We then sought to quantify the association of poor physical function with physical activity (PA), walking, and lower limb lymphedema (LLL), among women with a history of uterine cancer.
Methods
Physical function was quantified using the SF-12 questionnaire. PA, walking, and LLL were measured using self-report questionnaire. PA was calculated using metabolic equivalent hours per week (MET-hrs·wk−1), and walking was calculated using blocks per day (blocks·d−1). Logistic regression estimated odds ratios (OR) and 95% confidence intervals (95% CI).
Results
Among the 213 uterine cancer survivors in our survey (43% response rate), 35% self-reported poor physical function. Compared to participants who reported <3.0 MET-hrs·wk−1 of PA, participants who reported ≥18.0 MET-hrs·wk−1 of PA were less likely to have poor physical function (OR: 0.03, 95% CI: 0.01–0.10; Ptrend<0.0001). Compared to participants who reported <4.0 blocks·d−1 of walking, participants who reported ≥12.0 blocks·d−1 of walking were less likely to have poor physical function (OR: 0.07, 95% CI: 0.03–0.19; Ptrend<0.0001). Compared to participants who did not have LLL, participants with LLL were more likely to have poor physical function (OR: 5.25, 95% CI: 2.41–11.41; P<0.0001).
Conclusion
Higher levels of PA and walking associate with a lower likelihood of reporting poor physical function. The presence of LLL associates with a higher likelihood of reporting poor physical function. These findings are hypothesis-generating, and should be evaluated in future prospective studies.
Keywords: functional impairment, SF-12, disability, edema, exercise, quality of life
INTRODUCTION
Uterine cancer is the most common gynecologic cancer among women in the United States, accounting for 40,000 new diagnoses each year [1]. Uterine cancer frequently exhibits early signs and symptoms, such as post-menopausal bleeding [2]. As a result of these signs and symptoms, 70% of women are diagnosed with early-stage disease [1]. Five-year survival rates among women with early-stage uterine cancer exceed 85% [1]. Despite favorable five-year survival rates, uterine cancer survivors may develop deleterious sequelae associated with cancer treatment. For example, the removal of abdominal lymph nodes used for cancer staging increases the risk of lower limb lymphedema (LLL) [3]. LLL is a chronic condition characterized by the pooling of protein rich fluid in the lower extremities that affects 36-47% of uterine cancer survivors [4–6]. In addition to treatment-related sequelae, over 38% of uterine cancer survivors are obese, and 70% are physically inactive [7]. Obesity and physical inactivity are risk-factors for premature mortality among uterine cancer survivors [8], and associate with poor physical function among older adults [9–11]. For the purposes of our study, poor physical function was broadly characterized by reporting difficulty completing moderate-intensity activities and difficulty climbing several flights of stairs [12].
Population-based cohort studies suggest cancer survivors are two-fold more likely to self-report poor physical function relative to age-matched peers without a history of cancer [13]. However, these studies have not quantified the prevalence and correlates of poor physical function among specific types of cancer, such as uterine cancer. Uterine cancer survivors are a largely understudied subset of the cancer survivorship population [14]. It is important to address this knowledge gap because poor physical function may persist for decades after cancer treatment [15], and deteriorate quality of life [16]. In addition to decreased quality of life, poor physical function signifies a considerable barrier to functional independence, and predicts hospital admissions and premature mortality [17].
The proportion of uterine cancer survivors who self-report poor physical function is unknown. It is uncertain if behaviors thought to preserve physical function, such as physical activity (PA) and walking, associate with a lower likelihood of reporting poor physical function among uterine cancer survivors. Uterine cancer survivors report that walking is their preferred modality of PA [18]. Conversely, it is uncertain if side effects of cancer treatment, such as LLL, associate with a higher likelihood of reporting poor physical function among uterine cancer survivors. Therefore, the purpose of this hypothesis-generating study was two-fold. First, we sought to quantify the proportion of uterine cancer survivors with poor physical function. Second, we sought to explore the association of poor physical function with self-reported PA, walking, and LLL, among women with a history of uterine cancer.
METHODS
Participants and procedures
We conducted a survey of patients with uterine cancer who received care at the University of Pennsylvania in Philadelphia, Pennsylvania. Participants included women ≥20 years old, with a history of uterine cancer. Potentially eligible participants were identified using fellow surgical case logs from 2008–2010, and ICD-9 diagnosis codes 179.0 and 182.0–182.8, from 2006–2010. ICD-9 codes 179.0 and 182.0–182.8 are the primary codes used to classify cancers of the uterus. Participants who met the study inclusion criteria were sent a letter by their oncologist explaining the purpose of the study. Participants who did not wish to participate were provided the option to decline participation within two-weeks of receiving the letter from their oncologist. Those who did not decline participation were sent the study survey. After two-weeks, a second survey was sent to those who did not reply to the first mailed survey. This protocol was approved by the University of Pennsylvania Institutional Review Board, and the University of Pennsylvania cancer center. Women who mailed back a completed survey were classified as having provided their informed consent.
Physical function
The Medical Outcomes Study 12-Item Short-Form Health Survey (SF-12) was used to assess physical function. The SF-12 is a self-report measure that evaluates eight domains of health, including one domain specific to physical functioning [12]. The physical functioning domain of the SF-12 associates with objective measures of lower extremity physical function including walking speed and chair stand time [19]. We used the two questions in the physical functioning domain to quantify physical function. Each of the two questions represents validated outcomes in the International Classification of Functioning Disability, and Health (ICF) framework [20–22]. The ICF was developed by the World Health Organization for use in clinical and research settings for functional status assessment, goal setting, treatment planning, and outcome measurement [21,22]. The two questions ask about difficulty: 1) completing moderate-intensity activities; and 2) climbing several flights of stairs. For each question, participants were provided with three response options: 1) ‘not limited at all’; 2) ‘limited a little’; and 3) ‘limited a lot’. Participants who reported being ‘limited a little’ or ‘limited a lot’ completing both moderate-intensity activities and climbing several flights of stairs were classified with poor physical function [20].
Physical activity questionnaire
The Paffenbarger PA Questionnaire (PPAQ) was used to assess participation in leisure time PA and daily walking [23]. The PPAQ is correlated with maximal oxygen consumption among women (r=0.53; P<0.01) [24], and has been used previously among uterine cancer survivors [5]. Participants were asked to list any PA that they participated in during the past year, and the frequency and duration of each PA. Research staff converted the PA to metabolic equivalents (MET), using the compendium of PA [25]. For each participant, an aggregate measure of MET-hrs·wk−1 was created by summing the MET-hrs·wk−1 for each activity described by the participant. We created categories of MET-hrs·wk−1, defined as <3.0, 3.0–8.9, 9.0–17.9, and ≥18.0 that correspond to <1.0, 1.0–2.9, 3.0–5.9, and ≥6.0 hours per week of moderate-intensity PA, consistent with prior analyses among uterine cancer survivors [5]. Participants were asked to report how many blocks they walked on an average day during the past year. We created categories of blocks per day (blocks·d−1) of walking, defined as <4.0, 4.0–11.9, and ≥12 blocks·d−1, which correspond to <¼, ¼ to <1, and ≥1 mile of walking per day, consistent with prior analyses among older adults [26], and uterine cancer survivors [5].
Lower limb lymphedema questionnaire
The Gynecologic Cancer Lymphedema Questionnaire (GCLQ) was used to assess symptoms associated with LLL [27]. The GCLQ is a validated self-report measure that assesses seven domains of symptoms in both lower extremities. The seven domains include heaviness, general swelling, limb-related swelling, infection, aching, numbness, and physical function. Participants reporting ≥5 symptoms of the lower extremities within the seven above-listed domains were classified as having LLL [27].
Covariates
Information on covariates came from self-report or electronic medical records. Variables collected from self-report included age, marital status, race, education, employment, body mass index (BMI). We used the age-adjusted Charlson Comorbidity Index to predict mortality based on number and severity of comorbid illnesses [28,29]. Variables collected from the electronic medical record included pathology of the cancer, stage of the cancer, time since diagnosis, and cancer treatment history.
Statistical analysis
We performed descriptive statistics and bivariate analyses on all study variables using the Wilcoxon Rank-Sum test for continuous variables and Fishers exact test for categorical variables. We used logistic regression models to estimate the odds ratio (OR) of reporting poor physical function with 95% confidence intervals (95% CI). The P value for the linear trend test across categories (Ptrend) was calculated using the median value for each category as a continuous variable in a logistic regression model. We examined unadjusted regression models, then adjusted for age and BMI, and subsequently built a multivariable regression model adjusting for demographic and clinical characteristics. Statistical tests were two-sided and P < 0.05 was the threshold for statistical significance.
RESULTS
Mailed survey results
We identified 531 participants using the fellow surgical case logs and ICD-9 codes. Among the 531 mailed letters, we had a 43% response rate. Sixty-seven potentially eligible participants were not interested in participating in our study, and 213 potentially eligible participants did not respond to either the letter or the mailed survey. There were 19 letters returned by the post office, labeled as undeliverable, and an additional seven people died. A total of 225 participants returned surveys, and 12 were subsequently identified as not meeting inclusion criteria (i.e., 10 diagnosed with cancer before 2006, and two misclassified (diagnosed with other gynecologic cancers)). The remaining 213 eligible participants replied to our survey and were included in the analyses described herein.
Participant Characteristics
Demographic characteristics of the study participants are depicted in Table 1. The age of the 213 participants ranged from 29–94 years. Clinical characteristics of the study participants are depicted in Table 2. The BMI of study participants ranged from 14–67 kg/m2. We identified no demographic or clinical characteristics associated with volume of self-reported PA (data not shown). Women who self-reported higher levels of daily walking were marginally more likely to be retired (P=0.07), and have lower BMI (P=0.06; data not shown). Demographic and clinical characteristics associated with LLL have been reported previously [5].
Table 1.
Demographic characteristics stratified by physical function status
| Variable | Total Sample (n=213) |
Low (poor) physical function (n=74) |
High physical function (n=139) |
Pa |
|---|---|---|---|---|
| Age — yr | 63.6±10.6 | 64.2±12.4 | 63.2±9.6 | 0.57 |
| Marital status — no. (%) | 0.87 | |||
| Never married | 20 (9%) | 7 (10%) | 13 (9%) | |
| Married | 128 (60%) | 45 (61%) | 83 (60%) | |
| Divorced or separated | 31 (15%) | 9 (12%) | 22 (16%) | |
| Widowed | 33 (16%) | 13 (18%) | 20 (14%) | |
| Self-reported race — no. (%) | 0.12 | |||
| White | 177 (84%) | 61 (82%) | 116 (84%) | |
| Black | 28 (13%) | 8 (11%) | 20 (14%) | |
| Other | 7 (3%) | 5 (7%) | 2 (2%) | |
| Education — no. (%) | 0.28 | |||
| High school or less | 46 (22%) | 12 (16%) | 34 (25%) | |
| Some college | 51 (24%) | 17 (23%) | 34 (25%) | |
| College degree or more | 114 (54%) | 45 (61%) | 69 (50%) | |
| Employment — no. (%) | 0.92 | |||
| Retired | 94 (45%) | 32 (43%) | 62 (45%) | |
| Unemployed | 7 (3%) | 2 (3%) | 5 (4%) | |
| Homemaker | 16 (8%) | 7 (9%) | 9 (7%) | |
| Other | 14 (7%) | 4 (5%) | 10 (7%) | |
| Full time | 80 (38%) | 29 (39%) | 51 (37%) |
By Wilcoxon rank sum, or Fishers Exact test. Values may not sum to 213 or 100% due to rounding error and item non-response.
Table 2.
Clinical characteristics stratified by physical function status
| Variable | Total Sample (n=213) |
Low (poor) physical function (n=74) |
High physical function (n=139) |
Pa |
|---|---|---|---|---|
| Pathology type — no. (%) | 0.49 | |||
| Endometroid Adenocarcinoma | 158 (75%) | 56 (77%) | 102 (73%) | |
| Papillary serous or Clear Cell | 35 (17%) | 14 (19%) | 21 (15%) | |
| Sarcoma | 8 (4%) | 2 (3%) | 6 (4%) | |
| Carcinosarcoma | 8 (4%) | 1 (1%) | 7 (5%) | |
| Other (Undifferentiated) | 3 (1%) | 0 (0%) | 3 (2%) | |
| Stage — no. (%) | 0.63 | |||
| 1 | 157 (74%) | 54 (73%) | 103 (74%) | |
| 2 | 13 (6%) | 5 (7%) | 8 (6%) | |
| 3 | 26 (12%) | 11 (15%) | 15 (11%) | |
| 4 | 5 (2%) | 2 (3%) | 3 (2%) | |
| Unknown | 12 (6%) | 2 (3%) | 10 (7%) | |
| Treatment Modalities — no. (%) | 0.44 | |||
| Surgery | 100 (47%) | 31 (42%) | 69 (50%) | |
| Surgery, Chemotherapy | 37 (17%) | 16 (22%) | 21 (15%) | |
| Surgery, Radiation | 47 (22%) | 14 (19%) | 33 (24%) | |
| Surgery, Chemotherapy, Radiation | 22 (10%) | 10 (14%) | 12 (9%) | |
| None or Unknown | 7 (3%) | 3 (4%) | 4 (3%) | |
| No. of nodes removed | 8.9±10.2 | 7.2±9.2 | 9.8±10.6 | 0.74 |
| Time since diagnosis — no. (%) | 0.08 | |||
| 0–2 yrs | 69 (32%) | 18 (24%) | 51 (37%) | |
| 3–4 yrs | 94 (44%) | 33 (45%) | 61 (44%) | |
| 5–6 yrs | 50 (23%) | 23 (31%) | 27 (19%) | |
| BMI — kg/m2 | 31.1±8.9 | 29.8±9.7 | 31.7±8.4 | 0.13 |
| Predicted 10-year mortalityb | 20.4±25.0% | 20.9±24.4% | 19.3±26.3% | 0.66 |
By Wilcoxon rank sum, or Fishers Exact test. Values may not sum to 213 or 100% due to rounding error and item non-response.
Predicted probability from the Charlson Comorbidity Index.
Characteristics between participants with versus without poor physical function
Among the 213 participants, 74 (35%) were classified with poor physical function, defined by self-reporting difficulty completing moderate-intensity activities and climbing several flights of stairs. There existed no significant differences in demographic or clinical characteristics between women with versus without poor physical function.
Physical function by level of physical activity
Among the 213 study participants, 40%, 13%, 13%, and 35% reported participating in <3.0, 3.0–8.9, 9.0–17.9, and ≥18.0 MET-hrs·wk−1 of PA, respectively (Table 3). In all analyses, the odds of reporting poor physical function decreased as MET-hrs·wk−1 of PA increased (Figure 1A; Ptrend< 0.0001). Compared with participants who reported <3.0 MET-hrs·wk−1 of PA, participants who reported ≥18.0 MET-hrs·wk−1 of PA had an OR of 0.03 (95% CI: 0.01–0.10), in the fully multivariable-adjusted regression model. The most common PA reported was walking (42%), aerobic gym-based activities including the recumbent bicycle and elliptical machine (11%), and swimming (8%).
Table 3.
Cases of poor physical function by level of physical activity, walking distance, and presence of lower limb lymphedema
| Physical Activity (MET-hrs·wk−1) | Total in category | Cases of poor physical function | Model 1a OR (95% CI) | Model 2b OR (95% CI) | Model 3c OR (95% CI) |
| <3.0 | 85 | 51 (60%) | 1 — Referent | 1 — Referent | 1 — Referent |
| 3.0–8.9 | 27 | 9 (33%) | 0.33 (0.13–0.83) | 0.32 (0.12–0.83) | 0.35 (0.11–1.19) |
| 9.0–17.9 | 27 | 8 (30%) | 0.28 (0.11–0.71) | 0.28 (0.11–0.72) | 0.11 (0.03–0.51) |
| ≥18.0 | 74 | 6 (8%) | 0.06 (0.03–0.15) | 0.06 (0.03–0.15) | 0.03 (0.01–0.10) |
| Ptrend | — | — | <0.0001 | <0.0001 | <0.0001 |
| Walking (blocks·d−1) | Total in category | Cases of poor physical function | Model 1a OR (95% CI) | Model 2b OR (95% CI) | Model 3c OR (95% CI) |
| <4.0 | 75 | 50 (67%) | 1 — Referent | 1 — Referent | 1 — Referent |
| 4.0–11.9 | 53 | 11 (21%) | 0.13 (0.06–0.30) | 0.13 (0.06–0.31) | 0.15 (0.05–0.43) |
| ≥12.0 | 78 | 12 (15%) | 0.09 (0.04–0.20) | 0.09 (0.04–0.20) | 0.07 (0.03–0.19) |
| Ptrend | — | — | <0.0001 | <0.0001 | <0.0001 |
| Lower Limb Lymphedema | Total in category | Cases of poor physical function | Model 1a OR (95% CI) | Model 2b OR (95% CI) | Model 3c OR (95% CI) |
| Absent | 136 | 31 (23%) | 1 — Referent | 1 — Referent | 1 — Referent |
| Present | 77 | 43 (56%) | 4.28 (2.34–7.82) | 4.06 (2.19–7.51) | 5.25 (2.41–11.41) |
| P | — | — | <0.0001 | <0.0001 | <0.0001 |
Model 1 is the crude (unadjusted) odds ratio and 95% confidence interval.
Model 2 is the age and BMI adjusted odds ratio and 95% confidence interval.
Model 3 is the fully adjusted (multivariable) odds ratio and 95% confidence interval, controlling for age, marital status, race, education, employment, pathology type, stage, treatment, no. of nodes removed, time since diagnosis, body mass index, and Carlson Comorbidity Index.
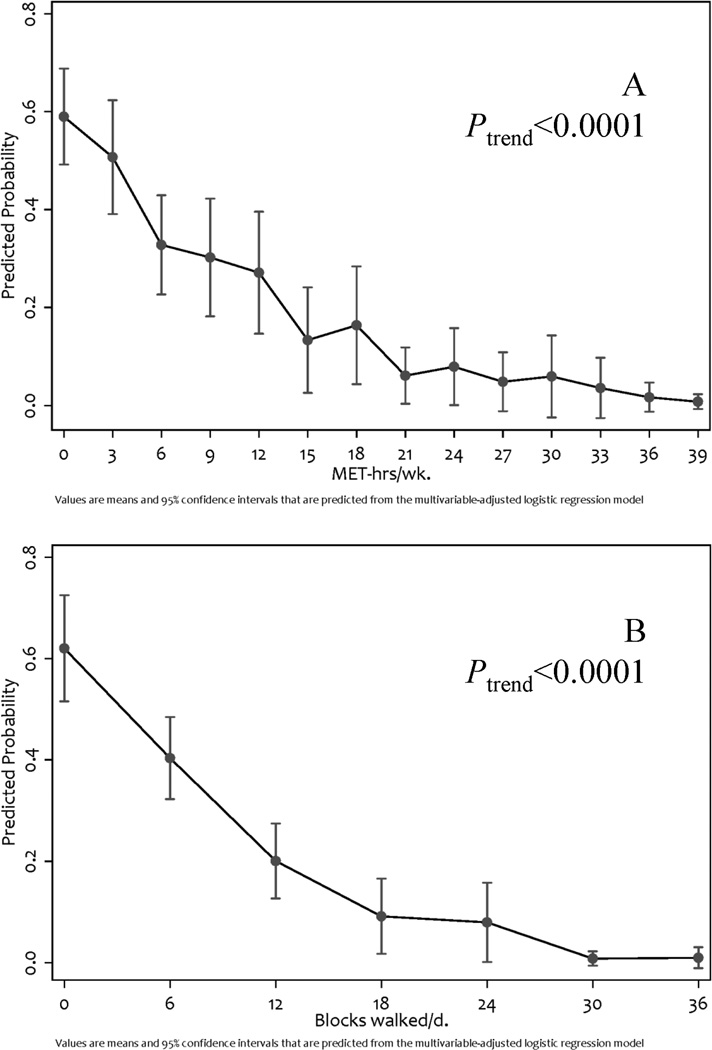
Figure 1.
Predicted probability of poor physical function and relationship of: A) increasing MET-hrs·wk−1 of physical activity and; B) increasing blocks walked per day.
Physical function by level of daily walking distance
Among the 213 study participants, 36%, 26%, and 38% reported walking <4.0, 4.0–11.9, and ≥12 blocks·d−1, respectively (Table 3). In all analyses, the odds of reporting poor physical function decreased as the blocks·d−1 of walking increased (Figure 1B; Ptrend < 0.0001). Compared with participants who reported <4.0 blocks·d−1 of walking, participants who reported ≥12.0 blocks·d−1 of walking had an OR of 0.07 (95% CI: 0.03–0.19), in the fully multivariable-adjusted regression model.
Physical function by LLL
Among the 213 study participants, 36% reported symptoms in the lower extremities sufficient to be classified as LLL. In all analyses, the odds of reporting poor physical function were increased in the presence of LLL (P < 0.0001). Compared with participants who did not have LLL, participants with LLL had an OR of 5.25 (95% CI: 2.41–11.41), in the fully multivariable-adjusted regression model. BMI was not associated with LLL [5], and was not associated with poor physical function as a continuous variable (P = 0.88) or a categorical variable (i.e., <25, 25–30, ≥30; P = 0.47) in multivariable-adjusted logistic regression models.
Joint effects of LLL and physical activity or walking distance on physical function
We assessed the joint effects of LLL with PA, and LLL with walking (Table 4) to determine if the association between PA, walking, and poor physical function differed among women with and without LLL. The interaction for PA was not statistically significant (Pinteraction = 0.61), and stratified analyses suggested the association of PA and reporting poor physical function existed among women with and without LLL (both Ptrend < 0.0001). The interaction for walking was not statistically significant (Pinteraction = 0.83), and stratified analyses suggested the association of walking and reporting poor physical function existed among women with and without LLL (both Ptrend < 0.0001).
Table 4.
Multivariable-adjusted cases of poor physical function by level of physical activity and walking distance, stratified by presence of lower limb lymphedemaa.
| Physical Activity (MET-hrs·wk−1) | LLL Present (n=77) | LLL Absent (n=136) | Pinteraction |
| <3.0 | 1 — Referent | 1 — Referent | 0.61 |
| 3.0–8.9 | 0.18 (0.03–1.28) | 0.35 (0.03–4.91) | |
| 9.0–17.9 | 0.28 (0.04–2.16) | 0.03 (0.01–0.48) | |
| ≥18.0 | 0.02 (0.01–0.16) | 0.02 (0.01–0.20) | |
| Ptrend | <0.0001 | <0.0001 | |
| Walking (blocks·d−1) | LLL Present (n=77) | LLL Absent (n=136) | Pinteraction |
| <4.0 | 1 — Referent | 1 — Referent | 0.83 |
| 4.0–11.9 | 0.04 (0.01–0.29) | 0.18 (0.04–0.78) | |
| ≥12.0 | 0.02 (0.01–0.14) | 0.09 (0.02–0.33) | |
| Ptrend | <0.0001 | <0.0001 | |
Fully adjusted (multivariable) odds ratio and 95% confidence interval controlling for age, marital status, race, education, employment, pathology type, stage, treatment, no. of nodes removed, time since diagnosis, body mass index, and Carlson Comorbidity Index.
DISCUSSION
The first finding of this study is that 35% of uterine cancer survivors reported poor physical function, defined by difficulty participating in moderate-intensity activities and climbing several flights of stairs. The second finding of this study is that higher levels of self-reported PA and walking associate with a lower likelihood of reporting poor physical function. Conversely, the presence of LLL associates with a higher likelihood of reporting poor physical function. Our estimate that 35% of uterine cancer survivors have poor physical function is similar to that of the Iowa Women’s Health Study [15], and the National Health Interview Study [30], which concluded that 37% and 34% of cancer survivors have functional limitations, respectively.
Physical function is an important clinical measure [31]. Physical function predicts mortality among women with gynecologic cancer [32]. Many studies among cancer survivors have focused on overall quality of life rather than physical function-specific outcomes [33]. Furthermore, studies that do report physical function-specific outcomes commonly use continuous measures, such as means and standard deviations, which may limit their clinical interpretability [34]. We purposefully created a binary endpoint that was derived from the SF-12 survey to promote the clinical utility of this measure [20]. This endpoint identified women with difficulty completing moderate-intensity activities, such as moving a table or pushing a vacuum, and climbing several flights of stairs.
Though our study was cross-sectional, our data suggest higher levels of PA and walking associate with a lower likelihood of self-reporting poor physical function. Examining the outcomes of PA and daily walking as continuous variables revealed a negative trend with a floor effect, such that women who reported ≥15 MET-hrs·wk−1 of PA or ≥18 blocks·d−1 of walking had the lowest predicted probability of reporting poor physical function (see Figure). The dose-response relationship between PA and physical function has been reported among older community-dwelling men [35], women [11,35], and colorectal cancer survivors [36]. The observed dose-response relationship suggests that uterine cancer survivors may derive health benefits from PA even if they do not meet the PA recommendations for cancer survivors. This hypothesis is consistent with the ACSM guidelines that suggest all cancer survivors should avoid inactivity [14]. It has been noted that even small increases in PA may yield improvement in health outcomes, particularly among those who are sedentary at baseline and modestly increase PA levels [37].
LLL affects up to 47% of the 40,000 women diagnosed with uterine cancer each year in the United States [6]. In our study, 36% of women reported symptoms consistent with LLL [5]. LLL is described as an accumulation of protein-rich fluid that results in swelling of the lower limbs [38,39], and impairs quality of life [40,41]. Objective measures of physical function using the 6-minute walk test among cancer survivors with LLL are 30% below normative values compared to adults of a similar age without a history of cancer [42]. This supports our finding that LLL is associated with poor self-reported physical function. It is noteworthy that our interaction analyses suggested the association between PA and poor physical function and walking and poor physical function did not vary according to LLL status. Physical activity has been hypothesized as a possible intervention to alleviate symptom burden among cancer survivors with LLL [4,5]. Higher levels of PA, such as walking, associate with a lower likelihood of reporting symptoms sufficient for a diagnosis of LLL among uterine cancer survivors [5]. To date, a randomized trial examining a structured PA program, such as progressive treadmill walking, on LLL outcomes has not been reported. In addition to aerobic exercise, a non-randomized trial of weightlifting among cancer survivors with LLL improved upper- and lower-extremity muscular strength and objective measures of physical function, without significantly worsening limb volume [42]. A randomized trial is necessary to confirm the efficacy and clarify the safety of exercise among cancer survivors with LLL. The development of trials that examine PA or exercise such as treadmill walking or weightlifting should be considered a research priority, given that limited efficacious therapies exist to manage LLL, and the incidence of LLL is predicted to increase among gynecologic cancer survivors [4]. We encourage investigators to consider designing interventions that are not only safe and efficacious, but are disseminable and sustainable in clinical practice [43].
Contrary to our hypothesis, BMI was not associated with poor physical function in our multivariable-adjusted regression analyses. We analyzed BMI as a continuous variable and as a categorical variable using established thresholds. Obesity is a risk-factor for poor physical function among uterine cancer survivors [44], and among older adults [9,10]. It is unclear why we did not observe an association between BMI and physical function. In our study sample, BMI ranged from 14 to 67 kg/m2, therefore it is unlikely that there was insufficient variability in BMI to observe an association with poor physical function. Perhaps one reason we failed to observe an association is because BMI was self-reported. It is plausible that study participants misreported their BMI sufficiently to bias the observed relationship with physical functioning. It is known that BMI is historically underreported when contrasted with objective measures, particularly among women with BMI’s ≥40 kg/m2 [45]. This differential misclassification would thereby obscure the true association between BMI and physical functioning.
The major limitation of this study is the cross-sectional design which it is impossible to determine causal associations. It is plausible that uterine cancer survivors who engage in more PA or walking subsequently report more favorable physical function. Conversely, it is plausible that uterine cancer survivors with poor physical function may be physically unable to engage in PA or walking. Longitudinal studies are now necessary to delineate the direction of the observed associations. If poor physical function limits participation in PA, then it is necessary to identify rehabilitative interventions, such as physical therapy, that will improve or restore levels of physical function sufficient to allow participation in PA. Given the cross-sectional design, a similar relationship exists between impaired physical function and LLL. We used the SF-12 questionnaire to quantify poor physical function in this study. The SF-12 is a valid and reliable subjective measure of physical function [12]. However, it is likely that the two questions used in the SF-12 did not fully capture physical function. For example, participants may have experienced challenges in instrumental activities of daily living that negatively impact quality of life such as bathing or toileting. Therefore, our study may underestimate the prevalence of poor physical function among uterine cancer survivors. The Working Group on Health Outcomes for Older Persons with Multiple Chronic Conditions recommends the use of a self-reported questionnaire of general health (such as the SF-36 or SF-12), with follow-on objective measures, such as gait speed, for clinical and research activities [46]. PA in our study was self-reported. Self-reported PA is valid and correlated with objective measures of PA [47], however it is plausible that participants in our study may have misreported their PA due to inaccurate recall and subsequent reporting bias. Another limitation to our study was LLL was self-reported [5]. The current gold-standard method to diagnosis LLL is circumferential measures of the lower limbs. However, this method has not been adopted for use in routine clinical care [4]. Our method to assess LLL relied on symptoms using a self-report questionnaire that was validated against circumferential measures of the lower limbs, and had excellent psychometric characteristics [27].
Conclusion
Using a validated self-report questionnaire [12], 35% of uterine cancer survivors had difficulty completing moderate-intensity activities and climbing several flights of stairs. Higher levels of PA and walking associate with a lower likelihood of reporting poor physical function in dose-response fashion, such that the group who engaged in the highest levels of PA or walking reported the smallest proportion of cases of poor physical function. Interventions designed to improve physical function through rehabilitation, exercise, and physical activity should be considered and investigated in future studies. Furthermore, the presence of LLL associates with a higher likelihood of reporting poor physical function. Interventions to reduce the incidence of LLL, and improve physical function among those with LLL should also be investigated in future studies.
Footnotes
Conflicts of Interest
The authors declare there are no conflicts of interest.
REFERENCES
- 1.Siegel R, Desantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 2.Gredmark T, Kvint S, Havel G, Mattsson L. Histopathological findings in women with postmenopausal bleeding. BJOG: An International Journal of Obstetrics & Gynaecology. 1995;102:133–136. doi: 10.1111/j.1471-0528.1995.tb09066.x. [DOI] [PubMed] [Google Scholar]
- 3.Brown JC, Winters-Stone K, Lee A, Schmitz KH. Cancer, Physical Activity, and Exercise. Compr Physiol. 2012;2(4):2775–2809. doi: 10.1002/cphy.c120005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown JC, Chu CS, Cheville AL, Schmitz KH. The prevalence of lymphedema symptoms among survivors of long-term cancer with or at risk for lower limb lymphedema. Am J Phys Med Rehabil. 2013;92:223–231. doi: 10.1097/PHM.0b013e31826edd97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown JC, John GM, Segal S, Chu CS, Schmitz KH. Physical activity and lower limb lymphedema among uterine cancer survivors. Med Sci Sports Exerc. 2013;45:2091–2097. doi: 10.1249/MSS.0b013e318299afd4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finnane A, Hayes SC, Obermair A, Janda M. Quality of life of women with lower-limb lymphedema following gynecological cancer. Expert Rev Pharmacoecon Outcomes Res. 2011;11:287–297. doi: 10.1586/erp.11.30. [DOI] [PubMed] [Google Scholar]
- 7.Courneya KS, Karvinen KH, Campbell KL, Pearcey RG, Dundas G, Capstick V, et al. Associations among exercise, body weight, and quality of life in a population-based sample of endometrial cancer survivors. Gynecol Oncol. 2005;97:422–430. doi: 10.1016/j.ygyno.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Arem H, Park Y, Pelser C, Ballard-Barbash R, Irwin ML, Hollenbeck A, et al. Prediagnosis body mass index, physical activity, and mortality in endometrial cancer patients. J Natl Cancer Inst. 2013;105:342–349. doi: 10.1093/jnci/djs530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woo J, Leung J, Kwok T. BMI, body composition, and physical functioning in older adults. Obesity. 2007;15:1886–1894. doi: 10.1038/oby.2007.223. [DOI] [PubMed] [Google Scholar]
- 10.Hardy R, Cooper R, Sayer AA, Ben-Shlomo Y, Cooper C, Deary IJ, et al. Body mass index, muscle strength and physical performance in older adults from eight cohort studies: the HALCyon programme. PloS one. 2013;8:e56483. doi: 10.1371/journal.pone.0056483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brach JS, FitzGerald S, Newman AB, Kelsey S, Kuller L, VanSwearingen JM, et al. Physical activity and functional status in community-dwelling older women: a 14-year prospective study. Arch Intern Med. 2003;163:2565–2571. doi: 10.1001/archinte.163.21.2565. [DOI] [PubMed] [Google Scholar]
- 12.Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. J Clin Epidemiol. 1998;51:1171–1178. doi: 10.1016/s0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 13.Ness KK, Wall MM, Oakes JM, Robison LL, Gurney JG. Physical performance limitations and participation restrictions among cancer survivors: a population-based study. Ann Epidemiol. 2006;16:197–205. doi: 10.1016/j.annepidem.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 15.Sweeney C, Schmitz KH, Lazovich D, Virnig BA, Wallace RB, Folsom AR. Functional limitations in elderly female cancer survivors. J Natl Cancer Inst. 2006;98:521–529. doi: 10.1093/jnci/djj130. [DOI] [PubMed] [Google Scholar]
- 16.Lutgendorf SK, Anderson B, Ullrich P, Johnsen EL, Buller RE, Sood AK, et al. Quality of life and mood in women with gynecologic cancer. Cancer. 2002;94:131–140. doi: 10.1002/cncr.10155. [DOI] [PubMed] [Google Scholar]
- 17.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 18.Karvinen KH, Courneya KS, Campbell KL, Pearcey RG, Dundas G, Capstick V, et al. Exercise preferences of endometrial cancer survivors: a population-based study. Cancer Nurs. 2006;29:259–265. doi: 10.1097/00002820-200607000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Hall SA, Chiu GR, Williams RE, Clark RV, Araujo AB. Physical function and health-related quality-of-life in a population-based sample. The Aging Male. 2011;14:119–126. doi: 10.3109/13685538.2010.502267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayo NE, Poissant L, Ahmed S, Finch L, Higgins J, Salbach NM, et al. Incorporating the International Classification of Functioning, Disability, and Health (ICF) into an electronic health record to create indicators of function: proof of concept using the SF-12. Journal of the American Medical Informatics Association. 2004;11:514–522. doi: 10.1197/jamia.M1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Üstün TB, Chatterji S, Bickenbach J, Kostanjsek N, Schneider M. The International Classification of Functioning, Disability and Health: a new tool for understanding disability and health. Disability & Rehabilitation. 2003;25:565–571. doi: 10.1080/0963828031000137063. [DOI] [PubMed] [Google Scholar]
- 22.Stucki G, Cieza A, Ewert T, Kostanjsek N, Chatterji S, ÜstÜn TB. Application of the International Classification of Functioning, Disability and Health (ICF) in clinical practice. Disability & Rehabilitation. 2002;24:281–282. doi: 10.1080/09638280110105222. [DOI] [PubMed] [Google Scholar]
- 23.Paffenbarger RS, Jr, Hyde RT, Wing AL. Physical activity and incidence of cancer in diverse populations: a preliminary report. Am J Clin Nutr. 1987;45:312–317. doi: 10.1093/ajcn/45.1.312. [DOI] [PubMed] [Google Scholar]
- 24.Ainsworth BE, Leon AS, Richardson MT, Jacobs DR, Paffenbarger RS., Jr Accuracy of the College Alumnus Physical Activity Questionnaire. J Clin Epidemiol. 1993;46:1403–1411. doi: 10.1016/0895-4356(93)90140-v. [DOI] [PubMed] [Google Scholar]
- 25.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 26.Hardy SE, Kang Y, Studenski SA, Degenholtz HB. Ability to walk 1/4 mile predicts subsequent disability, mortality, and health care costs. J Gen Intern Med. 2011;26:130–135. doi: 10.1007/s11606-010-1543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter J, Raviv L, Appollo K, Baser RE, Iasonos A, Barakat RR. A pilot study using the Gynecologic Cancer Lymphedema Questionnaire (GCLQ) as a clinical care tool to identify lower extremity lymphedema in gynecologic cancer survivors. Gynecol Oncol. 2010;117:317–323. doi: 10.1016/j.ygyno.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 30.Yabroff KR, Lawrence WF, Clauser S, Davis WW, Brown ML. Burden of illness in cancer survivors: findings from a population-based national sample. J Natl Cancer Inst. 2004;96:1322–1330. doi: 10.1093/jnci/djh255. [DOI] [PubMed] [Google Scholar]
- 31.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA: the journal of the American Medical Association. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cesari M, Cerullo F, Zamboni V, Di Palma R, Scambia G, Balducci L, et al. Functional Status and Mortality in Older Women With Gynecological Cancer. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2013 doi: 10.1093/gerona/glt073. [DOI] [PubMed] [Google Scholar]
- 33.Fong DY, Ho JW, Hui BP, Lee AM, Macfarlane DJ, Leung SS, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ: British Medical Journal. 2012;344 doi: 10.1136/bmj.e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roila F, Cortesi E. Quality of life as a primary end point in oncology. Annals of oncology. 2001;12:S3–S6. doi: 10.1093/annonc/12.suppl_3.s3. [DOI] [PubMed] [Google Scholar]
- 35.Wendel-Vos G, Schuit A, Tijhuis M, Kromhout D. Leisure time physical activity and health-related quality of life: cross-sectional and longitudinal associations. Quality of Life Research. 2004;13:667–677. doi: 10.1023/B:QURE.0000021313.51397.33. [DOI] [PubMed] [Google Scholar]
- 36.Thraen-Borowski KM, Trentham-Dietz A, Edwards DF, Koltyn KF, Colbert LH. Dose–response relationships between physical activity, social participation, and health-related quality of life in colorectal cancer survivors. Journal of Cancer Survivorship. 2013;7:369–378. doi: 10.1007/s11764-013-0277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tudor-Locke C, Craig CL, Aoyagi Y, Bell RC, Croteau KA, De Bourdeaudhuij I, et al. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. 2011;8:80. doi: 10.1186/1479-5868-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langbecker D, Hayes SC, Newman B, Janda M. Treatment for upper-limb and lower-limb lymphedema by professionals specializing in lymphedema care. Eur J Cancer Care (Engl) 2008;17:557–564. doi: 10.1111/j.1365-2354.2007.00878.x. [DOI] [PubMed] [Google Scholar]
- 39.Jensen MR, Simonsen L, Karlsmark T, Bulow J. Lymphoedema of the lower extremities--background, pathophysiology and diagnostic considerations. Clin Physiol Funct Imaging. 2010;30:389–398. doi: 10.1111/j.1475-097X.2010.00969.x. [DOI] [PubMed] [Google Scholar]
- 40.Bergmark K, Avall-Lundqvist E, Dickman PW, Henningsohn L, Steineck G. Lymphedema and bladder-emptying difficulties after radical hysterectomy for early cervical cancer and among population controls. Int J Gynecol Cancer. 2006;16:1130–1139. doi: 10.1111/j.1525-1438.2006.00601.x. [DOI] [PubMed] [Google Scholar]
- 41.Ferrandina G, Mantegna G, Petrillo M, Fuoco G, Venditti L, Terzano S, et al. Quality of life and emotional distress in early stage and locally advanced cervical cancer patients: a prospective, longitudinal study. Gynecol Oncol. 2012;124:389–394. doi: 10.1016/j.ygyno.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 42.Katz E, Dugan NL, Cohn JC, Chu C, Smith RG, Schmitz KH. Weight lifting in patients with lower-extremity lymphedema secondary to cancer: a pilot and feasibility study. Arch Phys Med Rehabil. 2010;91:1070–1076. doi: 10.1016/j.apmr.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phillips SM, Alfano CM, Perna FM, Glasgow RE. Accelerating Translation of Physical Activity and Cancer Survivorship Research into Practice: Recommendations for a More Integrated and Collaborative Approach. Cancer Epidemiol Biomarkers Prev. 2014 doi: 10.1158/1055-9965.EPI-13-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basen-Engquist K, Scruggs S, Jhingran A, Bodurka DC, Lu K, Ramondetta L, et al. Physical activity and obesity in endometrial cancer survivors: associations with pain, fatigue, and physical functioning. Obstet Gynecol. 2009;200:288:e1–e288. e8. doi: 10.1016/j.ajog.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorber SC, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obesity reviews. 2007;8:307–326. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 46.Working Group on Health Outcomes for Older Persons with Multiple Chronic Conditions Universal health outcome measures for older persons with multiple chronic conditions. J Am Geriatr Soc. 2012;60:2333–2341. doi: 10.1111/j.1532-5415.2012.04240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]