SYNOPSIS
The adaptor protein ALIX links retroviruses to ESCRT machinery during retroviral budding. This function of ALIX requires its interaction with the ESCRT-III component CHMP4 at the N-terminal Bro1 domain and retroviral Gag proteins at the middle V domain. Since cytoplasmic or recombinant ALIX is unable to interact with CHMP4 or retroviral Gag proteins under non-denaturing conditions, we constructed ALIX truncations and mutations to define the intrinsic mechanism through which ALIX interactions with these partner proteins are prohibited. Our results demonstrate that an intramolecular interaction between Patch 2 in the Bro1 domain and the TSG101 docking site in the Pro-rich domain locks ALIX into a closed conformation that renders ALIX unable to interact with CHMP4 and retroviral Gag proteins. Relieving the intramolecular interaction of ALIX, by ectopically expressing a binding partner for one of the intramolecular interaction sites or by deleting one of these sites, promotes ALIX interaction with these partner proteins and facilitates ALIX association with the membrane. Ectopic expression of a GFP-ALIX mutant with a constitutively open conformation, but not the wild type protein increases EIAV budding from HEK293 cells. These findings predict that relieving the autoinhibitory intramolecular interaction of ALIX is a critical step for ALIX to participate in retroviral budding.
Keywords: ALIX, autoinhibition, intramolecular interaction, Src, TSG101, CHMP4, retroviral budding
INTRODUCTION
Apoptosis-linked gene-2 product (ALG-2) interacting protein X (ALIX) [1], also termed ALG-2 interacting protein 1 (AIP1) [2] and the human ortholog of Xp95 (Hp95) [3], is an abundant adaptor protein that is involved in diverse cellular processes, including actin-based cytoskeleton assembly [4], integrin-mediated cell adhesion [5] and ESCRT (endosomal sorting complexes required for transport)-mediated membrane invagination and abscission in cytokinesis and retroviral budding [6–10]. Participation of ALIX in actin-based cytoskeleton assembly requires direct interaction with F-actin and F-actin binding proteins [4]. In cytokinesis, ALIX interacts with membrane-associated ESCRT proteins and CEP55 (centrosome protein 55) at midbody [6, 10]. During retroviral budding, ALIX interacts with both ESCRT and retroviral Gag proteins at the membrane [7–9, 11, 12]. However, although cytoplasmic or recombinant ALIX is able to interact with F-actin and F-actin binding proteins, it fails to interact with ESCRT and viral proteins under non-denaturing conditions [13, 14]. These findings predict the existence of an autoinhibitory mechanism that prohibits the majority of ALIX from participating in ESCRT-mediated processes. Understanding this mechanism is a pre-requisite for elucidating the activating mechanism that specializes a portion of ALIX to function in ESCRT-mediated processes.
Two potential mechanisms may prohibit interaction of the native form of ALIX with ESCRT and viral proteins. One is the lack of functional domains/docking sites in the native conformation of ALIX; the other is the presence of an intramolecular interaction that renders the partner protein binding sites inaccessible. The N-terminal portion (residues 1–359) of ALIX has been shown to form a banana-shaped three-dimensional structure, called the Bro1 domain. The Bro1 domain has two hydrophobic regions, termed Patch 1 and Patch 2, on its surface [15, 16]. The three dimensional Patch 1 is the docking site for CHMP4, and the linear Patch 2 around Y319 is the docking site for the non-ESCRT protein Src. Neither of these docking sites is available in non-denatured cytoplasmic or recombinant ALIX [14]. The middle portion of ALIX (residues 360–701) forms a letter V shaped three-dimensional structure, hence called the V domain, with two arms of unequal lengths. The long arm of the V domain contains a highly hydrophobic pocket, called the F676 pocket, which is the docking site for retroviral Gag proteins such as the HIV-1 (human immunodeficiency virus type-1) p6Gag and EIAV (equine infectious anemia virus) p9Gag [16–18]. This docking site is unavailable to protein binding in cytoplasmic or recombinant ALIX under non-denaturing conditions [13]. The C-terminal portion of ALIX (residues 702–868) is proline-rich; thus it is often called the Pro-rich domain (PRD). Although multiple partner protein docking sites within the PRD have been identified, including the docking site for the ESCRT-I protein TSG101, neither the structure of the PRD nor the availability of the TSG101 docking site (TDS) in the native form of ALIX has been determined. In this study, we constructed specific ALIX domain deletions and mutations and examined their effects on the availability of the docking sites for CHMP4, Src, p6Gag/p9Gag and TSG101. Our results demonstrate that an intramolecular interaction between Patch 2 in the Bro1 domain and the TDS in the PRD locks ALIX in a closed conformation, prohibiting ALIX interaction with any of these partner proteins and, as a result, inhibits ALIX association with the membrane. We also provide the functional evidence that relieving the autoinhibitory intramolecular interaction of ALIX is a critical step for ALIX to participate in budding of EIAV from mammalian cells.
EXPERIMENTAL
Cell culture and cDNA transfection
HEK293 cells were maintained in Dulbecco’s Modification of Eagle’s Medium (DMEM) (Mediatech Inc, Herndon, VA, USA) supplemented with 2 mM L-glutamine and 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, CA, USA). The pCMV-based expression vectors for ALIXΔC and FLAG-ALIXBro1 were generated and used in our previous studies [4, 14]. The pIRES2-based mammalian expression vector for FLAG-TSG101 [12] was a generous gift from Dr. Wesley I. Sundquist (Salt Lake City, UT, USA). The mammalian expression vectors for the wild-type Src (Src-WT) and kinase-defective Src (Src-KD) [19] were generous gifts from Dr. Yun Qiu (Baltimore, MD, USA). The pCMV-based expression vector for FLAG-CHMP4b [20] and the pEGFP-C3-based expression vector for GFP-ALIX [21] were generously provided by Dr. Masatoshi Maki (Nagoya, Japan). The expression vectors for various mutant forms of GFP-ALIX, FLAG-tagged ALIXΔBro1, ALIXPRD and ALIX697–747, and the M95A mutant form of FLAG-TSG101 were generated by PCR-based cloning methods as described in Table S1. Subconfluent cultures of HEK293 cells in 35-mm or 60-mm culture dishes were transfected with cDNA using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instruction.
Production of recombinant proteins and GST pull-down assays
The pGEX-based bacterial expression vectors for GST-ALIX, GST-ALIXBro1, GST-ALIXΔC and GST-Xp95P3 were generated as described in our previous studies [4, 5, 14, 22]. The bacterial expression vectors for GST-p6Gag and GST-p9Gag [12] were generous gifts from Dr. Wesley I. Sundquist (Salt Lake City, UT, USA). The pGEX-4T-3 based expression vectors for various mutant forms of GST-ALIX were generated by PCR-based cloning methods as described in Table S1. GST and GST tagged proteins were produced and purified as previously described [23]. In GST pull-down assays, 0.5–1 μg of GST or GST fusion protein immobilized onto 10 μl glutathione-sepharose (Sigma-Aldrich, St Louis, MO, USA) was incubated with the cytosolic fraction of HEK293 cell lysates at 4°C for 2 hrs, and beads were washed and eluted as described for immunoprecipitation.
Protein extraction, fractionation, immunoblotting and immunoprecipitation
HEK293 cells in monolayer cultures were washed twice with ice-cold PBS (140 mM NaCl in 8 mM Na2HPO4/2 mM NaH2PO4, pH7.1) and scraped from culture dishes in the same buffer. After collected cells were centrifuged at 3,000 g for 5 min, cell pellets were resuspended in 10 volumes of ice-cold Tris-buffered saline (TBS) (150 mM NaCl in 50 mM Tris•HCl, pH7.4), which was supplemented with 1 mM PMSF (Sigma-Aldrich, St Louis, MO, USA) and 1 μg/ml each of leupeptin, pepstatin A and chymostatin (Roche, Basel, Switzerland). Cells in suspension were then lysed by sonication on ice with a Sonic Dismembrator (Fisher Scientific, Pittsburgh, PA, USA), and the cytosolic fraction of proteins was obtained by centrifugation of total cell lysates at 13,000 g for 30 min. Membrane flotation centrifugation of HEK293 cell lysates through a step sucrose gradient was performed as previously described [14]. The anti-ALIX monoclonal antibodies were described previously [4]. The 30-6A1 anti-capsid (CA) monoclonal antibody [24, 25] was a generous gift from Dr. Robert Mealey (Pullman, WA, USA). Other antibodies were purchased from commercial sources as described in Table S2. Prestained molecular weight standard proteins for SDS were purchased from Sigma-Aldrich (St Louis, MO, USA). Immunoblotting and immunoprecipitation were performed according to our standard procedures [4, 13]. Immunoblot signals were quantified by analyzing scanned images with NIH ImageJ 1.41o and plotting results against standard curves from immunoblotting of serially diluted samples. Standard deviations were calculated with Microsoft Office Excel 2003.
Analysis of the production of EIAV virus-like particles (VLPs)
The pEV53B-based mammalian expression vector for infection-defective EIAV [26] was a generous gift from Dr. John Olsen (Chapel Hill, NC, USA). Confluent HEK293 cells grown in 60-mm dishes were transfected with 12 μg of this vector and cultured further for 48 hrs. Production of virus-like particles (VLPs) of EIAV was measured as described by Fisher et al. [16] and Strack et al. [11]. The “fold” of VLP production represents the ratio of VLP production from GFP-ALIX expressing cells to that obtained from mock-treated cells.
RESULTS
The N-terminal portion of the PRD is required for the autoinhibition of ALIX
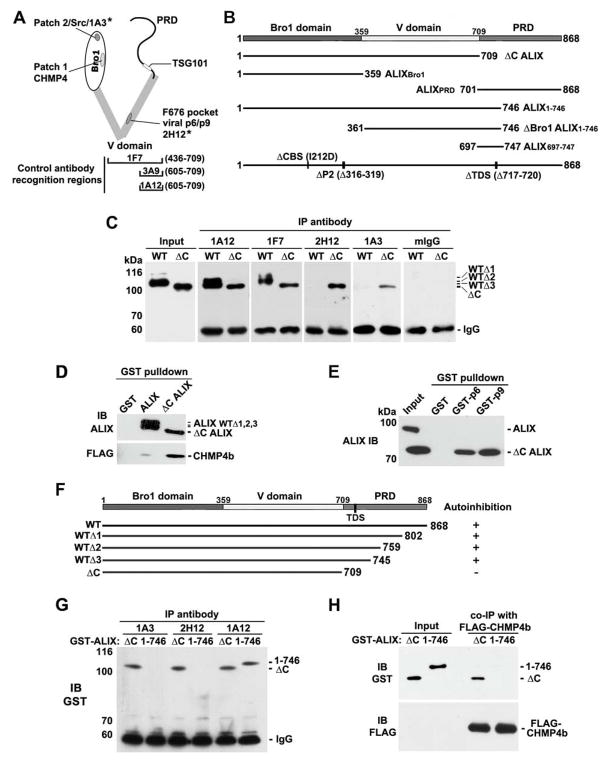
To understand the mechanism by which ALIX interaction with CHMP4, Src and p6Gag/p9Gag is prohibited, we constructed specific ALIX domain deletions and mutations and examined their effects on the availability of these partner protein docking sites in ALIX by immunoprecipitation with specific anti-ALIX monoclonal antibodies and/or ALIX interaction with partner proteins. The antibody recognition sites and partner protein docking sites are illustrated in Figure 1A. As established in our previous studies, the autoinhibition-sensitive 1A3 or 2H12 antibody specifically recognizes Patch 2 in the Bro1 domain or the p6Gag/p9Gag binding site (F676 pocket) in the V domain, whereas the 1F7 and 1A12/3A9 antibodies recognize the PRD adjacent 436–709 and 605–709 regions of ALIX, respectively. Figure 1B illustrates the various mutant forms of ALIX used in this study. Since 1A12, 1F7 and 3A9 monoclonal antibodies each specifically immunoblotted ALIX in HEK293 cell lysates (Figure S1), these three antibodies were used together in ALIX immunoblotting.
Figure 1. The N-terminal portion of the PRD is required for the autoinhibition of ALIX.
(A) Schematic illustration of ALIX, showing its structural domains, partner protein docking sites and recognition sites/regions for specific antibodies used for ALIX recognition. Antibodies that do not recognize the autoinhibited form of ALIX are indicated by stars.
(B) Schematic illustration of the different mutant forms of ALIX used in this study.
(C) WT GST-ALIX and ΔC GST-ALIX were immunoprecipitated with each of the indicated anti-ALIX antibodies or mouse IgG as a control, and immunocomplexes were immunoblotted with an anti-ALIX antibody mix (as described in Materials and Methods).
(D) FLAG-CHMP4b was ectopically expressed in HEK293 cells, after which cytosolic proteins from cell lysates were incubated with immobilized GST, GST-ALIX or ΔC GST-ALIX. Bound proteins were immunoblotted with an anti-ALIX antibody mix (upper panel) and anti-FLAG monoclonal antibody (lower panel).
(E) ΔC ALIX was ectopically expressed in HEK293 cells, after which cytosolic proteins from cell lysates were incubated with immobilized GST, GST-p6 or GST-p9. Bound proteins were immunoblotted with an anti-ALIX antibody mix.
(F) Summary of the autoinhibition states of ALIX fragments examined in (C–E).
(G) ΔC GST-ALIX and GST-ALIX1-746 were immunoprecipitated with each of the indicated anti-ALIX antibodies, and immunocomplexes were immunoblotted with anti-GST monoclonal antibody.
(H) FLAG-CHMP4b was ectopically expressed in HEK293 cells, after which cytosolic proteins from cell lysates were mixed with ΔC GST-ALIX or GST-ALIX1-746. FLAG-CHMP4b was then immunoprecipitated with polyclonal anti-FLAG antibodies, and input recombinant proteins and immunocomplexes were immunoblotted with anti-GST (upper panel) or anti-FLAG (lower panel) monoclonal antibodies, respectively.
In our initial experiments, we determined the role of the C-terminal PRD (709–868) in the autoinhibition of ALIX. As observed in our previous studies [4], GST-ALIX was C-terminally cleaved, and there were three products (WTΔ1, Δ2, Δ3) larger than the PRD-deleted GST-ALIX (ΔC GST-ALIX). Parallel immunoprecipitations of GST-ALIX and ΔC GST-ALIX with each of anti-ALIX antibodies showed that while 1A12 and 1F7 antibodies readily immunoprecipitated both proteins, 2H12 and 1A3 antibodies immunoprecipitated ΔC GST-ALIX but not the cleaved forms (WTΔ1, Δ2, Δ3) of GST-ALIX (Figure 1C). Examination of non-tagged ΔC ALIX ectopically expressed in HEK293 cells generated similar results (data not shown). When partner protein interaction was examined, FLAG-CHMP4b from HEK293 cell lysates interacted with ΔC GST-ALIX much more efficiently than with GST-ALIX (Figure 1D). GST-tagged HIV-1 p6Gag or EIAV p9Gag each interacted with ΔC ALIX but not with ALIX (Figure 1E). Together, these results demonstrate that the PRD is required for the autoinhibition of CHMP4 and Src docking sites in the Bro1 domain and the viral protein docking site in the V domain.
Since the WTΔ1, Δ2, Δ3 of ALIX were previously determined to contain the residues 1–802, 1–759 and 1–745 of ALIX, respectively [4] (Figure 1F), it seems likely that the 709–745 region of ALIX is responsible the autoinhibitory function of the PRD. To test this hypothesis, we produced a GST-tagged 1–746 fragment of ALIX (GST-ALIX1-746) and examined its autoinhibition status. While the 1A12 antibody immunoprecipitated both ΔC GST-ALIX and GST-ALIX1-746, the 2H12 and 1A3 antibodies specifically immunoprecipitated ΔC GST-ALIX (Figure 1G). Similarly, ectopically expressed FLAG-CHMP4b in HEK293 cell lysates interacted with ΔC GST-ALIX but not with GST-ALIX1-746 (Figure 1H). These results support the hypothesis that the autoinhibitory function of the PRD localizes within the 709–745 region.
Patch 2 of the Bro1 domain interacts with the PRD
Structural studies of ALIX’s domains have suggested that the V-shaped middle domain of ALIX may bring the Bro1 domain and the PRD into proximity [8, 16, 17]. To determine whether the autoinhibitory function of the N-terminal portion of the PRD is achieved through its interaction with the Bro1 domain, we first ectopically expressed the FLAG-tagged 697–747 fragment of ALIX (FLAG-ALIX697-747) in HEK293 cells and determined its ability to interact with GST-ALIXBro1. As shown in Figure 2A, FLAG-ALIX697-747 interacted with GST-ALIXBro1 but not with GST, demonstrating that the N-terminal portion of the PRD indeed interacts with the Bro1 domain. Then, we deleted the Bro1 domain from GST-ALIX1-746 to determine if the F676 pocket in the V domain becomes exposed in the Bro1 domain-truncated GST-ALIX1-746 (ΔBro1 GST-ALIX1-746). As shown in Figure 2B, the 2H12 antibody immunoprecipitated ΔBro1 GST-ALIX1-746 but not GST-ALIX1-746, whereas the positive control (1A12 and 1F7) antibodies immunoprecipitated both proteins. These results demonstrate that an intramolecular interaction between the Bro1 domain and the N-terminal portion of the PRD is responsible for ALIX autoinhibition.
Figure 2. Patch 2 of the Bro1 domain interacts with the PRD.
(A) Immobilized GST or GST-ALIXBro1 was incubated with cytosolic proteins from the lysates of HEK293 cells that ectopically expressed FLAG-ALIX697-747. Both input cytosolic proteins and bound proteins were immunoblotted with anti-FLAG (upper panel) or anti-GST (lower panel) monoclonal antibody.
(B) GST-ALIX1-746 and ΔBro1 GST-ALIX1-746 were immunoprecipitated with each of indicated anti-ALIX antibodies, and immunocomplexes were immunoblotted with anti-GST polyclonal antibodies.
(C) HEK293 cells were transfected with an expression vector for Src-WT, Src-KD or an empty vector (control), after which cytosolic proteins from cell lysates were immunoprecipitated with each of the indicated anti-ALIX antibodies. The input cytosolic proteins and resulting immunocomplexes were immunoblotted with an anti-ALIX antibody mix (upper panel) or anti-Src polyclonal antibodies (lower panel).
(D) FLAG-ALIXPRD was ectopically expressed in HEK293 cells, after which cytosolic proteins from cell lysates were incubated with immobilized WT or ΔP2 GST-ALIXBro1. Both input and bound proteins were immunoblotted with anti-FLAG monoclonal antibody (upper panel) or anti-GST polyclonal antibodies (lower panel).
(E) WT or ΔP2 GFP-ALIX was ectopically expressed in HEK293 cells. Following cell lysis, cytosolic proteins from cell lysates were immunoprecipitated with each of the indicated anti-ALIX antibodies. Immunocomplexes were immunoblotted with anti-GFP monoclonal antibody.
(F) WT or ΔP2 GFP-ALIX was ectopically expressed in HEK293 cells. Following cell lysis, cytosolic proteins from cell lysates were incubated with immobilized GST-p9 or GST-p6. Both input and bound proteins were immunoblotted with anti-GFP polyclonal antibodies.
To identify the site in the Bro1 domain that interacts with the N-terminal portion of the PRD, we deleted the N-terminal 160 residues of ALIX, and found that this deletion did not expose the 2H12 epitope (Figure S2). Since this deletion was predicted to disrupt the three-dimensional Patch 1 but not the linear Patch 2 in the Bro1 domain [15], we next tested the possibility that Patch 2 mediates the interaction of the Bro1 domain with the PRD. For this objective, we ectopically expressed in HEK293 cells wild type (WT) or kinase-defective (KD) Src, each of which could bind Patch 2, and determined the effect on the autoinhibition of cytosolic ALIX by immunoprecipitation with anti-ALIX antibodies. As shown in Figure 2C, the conformation-sensitive 2H12 antibody immunoprecipitated a portion of cytosolic ALIX when either Src-WT or Src-KD was expressed, whereas the positive control 1A12 antibody immunoprecipitated ALIX independently of whether Src was expressed. As a confirmation of the binding of Src to Patch 2, the Patch 2 specific 1A3 antibody did not immunoprecipitate cytosolic ALIX under any conditions. These results demonstrate that binding of Patch 2 by a specific partner protein (Src) prevents/relieves the autoinhibitory intramolecular interaction of cytosolic ALIX. Next, we deleted four amino acids within Patch 2 (316–319) from GST-ALIXBro1 and determined its effect on the ability of GST-ALIXBro1 to interact with FLAG-ALIXPRD. Although FLAG-ALIXPRD interacted with GST-ALIXBro1, it did not interact with Patch 2-deleted (ΔP2) GST-ALIXBro1 (Figure 2D), indicating that the N-terminal portion of the PRD interacts with Patch 2 in the Bro1 domain. Further, we produced ΔP2 GFP-ALIX and examined its autoinhibition. As shown in Figure 2E, Patch 2 deletion relieved the autoinhibition of the 2H12 epitope without affecting the accessibility of the 1A12 and 1F7 epitopes. Likewise, Patch 2 deletion allowed interaction of GFP-ALIX with GST-p6Gag and GST-p9Gag (Figure 2F). Taken together, these results demonstrate that the interaction of Patch 2 in the Bro1 domain with the N-terminal portion of the PRD is responsible for the autoinhibition of ALIX.
The TSG101 docking site in the PRD interacts with Patch 2 in the Bro1 domain
The TSG101 docking site (TDS, 717–720) localizes within the N-terminal portion of the PRD that interacts with Patch 2 of the Bro1 domain. As shown in Figure 3A, the non-ionic detergent NP-40, which was previously shown to relieve the autoinhibition of ALIX [13, 14], greatly increased the level of cytosolic ALIX that co-immunoprecipitated with ectopically expressed FLAG-TSG101. Figure 3B shows that FLAG-TSG101 interacted with ΔBro1 GST-ALIX1-746 (non-autoinhibited) but not GST-ALIX1-746 (autoinhibited). Figure 3C shows that FLAG-TSG101 interacted with ΔP2 GFP-ALIX but not GFP-ALIX. All these results demonstrate that the TDS is autoinhibited in the native form of ALIX, which raised the possibility that the TDS mediates the PRD interaction with Patch 2 in the Bro1 domain. To test this possibility, we first ectopically expressed FLAG-TSG101 or the ALIX binding-defective mutant form (M95A) [12] of this protein in HEK293 cells and determined the effect on the autoinhibition status of cytosolic ALIX by immunoprecipitation with anti-ALIX antibodies. As shown in Figure 3D, wild-type but not mutant form of FLAG-TSG101 allowed the conformation-sensitive 2H12 or 1A3 antibody to immunoprecipitate a small portion of cytosolic ALIX. As expected, the immunoprecipitation of the positive control 1A12 antibody was not affected. These results demonstrate that binding of the TDS by TSG101 prevents/relieves the autoinhibitory intramolecular interaction of cytosolic ALIX. Next, we determined whether deletion of the TDS in FLAG-ALIXPRD abolishes its interaction with GST-ALIXBro1. As shown in Figure 3E, ΔTDS FLAG-ALIXPRD indeed does not interact with GST-ALIXBro1, demonstrating that the TDS mediates the interaction of the PRD with the Bro1 domain. For further confirmation, we ectopically expressed the wild type and ΔTDS GFP-ALIX in HEK293 cells, and determined the effect of the TDS deletion on the autoinhibition of ALIX. While 1A12 and 1F7 antibodies readily immunoprecipitated both WT GFP-ALIX and ΔTDS GFP-ALIX, 2H12 and 1A3 antibodies immunoprecipitated ΔTDS GFP-ALIX but not WT GFP-ALIX (Figure 3F). Similar results were obtained when the wild type and ΔTDS GST-ALIX1-746 were immunoprecipitated with these anti-ALIX antibodies (Figure S3). Consistent with these results, GST-tagged p6Gag and p9Gag each interacted with ΔTDS GFP-ALIX but not GFP-ALIX (Figure 3G). FLAG-CHMP4b interacted with ΔTDS GST-ALIX1-746 but not GST-ALIX1-746 (Figure 3H). These results demonstrate that the intramolecular interaction between Patch 2 in the Bro1 domain and the TDS in the PRD is responsible for the autoinhibition of ALIX.
Figure 3. The TSG101 docking site in the PRD interacts with Patch 2 of the Bro1 domain.
(A) FLAG-TSG101 was ectopically expressed in HEK293 cells. Following cell lysis, cytosolic proteins from cell lysates were immunoprecipitated with polyclonal anti-FLAG antibodies in TBS or TBS supplemented with 1% NP-40. Immunocomplexes were immunoblotted with an anti-ALIX antibody mix (upper) or anti-FLAG monoclonal antibody (lower panel).
(B) FLAG-TSG101 was ectopically expressed in HEK293 cells. Following cell lysis, cytosolic proteins from cell lysates were mixed with GST-ALIX1-746 or ΔBro1 GST-ALIX1-746. FLAG-TSG101 was then immunoprecipitated with polyclonal anti-FLAG antibodies, and input recombinant proteins and immunocomplexes were immunoblotted with anti-GST (upper panel) or anti-FLAG (lower panel) monoclonal antibody.
(C) FLAG-TSG101 was co-expressed with either WT or ΔP2 GFP-ALIX in HEK293 cells. Following cell lysis, cytosolic proteins from cell lysates were immunoprecipitated with anti-FLAG monoclonal antibody, and input proteins and immunocomplexes were immunoblotted with anti-GFP (upper panel) or anti-FLAG polyclonal antibodies (lower panel).
(D) HEK293 cells were transfected with an expression vector for WT or the M95A mutant form (mut) of FLAG-TSG101 or an empty vector as a control. Following cell lysis, cytosolic proteins from cell lysates were immunoprecipitated with each of the indicated anti-ALIX antibodies, and input cytosolic proteins and immunocomplexes were immunoblotted with an anti-ALIX antibody mix (upper panel) or anti-FLAG polyclonal antibodies (lower panel).
(E) The wild type (WT) or TDS-deleted (ΔTDS) FLAG-ALIXPRD was ectopically expressed in HEK293 cells. Following cell lysis, cytosolic proteins from cell lysates were incubated with immobilized GST-ALIXBro1, and input and bound proteins were immunoblotted with anti-FLAG (upper panel) or anti-GST monoclonal antibody (lower panel).
(F) WT or ΔTDS GFP-ALIX was ectopically expressed in HEK293 cells. Following cell lysis, cytosolic proteins from each transfectant were split equally into four samples, which were immunoprecipitated with each of the indicated anti-ALIX antibodies. The immunocomplexes were immunoblotted with anti-GFP monoclonal antibody.
(G) WT or ΔTDS GFP-ALIX was ectopically expressed in HEK293 cells. Following cell lysis, cytosolic proteins were incubated with immobilized GST-p9 or GST-p6, and input and bound proteins were immunoblotted with anti-GFP monoclonal antibody.
(H) FLAG-CHMP4b was ectopically expressed in HEK293 cells. Following cell lysis, cytosolic proteins from cell lysates were immunoprecipitated with anti-FLAG polyclonal antibodies. Then, the FLAG-CHMP4b immunocomplex was incubated with WT or ΔTDS GST-ALIX1–746, and input recombinant proteins and bound proteins were immunoblotted with anti-GST (upper panel) or anti-FLAG monoclonal antibody (lower panel).
Relieving the intramolecular interaction of ALIX promotes ALIX association with membrane
Previous studies demonstrated that ALIX is able to localize to the membrane through its interaction with membrane-associated CHMP4 [15, 27]. Since CHMP4 interacts with only the open conformation of ALIX, relieving the autoinhibition of ALIX is likely to play a rate-limiting role in ALIX association with membrane. To test this hypothesis, we ectopically expressed Src-KD or FLAG-TSG101 in HEK293 cells (to relieve the autoinhibition of ALIX) and determined the effect on ALIX association with membrane by membrane flotation centrifugation [28, 29]. As shown in Figure 4A–D, ~10% of ALIX in control cells co-distributed with the membrane marker protein EEA1 (early endosomal antigen 1), and the percentage increased to >40% of ALIX in Src-KD or FLAG-TSG101 expressing cells. In a complimentary approach, we ectopically expressed the wild type or two constitutively opened forms (ΔP2 and ΔTDS) of GFP-ALIX in HEK293 cells and determined the membrane association of these proteins. As shown in Figure 5A–D, ~15% of the wild type GFP-ALIX was recovered in the membrane fraction; whereas, >50% of ΔP2 or ΔTDS GFP-ALIX was membrane bound. The increased association of ALIX with membrane occurred without redistribution of CHMP4. Thus, these results demonstrate that relieving the intramolecular interaction of ALIX is critical to ALIX association with the membrane.
Figure 4. Ectopic expression of Src-KD or TSG101 increases ALIX association with the membrane.
(A–C) HEK293 cells were transfected with an empty vector (A); an expression vector for Src-KD (B) or FLAG-TSG101 (C). Crude cell lysates were fractionated by membrane flotation centrifugation, and proteins in collected fractions were immunoblotted with antibodies for each of the indicated proteins. M indicates membrane protein fractions; S indicates soluble protein fractions.
(D) ALIX in M and S fractions were quantified, and the percentages of total ALIX in each fraction were calculated. Error bars represent standard deviations from three separate experiments.
Figure 5. Deletion of one of the intramolecular interaction sites in ALIX increases ALIX association with the membrane.<.
br>(A–C) HEK293 cells were transfected with an expression vector for WT (A), ΔP2 (B) or ΔTDS (C) GFP-ALIX. Crude cell lysates were fractionated by membrane flotation centrifugation, and proteins in collected fractions were immunoblotted with antibodies for each of the indicated proteins. M indicates membrane protein fractions; S indicates soluble protein fractions.
(D) ALIX in M and S fractions were quantified, and the percentages of total ALIX in each fraction were calculated. Error bars represent standard deviations from three separate experiments.
Relieving the intramolecular interaction of ALIX promotes EIAV budding
Previous studies have demonstrated that the budding of EIAV (equine infectious anemia virus) from infected mammalian cells depends on ALIX interaction with both CHMP4 and EIAV p9Gag [11, 12, 16–18, 27, 30]. Thus, to further establish the physiological significance of the autoinhibitory intramolecular interaction of ALIX, we determined the effect of relieving the autoinhibition of ALIX on ALIX’s ability to facilitate EIAV budding from HEK293 cells. For these studies, a pEV53B-based mammalian expression vector for infection-defective EIAV was co-transfected into HEK293 cells with the expression vector for the wild type, ΔP2, ΔTDS or ΔTDSΔCBS GFP-ALIX, and release of EIAV virus-like particles (VLPs) was measured by immunoblotting cell lysates and VLP proteins with an anti-viral capsid protein (CA) monoclonal antibody [24, 25]. While the unprocessed Gag precursor protein containing CA domain is 55 kDa, the released CA associated with newly produced VLPs is 26 kDa (Figure 6A). As shown in Figure 6B and 6C, ectopic expression of GFP-ALIX had little effect on the production of EIAV VLPs. In contrast, ectopic expression of similar levels of ΔP2 or ΔTDS GFP-ALIX increased the production of EIAV VLPs by >2.5 fold, in the absence of changes in the expression of ALIX and viral Gag proteins. However, the ΔCBS form of ΔTDS GFP-ALIX, which cannot not interact with CHMP4, did not promote production of EIAV VLPs. These results demonstrate that ALIX promotes ESCRT-mediated retroviral budding when it is in the open conformation.
Figure 6. Relieving the intramolecular interaction of ALIX promotes EIAV budding.
(A) Schematic illustrations of unprocessed EIAV Gag precursor protein (p55) and processed capsid (CA) protein (p26).
(B) HEK293 cells were co-transfected with EIAV pEV53B expression vector as well as an expression vector for each of the indicated forms of GFP-ALIX. Both crude cell lysates and released virus-like particles (VLPs) were immunoblotted with antibodies for each of the indicated proteins.
(C) Relative levels of CA in the VLPs released from GFP-ALIX-expressing cells were quantified and normalized against the level obtained from mock-treated cells. Fold change represents the ratio of the VLP production from GFP-ALIX expressing cells as compared to mock-treated cells; error bars represent standard deviations from three separate experiments.
DISCUSSION
ALIX interacts with both CHMP4 and late domains of viral Gag proteins in retroviral budding, and these interactions connect newly produced viruses to the ESCRT machinery. Our previous studies suggested an intrinsic mechanism that prohibits ALIX interaction with CHMP4, Src and viral proteins. In this study, we demonstrate that an intramolecular interaction between the Src docking site (Patch 2) in the N-terminal Bro1 domain and the TSG101 docking site in the C-terminal PRD renders ALIX into a closed conformation that simultaneously prohibits ALIX interaction with CHMP4, Src, viral Gag proteins and TSG101 (Figure 7). Relieving the intramolecular interaction of ALIX by ectopically expressing Src-KD or TSG101 or deleting one of the intramolecular interaction sites in ALIX promotes both ALIX interaction with these partner proteins and ALIX association with the membrane. Moreover, ectopic expression of mutant forms of ALIX that assume a constitutively open conformation promotes EIAV budding from HEK293 cells. These findings predict that any molecular event that prevents/relieves the intramolecular interaction of ALIX is a potential mechanism that allows ALIX to participate in ESCRT-mediated processes.
Figure 7. Diagrammatic representations of the autoinhibitory intramolecular interaction of ALIX and three potential mechanisms that lead to an open conformation.

In the “Competitive binding” mechanism, an intermolecular interaction of proteins labeled as “X” or “Y” at or near the Src docking site or TDS competes with the intramolecular interaction to relieve the autoinhibition of ALIX. In the “Posttranslational modification” mechanism, circled “P” indicates phosphorylation(s) or other protein modification(s) in or near the regions of the intramolecular interaction of ALIX, which prevent the intramolecular interaction. In the “Activator-induced conformational change” mechanism, binding of an activator, indicated as “Z”, to unmasked regions in ALIX leads to a global conformational change, disrupting the intramolecular interaction of ALIX.
Since ectopically expression of high levels of Src-KD or TSG101 that occupies one of the intramolecular interaction sites in ALIX relieves the autoinhibition of a portion of ALIX, it is conceivable that a competitive intermolecular interaction at or near the Src docking site or the TDS relieves the autoinhibition of ALIX (Figure 7, see “Competitive binding” mechanism). Accordingly, the abundance or affinity of intermolecular binding partners may determine the level of ALIX that assumes an open confirmation. Although physiological levels of Src and/or TSG101 alone may not be sufficient to relieve the autoinhibition of ALIX, other higher affinity or higher level binding partners for these intramolecular interaction sites may exist and function as an activator of ALIX function in ESCRT-mediated processes.
As deleting the Src docking site or the TDS in ALIX relieves the autoinhibitory intramolecular interaction of ALIX, posttranslational modifications, such as phosphorylation, in or near either of these sites that change the electrostatic or hydrophobic state of the site may also inhibit the intramolecular interaction of ALIX (Figure 7, see “Posttranslational modification” mechanism). Our previous studies demonstrated that both ALIX and Xp95 are phosphorylated within the N-terminal portion of the PRD in M phase and that the phosphorylation correlates with ALIX/Xp95 recruitment of multiple partner proteins, including AMSH [22]. Based on the findings in this study, it is conceivable that phosphorylation of the N-terminal portion of the PRD prevents its intramolecular interaction with the Bro1 domain, thus allowing ALIX interaction with new partner proteins.
In addition to the above two potential mechanisms, the intramolecular interaction of ALIX may also be relieved by a global conformational change in ALIX induced by binding of an activator (Figure 7, see “Activator-induced conformational change”). In this scenario, the autoinhibitory intramolecular interaction of ALIX is very similar to the autoinhibitory intramolecular interaction of CHMP1-6 proteins in ESCRT-III [31, 32]. CHMP1-6 proteins in common consist of an N-terminal basic domain and a C-terminal acidic domain. The intramolecular interaction between the N-terminal and C-terminal domains of these proteins prevents their membrane binding and the abilities to assemble of the ESCRT-III complex [31–34]. Both events are required for endosomal sorting, viral budding and cytokinesis [35–39]. In the endosomal sorting pathway, the autoinhibition of CHMP6/Vps20 is relieved by binding of the upstream ESCRT-II protein Vps25/EAP20 to the exposed α1 helix of the N-terminal basic domain [32, 40]. This binding induces a conformational change that disrupts the intramolecular interaction and relieves the autoinhibition of CHMP6 [35, 37, 40, 41]. Based on this precedence, it is conceivable that the intramolecular interaction of ALIX may be relieved by binding of an activator to a non-autoinhibited site in ALIX. In this context, it is interesting that outside the N-terminal portion of the PRD required for the autoinhibition of ALIX localize multiple partner protein interaction sites, including the ones for SETA, endophilin, CEP55 and ALG-2 [6, 10, 42, 43]. Further studies will determine whether any of these proteins may be an activator of ALIX function in ESCRT-mediated processes.
In summary, defining the intrinsic mechanism that prohibits ALIX interaction with ESCRT and viral proteins has generated three potential mechanisms that relieve the autoinhibition of ALIX. Identifying the actual mechanisms that specialize a small portion of ALIX to function in retroviral budding and other ESCRT-mediated processes is a critical step toward understanding the physiological regulation and therapeutic intervention of these processes.
Supplementary Material
Acknowledgments
This work was supported by American Cancer Society grant RPG-00-071-01-DDC, NIH/NCI grant 1 RO1 CA93941 and UTMDACC Center for Targeted Therapy CTT-TI3D grant awarded to J. Kuang, and a Hamill and Beimfohr Foundation grant awarded to G.E. Gallick. DNA sequencing was performed by the DNA Analysis Facility of UT M.D. Anderson Cancer Center, which is supported by NCI Grant CA-16672. We thank Drs. M. Maki, R. Mealey, J. Olsen, Y. Qiu and W.I. Sundquist for generously providing published reagents.
ABBREVIATIONS FOOTNOTE
- ALG-2
apoptosis-linked-gene-2 product
- ALIX
ALG-2-interacting protein X
- AIP1
ALG-2-interacting protein 1
- CA
capsid
- CBS
CHMP4 binding site
- CEP55
centrosome protein 55
- CHMP4
charged multivesicular body protein 4
- EEA1
early endosomal antigen 1
- EIAV
equine infectious anemia virus
- ESCRT
endosomal sorting complex required for transport
- GFP
green fluorescent protein
- GST
glutathione S-transferase
- Hp95
human ortholog of Xp95
- HEK293
human embryonic kidney 293 cell line
- HIV-1
human immunodeficiency virus type-1
- PRD
proline-rich domain
- P2
Patch 2
- TBS
Tris-buffered saline
- TSG101
tumor susceptibility gene 101 protein
- TDS
TSG101 docking site
- Vps
vacuolar protein sorting
- WT
wild type
References
- 1.Missotten M, Nichols A, Rieger K, Sadoul R. Alix, a novel mouse protein undergoing calcium-dependent interaction with the apoptosis-linked-gene 2 (ALG-2) protein. Cell Death Differ. 1999;6:124–129. doi: 10.1038/sj.cdd.4400456. [DOI] [PubMed] [Google Scholar]
- 2.Vito P, Pellegrini L, Guiet C, D’Adamio L. Cloning of AIP1, a novel protein that associates with the apoptosis-linked gene ALG-2 in a Ca2+-dependent reaction. J Biol Chem. 1999;274:1533–1540. doi: 10.1074/jbc.274.3.1533. [DOI] [PubMed] [Google Scholar]
- 3.Wu Y, Pan S, Che S, He G, Nelman-Gonzalez M, Weil MM, Kuang J. Overexpression of Hp95 induces G1 phase arrest in confluent HeLa cells. Differentiation. 2001;67:139–153. doi: 10.1046/j.1432-0436.2001.670406.x. [DOI] [PubMed] [Google Scholar]
- 4.Pan S, Wang R, Zhou X, He G, Koomen J, Kobayashi R, Sun L, Corvera J, Gallick GE, Kuang J. Involvement of the adaptor protein Alix in actin cytoskeleton assembly. J Biol Chem. 2006;285:34640–34650. doi: 10.1074/jbc.M602263200. [DOI] [PubMed] [Google Scholar]
- 5.Pan S, Wang R, Zhou X, Kloc M, Corvera J, Koomen J, Kobayashi R, Sifers R, Gallick GE, Lin SH, Kuang J. Extracellular Alix Regulates Integrin-Mediated Cell Adhesions And Extracellular Matrix Assembly. EMBO J. 2008;27(15):2077–2090. doi: 10.1038/emboj.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlton JG, Martin-Serrano J. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science. 2007;316:1908–1912. doi: 10.1126/science.1143422. [DOI] [PubMed] [Google Scholar]
- 7.Fujii K, Hurley JH, Freed EO. Beyond Tsg101: the role of Alix in ‘ESCRTing’ HIV-1. Nat Rev Microbiol. 2007;5:912–916. doi: 10.1038/nrmicro1790. [DOI] [PubMed] [Google Scholar]
- 8.Gottlinger HG. How HIV-1 hijacks ALIX. Nat Struct Mol Biol. 2007;14:254–256. doi: 10.1038/nsmb0407-254. [DOI] [PubMed] [Google Scholar]
- 9.Martin-Serrano J, Marsh M. ALIX catches HIV. Cell Host & Microbe. 2007;1:5–7. doi: 10.1016/j.chom.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Morita E, Sandrin V, Chung HY, Morham SG, Gygi SP, Rodesch CK, Sundquist WI. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J. 2007;26:4215–4227. doi: 10.1038/sj.emboj.7601850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strack B, Calistri A, Craig S, Popova E, Gottlinger HG. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell. 2003;114:689–699. doi: 10.1016/s0092-8674(03)00653-6. [DOI] [PubMed] [Google Scholar]
- 12.von Schwedler UK, Stuchell M, Muller B, Ward DM, Chung HY, Morita E, Wang HE, Davis T, He GP, Cimbora DM, Scott A, Krausslich HG, Kaplan J, Morham SG, Sundquist WI. The protein network of HIV budding. Cell. 2003;114:701–713. doi: 10.1016/s0092-8674(03)00714-1. [DOI] [PubMed] [Google Scholar]
- 13.Zhou X, Pan S, Sun L, Corvera J, Lin SH, Kuang J. The HIV-1 p6/EIAV p9 docking site in Alix is autoinhibited as revealed by a conformation-sensitive anti-Alix monoclonal antibody. Biochem J. 2008;414:215–220. doi: 10.1042/BJ20080642. [DOI] [PubMed] [Google Scholar]
- 14.Zhou X, Pan S, Sun L, Corvera J, Lee YC, Lin SH, Kuang J. The CHMP4b and Src docking sites in the Bro1 domain are autoinhibited in the native state of Alix. Biochem J. 2009;418:277–284. doi: 10.1042/BJ20081388. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Sitaraman S, Hierro A, Beach BM, Odorizzi G, Hurley JH. Structural basis for endosomal targeting by the Bro1 domain. Dev Cell. 2005;8:937–947. doi: 10.1016/j.devcel.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher RD, Chung HY, Zhai Q, Robinson H, Sundquist WI, Hill CP. Structural and biochemical studies of ALIX/AIP1 and its role in retrovirus budding. Cell. 2007;128:841–852. doi: 10.1016/j.cell.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 17.Lee S, Joshi A, Nagashima K, Freed EO, Hurley JH. Structural basis for viral late-domain binding to Alix. Nat Struct Mol Biol. 2007;14:194–199. doi: 10.1038/nsmb1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhai Q, Fisher RD, Chung HY, Myszka DG, Sundquist WI, Hill CP. Structural and functional studies of ALIX interactions with YPX(n)L late domains of HIV-1 and EIAV. Nat Struct Mol Biol. 2008;15:43–49. doi: 10.1038/nsmb1319. [DOI] [PubMed] [Google Scholar]
- 19.Chen R, Kim O, Yang J, Sato K, Eisenmann KM, McCarthy J, Chen H, Qiu Y. Regulation of Akt/PKB activation by tyrosine phosphorylation. J Biol Chem. 2001;276:31858–31862. doi: 10.1074/jbc.C100271200. [DOI] [PubMed] [Google Scholar]
- 20.Katoh K, Shibata H, Suzuki H, Nara A, Ishidoh K, Kominami E, Yoshimori T, Maki M. The ALG-2-interacting protein Alix associates with CHMP4b, a human homologue of yeast Snf7 that is involved in multivesicular body sorting. J Biol Chem. 2003;278:39104–39113. doi: 10.1074/jbc.M301604200. [DOI] [PubMed] [Google Scholar]
- 21.Shibata H, Yamada K, Mizuno T, Yorikawa C, Takahashi H, Satoh H, Kitaura Y, Maki M. The penta-EF-hand protein ALG-2 interacts with a region containing PxY repeats in Alix/AIP1, which is required for the subcellular punctate distribution of the amino-terminal truncation form of Alix/AIP1. J Biochem (Tokyo) 2004;135:117–128. doi: 10.1093/jb/mvh014. [DOI] [PubMed] [Google Scholar]
- 22.Dejournett RE, Kobayashi R, Pan S, Wu C, Etkin LD, Clark RB, Bogler O, Kuang J. Phosphorylation of the proline-rich domain of Xp95 modulates Xp95 interaction with partner proteins. Biochem J. 2007;401:521–531. doi: 10.1042/BJ20061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Che S, Weil MM, Nelman-Gonzalez M, Ashorn CL, Kuang J. MPM-2 epitope sequence is not sufficient for recognition and phosphorylation by ME kinase-H. FEBS Lett. 1997;413:417–423. doi: 10.1016/s0014-5793(97)00948-4. [DOI] [PubMed] [Google Scholar]
- 24.McGuire TC, O’Rourke KI, Baszler TV, Leib SR, Brassfield AL, Davis WC. Expression of functional protease and subviral particles by vaccinia virus containing equine infectious anaemia virus gag and 5′ pol genes. J Gen Virol. 1994;75 (Pt 4):895–900. doi: 10.1099/0022-1317-75-4-895. [DOI] [PubMed] [Google Scholar]
- 25.Mealey RH, Leib SR, Littke MH, Wagner B, Horohov DW, McGuire TC. Viral load and clinical disease enhancement associated with a lentivirus cytotoxic T lymphocyte vaccine regimen. Vaccine. 2009;27:2453–2468. doi: 10.1016/j.vaccine.2009.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsen JC. Gene transfer vectors derived from equine infectious anemia virus. Gene therapy. 1998;5:1481–1487. doi: 10.1038/sj.gt.3300768. [DOI] [PubMed] [Google Scholar]
- 27.McCullough J, Fisher RD, Whitby FG, Sundquist WI, Hill CP. ALIX-CHMP4 interactions in the human ESCRT pathway. Proc Natl Acad Sci USA. 2008;105:7687–7691. doi: 10.1073/pnas.0801567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spearman P, Horton R, Ratner L, Kuli-Zade I. Membrane binding of human immunodeficiency virus type 1 matrix protein in vivo supports a conformational myristyl switch mechanism. J Virol. 1997;71:6582–6592. doi: 10.1128/jvi.71.9.6582-6592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ono A, Freed EO. Binding of human immunodeficiency virus type 1 Gag to membrane: role of the matrix amino terminus. J Virol. 1999;73:4136–4144. doi: 10.1128/jvi.73.5.4136-4144.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Usami Y, Popov S, Gottlinger HG. Potent rescue of human immunodeficiency virus type 1 late domain mutants by ALIX/AIP1 depends on its CHMP4 binding site. J Virol. 2007;81:6614–6622. doi: 10.1128/JVI.00314-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shim S, Kimpler LA, Hanson PI. Structure/function analysis of four core ESCRT-III proteins reveals common regulatory role for extreme C-terminal domain. Traffic. 2007;8:1068–1079. doi: 10.1111/j.1600-0854.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 32.Bajorek M, Schubert HL, McCullough J, Langelier C, Eckert DM, Stubblefield WM, Uter NT, Myszka DG, Hill CP, Sundquist WI. Structural basis for ESCRT-III protein autoinhibition. Nat Struct Mol Biol. 2009;16:754–762. doi: 10.1038/nsmb.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zamborlini A, Usami Y, Radoshitzky SR, Popova E, Palu G, Gottlinger H. Release of autoinhibition converts ESCRT-III components into potent inhibitors of HIV-1 budding. Proc Natl Acad Sci USA. 2006;103:19140–19145. doi: 10.1073/pnas.0603788103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lata S, Roessle M, Solomons J, Jamin M, Gottlinger HG, Svergun DI, Weissenhorn W. Structural basis for autoinhibition of ESCRT-III CHMP3. J Mol Biol. 2008;378:816–825. doi: 10.1016/j.jmb.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saksena S, Wahlman J, Teis D, Johnson AE, Emr SD. Functional reconstitution of ESCRT-III assembly and disassembly. Cell. 2009;136:97–109. doi: 10.1016/j.cell.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carlton JG, Agromayor M, Martin-Serrano J. Differential requirements for Alix and ESCRT-III in cytokinesis and HIV-1 release. Proc Natl Acad Sci USA. 2008;105:10541–10546. doi: 10.1073/pnas.0802008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wollert T, Wunder C, Lippincott-Schwartz J, Hurley JH. Membrane scission by the ESCRT-III complex. Nature. 2009;458:172–177. doi: 10.1038/nature07836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teis D, Saksena S, Emr SD. Ordered assembly of the ESCRT-III complex on endosomes is required to sequester cargo during MVB formation. Dev Cell. 2008;15:578–589. doi: 10.1016/j.devcel.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 39.Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464:864–869. doi: 10.1038/nature08849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Im YJ, Wollert T, Boura E, Hurley JH. Structure and function of the ESCRT-II-III interface in multivesicular body biogenesis. Dev Cell. 2009;17:234–243. doi: 10.1016/j.devcel.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teis D, Saksena S, Judson BL, Emr SD. ESCRT-II coordinates the assembly of ESCRT-III filaments for cargo sorting and multivesicular body vesicle formation. EMBO J. 2010;29(5):871–83. doi: 10.1038/emboj.2009.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Odorizzi G. The multiple personalities of Alix. J Cell Sci. 2006;119:3025–3032. doi: 10.1242/jcs.03072. [DOI] [PubMed] [Google Scholar]
- 43.Lee HH, Elia N, Ghirlando R, Lippincott-Schwartz J, Hurley JH. Midbody targeting of the ESCRT machinery by a noncanonical coiled coil in CEP55. Science. 2008;322:576–580. doi: 10.1126/science.1162042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.