Abstract
This study determined the effects of the peripherally restricted µ-opiate receptor (µ-OR) antagonist, naloxone methiodide (NLXmi) on fentanyl (25 µg/kg, i.v.)-induced changes in (1) analgesia, (2) arterial blood gas chemistry (ABG) and alveolar-arterial gradient (A-a gradient), and (3) ventilatory parameters, in conscious rats. The fentanyl-induced increase in analgesia was minimally affected by a 1.5 mg/kg of NLXmi but was attenuated by a 5.0 mg/kg dose. Fentanyl decreased arterial blood pH, pO2 and sO2 and increased pCO2 and A-a gradient. These responses were markedly diminished in NLXmi (1.5 mg/kg)-pretreated rats. Fentanyl caused ventilatory depression (e.g., decreases in tidal volume and peak inspiratory flow). Pretreatment with NLXmi (1.5 mg/kg, i.v.) antagonized the fentanyl decrease in tidal volume but minimally affected the other responses. These findings suggest that (1) the analgesia and ventilatory depression caused by fentanyl involve peripheral µ-ORs and (2) NLXmi prevents the fentanyl effects on ABG by blocking the negative actions of the opioid on tidal volume and A-a gradient.
Keywords: Fentanyl, Naloxone methiodide, Ventilation, Arterial blood gases, Analgesia, Rats
1. Introduction
The peripheral administration of opioids such as morphine elicits analgesia in humans (DeHaven-Hudkins and Dolle, 2004; Stein and Lang, 2009) and rodents (Reichert et al., 2001; Lewanowitsch and Irvine, 2002; Lewanowitsch et al., 2006; Stein et al., 2009) via activation of opiate receptors (ORs) in the central nervous system (CNS) and on peripheral nociceptive afferents. Analgesic doses of opioids are associated with a significant incidence of ventilatory depression in humans and rodents via activation of ORs within the CNS and also key peripheral structures such as the carotid bodies and neuromuscular components of the chest-wall and diaphragm (Dahan et al., 2010). In addition, opiates increase pulmonary vascular resistance suggesting decreased perfusion of alveoli (Schurig et al., 1978; Hakim et al., 1992).
Fentanyl is a high-potency opiate that is widely prescribed to treat acute and chronic pain (Nelson and Schwaner, 2009; Johnston, 2010). Abuse or misuse lead to significant consequences, including death via depression of ventilation (Nelson and Schwaner, 2009). The mechanisms responsible for the analgesic and ventilatory depressant effects of fentanyl and analogs have been extensively investigated (Dahan et al., 1998; Sarton et al., 1999; Stein et al., 2009). Although fentanyl is thought of as a selective µ-OR agonist (Trescot et al., 2008; Hajiha et al., 2009) and has high affinity for µ-ORs (Raynor et al., 1994; Huang et al., 2001), it also activates δ- and κ-ORs with affinities/intrinsic activities of biological significance (Yeadon and Kitchen, 1990; Zhu et al., 1996, Butelman et al., 2002; Gharagozlou et al., 2006). For example, whereas fentanyl has low affinity for κ-ORs it has a remarkably high efficacy at these ORs (Gharagozlou et al., 2006).
The relative contributions of central and peripheral ORs in the analgesic and ventilatory effects of fentanyl have received little attention. One approach to evaluating these contributions is to compare the effects of centrally-penetrant and -impenetrant OR antagonists on the fentanyl-induced responses. Naloxone (NLX) is an OR antagonist that readily enters the CNS (DeHaven-Hudkins and Dolle, 2004; Stein et al., 2009). NLX is an effective antagonist of µ-, δ- and κ-ORs although it has ≈twice the affinity for µ-ORs than for δ-ORs and ≈15 times greater affinity for µ-ORs than κ-ORs (see Lewanowitsch and Irvine, 2003). In contrast, it appears that naloxone methiodide (NLXmi) does not cross the blood–brain barrier in rodents (Lewanowitsch and Irvine, 2002; Lewanowitsch et al., 2006; Inglis et al., 2008; He et al., 2009). NLXmi has lower affinities for µ-, δ- and κ-ORs than NLX (Lewanowitsch and Irvine, 2003). For example: (1) NLXmi has ≈20 times lower affinity for µ-ORs in rat brain membranes than NLX (Bianchetti et al., 1983; Valentino et al., 1983), (2) NLXmi had ≈10 times lower affinity for µ-, κ- and δ-ORs in guinea pig brain homogenates than NLX (Magnan et al., 1982), and (3) binding affinities for NLX versus NLXmi in mouse brain homogenates was 15:1 for µ-, 6:1 for κ- and 330:1 for δ-ORs (Lewanowitsch and Irvine, 2003). Evidence that the analgesic and ventilatory depressant effects of morphine, methadone and heroin in mice were reversed by NLXmi (Lewanowitsch and Irvine, 2002; Lewanowitsch et al., 2006) provides evidence that peripheral ORs are involved in the effects of these opioids.
Since the relative contributions of central and peripheral ORs in the analgesic and ventilatory effects of fentanyl are not known, the aims of this study were to use conscious rats to determine (1) the effects of NLXmi (1.5 mg/kg, i.v.) on fentanyl-induced changes in analgesia status, arterial blood-gas chemistry (ABG) and Alveolar-arterial (A–a) gradient, an index of ventilation–perfusion (Stein et al., 1995; Story, 1996) and (2) the effects of 1.5 mg/kg doses of NLXmi or NLX on fentanyl-induced changes in ventilatory parameters. These studies were designed to discern which of the ventilatory responses and/or changes in A-a gradient were responsible for the fentanyl-induced changes in ABG chemistry, and the role of peripheral ORs in these responses.
2. Methods
2.1. Rats and surgeries
All studies were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23) revised in 1996. The protocols were approved by the Animal Care and Use Committee of the University of Virginia. Adult male Sprague-Dawley rats were purchased from Harlan (Madison, WI) with jugular vein catheters and/or femoral artery catheters (surgeries performed under ketamine-xylazine). The rats were allowed 5 days to recover from surgery and transport and were used for the blood-gas and analgesia studies. Other adult male Sprague-Dawley rats were purchased from Harlan and venous catheters were implanted under 2% isoflurane anesthesia. These rats were given 4 days to recover from surgery and were used for the plethysmography and body temperature (TB) studies.
2.2. Protocols for blood gas measurements and determination of Arterial-alveolar gradient
Study 1: Arterial blood samples (200 µL) were taken before and 5 and 12 min after injection of vehicle (saline, i.v.; n = 6 rats; 319 ± 3 g) or NLXmi (1.5 mg/kg, i.v.; n = 6 rats; 323 ± 4 g) and 1, 5 and 9 min after injection of fentanyl (25 µg/kg, i.v.). Study 2: Arterial blood samples (200 µL) were taken before and 5, 10 and 15 min after injection of vehicle (n = 6 rats; 312 ± 2 g) or NLXmi (1.5 mg/kg, i.v.; n = 6 rats; 310 ± 3 g) and 5, 10 and 15 min after injection of fentanyl (50 µg/kg, i.v.). The pH, pCO2, pO2 and sO2 of arterial blood samples (100 µL) were measured by a Radiometer blood-gas machine (ABL800 FLEX). The A-a gradient measures the difference between alveolar and arterial blood concentrations of O2 (Stein et al., 1995; Story, 1996). A decrease in PaO2, without a change in A-a gradient is caused by hypoventilation whereas a decrease in PaO2 with an increase in A-a gradient indicates ventilation–perfusion mismatch or shunting (Stein et al., 1995). A-a gradient = PAO2 − PaO2, where PAO2 is the partial pressure of alveolar O2 and PaO2 is pO2 in arterial blood. PAO2 = [(FiO2×(Patm−PH2O)−(PaCO2/respiratory quotient)], where FiO2 is the fraction of O2 in inspired air; Patm is atmospheric pressure; PH2O is the partial pressure of water in inspired air; PaCO2 is pCO2 in arterial blood; and respiratory quotient (RQ) is the ratio of CO2 eliminated/O2 consumed. We took FiO2 of room-air to be 21% = 0.21, Patm to be 760 mmHg, and PH2O to be 47 mmHg (see Stein et al., 1995; Story, 1996). We took the RQ value of our adult male Sprague-Dawley rats to be 0.9 (Stengel et al., 2010; Chapman et al., 2012).
2.3. Antinociception protocols
Antinociception was determined by the radiant heat tail-flick (TF) assay (Lewis et al., 1991). The intensity of the light was adjusted so that baseline TF latencies were ≈3 s. A cutoff time of 12 s was set to minimize damage to the tail. Rats were injected with vehicle (saline, i.v.; n = 4 rats; 300 ± 1 g) or NLXmi (1.5 mg/kg, i.v.; n = 4; 290 ± 2 g) and after 15 min the rats received fentanyl (25 µg/kg, i.v.). Other rats received vehicle (saline; n = 5 rats; 315 ± 7 g) or NLXmi (5 mg/kg, n = 5 rats; 317 ± 5 g) and after 15 min, an injection of fentanyl (25 µg/kg, i.v.). The rats were tested for antinociception at 15, 45, 60, 90 and 120 min post-fentanyl. Data are presented as TF latencies (s) and as “maximum possible effect” (%MPE) using the formula, %MPE = [(post-injection TF latency − baseline TF latency)/(12 − baseline TF latency)] × 100.
2.4. Body temperature (TB) protocols
Changes in TB can significantly impact the magnitude of recorded flow-related variables in plethysmography chambers (Mortola and Frappell, 1998). Although our plethysmography chambers are not equipped to continuously monitor TB, it remains imperative to record TB to better understand the influences of NLXmi and NLX on fentanyl-induced changes in ventilation and RQ. Adult male Sprague-Dawley rats of approximately 300 g were placed in separate open plastic boxes and allowed 60–90 min to acclimatize. TB was recorded as described previously (Kregel et al., 1997). In brief, a thermistor probe was inserted 5–6 cm into the rectum to allow regular recording of TB. A 2–3 in. length of the probe cable, which was connected to a telethermometer (Yellow Springs Instruments, South Burlington, Vermont), was taped to the tail. TB was recorded every 5 min during the acclimatization period to establish baseline values. One group of rats (n = 6) received an injection of vehicle (saline, i.v.) whereas other groups (n = 6 rats per group) received either NLX (1.5 mg/kg, i.v.) or NLXmi (1.5 or 5.0 mg/kg, i.v.). After 15 min, the four groups of rats received fentanyl (25 µg/kg, i.v.). TB was recorded 5, 10 and 15 min after injection of vehicle or the OR antagonists, and 5, 10, 15 and 20 min after injection of fentanyl. These and all rats used in the other described studies were not fasted prior to use in the experiments.
2.5. Ventilatory protocols
Ventilatory parameters were continuously recorded in rats using a whole body plethysmography system (PLY 3223; Buxco Inc., Wilmington, NC, USA), as described previously (Kanbar et al., 2010; Young et al., 2013). The parameters were (1) frequency of breathing (fR), (2) tidal volume (VT), (3) minute ventilation (V˙ = fR × VT), (4) inspiratory time (TI), (5) expiratory time (TE), (6) end inspiratory pause (EIP), time between end of inspiration and start of expiration, (7) peak inspiratory flow (PIF), and (8) peak expiratory flow (PEF). Provided software constantly corrected digitized values for changes in chamber temperature and humidity and a rejection algorithm was included in the breath-by-breath analysis to exclude nasal breathing. The rats were placed in the plethysmography chambers and allowed 45–60 min to acclimatize. One group of rats received a bolus injection of vehicle (saline, i.v.) whereas another group received an injection of NLX (1.5 mg/kg, i.v.). After 15 min, both groups of rats received an injection of fentanyl (25 µg/kg, i.v.) and parameters recorded for a further 20 min. In another study, one group of rats received an injection of vehicle (saline, i.v.) and another received an injection of NLXmi (1.5 mg/kg, i.v.). After 15 min, both groups of rats received an injection of fentanyl (25 µg/kg, i.v.) and parameters recorded for a further 20 min. The body weights of the four groups of rats (n = 6 rats per group) were 328 ± 3, 331 ± 3, 334 ± 3 and 329 ± 5 g, respectively (P > 0.05, for all between-group comparisons). Due to the closeness of these body weights, ventilatory data are presented without body weight corrections.
2.6. Statistics
All data are presented as mean ± SEM and were analyzed by one-way or two-way analysis of variance followed by Student's modified t test with Bonferroni corrections for multiple comparisons between means using the error mean square terms from the analyses of variance (Wallenstein et al., 1980). A value of P < 0.05 was taken to denote statistical significance.
3. RESULTS
3.1. Tail-flick latencies
Fentanyl (25 µg/kg) elicited a robust antinociception of at least 2h in duration in vehicle-treated (VEH) rats and smaller but not statistically different responses in NLXmi (1.5 mg/kg)-treated (NLXmi) rats (Fig. 1). Pretreatment with a higher dose of NLXmi (5 mg/kg) markedly attenuated but did not abolish the antinociceptive effects of the 25 µg/kg dose of fentanyl (Fig. 1).
Fig. 1.
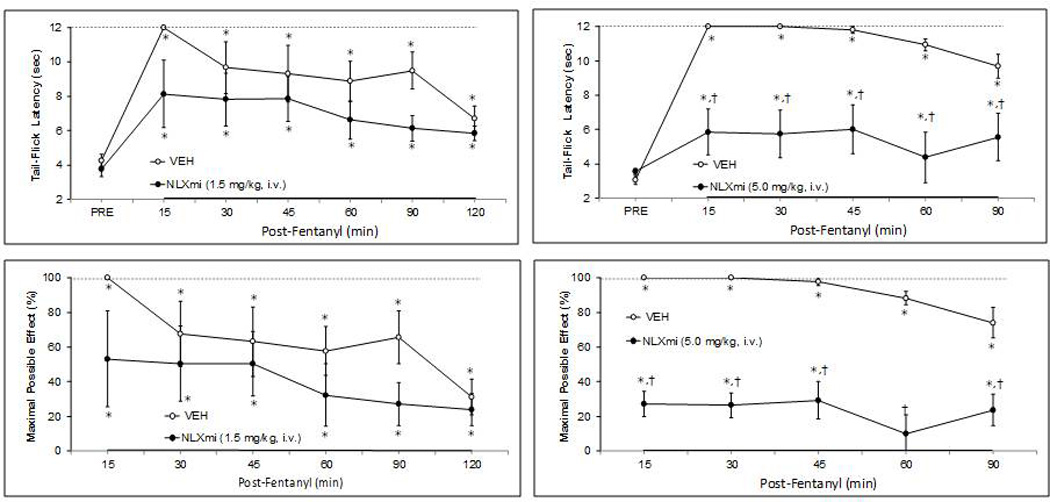
Effects of fentanyl (25 µg/kg, i.v.) on Tail-Flick latencies in rats pretreated with (a) vehicle (n=4) or naloxone methiodide (NLXmi; 1.5 mg/kg, i.v., n=4) (upper-left panel) or (b) vehicle (n=5) or naloxone methiodide (NLXmi; 5.0 mg/kg, i.v., n=5) (upper-right panel). The data are also expressed as maximal possible effect (%MPE) (lower panels). All data are presented as mean ± SEM. *P < 0.05, significant change from Post-Drug value. †P < 0.05, NLXmi versus vehicle.
3.2. Arterial blood gas chemistry
The effects of fentanyl (25 µg/kg) on ABG chemistry and A-a gradients in VEH or NLXmi (1.5 mg/kg) rats are summarized in Fig. 2. The terms F1, F5 and F9 on the x-axis refer to the values that were recorded 1, 5 and 9 min after the injection of fentanyl. Neither VEH nor NLXmi affected ABG chemistry or A-a gradients. In VEH rats, fentanyl decreased pH, pO2 and sO2 values whereas it elevated pCO2 and A-a gradient values. These fentanyl responses were virtually absent in NLXmi rats. The effects of a higher dose of fentanyl (50 µg/kg) on ABG and A-a gradient in VEH or NLXmi (1.5 mg/kg) rats are summarized in Table 1. This higher dose of fentanyl elicited (1) a decrease in pH at 5, 10 and 15 min, (2) an increase in pCO2 at 5, 10 and 15 min, (3) a decrease in pO2 at 5, 10 and 15 min, (4) a decrease in sO2 at 5 and 10 but not 15 min, and (5) an increase in A-a gradient at 5, 10 and 15 min. The fentanyl responses were markedly attenuated but not abolished by NLXmi.
Fig. 2.
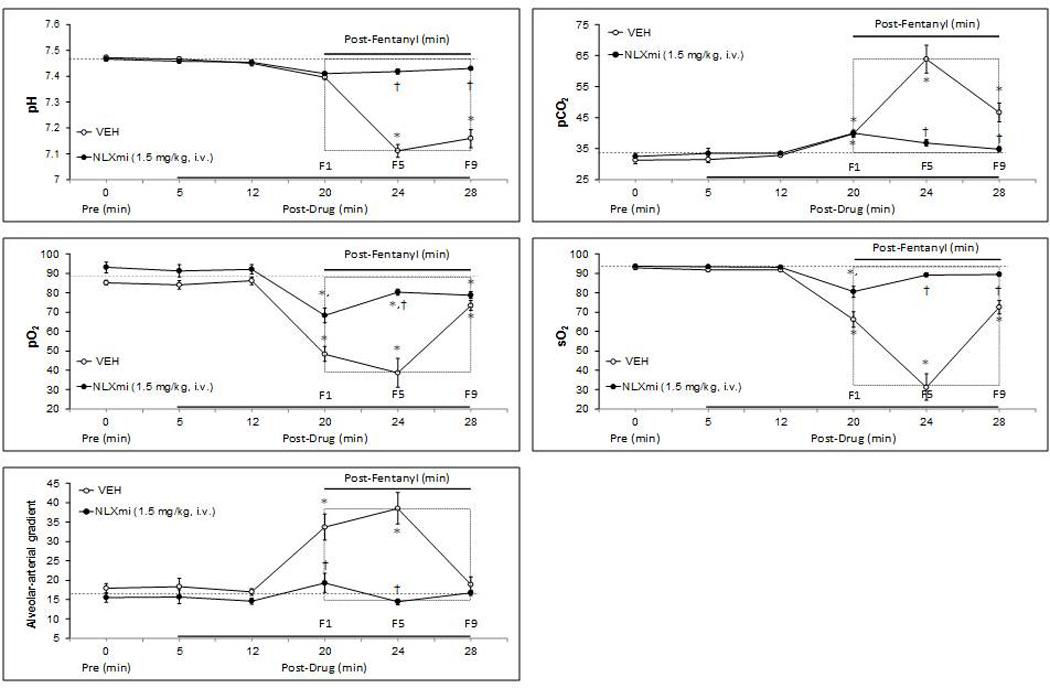
Effects of fentanyl (25 µg/kg, i.v.) on arterial blood pH, pO2, pCO2 and sO2 values and Alveolar-arterial gradients in conscious rats pretreated with vehicle (saline) or naloxone methiodide (NLXmi; 1.5 mg/kg, i.v.). The terms F1, F5 and F9 refer to values recorded 1, 5 and 9 min after administration of fentanyl. The data are presented as mean ± SEM. There were 6 rats in each group. *P < 0.05, significant change from post-drug value. †P < 0.05, NLXmi versus vehicle.
Table 1.
Effects of fentanyl on arterial blood gas values and Arterial-alveolar (A–a) gradients in rats pretreated with vehicle (saline) or naloxone methiodide
| Post-Treatment (min) |
Post-Fentanyl (min) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Group | Pre | T5 | T10 | T15 | F5 | F10 | F15 |
| pH | Vehicle | 7.45 ± 0.01 | 7.46 ± 0.01 | 7.45 ± 0.02 | 7.46 ± 0.02 | 7.15 ± 0.02* | 7.21 ± 0.02* | 7.33 ± 0.01* |
| NLXmi | 7.46 ± 0.02 | 7.43 ± 0.04 | 7.45 ± 0.02 | 7.45 ± 0.02 | 7.35 ± 0.01*,† | 7.38 ± 0.02† | 7.44 ± 0.01† | |
| pCO2 | Vehicle | 35.9 ± 0.5 | 36.3 ± 0.5 | 36.1 ± 0.5 | 35.8 ± 0.7 | 61.2 ± 2.5* | 49.6 ± 1.8* | 43.3 ± 1.4* |
| NLXmi | 36.2 ± 0.6 | 36.5 ± 0.5 | 36.4 ± 0.4 | 36.1 ± 0.5 | 38.0 ± 0.8† | 36.0 ± 0.4† | 35.5 ± 0.7† | |
| pO2 | Vehicle | 94.3 ± 1.2 | 92.6 ± 1.1 | 94.2 ± 1.5 | 94.1 ± 1.2 | 52.2 ± 1.2* | 59.7 ± 1.8* | 76.1 ± 1.0* |
| NLXmi | 93.0 ± 0.5 | 92.2 ± 1.6 | 92.8 ± 0.8 | 92.4 ± 1.6 | 88.7 ± 2.2† | 93.3 ± 1.0† | 95.6 ± 1.1† | |
| sO2 | Vehicle | 99.2 ± 1.0 | 99.5 ± 1.3 | 98.3 ± 0.7 | 99.0 ± 1.0 | 55.8 ± 1.4* | 70.8 ± 2.9* | 92.5 ± 2.2 |
| NLXmi | 98.7 ± 0.8 | 99.7 ± 0.9 | 99.2 ± 0.7 | 99.3 ± 0.9 | 90.2 ± 1.7*,† | 94.3 ± 2.1† | 98.8 ± 1.8 | |
| A-a gradient | Vehicle | 15.5 ± 2.6 | 16.8 ± 2.5 | 15.4 ± 3.2 | 15.9 ± 2.8 | 29.5 ± 4.3* | 34.9 ± 4.4* | 25.5 ± 2.6* |
| NLXmi | 16.5 ± 1.5 | 17.0 ± 1.1 | 16.5 ± 1.9 | 17.2 ± 1.5 | 18.8 ± 3.3† | 16.4 ± 2.2† | 14.7 ± 1.6† | |
Data are shown as mean ± SEM. There were 6 rats in each group. The dose of fentanyl was 50 µg/kg, i.v. The dose of naloxone methiodide (NLXmi) was 1.5 mg/kg, i.v.). The terms T5, T10 and T15 refer to values taken 5, 10 and 15 min after administration of vehicle or NLXmi. The terms F5, F10 and F20 refer to values taken 5, 10 and 20 min after administration of fentanyl.
3.3. Effects of vehicle, NLX and NLXmi on baseline ventilatory parameters
Resting TB was similar in the four groups of rats before drug injection (Table 2). The injection of vehicle, NLX (5.0 mg/kg, i.v.) or NLXmi (1.5 or 5 mg/kg, i.v.) did not affect TB as recorded at +15 min (Table 2). The changes in TB elicited by fentanyl (25 µg/kg) in these rats are summarized in Fig. 3. Fentanyl elicited relatively minor changes in TB in vehicle-treated rats over the 20 min recording period. Pretreatment with the 1.5 or 5 mg/kg doses of NLXmi did not affect these increases in TB whereas pretreatment with NLX abolished the hyperthermic responses.
Table 2.
Effects of treatments on body temperatures.
| Body temperature (°C) |
||||
|---|---|---|---|---|
| Group | Body weight (g) | Pre | Post | Change (°C) |
| Vehicle | 332 ± 3 | 37.8 ± 0.1 | 37.8 ± 0.1 | +0.02 ± 0.09 |
| NLX – 1.5 mg/kg | 330 ± 4 | 37.9 ± 0.1 | 37.8 ± 0.1 | −0.05 ± 0.08 |
| NLXmi – 1.5 mg/kg | 328 ± 3 | 37.7 ± 0.1 | 37.8 ± 0.1 | +0.04 ± 0.10 |
| NLXmi – 1.5 mg/kg | 334 ± 2 | 37.8 ± 0.1 | 37.8 ± 0.1 | +0.03 ± 0.06 |
Data are presented as mean ± SEM. NLX, naloxone. NLXmi, naloxone methiodide. There were 6 rats in each group. There were no between-group differences in body weights or body temperatures (P > 0.05, for all comparisons). None of the treatments affected baseline body temperatures (P > 0.05, for all comparisons).
Fig. 3.
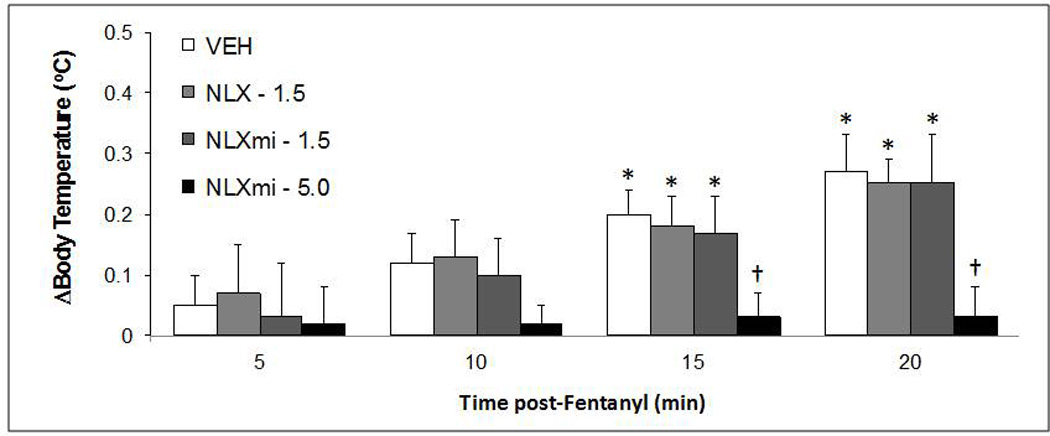
Effects of fentanyl (25 µg/kg, i.v.) on body temperatures (arithmetic change) in conscious rats pretreated with vehicle (saline), naloxone methiodide (NLXmi; 1.5 or 5.0 mg/kg, i.v.) or naloxone (NLX, 1.5 mg/kg, i.v.). The data are presented as mean ± SEM. There were 6 rats in each group. *P < 0.05, significant change from post-drug value. †P < 0.05, NLXmi or NLX versus vehicle.
3.4. Effects of NLX or NLXMI on fentanyl-induced changes in ventilatory parameters
Resting parameters before injection of VEH, NLX or NLXmi are summarized in Table 3. There were no between-group differences in any of these parameters. As seen in Figs. 4–6, the injection of VEH elicited transient changes in some ventilatory parameters (i.e., fR, V˙, TI, TE, PIF and PEF) but not others (VT or EIP). These responses resolved before the injection of fentanyl (25 µg/kg). The injections of NLX or NLXmi elicited responses that were similar to the vehicle responses (Figs. 4–6).
Table 3.
Baseline values in study groups prior to injection of vehicle, naloxone or naloxone methiodide
| Naloxone Study |
Naloxone methiodide Study |
|||
|---|---|---|---|---|
| Parameter | Vehicle | NLX | Vehicle | NLXmi |
| Frequency (breaths/min) | 124 ± 13 | 112 ± 10 | 122 ± 17 | 94 ± 11 |
| Tidal Volume (mls) | 2.11 ± 0.19 | 2.29 ± 0.14 | 2.29 ± 0.13 | 2.45 ± 0.12 |
| Minute Ventilation (mls/min) | 246 ± 23 | 252 ± 27 | 274 ± 31 | 235 ± 34 |
| Inspiratory Time (sec) | 0.17 ± 0.01 | 0.18 ± 0.01 | 0.17 ± 0.01 | 0.21 ± 0.03 |
| Expiratory Time (sec) | 0.37 ± 0.04 | 0.40 ± 0.04 | 0.37 ± 0.05 | 0.46 ± 0.05 |
| End Inspiratory Pause (msec) | 6.9 ± 0.4 | 7.6 ± 0.5 | 8.7 ± 0.7 | 8.6 ± 0.5 |
| Peak Inspiratory Flow (mls/sec) | 19.1 ± 1.7 | 19.9 ± 1.5 | 21.7 ± 1.9 | 19.7 ± 3.1 |
| Peak Expiratory Flow (mls/sec) | 14.0 ± 1.0 | 13.0 ± 0.8 | 13.4 ± 1.2 | 11.8 ± 1.4 |
Data are shown as mean ± SEM. NLX, naloxone. NLXmi, naloxone methiodide. There were 6 rats in each group. There were no significant between-group differences for any parameter (P > 0.05, for all comparisons).
Fig. 4.
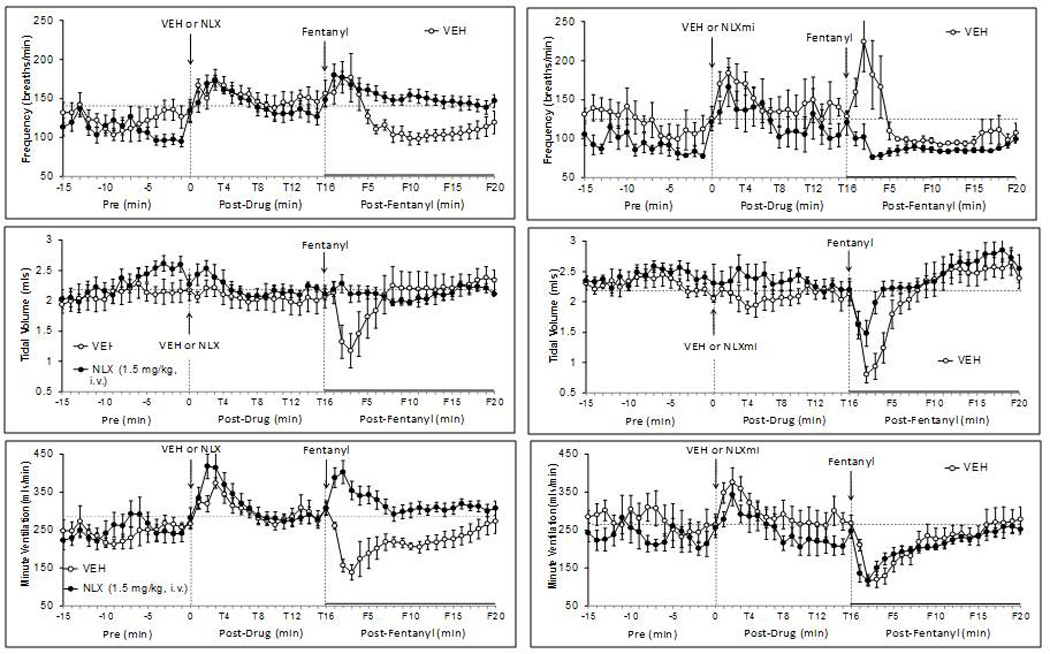
Effects of fentanyl (25 µg/kg, i.v.) on frequency of breathing (top panels), tidal volume (middle panels) and minute volume (bottom panels) in rats pretreated with either vehicle or naloxone (NLX; 1.5 mg/kg, i.v.) (left-hand panels) or vehicle or naloxone methiodide (NLXmi; 1.5 mg/kg, i.v.) (right-hand panels). The stippled horizontal line denotes average resting values immediately before injection of fentanyl. The data are presented as mean ± SEM. There were 6 rats in each group.
Fig. 6.
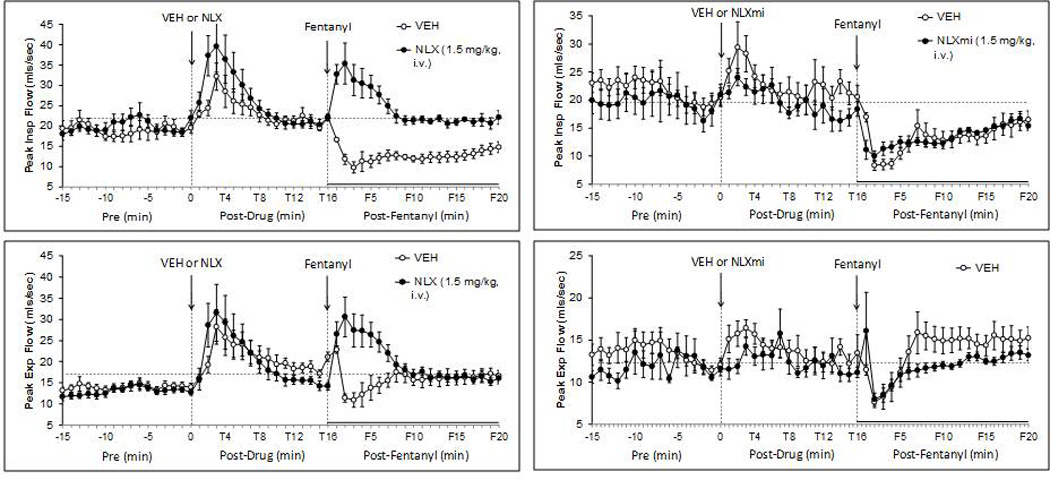
Effects of fentanyl (25 µg/kg, i.v.) on peak inspiratory flow (top panels) and peak expiratory flow (bottom panels) in rats pretreated with either vehicle or naloxone (NLX; 1.5 mg/kg, i.v.) (left-hand panels) or vehicle or naloxone methiodide (NLXmi; 1.5 mg/kg, i.v.) (right-hand panels). The stippled horizontal line denotes average resting values immediately before injection of fentanyl. The data are presented as mean ± SEM. There were 6 rats in each group.
3.5. Effects of NLX or NLXMI on fentanyl-induced changes in ventilatory parameters
3.5.1. fr, VT and V˙
As seen in Fig. 4, fentanyl (25 µg/kg) elicited an increase in fR of about 5 min in duration (F1–F5) in VEH rats, which was followed by a more sustained decrease (F6–F20). The increases in fR were not affected by NLX whereas they were reversed to a decrease in NLXmi rats. The fentanyl-induced decreases in fR were absent in NLX rats but were not affected by NLXmi. Fentanyl decreased VT for about 5 min in VEH rats (F1–F5) with minor F6–F20 changes (Fig. 4). The decreases in VT were abolished by NLX and markedly attenuated by NLXmi. The minor F6–F20 changes in VT were not affected by NLX or NLXmi. Fentanyl decreased VT for about 15 min in VEH rats (Fig. 4). The F1–F5 decreases in V˙ were converted to increases by NLX whereas the F6–F20 responses were abolished. None of the fentanyl changes in V˙ were affected by NLXmi. Response areas (RA, cumulative arithmetic change from pre) for the F1–F5 (Table 4) and F6–F20 (Table 5) time periods confirm that (1) the fentanyl-induced increases in fR were attenuated by NLXmi but not NLX whereas the fentanyl decreases in fR were attenuated by NLX but not NLXmi, (2) the fentanyl-induced decreases in VT (F1–F6) were eliminated by NLX and markedly attenuated by NLXmi whereas the F6–F20 responses were unaffected by NLX or NLXmi, and (3) the fentanyl-induced decreases in V˙ in NLX rats were reversed to increases at F1–F6 and were abolished at F6–F20, and that NLXmi was without effect.
Table 4.
Cumulative responses recorded 1–5 min after injection of fentanyl in vehicle-, naloxone- or naloxone-methiodide-treated rats
| NLX Study |
NLXmi Study |
|||
|---|---|---|---|---|
| Parameter | Vehicle | NLX | Vehicle | NLXmi |
| Frequency (breaths/min) x min | 88 ± 18* | 196 ± 19*, † | 200 ± 39* | −164 ± 18*,† |
| Tidal Volume (mls) x min | −2.53 ± 0.43* | 0.26 ± 0.14† | −4.27 ± 0.52* | −1.49 ± 0.30*,† |
| Minute Ventilation (mls/min) x min | −454 ± 58* | 441 ± 36*,† | −604 ± 73* | −471 ± 66* |
| Inspiratory Time (sec) x min | 0.30 ± 0.08 | −0.27 ± 0.03*,† | 0.26 ± 0.06* | 0.68 ± 0.09*,† |
| Expiratory Time (sec) x min | −0.00 ± 0.06 | −0.12 ± 0.06 | −0.19 ± 0.08 | 0.20 ± 0.13 |
| End Inspiratory Pause (msec) x min | 19 ± 3* | −8 ± 3† | 13 ± 1 | 16 ± 2 |
| Peak Inspiratory Flow (mls/sec) x min | −50 ± 5* | 56 ± 9*,† | −53 ± 6* | −38 ± 5* |
| Peak Expiratory Flow (mls/sec) x min | −23 ± 8 | 68 ± 10*,† | −19 ± 7 | −16 ± 8 |
Data are presented as mean ± SEM. NLX, naloxone. NLXmi, naloxone methiodide. There were 6 rats in each group.
P < 0.05, significant Response Area.
P < 0.05, NLX or NLXmi versus relevant vehicle.
Table 5.
Cumulative responses recorded 6–20 min after injection of fentanyl in vehicle-, naloxone- or naloxone-methiodide-treated rats
| Naloxone Study |
Naloxone methiodide Study |
|||
|---|---|---|---|---|
| Parameter | Vehicle | NLX | Vehicle | NLXmi |
| Frequency (breaths/min) x min | −582 ± 69* | 246 ± 38*,† | −435 ± 51* | −502 ± 60* |
| Tidal Volume (mls) x min | 2.14 ± 0.56 | −0.13 ± 0.18 | 4.06 ± 0.52* | 5.04 ± 0.58* |
| Minute Ventilation (mls/min) x min | −679 ± 73* | 455 ± 67*,† | −444 ± 53* | −337 ± 46* |
| Inspiratory Time (sec) x min | 2.27 ± 0.36* | −0.25 ± 0.08† | 2.25 ± 0.37* | 1.80 ± 0.29* |
| Expiratory Time (sec) x min | 0.13 ± 0.06 | −0.16 ± 0.08 | −0.15 ± 0.06 | −0.20 ± 0.07 |
| End Inspiratory Pause (msec) x min | 121 ± 17* | −9 ± 6 | 109 ± 15* | 66 ± 9*,† |
| Peak Inspiratory Flow (mls/sec) x min | −141 ± 13* | 20 ± 3*,† | −91 ± 9* | −78 ± 9* |
| Peak Expiratory Flow (mls/sec) x min | 24 ± 3* | 1 ± 6*,† | 28 ± 4* | 18 ± 4* |
Data are presented as mean ± SEM. NLX, naloxone. NLXmi, Naloxone methiodide. There were 6 rats in each group. P < 0.05, significant Response Area.
P < 0.05, NLX or NLXmi versus relevant vehicle.
3.5.2. TI, TE and EIP
Fentanyl (25 µg/kg) elicited sustained increases in TI in VEH rats (Fig. 5). The F1–F5 increases in TI were reversed to decreases by NLX but were augmented by NLXmi. The F6–F20 increases in TI were abolished by NLX but were unaffected by NLXmi. Fentanyl elicited minor changes in TE in VEH or NLX rats (Fig. 5). The F1–F5 and F6–20 TE values in NLXmi rats were higher than in VEH rats. These responses were absent in NLX rats. Fentanyl increased EIP for at least 20 min in VEH rats (Fig. 5). The increases in EIP at F1–F10 were similar whereas the F11–F20 values were smaller in NLXmi rats. RAs for the F1–F5 (Table 4) and F6–F20 (Table 5) periods confirm that (1) the fentanyl F1–F5 increases in TI were reversed to decreases by NLX but were augmented by NLXmi, and that the F6–F20 increases in TI were blocked by NLX only, (2) fentanyl elicited minimal effects on TE in VEH, NLX and NLXmi rats, and (3) the fentanyl increases in EIP were eliminated by NLX and that the latter time-points were attenuated by NLXmi.
Fig. 5.
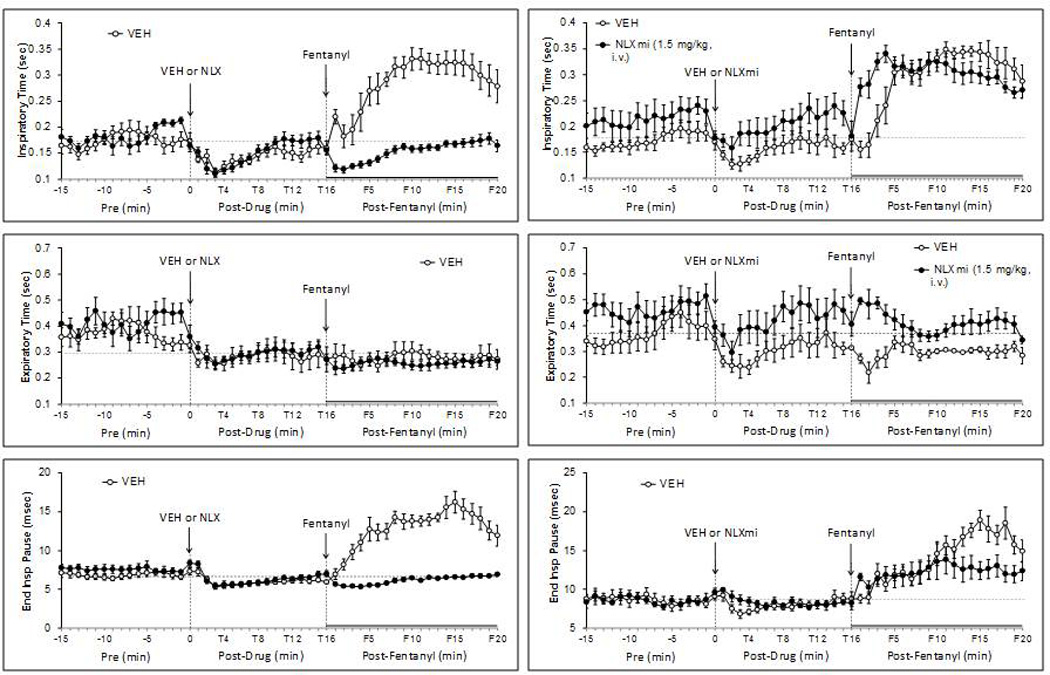
Effects of fentanyl (25 µg/kg, i.v.) on inspiratory time (top panels), expiratory time (middle panels) and end inspiratory pause (bottom panels) in rats pretreated with either vehicle or naloxone (NLX; 1.5 mg/kg, i.v.) (left-hand panels) or vehicle or naloxone methiodide (NLXmi; 1.5 mg/kg, i.v.) (right-hand panels). The stippled horizontal line denotes average resting values immediately before injection of fentanyl. The data are presented as mean ± SEM. There were 6 rats in each group.
3.5.3. PIF and PEF
Fentanyl decreased PIF for at least 20 min in VEH rats (Fig. 6). The F1–F5 decreases in PIF were converted to increases in the presence of NLX whereas F6–F20 responses were abolished. These fentanyl responses were not affected by NLXmi. Fentanyl decreased in PEF for about 5 min in VEH rats (Fig. 6). The F1–F5 decreases in PEF were converted to increases by NLX but were unaffected by NLXmi. The F6–F20 values were similar in VEH or NLX rats whereas these values tended to be lower in NLXmi rats. RAs for the F1–F5 (Table 4) and F6–F20 (Table 5) periods confirm that (1) the fentanyl-induced decreases in PIF were reversed to increases at F1–F6 and were abolished at F6–F20 in NLX rats, and that NLXmi was without effect and (2) the fentanyl decreases in PEF were reversed to increases at F1–F5 in NLX rats and that NLXmi was without effect.
4. DISCUSSION
The present study provides compelling evidence that the ventilatory depressant and analgesic effects of fentanyl involve the activation of peripheral µ-ORs that were sensitive to blockade with NLXmi. On the basis of our findings with NLXmi, it was apparent that the key mechanisms responsible for the deleterious effects of fentanyl on ABG chemistry were mainly dependent on peripheral µ-OR-mediated reductions in VT and mismatch in ventilation–perfusion.
4.1. NLXmi and fentanyl-induced changes in antinociception
A major finding of this study was that although the analgesic action of fentanyl was minimally affected by 1.5 mg/kg of NLXmi, it was markedly affected by a 5 mg/kg dose of this peripherally restricted OR antagonist. These findings support evidence that peripheral ORs participate in the analgesic actions of other opiates (Stein et al., 2009). In contrast to the analgesia, the lower dose of NLXmi virtually eliminated the effects of fentanyl on ABG chemistry and A-a gradient (see below). As such, NLXmi may have greater access to and/or greater affinity/intrinsic activity at OR sub-types mediating the ventilatory depressant effects of fentanyl than for those mediating the analgesic actions of this opioid. This scenario could arise if the relative roles of µ-, δ-and κ-ORs in the ventilatory depressant effects of 25 µg/kg fentanyl differ from those mediating the analgesia. Indeed, µ1-, µ2- and δ-ORs play different roles in morphine-induced (Ling et al., 1985; Su et al., 1998) and sufentanil-induced (Verborgh and Meert, 1999a,b; Verborgh et al., 1997; Latasch and Freye, 2002) analgesia and respiratory depression. Based on the known pharmacology of NLXmi, it could be argued that the effects of the 1.5 mg/kg dose of NLXmi are due mainly to blockade of µ-ORs, lesser blockade of δ-ORs and minimal blockade of κ-ORs. As such, if the ventilatory depressant effects of fentanyl (especially those mediating the changes in ABG and A-a gradient) are mediated primarily via µ-ORs and δ-ORs whereas the analgesic actions are primarily via µ-ORs and κ-ORs, then the 1.5 mg/kg dose of NLXmi would be expected to have relatively greater effects on the ventilatory depressant effects than the analgesic effects of fentanyl.
4.2. Effects of NLX/NLXmi on fentanyl-induced changes in TB
The observation that the injections of NLX and NLXmi had minimal effects on resting TB is consistent with evidence that blockade of peripheral and central µ-ORs has minimal effects on TB in rats (Geller et al., 1983; Colman and Miller, 2002; Cao and Morrison, 2005). The administration of fentanyl elicits a gradual hyperthermia in rats with maximal responses (0.5–0.7 °C) occurring between 45 and 60 min after administration (Geller et al., 1983; Colman and Miller, 2002; Cao and Morrison, 2005; Savić Vujović et al., 2013). The findings that the 25 µg/kg dose of fentanyl elicited minor responses during the 20 min post-injection period is consistent with the above findings. Moreover, our finding that this minor hyperthermia was blocked by NLX but not NLXmi (and therefore likely to be of central origin) is also consistent with previous findings (Geller et al., 1983; Colman and Miller, 2002; Cao and Morrison, 2005). It is important to note that the minor changes in TB observed in this study (about 0.25 °C) are likely to have a minimal impact (less than 2%) effect on the flow-dependent variables (e.g., VT, PIF, and PEF) recorded in the plethysmography chambers (Mortola and Frappell, 1998). Moreover, these minor changes in TB would not be expected to have major effects on RQ (van Klinken et al., 2013).
4.3. Effects of fentanyl on ventilatory parameters, ABG chemistry and A-a gradient
The 25 µg/kg dose of fentanyl elicited (1) a decrease in V˙ via reductions in fR and VT, (2) increases in TI and EIP with no effect on TE, and (3) decreases in PIF and PEF (see Table 6). The decreases in VT and PEF were relatively transient whereas the decreases in fR and therefore V˙ were more sustained. The increases in TI, EIP and PIF lasted for at least 20 min. As such, it is evident that this dose of fentanyl diminished View the V˙ by a combination of reduced rate and efficiency of breathing. The finding that fentanyl markedly affected TI and PIF whereas it elicited relatively minor effects on TE and PEF suggests that fentanyl primarily affected active inspiration rather than passive expiration. In addition, the 25 and 50 µg/kg doses of fentanyl produced changes in ABG chemistry and A-a gradient that were consistent with hypoventilation and mismatch of ventilation-perfusion. Specifically, fentanyl decreased pH, increased pCO2, decreased pO2 and sO2, and increased A-a gradient. The above effects of fentanyl are likely to involve activation of ORs on neurons in ventilatory control regions of the brainstem and spinal cord (Laferriere et al., 1999; Wang et al., 2002; Haji et al., 2003; Lonergan et al., 2003a; Lonergan et al., 2003b) and peripheral tissues such as the carotid bodies, pulmonary arteries and neuromuscular components of the chest-wall and diaphragm (Schurig et al., 1978; Shook et al., 1990; Hakim et al., 1992; Haji et al., 2000; Dahan et al., 2010).
Table 6.
Summary of the effects of naloxone and naloxone methiodide on fentanyl-induced responses
| Fentanyl Responses |
Effects of Antagonists |
|||
|---|---|---|---|---|
| Parameter | Direction | Duration (min) | NLX | NLXmi |
| Frequency | ↑*↓ | 1–5, 6–20 | +++ | 0 |
| Tidal Volume | ↓ | 5 | +++ | ++ |
| Minute Ventilation | ↓ | 20 | +++ | 0 |
| Inspiratory Time | ↑ | 20 | +++ | 0 |
| Expiratory Time | ≈0 | n.a. | n.a. | n.a. |
| End Inspiratory Pause | ↑ | > 20 | +++ | + |
| Peak Inspiratory Flow | ↓ | 20 | +++ | 0 |
| Peak Expiratory Flow | ↓ | 4 | +++ | 0 |
NLX, naloxone. NLXmi, naloxone methiodide. +++ to +, complete to partial blockade; 0, no effect; n.a., not applicable.
The novel finding that fentanyl increased A-a gradient suggests that it increased pulmonary vascular resistance and/or exacerbated the hypoxic pulmonary vasoconstriction due to the fentanyl-induced reduction in ventilation and the concomitant decreases in arterial blood pO2. These findings are consistent with evidence that other opiates increase pulmonary vascular resistance in humans (e.g., Popio et al., 1978; Mitaka et al., 1985) and animals (Schurig et al., 1978; Gentil et al., 1989; Hakim et al., 1992). ORs exist on alveolar walls, smooth muscle of the trachea and bronchi, and on autonomic nerve terminals and vagal afferents in the lungs (Cabot et al., 1996; Zebraski et al., 2000; Groneberg and Fischer, 2001). As such, the increase in A-a gradient elicited by fentanyl is also likely to involve increases in upper and/or lower airways resistance thereby diminishing O2 delivery to alveoli. Indeed, fentanyl increases airway resistance in rats (Willette et al., 1982, 1983, 1987; Bennett et al., 1997) and in rabbits (Taguchi et al., 1986). In humans, fentanyl and sufentanil increase overall airway resistance (Cohendy et al., 1992; Ruiz Neto and Auler Júnior, 1992) via tracheal constriction (Yasuda et al., 1978) and bronchoconstriction (Ruiz Neto and Auler Júnior, 1992). Moreover, fentanyl increases the tendency for airway obstruction at glottic and supraglottic levels and produces rigidity of chest wall and abdominal musculature (Niedhart et al., 1989; Bennett et al., 1997; Bowdle, 1998).
4.4. Effects of NLX/NLXmi on fentanyl changes in ventilation, ABG chemistry, and A-a gradient
The finding that NLX and NLXmi did not elicit ventilatory responses or changes in ABG chemistry or A-a gradients that were different to those of VEH, is consistent with evidence that blockade of endogenous µ1,2-OR-dependent pathways does not have major effects on these parameters (Trescot et al., 2008; Stein and Lang, 2009; Stein et al., 2009; Dahan et al., 2010). As expected, pretreatment with NLX blocked the effects of fentanyl on ABG chemistry and A-a gradient. NLX also blocked some of the ventilatory depressant effects of fentanyl (i.e., the decreases in fR and increases in EIP) whereas it actually reversed (i.e., decrease converted to an increase) many of the depressant effects of fentanyl (i.e., uncovered a facilitatory response) including V˙, TI, PIF and PEF. Since NLXmi did not uncover facilitatory response to fentanyl, the ability of NLX to elicit such responses to fentanyl suggests that blockade of central µ-ORs allows κ- and δ-ORs, ORL1, and/or non-OR-dependent mechanisms to facilitate ventilation (see below).
A key conclusion from the NLXmi studies is that the major mechanisms responsible for fentanyl-induced disturbances in ABG chemistry are the peripheral OR-mediated reductions in VT and the mismatch in ventilation–perfusion (as denoted by the increase in A-a gradient). More specifically, our study demonstrated that the ability of the low dose of NLXmi (1.5 mg/kg) to attenuate the effects of fentanyl on ABG chemistry was correlated with attenuation of the negative effects of fentanyl on (1) A-a gradient, and (2) VT specifically, since NLXmi did not blunt the effects of fentanyl on the other ventilatory parameters except for a minor effect on EIP (Table 6). This raises the possibility that the primary mechanisms by which fentanyl disturbs ABG chemistry is via activation of peripheral ORs on structures regulating VT including the carotid bodies and neuromuscular elements of the chest wall and diaphragm, and ORs within the lungs and pulmonary vasculature (decreased blood flow to alveoli and an increase in upper/lower airways resistance). The findings that NLXmi did not uncover ventilatory excitatory responses to fentanyl as effectively as NLX gives confidence to ascribing the effects of fentanyl to central and peripheral ORs and further supports evidence that NLXmi is peripherally restricted in the rat.
It should be noted that the accurate estimation of A-a gradient would normally require a detailed analysis of the changes in RQ. This quotient can be affected by numerous factors (e.g., changes in TB, hypoxia as elicited by opioids) that directly or indirectly affect metabolic rate and carbohydrate and lipid metabolism (see Mendoza et al., 2013). A switch to carbohydrate metabolism would increase RQ whereas a switch to lipid metabolism would decrease RQ (see Mendoza et al., 2013; van Klinken et al., 2013). The minor changes in BT observed in this study would minimally affect RQ. In addition, the majority of relevant studies provide compelling evidence that fentanyl in similar or higher doses used in the present study have minimal direct effects on carbohydrate and lipid metabolism, which is a major determinant of RQ (van Klinken et al., 2013). For example, (1) fentanyl (50 and 100 µg/kg, i.v.) or sufentanil (10–200 µg/kg, i.v.) had minimal effect on cerebral metabolism/energy status in dogs (Milde et al., 1989, 1990), (2) high-dose fentanyl (100 µg/kg, i.v. loading dose followed by an infusion of 200 µg/kg/h, i.v.), did not alter glucose, lactate or high-energy metabolite levels in the brains of rats (Keykhah et al., 1988), (3) intra-coronary fentanyl produced minor effects on left-ventricular mechanoenergetics in dog hearts over a wide range of blood concentration (Kohno et al., 1997), and (4) fentanyl was devoid of major effects on myocardial metabolism in isolated rat hearts (Blaise et al., 1990). Although several studies have provided evidence that morphine can either enhance or decrease lipid metabolism (see Mendoza et al., 2013). There is little direct evidence as to the effects of fentanyl on lipid metabolism. However, Reneman and Van der Vusse (1982) reported that fentanyl (25 µg/kg, i.v.) decreased fatty acid metabolism slightly in the ischemic myocardium of open-chest dogs. A decrease in fat metabolism, would shift RQ to a value greater than 0.9, which would increase the calculated A-a gradient (van Klinken et al., 2013). As such, keeping to an RQ of 0.9 in the calculations of A-a gradient, would underestimate the negative effects of morphine on gas exchange in the lungs.
5. Summary
This study provided evidence that the fentanyl-induced changes in ABG chemistry are closely related to the temporal decreases in VT and the increases in A-a gradient. The finding that the ability of NLXmi to block the effects of fentanyl on ABG chemistry was strongly correlated with its ability to diminish the fentanyl-induced decrease in VT and the increase in A-a gradient, provides novel evidence that fentanyl exerts detrimental ventilatory responses via activation of peripheral µ1,2-ORs. The importance of peripheral µ1,2-ORs in the ventilatory depressant effects of fentanyl in our rats is consistent with evidence that NLXmi attenuates the inhibitory effects of morphine in mice (Lewanowitsch and Irvine, 2002; Lewanowitsch et al., 2006). It is tempting to assume that the ability of NLXmi to blunt some of the ventilatory effects of fentanyl (i.e., depression of VT and increase in EIP) is due to blockade of µ-ORs in peripheral structures such as the carotid bodies, pulmonary circulation and the chest and diaphragm. However, an extensive search of the literature could not confirm that direct evidence (e.g., analyses of brain levels after peripheral administration) exists as to whether NLXmi actually does enter the brain. In addition, NLXmi has at least 10 times lower affinity for opioid receptors than NLX (e.g., Lewanowitsch and Irvine, 2003) and we have not found any studies that compare the effects of, for example, a 1.5 mg/kg a dose of intravenous NLX with a 10 times higher dose of NLXmi to provide more definitive evidence as to whether NLXmi is centrally-active. Moreover, the ventilatory effects of systemically injected opiates may involve ORs in brain structures devoid of a blood–brain barrier, sites that would be readily accessible to NLXmi. These circumventricular organs of the brain, which include the area postrema (Johnson and Gross, 1993), express µ-, δ-and κ-ORs (Pert et al., 1976; Atweh and Kuhar, 1977; Guan et al., 1999), and direct injections of OR agonists into these structures elicit physiological responses (Hattori et al., 1991; Bhandari et al., 1992; Mosqueda-Garcia et al., 1993).
Although fentanyl inhibits the carotid chemoreceptor reflex in conscious dogs (Mayer et al., 1989), it is not known whether this involves activation of µ1,2-ORs in the carotid bodies or whether fentanyl depresses the responses of carotid body chemoafferents upon exposure to hypoxia and/or hypercapnia. The relative contribution of central and peripheral ORs in the analgesic and ventilatory depressant effects of opiates in humans is still open to question. However, peripherally restricted µ-OR antagonists (e.g., methyl-naltrexone) attenuate opiate-induced constipation and pruritus while preserving (the presumably) centrally mediated analgesia (Moss and Rosow, 2008). Of interest is that these antagonists also attenuate opiate-induced nausea, vomiting and urinary retention, which are traditionally thought to be due to central actions of opiates (Moss and Rosow, 2008).
Acknowledgements
This work was supported by grants from Galleon Pharmaceuticals.
Footnotes
Conflicts of Interest
None
References
- Atweh SF, Kuhar MJ. Autoradiographic localization of opiate receptors in rat brain. III. The telencephalon. Brain Res. 1977;134:393–405. doi: 10.1016/0006-8993(77)90817-4. [DOI] [PubMed] [Google Scholar]
- Bennett JA, Abrams JT, Van Riper DF, Horrow JC. Difficult or impossible ventilation after sufenidil-induced anaesthesia is caused primarily by vocal cord closure. Anesthesiology. 1997;87:1070–1074. doi: 10.1097/00000542-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Bhandari P, Bingham S, Andrews PL. The neuropharmacology of loperamide-induced emesis in the ferret: the role of the area postrema, vagus, opiate and 5-HT3 receptors. Neuropharmacology. 1992;31:735–742. doi: 10.1016/0028-3908(92)90034-m. [DOI] [PubMed] [Google Scholar]
- Bianchetti A, Nisato D, Sacilotto R, Dragonetti M, Picerno N, Tarantino A, Manara L, Angel LM, Simon EJ. Quaternary derivatives of narcotic antagonists: stereochemical requirements at the chiral nitrogen for in vitro and in vivo activity. Life Sci. 1983;33(Suppl 1):415–418. doi: 10.1016/0024-3205(83)90530-1. [DOI] [PubMed] [Google Scholar]
- Blaise GA, Witzeling TM, Sill JC, Vinay P, Vanhoutte PM. Fentanyl is devoid of major effects on coronary vasoreactivity and myocardial metabolism in experimental animals. Anesthesiology. 1990;72:535–541. doi: 10.1097/00000542-199003000-00023. [DOI] [PubMed] [Google Scholar]
- Bowdle TA. Adverse effects of opioid agonists and agonist-antagonists in anaesthesia. Drug Saf. 1998;19:189–193. doi: 10.2165/00002018-199819030-00002. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Ball JW, Kreek MJ. Comparison of the discriminative and neuroendocrine effects of centrally penetrating kappa-opioid agonists in rhesus monkeys. Psychopharmacology (Berl) 2002;164:115–120. doi: 10.1007/s00213-002-1195-y. [DOI] [PubMed] [Google Scholar]
- Cao WH, Morrison SF. Brown adipose tissue thermogenesis contributes to fentanyl-evoked hyperthermia. Am J Physiol Regul Integr Comp Physiol. 2005;288:R723–R732. doi: 10.1152/ajpregu.00669.2004. [DOI] [PubMed] [Google Scholar]
- Cabot PJ, Cramond T, Smith MT. Quantitative autoradiography of peripheral opioid binding sites in rat lung. Eur J Pharmacol. 1996;310:47–53. doi: 10.1016/0014-2999(96)00363-9. [DOI] [PubMed] [Google Scholar]
- Chapman CD, Dono LM, French MC, Weinberg ZY, Schuette LM, Currie PJ. Paraventricular nucleus anandamide signaling alters eating and substrate oxidation. Neuroreport. 2012;23:425–429. doi: 10.1097/WNR.0b013e32835271d1. [DOI] [PubMed] [Google Scholar]
- Cohendy R, Lefrant JY, Laracine M, Rebiere T, Eledjam JJ. Effect of fentanyl on ventilatory resistances during barbiturate general anaesthesia. Brit J Anaesth. 1992;69:595–598. doi: 10.1093/bja/69.6.595. [DOI] [PubMed] [Google Scholar]
- Colman AS, Miller JH. µ-1 Opioid receptor stimulation decreases body temperature in conscious, unrestrained neonatal rats. Exp Biol Med. 2002;227:377–381. doi: 10.1177/153537020222700602. [DOI] [PubMed] [Google Scholar]
- Dahan A, Aarts L, Smith TW. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology. 2010;112:226–238. doi: 10.1097/ALN.0b013e3181c38c25. [DOI] [PubMed] [Google Scholar]
- Dahan A, Sarton E, Teppema L, Olievier C. Sex-related differences in the influence of morphine on ventilatory control in humans. Anesthesiology. 1998;88:903–913. doi: 10.1097/00000542-199804000-00009. [DOI] [PubMed] [Google Scholar]
- DeHaven-Hudkins DL, Dolle RE. Peripherally restricted opioid agonists as novel analgesic agents. Curr Pharm Des. 2004;10:743–757. doi: 10.2174/1381612043453036. [DOI] [PubMed] [Google Scholar]
- Geller EB, Hawk C, Keinath SH, Tallarida RJ, Adler MW. Subclasses of opioids based on body temperature change in rats: acute subcutaneous administration. J Pharmacol Exp Ther. 1983;225:391–398. [PubMed] [Google Scholar]
- Gentil B, Mavier IM, Harf A. Fentanyl-induced airway hyperreactivity in the guinea pig. Eur J Pharmacol. 1989;159:181–185. doi: 10.1016/0014-2999(89)90703-6. [DOI] [PubMed] [Google Scholar]
- Gharagozlou P, Hashemi E, DeLorey TM, Clark JD, Lameh J. Pharmacological profiles of opioid ligands at kappa opioid receptors. BMC Pharmacol. 2006;6:3. doi: 10.1186/1471-2210-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groneberg DA, Fischer A. Endogenous opioids as mediators of asthma. Pulm Pharmacol Ther. 2001;14:383–389. doi: 10.1006/pupt.2001.0305. [DOI] [PubMed] [Google Scholar]
- Guan JL, Wang QP, Nakai Y. Electron microscopic observation of mu-opioid receptor in the rat area postrema. Peptides. 1999;20:873–880. doi: 10.1016/s0196-9781(99)00075-3. [DOI] [PubMed] [Google Scholar]
- Haji A, Takeda R, Okazaki M. Neuropharmacology of control of respiratory rhythm and pattern in mature mammals. Pharmacol Ther. 2000;86:277–304. doi: 10.1016/s0163-7258(00)00059-0. [DOI] [PubMed] [Google Scholar]
- Haji A, Yamazaki H, Ohi Y, Takeda R. Distribution of mu receptors in the ventral respiratory group neurons; immunohistochemical and pharmacological studies in decerebrate cats. Neurosci Lett. 2003;351:37–40. doi: 10.1016/s0304-3940(03)00951-0. [DOI] [PubMed] [Google Scholar]
- Hajiha M, DuBord MA, Liu H, Horner RL. Opioid receptor mechanisms at the hypoglossal motor pool and effects on tongue muscle activity in vivo. J Physiol. 2009;587:2677–2692. doi: 10.1113/jphysiol.2009.171678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim TS, Grunstein MM, Michel RP. Opiate action in the pulmonary circulation. Pulm Pharmacol. 1992;5:159–165. doi: 10.1016/0952-0600(92)90036-g. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Katafuchi T, Koizumi K. Characterization of opioid-sensitive neurons in the anteroventral third ventricle region of polydipsic inbred mice in vitro. Brain Res. 1991;538:283–288. doi: 10.1016/0006-8993(91)90441-w. [DOI] [PubMed] [Google Scholar]
- He L, Kim J, Ou C, McFadden W, van Rijn RM, Whistler JL. Methadone antinociception is dependent on peripheral opioid receptors. J Pain. 2009;10:369–379. doi: 10.1016/j.jpain.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Kehner GB, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist. J Pharmacol Exp Ther. 2001;297:688–695. [PubMed] [Google Scholar]
- Inglis JJ, McNamee KE, Chia SL, Essex D, Feldmann M, Williams RO, Hunt SP, Vincent T. Regulation of pain sensitivity in experimental osteoarthritis by the endogenous peripheral opioid system. Arthritis Rheum. 2008;58:3110–3119. doi: 10.1002/art.23870. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993;7:678–686. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- Johnston KD. The potential for mu-opioid receptor agonists to be anti-emetic in humans: a review of clinical data. Acta Anaesthesiol Scand. 2010;54:132–140. doi: 10.1111/j.1399-6576.2009.02115.x. [DOI] [PubMed] [Google Scholar]
- Kanbar R, Stornetta RL, Cash DR, Lewis SJ, Guyenet PG. Photostimulation of Phox2b medullary neurons activates cardiorespiratory function in conscious rats. Am J Respir Crit Care Med. 2010;182:1184–1194. doi: 10.1164/rccm.201001-0047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keykhah MM, Smith DS, O’Neil JJ, Harp JR. The influence of fentanyl upon cerebral high-energy metabolites, lactate, and glucose during severe hypoxia in the rat. Anesthesiology. 1988;69:566–570. doi: 10.1097/00000542-198810000-00017. [DOI] [PubMed] [Google Scholar]
- Kohno K, Takaki M, Ishioka K, Nakayama Y, Suzuki S, Araki J, Namba T, Suga H. Effects of intracoronary fentanyl on left ventricular mechanoenergetics in the excised cross-circulated canine heart. Anesthesiology. 1997;87:658–666. doi: 10.1097/00000542-199709000-00028. [DOI] [PubMed] [Google Scholar]
- Kregel KC, Kenney MJ, Massett MP, Morgan DA, Lewis SJ. Role of nitrosyl factors in the hemodynamic adjustments to heat stress in the rat. Am J Physiol. 1997;273:H1537–H1543. doi: 10.1152/ajpheart.1997.273.3.H1537. [DOI] [PubMed] [Google Scholar]
- Laferriere A, Liu JK, Moss IR. Mu- and delta-opioid receptor densities in respiratory-related brainstem regions of neonatal swine. Brain Res Dev Brain Res. 1999;112:1–9. doi: 10.1016/s0165-3806(98)00149-7. [DOI] [PubMed] [Google Scholar]
- Latasch L, Freye E. Sufentanil-related respiratory depression and antinociception in the dog. Mediation by different receptor types. Arzneimittelforschung. 2002;52:870–876. doi: 10.1055/s-0031-1299983. [DOI] [PubMed] [Google Scholar]
- Lewanowitsch T, Irvine RJ. Naloxone methiodide reverses opioid-induced respiratory depression and analgesia without withdrawal. Eur J Pharmacol. 2002;445:61–67. doi: 10.1016/s0014-2999(02)01715-6. [DOI] [PubMed] [Google Scholar]
- Lewanowitsch T, Irvine RJ. Naloxone and its quaternary derivative, naloxone methiodide, have differing affinities for mu, delta, and kappa opioid receptors in mouse brain homogenates. Brain Res. 2003;964:302–305. doi: 10.1016/s0006-8993(02)04117-3. [DOI] [PubMed] [Google Scholar]
- Lewanowitsch T, Miller JH, Irvine RJ. Reversal of morphine, methadone and heroin induced effects in mice by naloxone methiodide. Life Sci. 2006;78:682–688. doi: 10.1016/j.lfs.2005.05.062. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Meller ST, Brody MJ, Gebhart GF. Reduced nociceptive effects of intravenous serotonin (5-HT) in the spontaneously hypertensive rat. Clin Exp Hypertens. 1991;A13:849–857. doi: 10.3109/10641969109042089. [DOI] [PubMed] [Google Scholar]
- Ling GS, Spiegel K, Lockhart SH, Pasternak GW. Separation of opioid analgesia from respiratory depression: evidence for different receptor mechanisms. J Pharmacol Exp Ther. 1985;232:149–155. [PubMed] [Google Scholar]
- Lonergan T, Goodchild AK, Christie MJ, Pilowsky PM. Presynaptic delta opioid receptors differentially modulate rhythm and pattern generation in the ventral respiratory group of the rat. Neuroscience. 2003a;121:959–973. doi: 10.1016/s0306-4522(03)00591-8. [DOI] [PubMed] [Google Scholar]
- Lonergan T, Goodchild AK, Christie MJ, Pilowsky PM. Mu opioid receptors in rat ventral medulla: effects of endomorphin-1 on phrenic nerve activity. Respir Physiol Neurobiol. 2003b;138:165–178. doi: 10.1016/s1569-9048(03)00173-3. [DOI] [PubMed] [Google Scholar]
- Magnan J, Paterson SJ, Tavani A, Kosterlitz HW. The binding spectrum of narcotic analgesic drugs with different agonist and antagonist properties. Naunyn Schmiedebergs Arch Pharmacol. 1982;319:197–205. doi: 10.1007/BF00495865. [DOI] [PubMed] [Google Scholar]
- Mayer N, Zimpfer M, Raberger G, Beck A. Fentanyl inhibits the canine carotid chemoreceptor reflex. Anesth Analg. 1989;69:756–762. [PubMed] [Google Scholar]
- Mendoza J, Passafaro R, Baby S, Young AP, Bates JN, Gaston B, Lewis SJ. L-Cysteine ethyl ester reverses the deleterious effects of morphine on arterial blood-gas chemistry in tracheotomized rats. Resp Physiol Neurobiol. 2013;189:136–143. doi: 10.1016/j.resp.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milde LN, Milde JH, Gallagher WJ. Cerebral effects of fentanyl in dogs. Brit J Anaesth. 1989;63:710–715. doi: 10.1093/bja/63.6.710. [DOI] [PubMed] [Google Scholar]
- Milde LN, Milde JH, Gallagher WJ. Effects of sufentanil on cerebral circulation and metabolism in dogs. Anesth Analg. 1990;70:138–146. doi: 10.1213/00000539-199002000-00002. [DOI] [PubMed] [Google Scholar]
- Mitaka C, Sakanishi N, Tsunoda Y, Mishima Y. Comparison of hemodynamic effects of morphine, butorphanol, buprenorphine and pentazocine on ICU patients. Bull Tokyo Med Dent Univ. 1985;32:31–39. [PubMed] [Google Scholar]
- Mortola JP, Frappell PB. On the barometric method for measurements of ventilation, and its use in small animals. Can J Physiol Pharmacol. 1998;76:937–944. doi: 10.1139/cjpp-76-10-11-937. [DOI] [PubMed] [Google Scholar]
- Mosqueda-Garcia R, Inagami T, Appalsamy M, Sugiura M, Robertson RM. Endothelin as a neuropeptide. Cardiovascular effects in the brainstem of normotensive rats. Circ Res. 1993;72:20–35. doi: 10.1161/01.res.72.1.20. [DOI] [PubMed] [Google Scholar]
- Moss J, Rosow CE. Development of peripheral opioid antagonists' new insights into opioid effects. Mayo Clin Proc. 2008;83:1116–1130. doi: 10.4065/83.10.1116. [DOI] [PubMed] [Google Scholar]
- Nelson L, Schwaner R. Transdermal fentanyl: pharmacology and toxicology. J Med Toxicol. 2009;5:230–241. doi: 10.1007/BF03178274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedhart P, Burgener MC, Schweiger J, Suter PM. Chest wall rigidity during fentanyl and midazolam-fentanyl induction: ventiltory and hemodynamic effects. Acta Anaesthesiol Scand. 1989;33:1–5. doi: 10.1111/j.1399-6576.1989.tb02849.x. [DOI] [PubMed] [Google Scholar]
- Pert CB, Kuhar MJ, Snyder SH. Opiate receptor: autoradiographic localization in rat brain. Proc Natl Acad Sci USA. 1976;73:3729–333. doi: 10.1073/pnas.73.10.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popio KA, Jackson DH, Ross AM, Schreiner BF, Yu PN. Hemodynamic and respiratory effects of morphine and butorphanol. Clin Pharmacol Ther. 1978;23:281–287. doi: 10.1002/cpt1978233281. [DOI] [PubMed] [Google Scholar]
- Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol Pharmacol. 1994;45:330–334. [PubMed] [Google Scholar]
- Reichert JA, Daughters RS, Rivard R, Simone DA. Peripheral and preemptive opioid antinociception in a mouse visceral pain model. Pain. 2001;89:221–227. doi: 10.1016/s0304-3959(00)00365-1. [DOI] [PubMed] [Google Scholar]
- Reneman RS, Van der Vusse GJ. Effect of fentanyl on myocardial metabolism during ischemia. Angiology. 1982;33:51–63. doi: 10.1177/000331978203300108. [DOI] [PubMed] [Google Scholar]
- Ruiz Neto PP, Auler Júnior JO. Respiratory mechanical properties during fentanyl and alfentanil anaesthesia. Can J Anaesth. 1992;39:458–465. doi: 10.1007/BF03008710. [DOI] [PubMed] [Google Scholar]
- Sarton E, Teppema L, Dahan A. Sex differences in morphine-induced ventilatory depression reside within the peripheral chemoreflex loop. Anesthesiology. 1999;90:1329–1338. doi: 10.1097/00000542-199905000-00017. [DOI] [PubMed] [Google Scholar]
- Savić Vujović KR, Vučković S, Srebro D, Ivanović M, Došen-Mićović L, Vučetić Č, Džoljić E, Prostran M. A comparison of the antinociceptive and temperature responses to morphine and fentanyl derivatives in rats. Arch Pharm Res. 2013;36:501–508. doi: 10.1007/s12272-013-0072-z. [DOI] [PubMed] [Google Scholar]
- Schurig JE, Cavanagh RL, Buyniski JP. Effect of butorphanol and morphine on pulmonary mechanics, arterial blood pressure and venous plasma histamine in the anesthetized dog. Arch Int Pharmacodyn Ther. 1978;233:296–304. [PubMed] [Google Scholar]
- Shook JE, Watkins WD, Camporesi EM. Differential roles of opioid receptors in respiration, respiratory disease, and opiate-induced respiratory depression. Am Rev Respir Dis. 1990;142:895–909. doi: 10.1164/ajrccm/142.4.895. [DOI] [PubMed] [Google Scholar]
- Stein C, Lang LJ. Peripheral mechanisms of opioid analgesia. Curr Opin Pharmacol. 2009;9:3–8. doi: 10.1016/j.coph.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Stein C, Clark JD, Oh U, Vasko MR, Wilcox GL, Overland AC, Vanderah TW, Spencer RH. Peripheral mechanisms of pain and analgesia. Brain Res Rev. 2009;60:90–113. doi: 10.1016/j.brainresrev.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PD, Goldhaber SZ, Henry JW. Alveolar-arterial oxygen gradient in the assessment of acute pulmonary embolism. Chest. 1995;107:139–143. doi: 10.1378/chest.107.1.139. [DOI] [PubMed] [Google Scholar]
- Stengel A, Coskun T, Goebel M, Wang L, Craft L, Alsina-Fernandez J, Rivier J, Taché Y. Central injection of the stable somatostatin analog ODT8-SST induces a somatostatin2 receptor-mediated orexigenic effect: role of neuropeptide Y and opioid signaling pathways in rats. Endocrinology. 2010;151:4224–4235. doi: 10.1210/en.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story DA. Alveolar oxygen partial pressure, alveolar carbon dioxide partial pressure, and the alveolar gas equation. Anesthesiology. 1996;84:1011. doi: 10.1097/00000542-199604000-00036. [DOI] [PubMed] [Google Scholar]
- Su YF, McNutt RW, Chang KJ. Delta-opioid ligands reverse alfentanil-induced respiratory depression but not antinociception. J Pharmacol Exp Ther. 1998;287:815–823. [PubMed] [Google Scholar]
- Taguchi H, Mima M, Imanishi T, Horiuchi T, Echikawa M, Uchida M. Effects of fentanyl on pulmonary airway dynamics in the rabbit. Masui. 1986;35:379–387. [PubMed] [Google Scholar]
- Trescot AM, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain Physician. 2008;11(2 Suppl):S133–S153. [PubMed] [Google Scholar]
- Valentino RJ, Katz JL, Medzihradsky F, Woods JH. Receptor binding, antagonist, and withdrawal precipitating properties of opiate antagonists. Life Sci. 1983;32:2887–2896. doi: 10.1016/0024-3205(83)90325-9. [DOI] [PubMed] [Google Scholar]
- van Klinken JB, van den Berg SA, van Dijk KW. Practical aspects of estimating energy components in rodents. Front Physiol. 2013;4:94. doi: 10.3389/fphys.2013.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verborgh CM, Camu F, Meert TF. Interaction between sufentanil and U-50488H with respect to antinociception and respiratory depression in rats. Acta Anaesthesiol Scand. 1997;41:895–902. doi: 10.1111/j.1399-6576.1997.tb04806.x. [DOI] [PubMed] [Google Scholar]
- Verborgh C, Meert TF. Antagonistic effects of naloxone and naloxonazine on sufentanil-induced antinociception and respiratory depression in rats. Pain. 1999a;1983:17–24. doi: 10.1016/s0304-3959(99)00068-8. [DOI] [PubMed] [Google Scholar]
- Verborgh C, Meert TF. The effects of intravenous naltrindole and naltrindole 5′-isothiocyanate on sufentanil-induced respiratory depression and antinociception in rats. Pharmacol Biochem Behav. 1999b;63:175–183. doi: 10.1016/s0091-3057(98)00238-x. [DOI] [PubMed] [Google Scholar]
- Wallenstein S, Zucker CL, Fleiss JL. Some statistical methods useful in circulation research. Circ Res. 1980;47:1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Wang QP, Zadina JE, Guan JL, Shioda S. Morphological evidence of endomorphin as an agonist for the mu-opioid receptor in the rat spinal cord. Neurosci Lett. 2002;341:107–110. doi: 10.1016/s0304-3940(03)00182-4. [DOI] [PubMed] [Google Scholar]
- Willette RN, Krieger AJ, Sapru HN. Opioids increase laryngeal resistance and motoneuron activity in the recurrent laryngeal nerve. Eur J Pharmacol. 1982;80:57–63. doi: 10.1016/0014-2999(82)90177-7. [DOI] [PubMed] [Google Scholar]
- Willette RN, Barcas PP, Krieger AJ, Sapru HN. Pulmonary resistance and compliance changes evoked by pulmonary opiate receptor stimulation. Eur J Pharmacol. 1983;91:181–188. doi: 10.1016/0014-2999(83)90463-6. [DOI] [PubMed] [Google Scholar]
- Willette RN, Evans DY, Doorley BM. The in situ isolated larynx for evaluating peripheral opiate receptor antagonists. J Pharmacol Methods. 1987;17:15–25. doi: 10.1016/0160-5402(87)90033-7. [DOI] [PubMed] [Google Scholar]
- Yasuda I, Hiyano T, Yusa T, Satoh M. Tracheal constriction by morphine and by fentanyl in man. Anesthesiology. 1978;49:117–119. doi: 10.1097/00000542-197808000-00012. [DOI] [PubMed] [Google Scholar]
- Yeadon M, Kitchen I. Multiple opioid receptors mediate the respiratory depressant effects of fentanyl-like drugs in the rat. Gen. Pharmacol. 1990;21:655–664. doi: 10.1016/0306-3623(90)91013-h. [DOI] [PubMed] [Google Scholar]
- Young AP, Gruber RB, Discala JF, May WJ, Palmer LA, Lewis SJ. Co-activation of µ- and δ-opioid receptors elicits tolerance to morphine-induced ventilatory depression via generation of peroxynitrite. Resp Physiol Neurobiol. 2013;186:255–264. doi: 10.1016/j.resp.2013.02.028. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebraski SE, Kochenash SM, Raffa RB. Lung opioid receptors: pharmacology and possible target for nebulized morphine in dyspnea. Life Sci. 2000;66:2221–2231. doi: 10.1016/s0024-3205(00)00434-3. [DOI] [PubMed] [Google Scholar]
- Zhu J, Xue JC, Law PY, Claude PA, Luo LY, Yin J, Chen C, Liu-Chen LY. The region in the mu opioid receptor conferring selectivity for sufentanil over the delta receptor is different from that over the kappa receptor. FEBS Lett. 1996;384:198–202. doi: 10.1016/0014-5793(96)00312-2. [DOI] [PubMed] [Google Scholar]


