Abstract
Tobacco smoking has been associated with the development and exacerbation of chronically painful conditions. Conversely, there is reason to believe that smokers may be motivated to use tobacco as a means of coping with their pain. To date, no controlled, experimental studies have tested for a causal relationship between pain and smoking motivation. The primary aim of the current study was to test the hypothesis that laboratory-induced cold-pressor pain would enhance smoking motivation, as measured by self-reported urge to smoke and observation of immediate smoking behavior. Smokers (N = 132) were randomly assigned to either pain or no pain conditions. Results indicated that situational pain increased urge ratings and produced shorter latencies to smoke. The relationship between pain and increased urge to smoke was partially mediated by pain-induced negative affect. The relationship between pain and shorter latency to smoke was fully mediated by pain-induced urge to smoke. This study provides the first experimental evidence that situational pain can be a potent motivator of smoking.
Keywords: Tobacco, smoking, pain, affect, cold-pressor
According to the American Pain Society (2003), over 75 million Americans are totally or partially disabled by serious pain, and more than 50 million suffer from chronic nonmalignant pain. Pain and tobacco smoking have been linked in both the clinical and empirical literature for decades. In fact, the prevalence of smoking among individuals in pain is approximately double that of the general population, indicating that more than half of chronic pain patients are smokers (e.g., Jamison, Stetson, & Parris, 1991).
Although a causal effect has yet to be demonstrated, a copious number of mostly cross-sectional studies provide evidence of an association between smoking and increased prevalence and aggravation of several chronically painful conditions, including: musculoskeletal pain, rheumatoid arthritis, fibromyalgia, oral pain, cluster headaches, and bodily pain in persons with HIV (e.g., Albano, Santana-Sahagun, & Weisman, 2001; Palmer, Syddall, Cooper, & Coggon, 2003; Patel et al., 2006; Riley, Tomar, & Gilbert, 2004; Yunus, Arslan, & Aldag, 2002). Smoking has also been associated with an increased use of opioids by post-surgical patients in pain (e.g., Creekmore, Lugo, & Weiland, 2004). Conversely, most controlled experimental pain induction studies have found evidence for a pain-inhibitory effect of smoking (e.g., Girdler et al., 2005; Jamner, Girdler, Shapiro, & Jarvik, 1998), and there is reason to believe that some smokers may be motivated to use tobacco as a means of coping with their pain.
Researchers have proposed that the avoidance, relief, or both, of pain is a powerful behavioral reinforcer that may be an important mechanism in the maintenance of smoking (e.g., Fertig, Pomerleau, & Sanders, 1986; Silverstein, 1982). For example, on questionnaires administered to smokers being treated for chronic low back pain, 57% of patients acknowledged a need to smoke when in pain, though only 9% of the sample stated that smoking directly affected their pain intensity (Jamison et al., 1991). Additionally, these patients were at greater risk for smoking when their pain was most severe. A more recent cross-sectional study found that smokers who suffered from significant pain in the previous week smoked more cigarettes per day than smokers who indicated no significant pain (Hahn, Rayens, Kirsh, & Passik, 2006). Moreover, 18% of the respondents who had experienced significant pain in the past week reported using cigarettes for pain relief, compared with only 4% who did not endure significant pain. There is also evidence of a positive relationship between daily cigarette consumption and the intensity, frequency, and duration of several chronically painful conditions (e.g., Saag et al., 1997; Scott, Goldberg, Mayo, Stock, & Poitras, 1999; Yunus et al., 2002).
The apparent covariance between pain and smoking may reflect either smokers’ use of tobacco to cope with pain, smoking's aggravation of painful conditions, or both. That is, the direction of causality is uncertain. To date, experimental research on pain and smoking has focused almost exclusively on tobacco's ability to influence the subjective experience of pain. To our knowledge, there have been no experimental investigations into the effect of pain on smoking motivation. Thus, the primary aim of the present study was to test the hypothesis that laboratory-induced cold-pressor pain would elicit greater reports of smoking urge and increases in immediate smoking behavior.
Method
Participants
Smokers (N = 132; 66 women) were recruited through newspaper advertisements and flyers. Inclusion criteria were: age 18-65 (M = 36.0; SD = 11.8), ≥ 20 cigarettes/day (M = 23.2; SD = 6.9), and expired carbon monoxide concentration ≥ 8ppm (M = 23.5; SD = 11.6). Exclusion criteria included contraindicative medical conditions (e.g., current pain, diabetes), and the use of prescription medications for pain management, heart problems, or blood circulation problems. The ethnic composition was 73% Caucasian, 20% African American, 5% American Indian or Alaska Native, and 2% other. Ten percent identified as Hispanic.
Design
Participants were randomly assigned to either pain- or no pain-induction conditions, stratified by gender, in this crossed factorial between-subjects design.1
Measures
Questionnaire of Smoking Urges-Brief (QSU-Brief; Cox, Tiffany, & Christen, 2001)
Our primary dependent measure, this 10-item urge measure consists of two factor-derived subscales (F1: urge to smoke for pleasure/reward, and F2: urge to smoke for the relief of negative affect), with higher scores indicating stronger smoking urges. The QSU-B demonstrated excellent internal consistency (α = .93), as did each of the two subscales (.92 and .89, respectively).
Visual Analogue Scale (VAS)
A single-item VAS was also used to assess smoking urge at four points throughout the study (VAS1, VAS2, VAS3, and VAS4). Participants were asked to rate their urge to smoke by marking along a 100mm line between the phrases “No Urge At All” and “Strongest Urge Ever.” VAS1 and VAS4, which complemented the QSU-Brief, were added to examine urge across the four time points using the same VAS scale. The VAS measures of urge were not available for the first three participants in the study.
Smoking behavior
As a behavioral index of smoking motivation, participants were given an opportunity to smoke following the manipulations. Smoking was recorded with a discrete video camera and was later independently scored by two trained raters using a specialized computer program. Latency to smoke (time until cigarette is first lit) was the smoking behavior of primary interest because experimental manipulations of negative affect and anxiety have typically resulted in decreased smoking latency (e.g., Conklin & Perkins, 2005).
Positive And Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988)
The PANAS is comprised of two orthogonal mood scales (positive and negative), each containing 10 items. This measure was primarily used to assess state-negative affect before and after the pain manipulation. Participants were asked to rate their mood on a 5-point Likert scale. The positive and negative affect scales were internally consistent (α = .91 and .87, respectively).
Numerical Rating Scale (NRS; Dworkin et al., 2005)
The NRS is an 11-point numerical rating scale of pain intensity. Following pain induction, participants were asked to circle the number that best described their pain, at its worst, since placing their hand in the water (0=“No pain”; 10=“Pain as bad as you can imagine”).
Pain Manipulation
Cold Pressor
The cold pressor method of pain stimulation is thought to share some subjective qualities frequently observed in clinical pain patients because of its potential to mimic the unpleasantness experienced by individuals with chronically painful conditions (Keogh, Hatton, & Ellery, 2000; Rainville, Feine, Bushnell, & Duncan, 1992). All participants were told that the maximum cold-pressor tolerance time would be limited to five minutes. Participants in the Pain (P) induction conditions were asked to immerse their non-dominant hand into a circulating cold-water bath (0-1° Celsius) until they felt it was too uncomfortable to continue. Participants in the No Pain (NP) conditions were asked to immerse their non-dominant hand into a room temperature bath until they felt it was too uncomfortable to continue. However, these NP participants were prompted to remove their hand after 100 seconds to approximate the tolerance times of participants in the P conditions (Mitchell, MacDonald, & Brodie, 2004). Both cold pressors were identical insulated cooler units consisting of a perforated screen (to separate the water and ice) and a 12-volt bilge pump (to circulate the water).
Procedure
This study was conducted in two parts during one session that lasted approximately 80 minutes. To standardize smoking behavior prior to the experiment, all participants were asked to smoke one cigarette one hour before their appointment, and none thereafter. As approved by the institutional review board, we employed a two-part consent process to ensure that anticipatory anxiety related to undergoing a cold pressor task would not influence baseline measures. Once the initial informed consent was obtained, participants completed baseline measures (e.g., QSU-B, VAS1, PANAS) and were paid $15 for completing the first part of the study. Participants were then informed that the second part of this study was designed to investigate their pain threshold and tolerance. The experimenter described the cold pressor procedure and provided rationale for conducting the study in two parts. None of the participants declined to proceed with the study. Once the second informed consent was obtained, the experimenter administered a second measure of smoking urge (VAS2) to assess the influence of anticipatory anxiety on smoking motivation. Participants were then randomly assigned to one of the four experimental conditions. Note that both experimenters and participants were blind to condition assignment until this point.
Following randomization, participants were escorted to an experimental room consisting of a table, a chair, and both cold pressor units. To standardize limb temperature and reduce the chance of alterations in vasoconstriction influencing the results, each participant first immersed his/her non-dominant hand in the room temperature bath for two minutes. Immediately afterward, the same hand was immersed to 7 cm above the wrist into either the cold-water bath (Pain conditions) or back into the room temperature bath (No Pain conditions). Participants were instructed to inform the experimenter when the sensations in their hand first became painful (pain threshold) and also indicate when they were no longer willing or able to tolerate the pain by removing their hand (pain tolerance). Immediately after participants in the P conditions reported reaching their pain threshold, the experimenter administered the VAS3 to assess craving during the experience of pain. Participants in the NP conditions were asked to complete VAS3 approximately 10 seconds after they submerged their hand back into the room temperature bath.
Once participants removed their hand from the water they were asked to lay their non-dominant hand across the cold pressor and immediately complete the post-test measures with their dominant hand (NRS, QSU-Brief, VAS4, PANAS). After these measures were collected, participants were provided with paper towels to dry their hand. Once the paper towels were disposed of, participants were told that they were welcome to smoke as much of one cigarette as they would like, but to please take at least one puff. The experimenter then left the room and the participant was videotaped smoking the cigarette. When the experimenter returned after approximately 10 minutes, participants provided a second CO sample, were debriefed, and were compensated an additional $15.
Results
Baseline Measures
There were no significant differences among experimental groups on baseline measures, including those related to smoking urge and negative affect (all ps > .05). Analyses did reveal unexpected group differences on VAS2, with participants in the P conditions reporting significantly greater urge to smoke than participants in the NP conditions, F(3, 125) = 30.30, p < .01. The reason for these group differences remains unclear, however, because both participants and experimenters were blind to condition assignment when the measure was administered. Accordingly, ANCOVAs were conducted in all subsequent analyses to statistically control for VAS2 differences that occurred prior to randomization.
Manipulation Checks: Pain Intensity and Negative Affect
As expected, participants in the P conditions reported much greater pain intensity (M = 7.79; SE = .19) than participants in the NP conditions (M = .46; SE = .19), F(3, 128) = 736.65, p < .001, f = 2.38. Also as expected, participants in the P conditions reported greater pain-induced negative affect (M = 16.86; SE = .71) than participants in the NP conditions (M = 14.47; SE = .71), F(3, 128) = 5.76, p = .02, f = .21.
Reported Urge to Smoke
To examine group differences on self-report measures of smoking urge, 2 X 2 ANCOVAs were conducted, with pre-manipulation urge to smoke (VAS2) as the covariate. Effect sizes, f, were calculated for significant F tests. According to Cohen (1988), f values of .10, .25, and .40 can be considered small, medium, and large, respectively. Analysis of post-test urge to smoke (QSU-Brief Total) indicated a main effect of the pain manipulation, F(4, 124) = 18.75, p < .001, f = .39, such that urge ratings were significantly higher for participants who experienced situational pain (P) than for participants who did not experience pain (NP). A main effect of the pain manipulation was also found for a secondary measure of post-test urge to smoke (VAS4), F(4, 124) = 5.70, p = .02, f = .21. In addition to the QSU-Brief Total, the two QSU-Brief factor-derived subscales (F1: urge to smoke for pleasure/reward, and F2: urge to smoke for the relief of negative affect) were examined for group differences. Analyses indicated a main effect of the pain manipulation for both QSU-Brief F1 [F(4, 124) = 15.99, p < .001, f = .36] and QSU-Brief F2 [F(4, 124) = 12.37, p = .001, f = .32], with greater urges reported by participants who underwent pain induction. VAS3 (urge during pain induction) also showed significant group differences, though significance was not maintained when controlling for VAS2. Unadjusted and covariate-adjusted means and standard errors are presented in Table 1.2
Table 1.
Unadjusted and Covariate-Adjusted Means (and Standard Errors) for Measures of Smoking Motivation
| Unadjusted | VAS2 Adjusted | |||
|---|---|---|---|---|
| P | NP | P | NP | |
| QSU-B T | 48.70 (1.61) | 36.08 (1.61) ** | 46.54 (1.33) | 38.34 (1.30) ** |
| QSU-B F1 | 28.91 (0.87) | 22.42 (0.87) ** | 27.71 (0.71) | 23.65 (0.70) ** |
| QSU-B F2 | 19.79 (0.91) | 13.65 (0.91) ** | 18.83 (0.83) | 14.69 (0.81) ** |
| VAS 3 | 65.08 (3.38) | 49.50 (3.30) * | 59.70 (2.50) | 54.60 (2.44) |
| VAS 4 | 71.18 (3.46) | 51.83 (3.38) ** | 65.87 (2.65) | 56.87 (2.58) * |
| Latency | 2.84 (0.51) | 4.53 (0.49) * | 2.87 (0.52) | 4.42 (0.42) * |
Note. P = pain induction conditions. NP = no pain induction conditions. QSU-B T = QSU-Brief Total measure of smoking urge. QSU-B F1 = Factor 1 of the QSU-Brief (urge to smoke for pleasure/reward). QSU-B F2 = Factor 2 of the QSU-Brief (urge to smoke for the relief of negative affect). VAS 3 = visual analogue scale of smoking urge administered during the pain manipulation. VAS 4 = visual analogue scale of smoking urge administered post-pain manipulation. Latency = mean latency to light a cigarette post-pain manipulation (in seconds).
p < .05
p < .001.
Reported Urge to Smoke and Negative Affect
Mediation analyses were conducted to examine whether situational pain indirectly enhanced smoking urge by increasing levels of state negative affect (NA), which has been found to be a potent influence upon measures of smoking motivation (Brandon, 1994). Formal significance tests of the indirect effect of NA were conducted by means of the Sobel test and a bootstrap approach (Preacher & Hayes, 2004). Results of both procedures indicated a significant indirect effect of pain induction on urge to smoke through increased NA (p < .05). To further examine the degree of mediation, a four-step, ordinary least squares approach was employed (Baron & Kenny, 1986). Step 1 indicated a significant total effect of pain induction on urge to smoke (ß = .43, R2 = .19, p < .001); Step 2 indicated a significant effect of pain induction on NA (ß = .21, R2 = .04, p = .02); and Step 3 indicated a significant effect of NA on urge to smoke, while controlling for pain induction (ß = .32, sr2 = .096, p < .001). Thus, the first three steps in establishing mediation were satisfied, supporting the results of our tests of the indirect effect. Step 4 revealed that although the total effect of pain induction on smoking urge decreased when controlling for NA, it remained significant (ß = .37, sr2 = .129, p < .001), indicating that NA partially mediated this relationship. These results demonstrate that pain induction and pain-induced NA, collectively, accounted for 28% of the variance (R2 = .28) in self-reported urge to smoke. Of this, pain induction uniquely accounted for 13%, NA uniquely accounted for 9.5%, and approximately 5.5% of the variance was shared. Thus, NA accounted for approximately 30% of the total effect of pain induction on urge to smoke (5.5% / 18.5%). Similar indirect effects of NA were observed for VAS3 (p = .01) and VAS4 (p = .001) urge measures.
Latency to Smoke
Similar 2 X 2 ANCOVAs were conducted. The data for 10 participants (7.5%) were excluded from the latency analysis either because of experimenter error (e.g., participants cued to smoke too early in the procedure; n = 4), or because the participant encountered a disruptive confound (e.g., broken cigarette or lighter; n = 6). These participants were fairly balanced across experimental conditions with six assigned to P conditions and four assigned to NP conditions. Analysis of latency to light a cigarette following the pain manipulation revealed a main effect of pain induction, F(4, 115) = 4.60, p = .03, f = .20, with participants in the P conditions demonstrating shorter latency to smoke than participants in the NP conditions (means and standard errors are presented in Table 1). Additional smoking topography variables (e.g., number of puffs taken) were unrelated to latency.
Latency to Smoke, Negative Affect, and Reported Urge to Smoke
Application of the previously described mediation techniques revealed no evidence that pain indirectly enhanced latency to smoke via increased NA (all ps > .11). However, when urge to smoke was tested as a mediator, a significant indirect effect of pain induction on latency to smoke through increased urge to smoke was revealed (all ps < .05). Step 4 of the ordinary least squares approach revealed that the total effect of pain induction on latency to smoke (ß = -.21, R2 = .04, p = .02) was no longer significant after controlling for urge (ß = -.10, sr2 = .009, p = .29), indicating that urge to smoke fully mediated the effect of pain on latency.
Discussion
The main goal of this study was to determine whether situational pain was sufficient to increase smoking motivation, as indexed by self-reported urge to smoke and observation of immediate smoking behavior. As hypothesized, participants who underwent pain induction reported significantly greater smoking urges and demonstrated shorter latency to smoke than participants who did not experience pain.
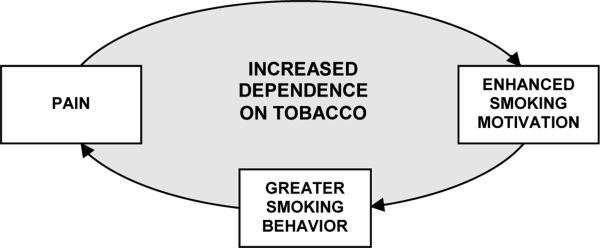
As reviewed earlier, most research into the relationship between tobacco use and pain has focused on the effects of smoking on pain (e.g., smoking exacerbating underlying pain conditions or inhibiting acute episodes of pain). An alternative approach is to examine the effects of pain on smoking. For example, the obvious health implications of smoking causing or exacerbating chronic pain could only be compounded if the pain itself further increases smoking behavior. Some cross-sectional evidence does indicate that smokers with chronic pain report a need to smoke when in pain, and this study provides the first experimental evidence that situational pain is a causal motivator of smoking. By integrating these two research directions, comprising the literature to date, we conceptualize a potentially reciprocal relationship between pain and smoking that acts as a positive feedback loop, with the end result being increases in both pain and smoking. As illustrated in Figure 1, we propose that smokers who are motivated to use tobacco to cope with or assuage pain may unwittingly aggravate their painful condition by increasing their cigarette consumption, thus engendering a vicious cycle that could lead to greater nicotine dependence.
Figure 1.
Conceptualization of the proposed reciprocal relationship between pain and smoking
Interestingly, the demonstrated causal relationship between pain and enhanced smoking motivation was only partially mediated by pain-induced state-negative affect. This finding suggests that pain may be a potent motivator of smoking, mostly independent of putative mood effects. Indeed, approximately 70% of the variance in the direct effect of pain induction on increased urge to smoke remains unexplained. It is plausible that this relationship may also be partially mediated by other psychological or physiological factors that were not measured or manipulated in the current study (e.g., the activation of smoking-related or pain-related self-efficacy and outcome expectancies, and the execution of pain-related coping behaviors).
The main limitation of the current study is that these findings do not necessarily generalize to individuals who suffer from chronic pain. Although the cold-pressor method of pain stimulation may share some subjective qualities frequently observed in clinical pain patients (Keogh et al., 2000; Rainville et al., 1992), there is no question that experimentally-induced acute pain is not equivalent to the daily pain endured by people with chronic conditions. Indeed, there currently exists no method of experimental pain induction that can be considered a perfect analogue to chronic pain. However, we felt that subjecting chronic pain patients to experimental pain induction at this early stage of hypothesis testing was unnecessary and possibly inappropriate. In addition to ethical concerns, a laboratory pain paradigm was selected to allow for increased experimental control and enhanced feasibility. Second, it is important to consider the modest effect of pain induction on state-NA (mean difference of 2.39) when interpreting the findings of our mediation analyses. It is possible that the PANAS did not adequately capture experimentally-induced pain-related distress. Also, the differential effects of acute vs. chronic pain on the magnitude and duration of pain-related negative mood must be considered when assessing the generalizability of these findings. A third limitation is the potential influence of demand effects. Whereas it is conceivable that participants may have recognized that self-reported urges to smoke were hypothesized to increase following pain induction, it is more difficult to attribute the observed group differences on the behavioral measure of smoking motivation (i.e., latency to smoke) to demand effects.
Future studies may wish to examine the potential for a dose-response relationship between pain and smoking motivation. For example, one investigation of the relationship between cold-pressor water temperature and pain tolerance and intensity concluded that small reductions in water temperature resulted in significantly reduced tolerance times and increased ratings of pain intensity (Mitchell et al., 2004). It would be interesting to know if smoking motivation increased as a function of pain intensity or duration of the painful experience.3 Another possibility is to examine differences in pain-induced smoking motivation as a function of pain modality (e.g., cold pressor, thermal heat, electrical stimulation, tourniquet ischemia), some of which may be closer analogues to chronic pain. Indeed, the nature of the noxious stimulus used to induce pain has been shown to impact pain sensitivity (Girdler et al., 2005; Rainville et al., 1992). Finally, future research should investigate the temporal relationship between pain and smoking motivation in naturalistic (i.e., real-world) settings, perhaps using ecological momentary assessment (Stone & Shiffman, 1994).
In summary, this study provides the first experimental evidence that situational pain is a potent motivator of smoking, partially mediated by pain-induced negative affect. That smokers are motivated to use tobacco in response to pain raises the possibility that smokers with painful conditions could develop unique dependence profiles. We believe that a systematic analysis of the causal link between pain and smoking motivation is an appropriate next step in this line of research. Smoking appears to be a prominent feature of painful conditions, and tobacco dependence may provide an invaluable model for research on addictive behaviors in the chronic pain population.
Acknowledgments
This study was supported in part by National Cancer Institute grant R01 CA94256 and the generous contribution of Drs. Dorothy and Edwin Sved. The authors thank Paul Jacobsen, David Drobes, and Cynthia Myers for their helpful suggestions; and Courtney Nichols, Melanie Jackson, William Grewe, and Gary Harding for their assistance in conducting the study.
Footnotes
A secondary aim of the current study was to test whether environmental smoking cues would interact with pain upon smoking outcomes. Therefore, all participants underwent the pain manipulation and completed post-test questionnaires with either a smoking cue or neutral cue in their view. Cue-type analyses revealed a main effect on urge to smoke (p = .04), and no effect on latency to smoke (p = .59). Due to the relatively weak effects of the cue manipulation, the presented analyses and discussion are restricted to those involving the pain manipulation (i.e., P vs. NP groups). However, cue-type was retained as a fixed factor for all ANCOVAs due to the significant variance accounted for by the cue manipulation. A full report describing this manipulation and associated findings is available from the corresponding author.
A range of individual difference measures (e.g., nicotine dependence scores) were tested as moderators of the relationship between the pain manipulation and smoking urge/latency, but no significant effects were found.
However, we found no significant correlations between magnitude of pain reactivity (i.e., ratings of pain intensity, pain threshold and tolerance times) and multiple indices of urge and smoking behavior.
Contributor Information
Joseph W. Ditre, Department of Psychology, University of South Florida, and the H. Lee Moffitt Cancer Center & Research Institute.
Thomas H. Brandon, Department of Psychology, University of South Florida, and the H. Lee Moffitt Cancer Center & Research Institute. Department of Interdisciplinary Oncology, University of South Florida.
References
- Albano SA, Santana-Sahagun E, Weisman MH. Cigarette smoking and rheumatoid arthritis. Seminars in Arthritis and Rheumatism. 2001;31:146–159. doi: 10.1053/sarh.2001.27719. [DOI] [PubMed] [Google Scholar]
- American Pain Society . Principles of analgesic use in the treatment of acute pain and cancer pain. 5th ed. Author; Glenview, IL: 2003. [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Brandon TH. Negative affect as motivation to smoke. Current Directions in Psychological Science. 1994;3:33–37. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd Ed. Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Conklin CA, Perkins KA. Subjective and reinforcing effects of smoking during negative mood induction. Journal of Abnormal Psychology. 2005;114:153–164. doi: 10.1037/0021-843X.114.1.153. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Creekmore FM, Lugo RA, Weiland KJ. Postoperative opiate analgesia requirements of smokers and nonsmokers. Annals of Pharmacotherapy. 2004;38:949–953. doi: 10.1345/aph.1D580. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Fertig JB, Pomerleau OF, Sanders B. Nicotine-produced antinociception in minimally deprived smokers and ex-smokers. Addictive Behaviors. 1986;11:239–248. doi: 10.1016/0306-4603(86)90052-3. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Maixner W, Naftel HA, Stewart PW, Moretz RL, Light KC. Cigarette smoking, stress-induced analgesia and pain perception in men and women. Pain. 2005;114:372–385. doi: 10.1016/j.pain.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Hahn EJ, Rayens MK, Kirsh KL, Passik SD. Brief report: Pain and readiness to quit smoking cigarettes. Nicotine & Tobacco Research. 2006;8:473–480. doi: 10.1080/14622200600670355. [DOI] [PubMed] [Google Scholar]
- Jamison RN, Stetson BA, Parris WC. The relationship between cigarette smoking and chronic low back pain. Addictive Behaviors. 1991;16:103–110. doi: 10.1016/0306-4603(91)90002-y. [DOI] [PubMed] [Google Scholar]
- Jamner LD, Girdler SS, Shapiro D, Jarvik ME. Pain inhibition, nicotine, and gender. Experimental and Clinical Psychopharmacology. 1998;6:96–106. doi: 10.1037//1064-1297.6.1.96. [DOI] [PubMed] [Google Scholar]
- Keogh E, Hatton K, Ellery D. Avoidance versus focused attention and the perception of pain: differential effects for men and women. Pain. 2000;85:225–230. doi: 10.1016/s0304-3959(99)00270-5. [DOI] [PubMed] [Google Scholar]
- Mitchell LA, MacDonald RA, Brodie EE. Temperature and the cold pressor test. Journal of Pain. 2004;5:233–237. doi: 10.1016/j.jpain.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Palmer KT, Syddall H, Cooper C, Coggon D. Smoking and musculoskeletal disorders: findings from a British national survey. Annals of the Rheumatic Diseases. 2003;62:33–36. doi: 10.1136/ard.62.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N, Talwar A, Reichert VC, Brady T, Jain M, Kaplan MH. Tobacco and HIV. Occupational and Environmental Medicine. 2006;5:193–207. xi. doi: 10.1016/j.coem.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Rainville P, Feine JS, Bushnell MC, Duncan GH. A psychophysical comparison of sensory and affective responses to four modalities of experimental pain. Somatosensory and Motor Research. 1992;9:265–277. doi: 10.3109/08990229209144776. [DOI] [PubMed] [Google Scholar]
- Riley JL, 3rd, Tomar SL, Gilbert GH. Smoking and smokeless tobacco: increased risk for oral pain. Journal of Pain. 2004;5:218–225. doi: 10.1016/j.jpain.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Saag KG, Cerhan JR, Kolluri S, Ohashi K, Hunninghake GW, Schwartz DA. Cigarette smoking and rheumatoid arthritis severity. Annals of the Rheumatic Diseases. 1997;56:463–469. doi: 10.1136/ard.56.8.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SC, Goldberg MS, Mayo NE, Stock SR, Poitras B. The association between cigarette smoking and back pain in adults. Spine. 1999;24:1090–1098. doi: 10.1097/00007632-199906010-00008. [DOI] [PubMed] [Google Scholar]
- Silverstein B. Cigarette smoking, nicotine addiction, and relaxation. Journal of Personality and Social Psychology. 1982;42:946–950. doi: 10.1037//0022-3514.42.5.946. [DOI] [PubMed] [Google Scholar]
- Stone AA, Shiffman S. Ecological Momentary Assessment (EMA) in behavioral medicine. Annals of Behavioral Medicine. 1994;16:199–202. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Yunus MB, Arslan S, Aldag JC. Relationship between fibromyalgia features and smoking. Scandinavian Journal of Rheumatology. 2002;31:301–305. doi: 10.1080/030097402760375214. [DOI] [PubMed] [Google Scholar]