Abstract
We previously reported that DHA-enriched fish oil (DFO) feeding altered B cell membrane organization and enhanced B cell function. The purpose of this study was to evaluate whether menhaden oil (MO) and EPA-enriched fish oil (EFO) alters B cell function/phenotype similarly. Mice were fed control, MO, EFO, or DFO diets for 5 weeks. We evaluated the fatty acid composition of B cell phospholipids, membrane microdomain organization, ex vivo B cell functionality, and in vivo B cell subsets. Red blood cells and B cells were found to be strongly (r > 0.85) and significantly (p < 0.001) correlated for major n-3 and n-6 LCPUFAs. Compared to CON, MO and DFO resulted in decreased clustering of membrane microdomains, whereas EFO increased clustering. All fish oil treatments had 1.12-1.60-times higher CD40 expression following stimulation; however, we observed 0.86-times lower MHCII expression and 0.7-times lower IL-6 production from EFO, but 3.25-times higher IFN-y from MO and 1.5-times higher IL-6 from DFO. By 90 min incubation, MO had 1.11-times higher antigen uptake compared to CON, whereas EFO was 0.86-times lower. All fish oil treatments resulted in decreasingly mature splenic and bone marrow B cell subsets. We conclude that diets high in n-3 LCPUFAs may elicit similar B cell phenotypes, but different organizational and functional outcomes. More specifically, these data suggest that the EPA and DHA content of a diet influences immunological outcomes, highlighting the importance of understanding how specific n-3 LCPUFAs modulate B cell development and function.
Keywords: B cell, fish oil, inflammation, immunomodulation, membrane
1 Introduction
Over 30 million people in the U.S. report using fish oil as a dietary supplement [1]. Fish oil is rich in the n-3 long chain polyunsaturated fatty acids (n-3 LCPUFAs): eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Lovaza, a prescription fish oil containing 465 mg EPA and 375 mg DHA per 1 g capsule, is currently prescribed for the treatment of hypertriglyceridemia and there is evidence that regular consumption of fish may result in cardiovascular benefits [2]. Indeed, fish oil has been investigated as a potential therapeutic for a wide range of illnesses ranging from mental health, such as depression, to physical health, such as inflammatory bowel diseases (IBD). It has been hypothesized that the inflammation-mediating effects of n-3 LCPUFAs may be responsible for their potential health effects.
As potent mediators of inflammation, the immunomodulatory effects of n-3 LCPUFAs on various immune cells have been studied. EPA and DHA alter inflammation through a variety of mechanisms, including production of eicosanoid and specialized proresolving lipid mediators, gene expression, and membrane organization, among others [3]. The bulk of research regarding fish oil immunomodulation has highlighted functional suppression of leukocytes, including T cells and monocytes [4].
Recent research has demonstrated that feeding n-3 LCPUFAs may significantly increase B cell activation, enhance B cell response to antigen, and increase total B cell number [5-10]. Despite the vital role of B cells in the immune system, there is still little research elucidating the effects of n-3 LCPUFAs on B cells. Specifically, animal models investigating the effects of fish oil on B cell function has thus far inadequately addressed the discrepancy between purified n-3 LCPUFA exposures in vitro compared to common dietary fish oil exposures in vivo. Indeed, Rockett et al. observed differential outcomes of B cells exposed to n-3 LCPUFAs in vitro compared to in vivo [7].
Research on fish oil immunomodulation is further confounded by demonstrating differences in the mechanistic and functional outcomes of EPA and DHA on immune cells [4]. Currently, the majority of animal models investigating the effect of n-3 LCPUFAs have used common fish oils (e.g. menhaden fish oil); whereas, therapeutics and dietary supplements have shifted toward EPA- and DHA-enriched formulations despite the lack of knowledge regarding immunomodulatory outcomes from specific mixtures of n-3 LCPUFAs.
We previously reported that feeding DHA-enriched fish oil to colitis-prone SMAD3-/- mice increased lymphoid tissue B cell populations and surface markers of activation in vivo and enhanced activation ex vivo after stimulation with LPS [5]. These observations are consistent with recently published observations elsewhere using C57BL/6 mice [7, 8]. The plethora of research demonstrating the different mechanisms by which EPA and DHA exert their effects [11] suggests a need to understand the immunological outcomes of dietary exposure to fish oils that are enriched with EPA or DHA. The objective of this study was to investigate the in vivo immunomodulatory effects of different fish oil composition on B cell function expounding on our previous observations using DHA-enriched fish oil in our colitis-prone model. We sought to compare the phenotype and function of B cells isolated from mice fed MO, EFO or DFO diets. To that end, we assessed the phospholipid fatty acid composition and microdomain organization of purified, splenic B cells. We also examined ex vivo B cell functionality, including the cytokine response to LPS-stimulation and antigen uptake. In addition, we characterized the effect of these diets on in vivo subsets of splenic and bone marrow B cells.
2 Materials and Methods
2.1 Materials and chemicals
ACK lysing buffer was purchased from Invitrogen (Carlsbad, CA, USA), RPMI-medium 1640 was purchased from Sigma-Aldrich (St. Louis, MO, USA), and FBS was purchased from Gibco (Gaithersburg, MD, USA). HPLC-grade water, toluene, and sulfuric acid were purchased from J.T. Baker (Phillipsburg, NJ, USA). 2-propanol and butyrated hydroxytoluene were purchased from Sigma-Aldrich. HPLC-grade chloroform and n-hexane were purchased from OmniSolv (Charlotte, NC, USA). Isolute-XL® SPE aminopropyl columns were purchased from Biotage (Charlotte, NC, USA). High purity methanol was purchased from Burdick & Jackson (Morristown, NJ, USA). Standards and the RT-2560 column for gas chromatography were purchased from Restek (Bellefonte, PA, USA). The following fluorescent antibodies (clone) were purchased from eBioscience (San Diego, CA, USA): B220 (RA3-6B2), MHCII (M5/114.15.2), CD40 (1C10), IgM (11/41), CD23 (B3B4), and CD21/CD35 (4E3). Purified CD16/CD32 (2.4G2) and biotinylated-CD24 (M1/69), as well as the following fluorescent antibodies (clone) / secondary fluorophores were purchased from BD Biosciences (San Diego, CA, USA): CD80 (16-10A1), CD86 (GL1), IgD (11-26c.2a), and Streptavidin PE-Cy7. Chicken ovalbumin conjugated to fluorescein (OVA-FITC) used for the antigen uptake assay was purchased from Molecular Probes (Eugene, OR. USA). Cholera toxin subunit B (CTxB) conjugated to fluorescein and anti-CTxB used for lipid microdomain staining was purchased from Life Technologies (Carlsbad, CA, USA). Lipopolysaccharide (LPS) for the stimulation assay was purchased from Sigma-Aldrich. The EPA-enriched fish oil (EPA4E1400 MEG-3 fish oil) and DHA-enriched fish oil (DHA4E1400 MEG-3 fish oil) were generously donated by Ocean Nutrition Canada (Dartmouth, Nova Scotia, Canada). The Menhaden Oil was purchased from Sigma Aldrich (St. Louis, MO, USA). A Certificate of Analysis was provided with each of the fish oils indicating food-grade quality of the fish oil and that it is free of contaminants and oxidation.
2.2 Murine model
The colitis-prone SMAD3-/- mouse model was utilized for these studies building upon previous work in our laboratory with this model. SMAD3+/- and SMAD3-/- breeder pairs (129-Smad3tm1Par/J) were generated in-house. Homozygous males and heterozygous females were mated to obtain SMAD3-/- pups. Genotypes were confirmed by PCR. Mice were housed under SPF conditions in 152.4 cm2 plastic cages (maximum of five adult mice/cage) with microisolator lids in an Association for Assessment and Accreditation of Laboratory Animal Care-approved facility at Michigan State University (East Lansing, MI, USA). SPF conditions were assured through quarterly serology testing by Charles River Laboratories International (Wilmington, MA, USA) and in-house testing for ectoparasites, endoparasites, and fecal Helicobacter species (PCR). Full necropsies (including culture and sensitivity) were performed at least yearly on rodent-breeding colonies. Animal rooms were maintained at 23.3 ± 2.2°C with a 12-h light-dark cycle. Mice were fed non-purified diets and sterile water ad libitum. Animal protocols were approved by the Michigan State University Institutional Animal Care and Use Committee.
2.3 Experimental design and diet composition
SMAD3-/- mice (age 4–7 weeks) were fed an AIN-93G soybean oil-based (CON) standard diet or one of three fish oil (MO, EFO, and DFO) substituted diets. The AIN-93G pre-mixed diet without fat was purchased from Dyets (Bethlehem, PA, USA) and formulated as previously described [5]. Differing only in fat composition, all diets contained 7% fat by weight, providing 17% kcal from fat (0.637 kcal/g). The CON diet contains 7% (wt) soybean oil, while the fish oil diets contain 1% (wt) corn oil (0.091 kcal/g) and 6% (wt) of the respective fish oils (0.546 kcal/g). Supplemental Table 1 shows the fatty analysis of the prepared diets, which was analyzed with gas chromatography as described below. Animals were fed the experimental diets ad libitum for 5 week. Ex vivo studies of B cells from mice fed MO, EFO, or DFO were performed on separate experiments with CON fed mice present in each experiment serving as the control. Experiments performed to identify the fatty acid composition of B cells and RBCs phospholipids, as well as phenotyping of B cell subsets were performed collectively (i.e. CON, MO, EFO, and DFO fed mice all initiated diet and were sacrificed at the same time). At the end of the experiment, mice were asphyxiated with CO2 and exsanguinated through cardiac puncture using a heparin-coated syringe. Blood was collected on ice and centrifuged to obtain a red blood cell fraction and a plasma fraction to assess plasma cytokines.
2.4 B cell purification
Spleens were harvested from SMAD3-/- mice, fed the CON, MO, EFO, and DFO diet and immediately placed in ice-cold RPMI supplemented with 10% FBS. Splenocytes were isolated and B cells were negatively selected for using BD IMag cell separation, per the manufacturer's protocol for their B Lymphocyte Enrichment Set system (BD Biosciences) as previously described [5].
2.5 Lipid extraction, phospholipid isolation, and preparation of FAMEs
Lipid extraction was performed using the Rose and Oaklander method [12], which utilizes a mixture of 2-propanol:chloroform (11:7 v/v) to minimize heme interferences within erythrocytes [13]. Briefly, under dim lighting, packed erythrocytes (100 – 150 mg) or pelleted B cells (approximately 6 million) were carefully transferred into borosilicate glass screw-capped tubes, immediately mixed with 2 mL of ice-cold HPLC-grade water, tightly capped and allowed to incubate on ice for 20 minutes. Lysates were then combined with 6 mL of 2-propanol (Sigma – Aldrich, St. Louis, MO) containing 100 μg/mL butyrated hydroxytoluene (BHT; Sigma – Aldrich), vortexed, and incubated for 1 h on ice with occasional mixing. Following incubation, 3.8 mL of HPLC-grade chloroform (OmniSolv, Charlotte, NC) was added and samples where incubated for an additional hour on ice with occasional mixing. Samples were centrifuged, the lower phase extracted, and dried under a gentle stream of nitrogen at 40°C. Phospholipid isolation using solid-phase extraction was performed according to the modified procedures of Agren et al. [14]. Briefly, Isolute-XL® SPE aminopropyl columns (500 mg; Biotage, Charlotte, NC) were conditioned twice with 5 mL acetone:water (7:1 v/v) and activated twice with 4 mL n-hexane (high purity solvent, OmniSolv, Charlotte, NC). Dried lipids were dissolved in 2 mL of n-hexane:chloroform:acetic acid (100:5:5 v/v/v) containing 100 μg/mL BHT. Lipid samples were then added to individual columns and monitored to prevent them from drying. To remove neutral lipids and non-esterified free fatty acids (NEFA), columns were washed with 2 mL of n-hexanes, followed by 5 mL of n-hexane:chloroform:ethyl acetate (100:5:5 v/v/v; 100 μg/mL BHT), and 5 mL methanol:chloroform:acetic acid (100:2:2 v/v/v; 100 μg/mL BHT). Fresh collection tubes were placed on ice under the columns and phospholipids were eluted using 2 × 4 mL washes of methanol:chloroform:water (100:5:4 v/v/v; 100 μg/mL BHT) and 1 mL high-purity methanol (Burdick & Jackson, Morristown, NJ) to remove any residual phospholipids [15]. Solvent fractions were dried under a gentle stream of nitrogen at 40°C. Fatty acid methyl esters (FAMEs) were prepared from isolated phospholipids fractions by incubation with acidified methanol, according to the methods of Burdge et al. [16]. Isolated phospholipid FAMEs were resuspended in 1-8 μL/mg tissue of n-hexane (100 μg/mL BHT), transferred to a GC autosampler vial, and stored under nitrogen at -80°C until analysis and analyzed as previously described [5].
2.6 B cell lipid microdomain staining and ex vivo stimulation
B cell GM1 lipid microdomain cross-linking and image acquisition, LPS-stimulation of purified B cells to assess cytokine production and surface marker expression, and OVA-FITC antigen uptake was performed as previously described [5].
2.7 Flow cytometry of splenic and bone marrow B cell subsets
Spleens and femurs were harvested from mice fed the CON, MO, EFO, and DFO diet and immediately placed in ice-cold RPMI supplemented with 10% FBS. The spleen was processed using a dounce homogenizer, pelleted, and washed in RPMI, as described previously [5]. Splenocytes were briefly resuspended in ACK lysing buffer for red blood cell lysis, washed twice in RPMI, and passed through 70 μm filters. Cell counts were performed with a hemacytometer using trypan blue exclusion and resuspended to a concentration of 2 × 10ˆ7 cells/mL media. Splenocytes were stained with B220, IgM, IgD, CD23, CD24, and CD21 to identify various B cell subsets. Splenic B cells (B220+) subsets were phenotyped as transitional 1 (T1) B cells (CD23- CD24Bright CD21-), transitional 2 (T2) B cells (CD23- CD24Bright CD21Bright/Dim and CD23+ CD24Bright/Dim CD21Bright), follicular (FO) B cells (CD23+/-CD24Dim CD21Dim), and marginal zone (MZ) B cells (CD23- CD24Bright/Dim CD21Bright) [17].
Femurs were harvested, cleaned, placed in RPMI on ice, and then flushed with RPMI. The single cell suspension from the bone marrow was briefly resuspended in ACK lysing buffer for red blood cell lysis and washed twice in RPMI. Cell counts were performed with a hemacytometer using trypan blue exclusion and resuspended to a concentration of 2 ×107 cells/mL media. Isolated cells from the bone marrow were stained with B220, IgM, IgD, CD24, and CD43 to identify various stages of B cell development. Bone marrow precursor and developmental B cells (B220+) subsets were phenotyped as pre-pro-B cells (IgD- IgM- CD24+ CD43-), pro-B cells (IgD- IgM- CD24+ CD43+), pre-B cells (IgD-IgM- CD24- CD43), immature B cells (IgM+ IgD-), and mature B cells (IgM+ IgD+). All flow cytometric analyses were performed on a FACSCanto II (BD Biosciences) and analyzed using FlowJo (TreeStar, Ashland, OR, USA).
2.8 Statistical analysis
All reported values are mean ± SEM. Statistical significance was set at P < 0.05. Normally distributed data (i.e., data that were assumed Gaussian and passed the Kolmogorov-Smirnov test) were statistically analyzed using a Student's t-test for significant differences in fatty acid analyses, lipid microdomain scoring, and cell surface marker expression staining between CON and MO, or EFO, or DFO fed mice. A Pearson's correlation was used to measure the linear correlation RBC and B cell fatty acids. Individual cells (10 cells/animal) were categorically scored as having either clustered, not clustered, or mixed clustering of lipid microdomains based on a qualitative scoring by a researcher blinded to the treatments as previously described [18]. For the lipid microdomain analysis, cells were scored and controls were aggregated; a one-way ANOVA was used to assess differences across the dietary treatments. A two-way, repeated measures ANOVA was used to assess the differences in antigen uptake between CON and MO, EFO, or DFO fed mice over time. As a result of non-normal distributions of the data obtained from the cytokine assay, Mann-Whitney U tests were used to test for significant differences in the supernatant cytokines of LPS- stimulated B cells between CON and MO, EFO, of DFO fed mice. A one-way ANOVA with a Dunnett's post-hoc test was used to assess the differences in splenic B cell subsets and bone marrow B cell developmental subsets from fish oil fed mice compared to the CON fed mice. All statistical analyses were performed using GraphPad Prism (GraphPad Software, La Jolla, CA, USA).
3 Results
3.1 Various fish oils different in EPA and DHA content increase RBC and B cell phospholipid n-3 LCPUFAs
We compared MO, DFO and EFO diets in the current study. We had previously measured changes in body composition between mice fed these experimental diets and found that there was no significant difference (data not shown). In this present study, we isolated the phospholipid fraction from the membrane of RBCs and B cells to confirm n-3 LCPUFAs incorporation into the phospholipid fraction of cellular membranes. Supplementary Table 1 shows the results from a gas chromatographic analysis of the lipid content of the differing diets.
To distinguish the lipid composition of the cellular membrane from other components containing lipid (e.g. lipoprotein contamination), we utilized a lipid extraction specific for phospholipids. This was an improvement upon our previous methodology because it more appropriately reflects changes in the membrane lipid composition by removing contaminating lipid from lipoproteins and non-membrane associated lipid compartments. The fatty acid composition of phospholipids from RBCs (Table 1) and B cells (Table 2) from SMAD3-/- mice fed CON, MO, EFO, of DFO diets for 5-wk are provided. Across all diets, the fatty acid profile of B cell phospholipids had increased levels palmitic acid (16:0) compared to RBCs; whereas there was a compensatory decrease in oleic acid (18:1), linoleic acid (18:2n-6), arachidonic acid (AA; 20:4n-6), and DHA (22:6n-3) in B cell phospholipids.
Table 1. Fatty acid analysis of phospholipids from red blood cells1.
| Fatty Acid | CON | EFO | DFO | MO |
|---|---|---|---|---|
| 16:0 | 31.57 ± 0.89 | 35.89 ± 0.58** | 36.17 ± 0.39** | 37.75 ± 0.80** |
| 16:1 | 0.29 ± 0.03 | 0.74 ± 0.02** | 0.28 ± 0.03 | 0.35 ± 0.03 |
| 18:0 | 12.72 ± 0.30 | 11.73 ± 0.06 | 11.56 ± 0.62 | 10.17 ± 0.21** |
| 18:1 trans | 0.24 ± 0.03 | 0.20 ± 0.01 | 0.25 ± 0.01 | 0.24 ± 0.01 |
| 18:1 cis | 11.04 ± 0.31 | 11.93 ± 0.33 | 12.79 ± 0.29** | 11.35 ± 0.34 |
| 18:2 (n-6) | 15.95 ± 0.73 | 6.37 ± 0.26** | 3.88 ± 0.21** | 5.13 ± 0.14** |
| 18:3 (n-6) | 0.07 ± 0.00 | 0.05 ± 0.00* | 0.03 ± 0.00** | 0.02 ± 0.01** |
| 18:3 (n-3) | 0.20 ± 0.01 | 0.06 ± 0.01** | 0.04 ± 0.01** | 0.03 ± 0.00** |
| 20:4 (n-6) | 11.85 ± 0.28 | 5.33 ± 0.14** | 5.51 ± 0.33** | 3.95 ± 0.15** |
| 20:5 (n-3) | 0.25 ± 0.03 | 6.11 ± 0.16** | 9.01 ± 0.35** | 6.36 ± 0.45** |
| 22:5 (n-3) | 0.77 ± 0.03 | 2.14 ± 0.04** | 3.98 ± 0.28** | 1.71 ± 0.04** |
| 22:6 (n-3) | 5.59 ± 0.31 | 10.61 ± 0.28** | 8.01 ± 0.38** | 13.52 ± 0.16** |
| ΣSFA | 47.88 ± 1.43 | 52.39 ± 1.17 | 52.25 ± 1.38 | 52.52 ± 1.28 |
| ΣMUFA | 13.53 ± 0.45 | 15.01 ± 0.44 | 15.75 ± 0.40** | 14.57 ± 0.46 |
| ΣPUFA (n-3) | 6.81 ± 0.38 | 18.92 ± 0.49** | 21.05 ± 1.02** | 21.62 ± 0.65** |
| ΣPUFA (n-6) | 31.79 ± 1.25 | 13.68 ± 0.48** | 10.95 ± 0.67** | 10.76 ± 0.38** |
Data are mean ± SEM reported as percent total, n = 4 mice/group.
A student's t test was used compare differences in fatty acid composition between CON and MO, EFO, or DFO diets.
Asterisks indicate significant difference:
P < 0.05
P < 0.01
Table 2. Fatty acid analysis of phospholipids from purified splenic B cells1.
| Fatty Acid | CON | EFO | DFO | MO |
|---|---|---|---|---|
| 16:0 | 33.22 ± 0.41 | 34.00 ± 0.42 | 31.53 ± 0.85 | 34.50 ± 0.81 |
| 16:1 | 0.19 ± 0.04 | 0.36 ± 0.05 | 0.19 ± 0.02 | 0.40 ± 0.08 |
| 18:0 | 36.73 ± 1.78 | 37.81 ± 0.95 | 36.66 ± 2.47 | 37.95 ± 2.67 |
| 18:1 trans | 1.52 ± 0.21 | 1.17 ± 0.32 | 1.58 ± 0.13 | 1.27 ± 0.07 |
| 18:1 cis | 6.21 ± 0.11 | 6.10 ± 0.26 | 6.04 ± 0.21 | 5.99 ± 0.31 |
| 18:2 (n-6) | 3.26 ± 0.21 | 2.21 ± 0.21* | 3.14 ± 0.36 | 1.61 ± 0.25** |
| 18:3 (n-6) | 0.04 ± 0.00 | 0.04 ± 0.01 | 0.05 ± 0.02 | 0.07 ± 0.02 |
| 18:3 (n-3) | 0.06 ± 0.01 | 0.03 ± 0.01 | 0.08 ± 0.01 | 0.11 ± 0.03 |
| 20:4 (n-6) | 9.51 ± 0.28 | 3.14 ± 0.20** | 4.35 ± 0.10** | 1.99 ± 0.29** |
| 20:5 (n-3) | 0.37 ± 0.07 | 3.10 ± 0.14** | 4.87 ± 0.23** | 1.51 ± 0.05** |
| 22:5 (n-3) | 0.72 ± 0.08 | 1.17 ± 0.17 | 2.18 ± 0.20** | 0.86 ± 0.05 |
| 22:6 (n-3) | 2.76 ± 0.41 | 4.82 ± 0.19** | 3.42 ± 0.41 | 7.61 ± 0.36** |
| ΣSFA | 71.35 ± 2.28 | 72.98 ± 1.50 | 69.79 ± 3.60 | 74.19 ± 3.76 |
| ΣMUFA | 10.14 ± 0.72 | 9.54 ± 0.88 | 10.48 ± 0.74 | 10.06 ± 0.87 |
| ΣPUFA (n-3) | 3.91 ± 0.57 | 9.12 ± 0.51** | 10.55 ± 0.86** | 10.09 ± 0.49** |
| ΣPUFA (n-6) | 15.83 ± 0.72 | 8.80 ± 0.66** | 10.38 ± 0.83** | 6.15 ± 0.72** |
Data are mean ± SEM reported as percent total, n = 4 mice/group.
A student's t test was used compare differences in fatty acid composition between CON and MO, EFO, or DFO diets.
Asterisks indicate significant difference:
P < 0.05
P < 0.01
In RBCs, MO, EFO, and DFO feeding resulted in increases of palmitic acid (16:0), EPA (20:5n-3), n-3 docosapentaenoic acid (DPAn-3; 22:5n-3), and DHA (22:6n-3), whereas linoleic acid (18:2n-6), α-linolenic acid (18:3n-3), and AA (20:4n-6) decreased compared to CON fed mice. In B cells, MO, EFO, and DFO feeding resulted in increases of EPA, and decreases of AA compared to CON fed mice; only EFO feeding resulted in increased DPAn-3, while MO and DFO feeding resulted in increased DHA.
The three experimental diets are high in EPA and/or DHA compared to the CON diet (Figure 1A). All LCPUFAs demonstrated strong, significant (all p < 0.0001) linear correlations between RBCs and B cells, as evidenced by Pearson's r coefficients for EPA (r = 0.849, Figure 1B), DHA (r = 0.892, Figure 1C), AA (r = 0.973, Figure 1D), and EPA+DHA (r = 0.927, Figure 1E). These data demonstrate that RBC phospholipid fatty acids are a robust biomarker for the phospholipid fatty acid composition of purified B cells.
Figure 1. n-3/6 LCPUFA composition of experimental diets and phospholipids from murine red blood cells and B cells.
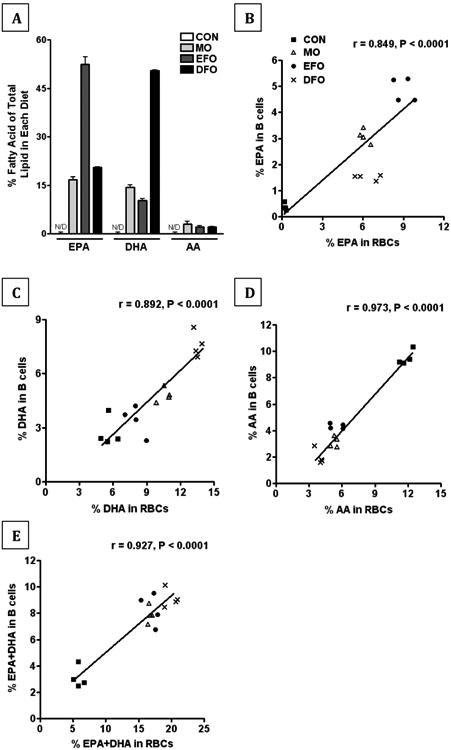
(A) The EPA, DHA, and AA content of the experimental diets (CON, MO, EFO, and DFO) were analyzed by gas chromatography. Triplicates were run on a single batch of each diet. Correlations of the (B) EPA, (C) DHA, (D) AA, and (E) EPA+DHA content between red blood cell phospholipids and B cell phospholipids from mice fed either CON, MO, EFO, or DFO diets was performed. A Pearson's r was used to assess the linear correlation between the two samples from each animal; n = 4 mice/group.
The diets supplemented with the different fish oils intentionally resulted in stark contrasts in the n-3 LCPUFA composition of the phospholipid fatty acids of B cells between the experimental groups. EPA was highest in the phospholipid fatty acids of B cells from mice fed EFO, followed by MO, and lowest in DFO; whereby DHA in the phospholipid fatty acids of B cells was highest in DFO, middle in MO, and lowest in EFO (Figure 1B-D).
Despite differences in individual content of EPA and DHA, we observed that all three experimental diets resulted in an increase in the omega-3 index, defined as the sum percentage of EPA+DHA over total lipid in RBCs [19], ranging between 15-22% in fish oil fed mice compared 5-7% in CON fed mice. The EPA+DHA content of B cells was much lower compared to RBCs, but we observed the same fold change between B cells of CON fed mice (2-4% EPA+DHA) compared to the fish oil treatments (7-10%). These data demonstrate a high correlation between the EPA+DHA content of RBCs and B cells providing evidence that the omega-3 index is useful to predict the EPA+DHA content of purified B cells.
3.2 DHA-rich oil alters the clustering of lipid microdomains
We assessed the presence or absence of lipid microdomain clustering using a blinded scoring analysis (Figure 2A-D). Purified B cells from mice fed MO and DFO diets had decreased clustering compared to B cells from CON and EFO mice, whereas B cells from EFO fed mice had increased clustering compared to CON, MO, and DFO fed mice (all p < 0.05, Figure 2E). As expected, this was observed in tandem with B cells from MO and DFO fed mice having more cells that were non-clustered (p < 0.05); however, there was no difference the amount of non-clustered lipid microdomains on B cells between CON and EFO fed mice (Figure 2F).
Figure 2. Clustering of lipid microdomains on murine B cells.
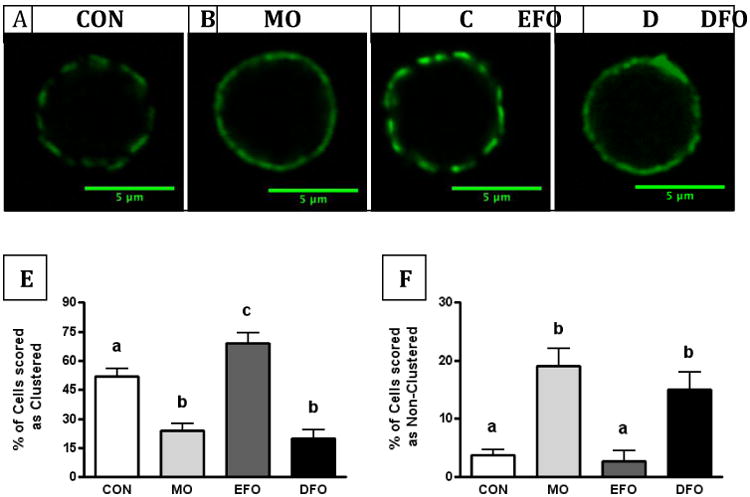
(A-D) Representative fluorescent images of lipid microdomains on purified, splenic B cells from SMAD3-/- mice fed CON, MO, EFO, or DFO diets. Cholera toxin subunit B conjugated to FITC was used to visualize GM1, an extensively used reporter of lipid rafts. Blinded scoring analysis of fluorescent lipid microdomains on cells as (E) clustered or (F) non-clustered. Data are represented as mean ± SEM, whereby 10 cells were scored per animal and n = 10-15 mice/group. Different letters denote statistically significant differences at the P < 0.05 level.
3.3 n-3 LCPUFAs differentially alter B cell response to stimulation
After LPS-stimulation, B cells from mice fed MO and EFO diets had increased CD40 expression compared to CON (Figure 3A). B cells from MO mice had greater CD40 expression in response to stimulation than those from EFO or DFO mice (Figure 3 A). Contrastingly, LPS-stimulated B cells from EFO fed mice had decreased MHCII expression compared to B cells from CON mice and responded unlike B cells from MO and DFO fed mice (Figure 3B).
Figure 3. Expression of B cell surface markers and secreted cytokines in LPS-stimulated murine B cells.
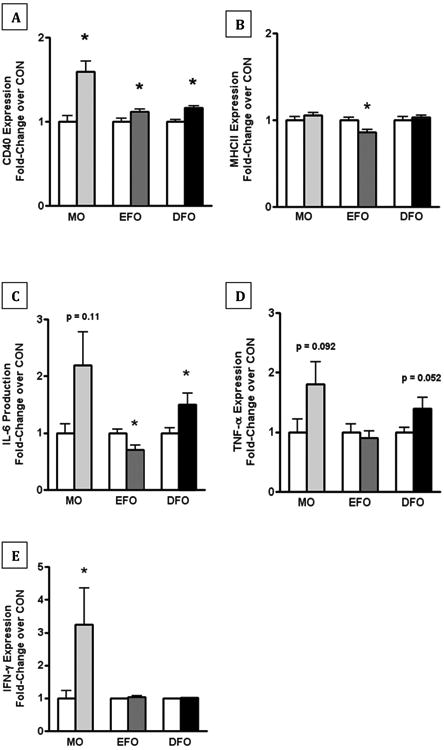
Purified, splenic B cells from SMAD3-/- mice fed CON, MO, EFO, or DFO diets were cultured for 24 h in the presence of 1μg/mL LPS. Flow cytometry on LPS-stimulated B cells was used to assess expression of B cell surface markers: (A) CD40 and (B) MHCII expression as a fold-change over CON. Flow-based multiplex assay was used to quantify secreted cytokines in the supernatants of LPS-stimulated B cells, including: (C) IL-6, (D) TNF-α, and (E) IFN-γ as a fold-change of cytokine production over CON. Data are represented as mean ± SEM; n = 5-15 mice/group. Each of the fish oil treatments were performed separately and therefore have their own controls. Different letters denote significant difference between treatment fold-changes in response to stimulation. Asterisks indicate significant differences compared to the CON diet: * P < 0.05
IL-6 production by LPS-stimulated B cells was increased from MO mice; however, it was not statistically significant (p = 0.11). There was a statistically significant increase in IL-6 production from DFO mice (p < 0.05), whereas, IL-6 production from LPS-stimulated B cells of EFO mice was decreased (p < 0.05) (Figure 3C). TNF-α production from LPS-stimulated B cells was increased in mice fed MO and DFO; however, these increases were not statistically significant (p = 0.092 and p = 0.052, respectively). There was no change in TNF-α reduction from LPS-stimulated mice fed EFO (Figure 3D). IFN-γ production from LPS-stimulated B cells was increased in mice fed MO (p < 0.05) with no change in EFO or DFO (Figure 3E).
3.4 MO increases, but EPA-enriched oil decreases antigen uptake
We previously reported that B cells from mice fed DFO had no alteration in antigen uptake using the model antigen, ovalbumin. In the current study, we observed increased antigen uptake in B cells from MO fed mice (p < 0.05) by 90 minutes of incubation with antigen, whereas B cells from EFO fed mice had significantly decreased antigen uptake by 90 minutes (p < 0.001) (Figure 4A-D).
Figure 4. Antigen uptake in murine B cells.
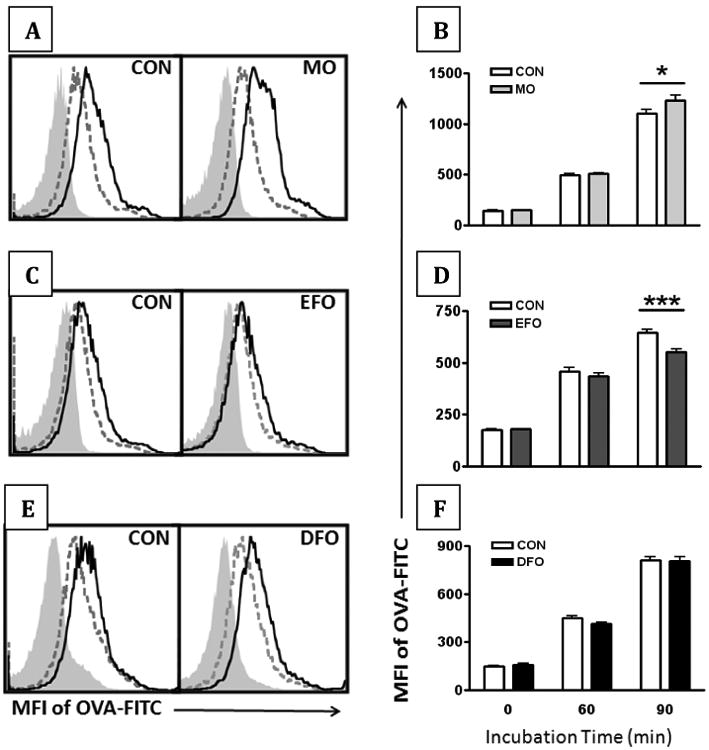
Uptake of ovalbumin conjugated to FITC (OVA-FITC) was used as a model antigen to assess B cell antigen uptake. Purified, splenic B cells from SMAD3-/- mice fed CON, MO, EFO, and DFO diets were incubated at 37°C for 0, 60, and 90 min. (A+C+E) Representative histograms demonstrate an increase in the MFI of OVA-FITC from 0 min (filled, light grey) to 60 min (dashed, dark grey) to 90 min (solid black). (B+D+F) Change over time of OVA-FITC MFI on purified B cells. Data are represented as mean ± SEM; n = 10-15 mice/group. Each of the fish oil treatments were performed separately and therefore have their own controls. Asterisks indicate significant differences compared to the CON diet: * P < 0.05 *** P < 0.001
3.5 Dietary fish oil decreases mature splenic and bone marrow B cell subsets
Despite having differential effects on splenic B cell function, MO, EFO, and DFO had few effects on splenic B cell subsets (Figure 5). There were reduced splenic marginal zone (MZ) B cells in the MO and EFO (p < 0.05) fed mice compared to CON fed mice (Figure 5A). There was a slight nonsignificant reduction in MZ B cells in DFO fed mice (Figure 5A). Fish oil diets did not significantly alter the splenic T1, T2, and mature follicular B cell populations (phenotype described in methodology section).
Figure 5. in vivo B cell subset phenotyping of spleen and bone marrow.
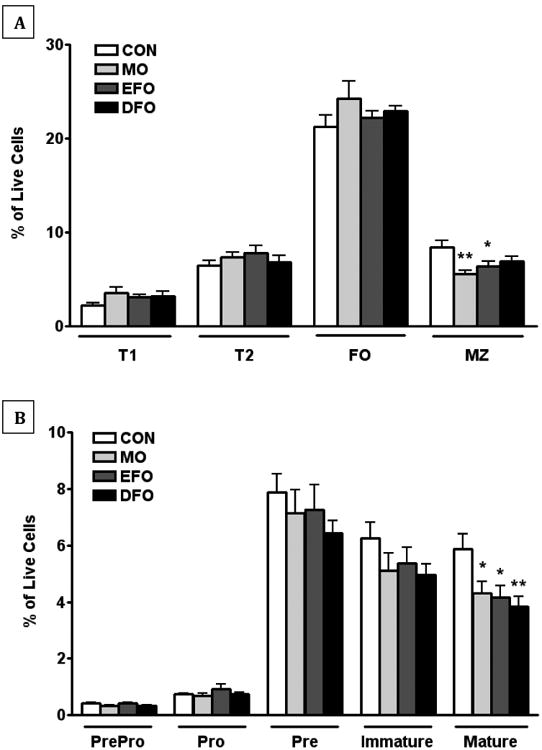
Flow cytometric phenotyping of B cell subsets was performed on spleen and bone marrow tissues in SMAD3-/- mice fed CON, MO, EFO, or DFO diets. (A) Splenic B cells (B220+) subsets were phenotyped as T1 B cells (CD23-CD24Bright CD21-), T2 B cells (CD23- CD24Bright CD21Bright/Dim and CD23+ CD24Bright/Dim CD21Bright), follicular B cells (CD23+/- CD24Dim CD21Dim), and marginal zone B cells (CD23- CD24Bright/Dim CD21Bright). (B) Bone marrow precursor and developmental B cells (B220+) subsets were phenotyped as pre-pro-B cells (IgD- IgM- CD24+ CD43-), pro-B cells (IgD- IgM- CD24+ CD43+), pre-B cells (IgD-IgM- CD24- CD43), immature B cells (IgM+ IgD-), and mature B cells (IgM+ IgD+). Data are represented as mean ± SEM; n = 10 mice/group. Asterisks indicate significant differences compared to the CON diet: * P < 0.05 ** P < 0.01
Similar to the spleen, MO, EFO, and DFO generally skew bone marrow B cell subsets in the same direction. Mature B cells in the bone marrow were decreased in MO, EFO and DFO fed mice compared to CON fed mice (p<0.05; Figure 5B). Bone marrow pre-pro-B cells, pro-B cells, pre-B cells and immature B cell populations were not significantly altered by fish oil diets.
4 Discussion
We observed that MO and DFO treatments elicit similar outcomes on B cell microdomain organization and function, whereas EFO treatment often resulted in opposite outcomes. The effects of MO and DFO include decreased microdomain clustering, increased activation and cytokine secretion in response to ex vivo stimulation, and increased antigen uptake (in the case of MO). Contrastingly, we observed that EFO increased microdomain clustering, but resulted in decreased activation, antigen uptake, and cytokine secretion following ex vivo stimulation compared to CON fed mice. The different fish oils influenced bone marrow and splenic B cell subsets in a similar direction.
We previously reported that DFO diminished the clustering micron-scale lipid microdomains on B cells [5], which agreed with the work of others using MO [18]. Using a blinded, semi-quantitative scoring method, B cells from mice fed control (CON) or fish oil diets were assessed as having clustered, non-clustered, or mixed clustering/non-clustering lipid microdomains. B cells from mice fed DFO and MO diets were found to have significantly decreased clustered microdomains and increased non-clustered microdomains (Figure 2). Contrasting the observed changes in the microdomain clustering of B cells from MO and DFO fed mice, EFO feeding resulted in increased clustering, suggesting EPA and DHA may differentially contribute to organization of membrane microdomains. Our observations corroborate in vitro studies demonstrating that DHA, but not EPA, is capable of increasing lipid raft size and decreasing clustering on EL4 cells [18]. These observations may be explained by more recent biophysical studies on the molecular organization of EPA and DHA in artificial rafts, demonstrating that DHA has a greater affinity for lipid microdomains than EPA [20]. Surprisingly, despite the highly disordered nature of DHA, Teague et al. found that DHA was capable of adapting to more highly ordered environments, becoming more ordered itself, even suggesting potential interaction with cholesterol [21]. These data provide evidence to support an appearance of increasing microdomain size due to DHA. Furthermore, our data are consistent with a recent study to show differences between EPA and DHA ethyl esters of B cell plasma membrane lipid packing after 5 weeks of dietary intervention in a model of diet-induced obesity [10].
The observations by us and others [22] that EPA and DHA may have differential effects on membrane organization, led us to hypothesize that fish oil composition will have differential effects on B cell function. Previous research on fish oil exposure using B cell lines in vitro resulted in decreased cytokine production and decreased inflammatory gene expression [7, 23, 24] and is suggestive that n-3 LCPUFAs may dampen B cell immunological responses. However, our previous observations that dietary DFO increased B cell response to stimulation and increased IgA secretion in the gut are in agreement with others' observations that dietary MO increases B cell activation and response to stimulation, suggesting that dietary n-3 LCPUFAs may increase activation of humoral immunity [6, 7]. We observed that MO elicited a similar immunological phenotype in B cells as DFO, including a significant increase in CD40 expression and increased IL-6, TNF-α, and IFN-γ in response to ex vivo LPS stimulation. While EFO also led to increase in CD40, the other immunomodulatory effects of EFO appear contradictory to MO and DFO, such as decreased MHCII expression and decreased IL-6 upon LPS stimulation.
B cells are capable of receptor-mediated pinocytosis/endocytosis and antigen presentation [25, 26] making antigen uptake an additional parameter of B cell function. We utilized ovalbumin conjugated to FITC (OVA-FITC) as the model antigen. We observed a significant decrease in OVA-FITC uptake in B cells from EFO fed mice but an increase in OVA-FITC in B cells from MO fed mice compared to CON. Interestingly, the decrease in MHCII on LPS-stimulated B cells from EFO fed mice appears congruent with our antigen uptake data. These data support the hypothesis that changes in the plasma membrane alter phagocytic capacity [27]. We did not measure the impact of n-3 PUFA on B cell MHC II antigen presentation to T cells in the immunological synapse, which has been shown to be suppressed with menhaden fish oil [28, 29]. Turk et al. corroborate differential outcomes of feeding EPA vs. DHA in mice [30]. While both EPA- and DHA-fed mice inhibited wound healing, the EPA-fed mice had increased mortality compared to corn oil or DHA-fed mice following colitis-induction using dextran sodium sulfate [30]. Taken together, these data highlight differential B cell immunomodulation depending on the composition of fish oil.
It is interesting to speculate that fish oil feeding is inducing emigration of transitional B cells from the bone marrow. Specifically, early transitional B cells, T1 B were increased in the spleen by MO, as well as EFO and DFO (Figure 5A). Our spleen T1 B cell data supports a recent publication by our collaborator [6]. Teague et al. observed an increase in T1 (IGM+ IGD- CD21-) B cells with an MO diet in the absence of antigen. In conjunction with sufficient B cell development in the bone marrow, increased T1 B cells indicates either increased emigration of transitional B cells from the bone marrow or proliferation of T1 B cells in the spleen. However, we observed a decrease in marginal zone (MZ) B cells in mice fed MO, EFO, and DFO. Our MZ B cell data contrasts Teague et al.'s observation that MZ B cells were increased in MO fed mice. T2 B cells are heterogeneous, some of these cells may seed the spleen directly from the bone marrow, give rise to mature follicular (FO) or MZ B cells, and are cycling in vivo [31]. We did not observe a difference in T2 B cells with fish oil feeding, but reduced MZ B cells could be the result of altered developmental program of T2 B cells from MZ to FO B cells.
Cells at the T2 B cell stage make the decision to become resident MZ B cells or circulating FO B cells based on signal strength of the B cell receptor and other signaling cascades. Hoek et al 2006 showed diacylglycerol responses were specific to spleen B cell subsets where diacylglycerol, IP3, PIP2, and phosphorylated PKC were constitutively expressed at higher levels in T2 B cells compared to T1 B cells [32]. Therefore, altered membrane composition and organization may alter the B cell subset cell survival and development. These observations are tangentially similar to a recent report by Monk et al., whereby they demonstrated in T cells that Th17 polarization was modified by fish oil; furthermore, they observed the same effect on development regardless of whether EPA alone, DHA alone, or EPA+DHA was provided in the diet [33]. As cells undergoing cell cycle and robust responses to B cell receptor stimulation [31, 34], T2 B cells may provide a target for acute dietary intervention such as fish oil feeding. Lipid microdomain reorganization and phospholipid content changes with fish oil may alter the kinetics of B cell development. Specific n-3 LCPUFAs may differentially alter signaling cascades in B cells resulting in our observed differences in functional outcomes B cells.
Recent epidemiological evidence demonstrates that high intakes of fish oil may not be unequivocally beneficial [35]. There is a greater need to understand how the omega-3 fatty acid composition of fish oil and fish oil supplements elicits their immunomodulatory effects. Fish oil supplements sold for therapeutic purposes are increasingly enriched in EPA, DHA, or both. Yet, the effect of increasing consumption of one or the other on immune function is poorly understood. Furthermore, differences between oil types beyond those of just EPA and DHA may also contribute toward functional differences, which we aim to investigate in the future. Findings from this study highlight the differential immunomodulatory outcomes of fish oils differing in fatty acid content on the activation of B cells and their subsequent function. These data and future similar research are critical when considering the clinical immunologic outcomes resulting from fish oil supplementation.
Supplementary Material
Acknowledgments
Financial support for this research was provided by: NIH R03CA162427 (to JIF) and NIH R15AT006122 (to SRS).
Abbreviations Used
- AA
Arachidonic acid
- CTxB
cholera toxin subunit B
- CON
control
- DFO
DHA-enriched fish oil
- EFO
EPA-enriched fish oil
- FACS
fluorescent activated cell sorting
- GC
gas chromatography
- IBD
inflammatory bowel disease
- LPS
lipopolysaccharide
- MO
Menhaden oil
- MHCII
major histocompatability complex class II
- n-3 LCPUFA
n-3 long chain polyunsaturated fatty acid
- NBF
neutral buffered formalin
- OVA
ovalbumin
- Smad3
mothers against decapentaplegic homolog-3
- SPF
specific pathogen-free
- TGF-β
transforming growth factor-beta
- TLR4
toll-like receptor 4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008:1–23. [PubMed] [Google Scholar]
- 2.Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ. Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis. 2008;197:12–24. doi: 10.1016/j.atherosclerosis.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Shaikh SR, Jolly CA, Chapkin RS. n-3 Polyunsaturated fatty acids exert immunomodulatory effects on lymphocytes by targeting plasma membrane molecular organization. Mol Aspects Med. 2012;33:46–54. doi: 10.1016/j.mam.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013;75:645–62. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurzell EA, Teague H, Harris M, Clinthorne J, Shaikh SR, Fenton JI. DHA-enriched fish oil targets B cell lipid microdomains and enhances ex vivo and in vivo B cell function. Journal of leukocyte biology. 2013;93:463–70. doi: 10.1189/jlb.0812394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teague H, Fhaner CJ, Harris M, Duriancik DM, Reid GE, Shaikh SR. n-3 PUFAs enhance the frequency of murine B-cell subsets and restore the impairment of antibody production to a T-independent antigen in obesity. Journal of lipid research. 2013;54:3130–8. doi: 10.1194/jlr.M042457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rockett BD, Salameh M, Carraway K, Morrison K, Shaikh SR. n-3 PUFA improves fatty acid composition, prevents palmitate-induced apoptosis, and differentially modifies B cell cytokine secretion in vitro and ex vivo. Journal of lipid research. 2010;51:1284–97. doi: 10.1194/jlr.M000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rockett BD, Teague H, Harris M, Melton M, Williams J, Wassall SR, et al. Fish oil increases raft size and membrane order of B cells accompanied by differential effects on function. Journal of lipid research. 2012;53:674–85. doi: 10.1194/jlr.M021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomasdottir V, Thorleifsdottir S, Vikingsson A, Hardardottir I, Freysdottir J. Dietary omega-3 fatty acids enhance the B1 but not the B2 cell immune response in mice with antigen-induced peritonitis. The Journal of nutritional biochemistry. 2014;25:111–7. doi: 10.1016/j.jnutbio.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Teague H, Harris M, Fenton J, Lallemand P, Shewchuk BM, Shaikh SR. Eicosapentaenoic and docosahexaenoic acid ethyl esters differentially enhance B-cell activity in murine obesity. Journal of lipid research. 2014;55:1420–33. doi: 10.1194/jlr.M049809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sijben JW, Calder PC. Differential immunomodulation with long-chain n-3 PUFA in health and chronic disease. Proc Nutr Soc. 2007;66:237–59. doi: 10.1017/S0029665107005472. [DOI] [PubMed] [Google Scholar]
- 12.Rose HG, Oklander M. Improved Procedure for Extraction of Lipids from Human Erythrocytes. Journal of lipid research. 1965;6:428–&. [PubMed] [Google Scholar]
- 13.Lydic TA, Renis R, Busik JV, Reid GE. Analysis of Retina and Erythrocyte Glycerophospholipid Alterations in a Rat Model of Type 1 Diabetes. Jala. 2009;14:383–99. doi: 10.1016/j.jala.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agren JJ, Julkunen A, Penttila I. Rapid Separation of Serum-Lipids for Fatty-Acid Analysis by a Single Aminopropyl Column. Journal of lipid research. 1992;33:1871–6. [PubMed] [Google Scholar]
- 15.Bondia-Pons I, Morera-Pons S, Castellote AI, Lopez-Sabater MC. Determination of phospholipid fatty acids in biological samples by solid-phase extraction and fast gas chromatography. Journal of Chromatography A. 2006;1116:204–8. doi: 10.1016/j.chroma.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 16.Burdge GC, Wright P, Jones AE, Wootton SA. A method for separation of phosphatidylcholine, triacylglycerol, non-esterified fatty acids and cholesterol esters from plasma by solid-phase extraction. British Journal of Nutrition. 2000;84:781–7. [PubMed] [Google Scholar]
- 17.Shaikh SR, Haas KM, Beck MA, Teague H. The effects of diet-induced obesity on B cell function. Clinical and experimental immunology. 2014 doi: 10.1111/cei.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaikh SR, Rockett BD, Salameh M, Carraway K. Docosahexaenoic acid modifies the clustering and size of lipid rafts and the lateral organization and surface expression of MHC class I of EL4 cells. The Journal of nutrition. 2009;139:1632–9. doi: 10.3945/jn.109.108720. [DOI] [PubMed] [Google Scholar]
- 19.Harris WS, Sands SA, Windsor SL, Ali HA, Stevens TL, Magalski A, et al. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients - Correlation with erythrocytes and response to supplementation. Circulation. 2004;110:1645–9. doi: 10.1161/01.CIR.0000142292.10048.B2. [DOI] [PubMed] [Google Scholar]
- 20.Williams JA, Batten SE, Harris M, Rockett BD, Shaikh SR, Stillwell W, et al. Docosahexaenoic and eicosapentaenoic acids segregate differently between raft and nonraft domains. Biophysical journal. 2012;103:228–37. doi: 10.1016/j.bpj.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teague H, Ross R, Harris M, Mitchell DC, Shaikh SR. DHA-fluorescent probe is sensitive to membrane order and reveals molecular adaptation of DHA in ordered lipid microdomains. The Journal of nutritional biochemistry. 2013;24:188–95. doi: 10.1016/j.jnutbio.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams Justin A, B SE, Mitchel Harris, Rockett Benjamin Drew, Shaikh Saame Raza, William Stillwell, Wassall Stephen R. Docosahexaenoic and Eicosapentaenoic Acids Segregate Differently between Raft and Nonraft Domains. Biophysical journal. 2012;103:228–37. doi: 10.1016/j.bpj.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verlengia R, Gorjao R, Kanunfre CC, Bordin S, de Lima TM, Martins EF, et al. Effects of EPA and DHA on proliferation, cytokine production, and gene expression in Raji cells. Lipids. 2004;39:857–64. doi: 10.1007/s11745-004-1307-2. [DOI] [PubMed] [Google Scholar]
- 24.Verlengia R, Gorjao R, Kanunfre CC, Bordin S, Martins De Lima T, Martins EF, et al. Comparative effects of eicosapentaenoic acid and docosahexaenoic acid on proliferation, cytokine production, and pleiotropic gene expression in Jurkat cells. The Journal of nutritional biochemistry. 2004;15:657–65. doi: 10.1016/j.jnutbio.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Nakashima M, Kinoshita M, Nakashima H, Habu Y, Miyazaki H, Shono S, et al. Pivotal Advance: Characterization of mouse liver phagocytic B cells in innate immunity. Journal of leukocyte biology. 2012;91:537–46. doi: 10.1189/jlb.0411214. [DOI] [PubMed] [Google Scholar]
- 26.Parra D, Rieger AM, Li J, Zhang YA, Randall LM, Hunter CA, et al. Pivotal Advance: Peritoneal cavity B-1 B cells have phagocytic and microbicidal capacities and present phagocytosed antigen to CD4(+) T cells. Journal of leukocyte biology. 2012;91:525–36. doi: 10.1189/jlb.0711372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kew S, Banerjee T, Minihane AM, Finnegan YE, Williams CM, Calder PC. Relation between the fatty acid composition of peripheral blood mononuclear cells and measures of immune cell function in health free-living subjects aged 25-72 y. American Journal of Clinical Nutrition. 2003;77:1278–86. doi: 10.1093/ajcn/77.5.1278. [DOI] [PubMed] [Google Scholar]
- 28.Rockett BD, Melton M, Harris M, Bridges LC, Shaikh SR. Fish oil disrupts MHC class II lateral organization on the B-cell side of the immunological synapse independent of B-T cell adhesion. The Journal of nutritional biochemistry. 2013;24:1810–6. doi: 10.1016/j.jnutbio.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim W, Fan YY, Barhomni R, Smith R, McMurray DN, Chapkin RS. n-3 Polyunsaturated Fatty Acids Suppress the Localization and Activation of Signaling Proteins at the Immunological Synapse in Murine CD4(+) T Cells by Affecting Lipid Raft Formation. Journal of immunology. 2008;181:6236–43. doi: 10.4049/jimmunol.181.9.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turk HF, Monk JM, Fan YY, Callaway ES, Weeks B, Chapkin RS. Inhibitory effects of omega-3 fatty acids on injury-induced epidermal growth factor receptor transactivation contribute to delayed wound healing. American journal of physiology Cell physiology. 2013;304:C905–17. doi: 10.1152/ajpcell.00379.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer-Bahlburg A, Andrews SF, Yu KO, Porcelli SA, Rawlings DJ. Characterization of a late transitional B cell population highly sensitive to BAFF-mediated homeostatic proliferation. The Journal of experimental medicine. 2008;205:155–68. doi: 10.1084/jem.20071088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoek KL, Antony P, Lowe J, Shinners N, Sarmah B, Wente SR, et al. Transitional B cell fate is associated with developmental stage-specific regulation of diacylglycerol and calcium signaling upon B cell receptor engagement. Journal of immunology. 2006;177:5405–13. doi: 10.4049/jimmunol.177.8.5405. [DOI] [PubMed] [Google Scholar]
- 33.Monk JM, Hou TY, Turk HF, McMurray DN, Chapkin RS. n3 PUFAs reduce mouse CD4+ T-cell ex vivo polarization into Th17 cells. The Journal of nutrition. 2013;143:1501–8. doi: 10.3945/jn.113.178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petro JB, Castro I, Lowe J, Khan WN. Bruton's tyrosine kinase targets NF-kappaB to the bcl-x promoter via a mechanism involving phospholipase C-gamma2 following B cell antigen receptor engagement. FEBS letters. 2002;532:57–60. doi: 10.1016/s0014-5793(02)03623-2. [DOI] [PubMed] [Google Scholar]
- 35.Brasky TM, Darke AK, Song X, Tangen CM, Goodman PJ, Thompson IM, et al. Plasma Phospholipid Fatty Acids and Prostate Cancer Risk in the SELECT Trial. J Natl Cancer Inst. 2013 doi: 10.1093/jnci/djt174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


