Abstract
Many studies on broccoli have analyzed the functional components and their functionality in terms of antioxidant and anticancer activities; however, these studies have focused on the florets of different varieties. Investigation of the functionality of broccoli by-products such as leaves, stems, and leaf stems from different cultivars and harvest dates might be valuable for utilizing waste materials as useful food components. Total phenolics and sulforaphane contents, and antioxidant and anticancer activities were measured in the leaves, leaf stems, and stems of early-maturing (Kyoyoshi), middle-maturing (Myeongil 96), and late-maturing broccoli (SK3-085) at different harvest dates. Total phenolics in the leaves of Kyoyoshi were about 1.8-fold to 12.1-fold higher than those in all of the other cultivars and parts. The sulforaphane content of Kyoyoshi was 2.8-fold higher in the stems than in the florets. Antioxidant activities using 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity and oxygen radical absorbance capacity were highest in Kyoyoshi, followed by Myeongil 96 and SK3-085, most notably in the leaves harvested at the immature stage. Inhibition activity of cell growth against the NCI-H1299 cell lines was highest in the leaves of all cultivars in decreasing order of florets, leaf stems, and stems. The leaves harvested in October (nonflowering stage) had the highest inhibition activity, while those harvested in January (mature broccoli) showed the lowest. The results of this study demonstrate that broccoli leaves and stems contain high levels of total phenolics, and high antioxidant and anticancer activities and can provide opportunities for early-maturing broccoli as functional fresh raw vegetables.
Keywords: broccoli by-products, total phenolics, sulforaphane, antioxidant and anticancer activities
INTRODUCTION
Broccoli (Brassica oleracea L. italica) has been marketed as a health-promoting food because it naturally has high content of bioactive phytochemicals such as glucosinolates, phenolic compounds, vitamin C, and mineral nutrients. Thus, a diet rich in broccoli plays a role in the prevention of chronic diseases, such as cardiovascular and carcinogenic pathologies, and breast and prostate cancers (1–4). Broccoli has also been found to exhibit antioxidant activity that prevents oxidative stress related to many diseases (5).
Broccoli parts being used for food are mostly florets, which make up 30% of the whole broccoli. Currently, the use of broccoli by-products such as leaves and stems is restricted to flour and fiber (6), but the potential use of these by-products as important sources of phytochemicals is now gaining more attention in the scientific community (7).
More than 72% of all broccoli produced in Korea is grown in Jeju. Broccoli is not only produced in large amounts, but it is also discarded in high amounts as by-products such as leaves and stems. This has a negative impact on agricultural environments, and recycling those by-products for physiologically active substances could help reduce environmental problems and increase farmers’ profits (8).
Many studies on broccoli have been performed on the antioxidant and anticancer activities of broccoli components, but most of the studies analyzed florets of different varieties (3–5,9). Domínguez-Perles et al. (8) determined the antioxidant activity of broccoli leaves and stalks of three different cultivars, but not at different harvest times. Furthermore, the determination of the anticancer activity has not been previously and simultaneously detailed for different broccoli parts at different harvest times.
The biological activities may be significantly different at various growth stages of broccoli leaves and stems from different cultivars. Thus, it might be valuable for the food industry to investigate the functionality of broccoli by-products such as stems and leaves as useful industrial materials, rather than only using the florets.
The purpose of this study was to evaluate total phenolics and sulforaphane contents, and antioxidant and anticancer activities of broccoli by-products from different cultivars and harvest dates of broccoli grown in Jeju, Korea.
MATERIALS AND METHODS
Reagents and chemicals
Folin-Ciocalteu phenol reagent, gallic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azobis(2-methyl-propan-imidamide) dihydrochloride (AAPH), 6-hydroxy-2,5,7,8-tetramethyl-2-carboxylic acid (trolox), and sulforaphane were purchased from Sigma Chemical Co. (St. Louis, MO, USA). RPMI-1640, fetal bovine serum (FBS), and penicillin/streptomycin were obtained from Gibco-BRL (Grand Island, NY, USA). Acetonitrile and dichloromethane (Sigma Chemical Co.) were of HPLC grade and other chemicals were of analytical grade.
Plant materials
Three different cultivars of broccoli including early-maturing (Kyoyoshi), middle-maturing (Myeongil 96), and late-maturing (SK3-085) crops were purchased from a private farm located in Jeju, Korea (Table 1). In particular, SK3-085 was harvested at four different growth stages including October (nonflowering stage), November (beginning of floret formation), December (mini broccoli), and January (fully developed broccoli). Broccoli was then divided into four parts including leaves, leaf stems, stems, and florets.
Table 1.
Sowing, planting, and harvest dates of broccoli from different cultivars
| Cultivars | Sowing date | Planting date | Harvest date | Growth stage |
|---|---|---|---|---|
| Kyoyoshi (Early season) | May | June | October | Mature broccoli |
| Myeongil 96 (Middle season) | June | July | November | Mature broccoli |
| SK3-085 (Late season) | July | August | October | Nonflowering stage |
| SK3-085 (Late season) | July | August | November | Beginning of floret formation |
| SK3-085 (Late season) | July | August | December | Mini broccoli |
| SK3-085 (Late season) | July | August | January | Mature broccoli |
Sample preparation
Broccoli leaves, leaf stems, stems, and florets were frozen at −70°C for 24 h and then freeze-dried. The freeze-dried powder was extracted twice with 80% methanol at room temperature for 24 h. The extracts were combined, filtered through a 0.45-μm membrane filter (Millipore Co., Bedford, MA, USA), concentrated under vacuum on a rotary evaporator (Rotavapor R-124, BUCHI Labortechnik AG, Flawil, Switzerland), and freeze-dried.
Total phenolics assay
Total phenolic contents in 80% methanol extracts were determined using the Folin-Denis method (10). Briefly, 100 μL of the extract (2 mg/mL) and 900 μL of distilled water were mixed with 100 μL of Folin-Ciocalteu phenol reagent. The mixture was allowed to stand at room temperature for 5 min, and 300 μL of 20% Na2CO3 was added and then filled up to 2 mL with distilled water. After standing at room temperature for 2 h, the absorbance was measured at 760 nm. Total phenolics were calculated as mg gallic acid equivalents (mg GAE) per 100 g dry weight of the extract.
Sulforaphane content assay
The sulforaphane contents in freeze-dried leaves, leaf stems, stems, and florets were measured by the method of Bertelli et al. (11) with a slight modification. Each conical tube containing 0.4 g of the freeze-dried sample was added to 2 mL distilled water and extracted with 4 mL methylene chloride at room temperature for 30 min. Each tube was centrifuged at 3,000 g for 10 min. The methylene chloride layer was collected, and this procedure was repeated three times. The combined methylene chloride extract was vacuum-evaporated and filled up to 4 mL with methylene chloride. The extract was purified using a Supelclean™ LC-Si SPE (Supelco Inc., Bellefonte, PA, USA) as follows: The solid-phase extraction was activated with 3 mL of methylene chloride and then loaded with 3 mL of the extract. The mixture was then washed with 3 mL of ethylacetate, which was discarded, and the sulforaphane was eluted with 3 mL of methanol. The methanol layer was evaporated and filled up to 3 mL with methanol.
The sulforaphane content in the extract was quantitatively analyzed using high-pressure liquid chromatography (1200 series, Agilent Technologies, Santa Clara, CA, USA). Separation was performed on an Xterra® RP18 column (4.6×250 mm, 5 μm film thickness; Waters, Maidstone, Kent, UK) using a solvent system of acetonitrile (A) and water (B): 0~15 min, 20~50% A (v/v); 15~20 min, 50~20% A. The flow rate was 0.8 mL/min, and the injection volume was 10 μL. The column temperature was 30°C, and the wavelength for detecting the sulforaphane was 240 nm. Sulforaphane content was expressed as μg per g freeze-dried sample.
DPPH radical scavenging activity assay
DPPH radical scavenging activity was determined using the Blois method (12) with a slight modification. Briefly, 100 μL of 80% methanol extract at different concentrations was added to 900 μL of DPPH solution, and the mixture was then allowed to stand in the absence of light for 30 min. The absorption was measured at 517 nm.
Oxygen radical absorbance capacity (ORAC) assay
The ORAC assay was performed using the protocol proposed by Ou et al. (13) with a slight modification. Briefly, 25 μL of 80% methanol extract (25 μg/mL) or trolox standard (200 μM) was mixed with 100 μL of fluorescein sodium salt (0.075 M K2HPO4: 0.075 M NaH2PO4=61.5:38.5, v/v) and 25 μL of 75 mM phosphate buffer in black-walled 96-well plates (Fisher Scientific, Hanover Park, IL, USA), and then 50 μL AAPH (41.6 mM) was added. Immediately, the plate was placed in a FLUOstar OPTIMA plate reader (BMG Lab-technologies, Offenburg, Germany) and set to an excitation filter at 485/25 nm and an emission filter at 538/35 nm; the absorbance was read every 3 min for 1 h at 37°C. The final fluorescence measurement was expressed relative to the initial reading. The result was calculated by comparing the net area under the fluorescein decay curve between the blank and the sample. The ORAC value was expressed as μM trolox equivalents (TE) per mg dry weight of the extract.
Anticancer activity assay
The human colon cancer HT-29 and the lung cancer NCI-H1299 cell lines were purchased from the Korean Cell Line Bank (Seoul, Korea). The cell lines were cultured in RPMI-1640 medium containing 10% FBS and maintained at 37°C in a 5% CO2 atmosphere. The viability of cells was assessed by the 3-(4,5-dimethylthia-zol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (14) as follows: HT-29 and NCI-H1299 cells were cultured in 96-well plates for 24 h at a concentration of 2×104 cells/well, and then 80% methanol extract (2 mg/mL) was added; the cells were cultured for 24 h and the MTT solution (200 μg/mL) was added. After 4 h, the supernatant was removed, the formazan crystals were dissolved in dimethyl sulfoxide, and the absorbance was measured at 540 nm. The percentage of cytotoxic cells was determined and compared to the control group.
Statistical analysis
The data were expressed as mean±SD of three replicates. The statistical significance was tested by Duncan’s multiple range test (Version 18.0, SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Total phenolic content
The total phenolic contents in 80% methanol extracts of broccoli by-products from different cultivars and harvest dates are shown in Table 2. Data showed significant differences in total phenolics among the different cultivars and by-products. Among the three cultivars, Kyoyoshi (early-maturing crop) was the highest in total phenolics followed by Myeongil 96 (middle-maturing crop) and SK3-085 (late-maturing crop) in most of the by-products. The leaves of all the cultivars had the highest total phenolic contents in decreasing order of florets, leaf stems, and stems. In particular, total phenolics in the leaves of Kyoyoshi were about 1.8-fold to 12.1-fold higher than those of all the other samples. Faller and Fialho (15) also showed that broccoli had different polyphenol contents depending on the parts in decreasing order of leaves, florets, and stems.
Table 2.
Total phenolic contents in 80% methanol extracts of broccoli by-products from different cultivars and harvest dates
| Cultivars/harvest date | Total phenolic content (mg GAE/100 g dried extract) | |||
|---|---|---|---|---|
|
| ||||
| Leaves | Stems | Leaf stems | Florets | |
| Kyoyoshi | 1,310.0±27.4dD | 215.6±4.4dA | 418.6±9.7fB | 528.9±8.4eC |
| Myeongil 96 | 537.6±10.1aD | 198.0±6.4cA | 275.4±6.0eB | 335.3±5.6dC |
| SK3-085/Jan | 604.2±12.3bD | 143.7±0.4aB | 108.2±1.1aA | 255.4±0.7bC |
| SK3-085/Dec | 545.3±5.6aD | 173.1±3.3bC | 117.8±1.2bA | 164.4±1.1aB |
| SK3-085/Nov | 739.2±27.2cC | 144.4±1.1aA | 146.6±0.8cA | 326.4±0.7cB |
| SK3-085/Oct | 706.8±17.3cC | 177.7±0.4bA | 191.5±0.4dB | N/A |
Values with different letters in the row (A–D) and in the column (a–f) are significantly different at P<0.05 according to Duncan’s multiple range tests.
N/A: The florets were too small and thus not analyzed.
SK3-085 was further tested for the effect of different harvest dates on total phenolic content (Table 2). The highest total phenolics were found in the parts of SK3-085 harvested in October (nonflowering stage) and November (beginning of floret formation).
The quantity of total phenolics in broccoli by-products varies significantly in accordance with different intrinsic and extrinsic factors such as plant cultivars and genetics, soil composition and growing conditions, and post-harvest conditions and maturity stage (1). The synthesis of the phenolics in plants is stimulated under stress such as ultraviolet (UV) exposure, air temperature, and pathogen attacks (16). In this study, the early-maturing crop Kyoyoshi was planted in June and harvested in October. Thus, high air temperatures or strong UV rays during the growing season could be the heavily stress-inducing factors that increased total phenolics contents in Kyoyoshi compared to Myeongil 96 and SK3-085.
Krumbein et al. (17) reported that quercetin and kaempferol in broccoli florets increased from the development stage to the maturity stage. However, in our study, the highest total phenolics were found in all of the parts of SK3-085 harvested at the development stage (October and November).
Sulforaphane content
Sulforaphane, a hydrolysis product of glucoraphanin in broccoli, is a physiologically active compound (6). The contents of sulforaphane in freeze-dried by-products from different cultivars and harvest dates were measured (data are not shown). With the exception of Kyoyoshi, florets had the highest sulforaphane contents in the other cultivars. SK3-085 harvested in January (mature stage) contained the highest sulforaphane content (715.3 μg/g), most notably in florets. However, the sulforaphane content of Kyoyoshi (early-maturing crop) was 2.8-fold higher in the stem (76.8 μg/g) than in the floret (26.8 μg/g). The sulforaphane contents of the remaining samples were less than 20 μg/g. Campas-Baypolia et al. (6) reported that the florets contained the highest quantity of sulforaphane in decreasing order of leaves and stems. In our study, the highest sulforaphane content was in the florets, while the lowest content was in the leaves.
DPPH radical scavenging activity and ORAC
Table 3 shows DPPH radical scavenging activities in terms of the 50% inhibitory concentration (IC50) in 80% methanol extracts of different broccoli by-products from different cultivars. Kyoyoshi, the early-maturing crop, had the highest DPPH radical scavenging activity. The DPPH radical scavenging activities were significantly different among the broccoli by-products. In all cultivars, the DPPH radical scavenging activity of the leaves was the highest and that of the stems was the lowest.
Table 3.
Antioxidant activities of 80% methanol extracts from broccoli by-products from different cultivars
| Cultivars | Leaves | Stems | Leaf stems | Florets | |
|---|---|---|---|---|---|
| DPPH radical scavenging activity (IC50, μg/mL) | Kyoyoshi | 408.1±21.1aA | >2,000aD | 1,432.7±55.3aC | 1,067.5±23.0aB |
| Myeongil 96 | 1,414.6±20.6cA | >2,000aC | >2,000bC | 1,814.9±55.6bB | |
| SK3-085 | 1,159.7±16.5bA | >2,000aB | >2,000bB | >2,000cB | |
| ORAC (mM TE/g dried extract) | Kyoyoshi | 699.1±0.8bD | 175.5±0.9cA | 244.3±0.7cB | 423.9±2.6cC |
| Myeongil 96 | 492.5±2.9aD | 137.7±1.2bA | 210.7±3.0bB | 254.2±0.1bC | |
| SK3-085 | 494.6±0.1aD | 97.3±1.7aB | 87.5±3.6aA | 206.4±1.9aC |
Values with different letters in the row (A–D) and in the column (a–c) within same method are significantly different at P<0.05 according to Duncan’s multiple range tests.
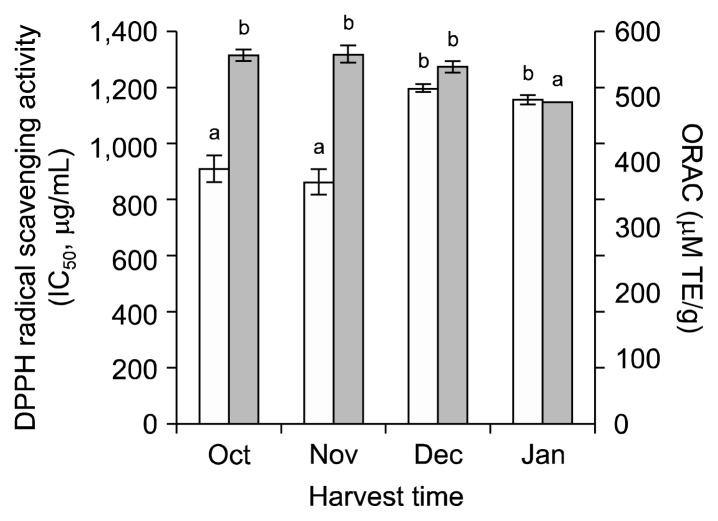
The leaves of SK3-085 with high total phenolics were further tested for DPPH radical scavenging activity at different harvest dates (Fig. 1). Higher activities were observed in the leaves harvested in October and November compared to the leaves harvested in December and January.
Fig. 1.
Antioxidant activities of 80% methanol extracts from the leaves of broccoli SK3-085 cultivar from different harvest dates. Results are expressed as DPPH radical scavenging activity (□) on the Y1 axis and ORAC (■) on the Y2 axis. The values within same property with different letters (a, b) are significantly different at P<0.05 by Duncan’s multiple range tests.
Table 3 also shows the antioxidant activity in 80% methanol extracts of different by-products from different cultivars analyzed using the ORAC assay. The ORAC value was highest in Kyoyoshi followed by Myeongil 96 and SK3-085. All cultivars had higher ORAC values in the leaves than in the florets, leaf stems, and stems.
The ORAC assay was further measured in the leaves of SK3-085 with high total phenolics and high DPPH free radical scavenging activities (Fig. 1). The leaves of broccoli harvested in October, November, and December had higher ORAC values than those harvested in January.
Inhibitory activity of cancer cell growth
Anticancer activities of 80% methanol extracts from different by-products with different cultivars and harvest dates were assessed against NCI-H1299 (human non-small lung carcinoma) cell lines using the MTT assay (Table 4). The extracts of florets, leaf stems, and stems from Kyoyoshi showed higher inhibition activities than those of Myeongil 96 and SK3-085 against NCI-H1299 cell lines. The extract of the leaves from Myeongil 96 showed the highest cell growth inhibition activity than all of the other by-products against NCI-H1299 cell lines. In particular, the leaves of all the cultivars had the highest inhibition activities of cell growth in decreasing order of florets, leaf stems, and stems.
Table 4.
Cell growth inhibitory activities of 80% methanol extracts (2 mg/mL) from broccoli by-products from different cultivars against human non-small lung carcinoma (NCI-H1229) and human colon adenocarcinoma grade II (HT-29) cell lines
| Cultivars | Cell growth inhibitory activity (%) | ||||
|---|---|---|---|---|---|
|
| |||||
| Leaves | Stems | Leaf stems | Florets | ||
| Human non-small lung carcinoma cell (NCI-H1229) | Kyoyoshi | 20.1±2.6aC | 8.6±1.6cA | 9.9±0.5cA | 15.6±0.2cB |
| Myeongil 96 | 32.5±2.3bD | 5.7±0.8bA | 8.3±0.6bB | 13.7±0.3bC | |
| SK3-085 | 21.1±1.9aC | 3.0±1.5aA | 6.5±1.1aB | 7.3±0.2aB | |
| Human colon adenocarcinoma grade II cell (HT-29) | Kyoyoshi | 11.6±2.4cC | 3.1±2.1aA | 5.2±0.8bA | 7.1±0.1aB |
| Myeongil 96 | 9.2±0.7bB | 5.5±1.5bA | 4.8±1.8bA | 9.3±0.3bB | |
| SK3-085 | 6.6±0.5aC | 3.4±0.1aB | 2.1±0.4aA | 6.4±0.4aC | |
Values with different letters in the row (A–D) and in the column (a–c) for each cell line are significantly different at P<0.05 according to Duncan’s multiple range tests.
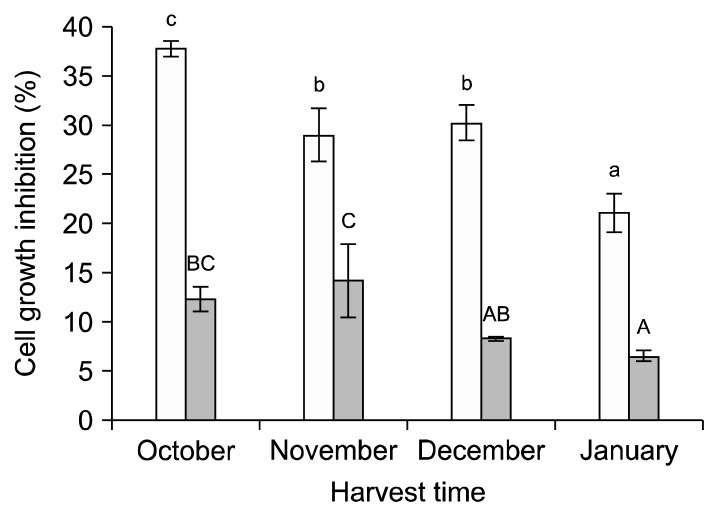
Anticancer activities against NCI-H1299 cell lines were further examined for the leaves of SK3-085 from different harvest dates (Fig. 2). All the leaves harvested at different dates were effective in reducing the viability of NCI-H1299 cell lines in the range of 20~40%. The leaves harvested in October had the greatest inhibitory activity, while those in January showed the lowest activity against NCI-H1299 cell lines.
Fig. 2.
Anticancer activity of 80% methanol extracts (2 mg/mL) from the leaves of broccoli K3-085 cultivar from different harvest dates. Results are expressed as inhibitory activities of cell growth against NCI-H1299 cell (□) and HT-29 (■) cell lines. The values with different letters in the same cell line are significantly different at P<0.05 by Duncan’s multiple range tests.
Anticancer activities of the extracts from different by-products from different cultivars and harvest dates were also measured against HT-29 (human colon adenocarcinoma) cell lines using the MTT assay (Table 4). All the leaves showed the potential for HT-29 growth inhibitory activity; however, significant differences between the leaves and florets were not observed. Overall, the inhibitory activities of the extracts from different by-products against HT-29 cell lines were less than 11.6%.
Correlation between active components, and antioxidant and anticancer activities
In our study, the correlation coefficient between total phenolic content and DPPH radical scavenging activity in broccoli by-products was high (r=0.880) as shown in Table 5. In contrast, the sulforaphane content in broccoli by-products showed no correlation with DPPH radical scavenging activity (r=−0.068). The antioxidant capacity using the ORAC assay was correlated with the total phenolic content (r=0.925), but not with the sulforaphane content (r=−0.095) as shown in Table 5.
Table 5.
Correlations among total phenolic content (TP), sulforaphane content (SF), DPPH radical scavenging activity (DPPH), oxygen radical absorbance capacity (ORAC), and cell growth inhibitory activities in NCI-H1299 and HT-29
| TP | SF | DPPH | ORAC | NCI-H1299 | HT-29 | |
|---|---|---|---|---|---|---|
| TP | 1.0 | −0.087 | 0.880* | 0.925* | 0.717* | 0.729* |
| SF | 1.0 | −0.068 | −0.095 | −0.156 | −0.029 | |
| DPPH | 1.0 | 0.926* | 0.834* | 0.738* | ||
| ORAC | 1.0 | 0.862* | 0.820* | |||
| NCI-H1299 | 1.0 | 0.718* | ||||
| HT-29 | 1.0 |
P<0.01.
Domínguez-Perles et al. (8) reported that total phenolics in broccoli were strongly correlated with DPPH radical scavenging activity. Im et al. (18) also showed that the relationship between total phenolic contents and DPPH radical scavenging activity in radish was a high positive linear correlation. In contrast, Domínguez-Perles et al. (8) reported that the correlation between sulforaphane content and DPPH radical scavenging activity in broccoli was low. Farag and Motaal (9) also showed that the sulforaphane content did not contribute to the antioxidant activity in terms of DPPH radical scavenging activity of broccoli and other cruciferous vegetables.
In this study, total phenolics showed positive correlations with anticancer activities against NCI-H1299 and HT-29 cell lines (r=0.717 and 0.729), respectively. In contrast, the correlation coefficients of the sulforaphane content with anticancer activities against NCI-H1299 and HT-29 cell lines were low (r=−0.156 and −0.029) (Table 5). It is known that various phenolic compounds have shown antiproliferative and cytotoxic effects, as well as pro-apoptotic activity in animal tumor models and several cancer cell lines (19). Numerous studies have shown that sulforaphane in broccoli is the major component with anticarcinogenic activity (20,21). However, in this study, the major factor that correlated with anticarcinogenic activity against NCI-H1299 and HT-29 cell lines was total phenolics rather than the sulforaphane content because the inherent sulforaphane content in broccoli by-products was low.
In conclusion, broccoli by-products such as leaves and stems from different cultivars and harvest dates contain high total phenolics and show high antioxidant and anti-cancer activities. Thus, these by-products can be used as functional food ingredients in the food industry.
ACKNOWLEDGEMENTS
This research was supported by the Ministry of Trade, Industry and Energy (MOTIE) and The Korea Institute for the Advancement of Technology (KIAT) through the Research and Development for Regional Industry.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Jeffery EH, Brown AF, Kurilich AC, Keck AS, Matusheski N, Klein BP, Juvik JA. Variation in content of bioactive components in broccoli. J Food Compos Anal. 2003;16:323–330. doi: 10.1016/S0889-1575(03)00045-0. [DOI] [Google Scholar]
- 2.Moreno DA, Carvajal M, López-Berenguer C, García-Viguera C. Chemical and biological characterisation of nutraceutical compounds of broccoli. J Pharm Biomed Anal. 2006;41:1508–1522. doi: 10.1016/j.jpba.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Kaur C, Kumar K, Anil D, Kapoor HC. Variations in antioxidant activity in broccoli (Brassica oleracea L.) cultivars. J Food Biochem. 2007;31:621–638. doi: 10.1111/j.1745-4514.2007.00134.x. [DOI] [Google Scholar]
- 4.Ares AM, Nozal MJ, Bernal J. Extraction, chemical characterization and biological activity determination of broccoli health promoting compounds. J Chromatogr A. 2013;1313:78–95. doi: 10.1016/j.chroma.2013.07.051. [DOI] [PubMed] [Google Scholar]
- 5.Borowski J, Szajdek A, Borowska EJ, Ciska E, Zieliński H. Content of selected bioactive components and antioxidant properties of broccoli (Brassica oleracea L.) Eur Food Res Technol. 2008;226:459–465. doi: 10.1007/s00217-006-0557-9. [DOI] [Google Scholar]
- 6.Campas-Baypoli ON, Sánchez-Machado DI, Bueno-Solano C, Ramírez-Wong B, López-Cervantes J. HPLC method validation for measurement of sulforaphane level in broccoli by-products. Biomed Chromatogr. 2010;24:387–392. doi: 10.1002/bmc.1303. [DOI] [PubMed] [Google Scholar]
- 7.Mahro B, Timm M. Potential of biowaste from the food industry as a biomass resource. Eng Life Sci. 2007;7:457–468. doi: 10.1002/elsc.200620206. [DOI] [Google Scholar]
- 8.Domínguez-Perles R, Martínez-Ballesta MC, Carvajal M, García-Viguera C, Moreno DA. Broccoli-derived by-products—a promising source of bioactive ingredients. J Food Sci. 2010;75:C383–C392. doi: 10.1111/j.1750-3841.2010.01606.x. [DOI] [PubMed] [Google Scholar]
- 9.Farag MA, Abdel Motaal AA. Sulforaphane composition, cytotoxic and antioxidant activity of crucifer vegetables. J Adv Res. 2010;1:65–70. doi: 10.1016/j.jare.2010.02.005. [DOI] [Google Scholar]
- 10.Zhang Q, Zhang J, Shen J, Silva A, Dennis DA, Barrow CJ. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J Appl Phycol. 2006;18:445–450. doi: 10.1007/s10811-006-9048-4. [DOI] [Google Scholar]
- 11.Bertelli D, Plessi M, Braghiroli D, Monzani A. Separation by solid phase extraction and quantification by reverse phase HPLC of sulforaphane in broccoli. Food Chem. 1998;63:417–421. doi: 10.1016/S0308-8146(98)00052-1. [DOI] [Google Scholar]
- 12.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 13.Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001;49:4619–4626. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari M, Fornasiero MC, Isetta AM. MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. J Immunol Methods. 1990;131:165–172. doi: 10.1016/0022-1759(90)90187-Z. [DOI] [PubMed] [Google Scholar]
- 15.Faller ALK, Fialho E. Polyphenol content and antioxidant capacity in organic and conventional plant foods. J Food Compos Anal. 2010;23:561–568. doi: 10.1016/j.jfca.2010.01.003. [DOI] [Google Scholar]
- 16.Dixon RA, Pavia NL. Stress-induced phenylpropanoid metabolism. Plant Cell. 1995;7:1085–1097. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krumbein A, Saeger-Fink H, Schonhof I. Changes in quercetin and kaempferol concentrations during broccoli head ontogeny in three broccoli cultivars. J Appl Bot Food Qual. 2007;81:136–139. [Google Scholar]
- 18.Im JS, Lee EH, Lee JN, Kim KD, Kim HY, Kim MJ. Sulforaphane and total phenolics contents and antioxidant activity of radish according to genotype and cultivation location with different altitudes. Kor J Hort Sci Technol. 2010;28:335–342. [Google Scholar]
- 19.Fresco P, Borges F, Marques MP, Diniz C. The anticancer properties of dietary polyphenols and its relation with apoptosis. Curr Pharm Des. 2010;16:114–134. doi: 10.2174/138161210789941856. [DOI] [PubMed] [Google Scholar]
- 20.Gamet-Payrastre L, Li P, Lumeau S, Cassar G, Dupont MA, Chevolleau S, Gasc N, Tulliez J, Tercé F. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in ht29 human colon cancer cells. Cancer Res. 2000;60:1426–1433. [PubMed] [Google Scholar]
- 21.Singh AV, Xiao D, Lew KL, Dhir R, Singh SV. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 2004;25:83–90. doi: 10.1093/carcin/bgg178. [DOI] [PubMed] [Google Scholar]