Abstract
Phenolic rich ethyl acetate fraction (EAF) from lotus leaves was prepared and its bioactive components, antioxidant and cytoprotective effects were investigated. EAF showed high total phenolic content and flavonoid content and contained rutin (11,331.3±4.5 mg/100 g EAF), catechin (10,853.8±5.8 mg/100 g EAF), sinapic acid (1,961.3±5.6 mg/100 g EAF), chlorogenic acid (631.9±2.3 mg/100 g EAF), syringic acid (512.3±2.5 mg/100 g EAF), and quercetin (415.0±2.1 mg/100 g EAF). EAF exerted the IC50 of 4.46 μg/mL and 5.35 μg/mL toward DPPH and ABTS cation radicals, respectively, and showed strong reducing power, which was better than that of ascorbic acid, a positive control. Additionally, EAF protected hydroxyl radical-induced DNA damage indicated by the conversion of supercoiled pBR322 plasmid DNA to the open circular form and inhibited lipid peroxidation of polyunsaturated fatty acid in a linoleic acid emulsion. In cultured hepatocytes, EAF exerted a cytoprotective effect against oxidative stress by inhibiting intracellular reactive oxygen species formation and membrane lipid peroxidation. In addition, depletion of glutathione under oxidative stress was remarkably restored by treatment with EAF. The results suggest that EAF have great potential to be used against oxidative stress-induced health conditions.
Keywords: lotus leaf, antioxidant, DNA damage, cytoprotection
INTRODUCTION
There is a growing interest in the development of natural antioxidants that suppress the excessive production of reactive oxygen species (ROS), which are strongly linked to numerous chronic diseases such as cancer, atherosclerosis, diabetes and cardiovascular diseases, as well as aging (1). Moreover, oxidative stress plays a key role in a number of liver disorders such as steatosis, impaired liver function, inflammation and fibrosis (2). To reduce oxidative stress, many antioxidant substances in our body such as superoxide dismutase, catalase, glutathione peroxidase, vitamin C, vitamin E, and glutathione effectively scavenge intracellular ROS, thereby preventing cell injury. However, abnormal conditions destroy the balance between production of ROS and the body’s defense capacity, leading to oxidative damage (3).
Consumption of food derived antioxidants such as phenolic compounds can reduce oxidative stress by scavenging ROS and free radical-mediated chain reactions and have beneficial effects on human health conditions. Several epidemiological studies have shown that phenolic compounds may have ameliorating effects with regard to hepatic damage, coronary heart disease and atherosclerosis (3–5). These phenolic compounds are extensively distributed in the plant kingdom, are major antioxidants from plant constituents, and their biological activities have been extensively investigated.
Lotus (Nelumbo nucifera) is an aquatic plant, and its leaves have a long history of usages as food and folk medicine in China and Korea. Catechin, quercetin, quercetin-3-O-glycoside, kampherol-3-O-glycoside, and myricetin-3-O-glucoside have been reported as part of its major components (6–8). Pharmacologic and physiological activities including antidiabetic, cytoprotective, antibacterial, antioxidant, and antiobesity effects have been documented from the extracts of lotus leaves, seed, and rhizome (4,6,7,9,10). However, limited information is available with regard to the evaluation of its antioxidant activity on cells, specifically on human hepatocyte. In the present study, ethyl acetate fraction (EAF) from lotus leaves was prepared and its phenolic composition determined. Further, antioxidant activities of EAF were measured using free radical scavenging activity, protection ability against DNA damage, and cytoprotective effect on hydrogen peroxide-induced hepatic damage in cultured human hepatocytes.
MATERIAS AND METHODS
Materials
Lotus leaves were purchased from a local farm in August 2012 (Muan, Korea). Linoleic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2-azino-bis(3-ethylbenzthiazoline)-6-sulfonic acid (ABTS), potassium ferricyanide, mushroom tyrosinase, acetylcholinesterase, acetylthiocholine chloride, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All other chemicals and reagents used in this study were of analytical grade and commercially available.
Preparation of ethyl acetate fraction (EAF) from lotus leaves
The dried powder from lotus leaves (20 g) were extracted thrice with 2 L of 80% ethanol at 80°C for 3 h. The extracts were combined and concentrated to dryness under reduced pressure. The ethanol extract (3.6 g) was suspended and fractionated in 360 mL of H2O using ethyl acetate (3×360 mL) to obtain the ethyl acetate fraction (EAF), which was concentrated in a vacuum evaporator, and finally freeze-dried to yield 0.25 g EAF. The dried EAF was kept in airtight bottles at −20°C until use.
Determination of total phenolic content (TPC) and total flavonoid content (TFC)
The TPC was determined by the method of Singleton et al. (11). Briefly, 40 μL of EAF (1 mg/mL) was mixed with 200 μL Folin-Ciocalteau reagent (2 N) and 1,160 μL of distilled water for 3 min, followed by addition of 600 μL sodium carbonate (20%, w/v). The mixture was shaken for 2 h at room temperature, and then a 200 μL aliquot of the mixture was added to each well of a 96-well microplate. Absorbance was measured at 760 nm using a microplate reader (SpectraMax® M2/M2e, Molecular Devices, LLC., Sunnyvale, CA, USA). Gallic acid was used as a standard and the results were expressed as mg gallic acid equivalents (GAE)/g EAF.
The TFC was determined using the method described by Meda et al. (12) with minor modifications. In brief, 5 mL of 2% AlCl3 was mixed with 5 mL of EAF. Absorbance at 415 nm was measured after 10 min against a blank sample consisting of 5 mL of sample solution and 5 mL of distilled water without AlCl3. The TFC was determined using a standard curve of quercetin and the results were expressed as mg quercetin equivalents (QUE)/g EAF.
Determination of phenolic composition by HPLC
To analyze the phenolic composition in EAF, 0.2 g of EAF was dissolved in 4 mL of methanol and then filtered using a 0.45 μm syringe filter. The standard stock solutions were prepared in the concentration range from 10 to 1,000 μg/mL. The components of EAF were separated using a reverse-phase column [Luna C18(2), 150×3.0 mm, 3 μm, Phenomenex Inc., Torrance, CA, USA] and analyzed by an UV detector at 270 nm. The mobile phase consisted of methanol (solvent A) and 0.1% formic acid (solvent B). The non-linear gradient elution used was as follow: A/B(10:90) to (15:85) at 5 min, (23:77) at 25 min, (50:50) at 30 min, and then hold for 5 min. The flow rate was 0.34 mL/min, and the injection volume was 20 μL. Phenolic composition was identified by the comparison of the retention time to the UV spectra of the standards.
Antioxidant activity of EAF
Measurement of DPPH scavenging activity
Antioxidant activity was evaluated by the DPPH scavenging assay modified from that of Blois (13). A 100 μL of DPPH solution (150 μM in methanol) was incubated with and without 100 μL of EAF, and the mixtures were then kept in the dark for 30 min. The absorbance was measured at 517 nm on a microplate reader. The DPPH scavenging activity was calculated by the following equation.
The IC50 value was defined as the concentration required for scavenging 50% of DPPH.
Measurement of ABTS+ radical scavenging activity
Prior to evaluating ABTS+ radical scavenging activity, ABTS+ radical stock solution was prepared by incubation of 7 mM ABTS with 2.4 mM potassium persulfate for 16 h in the dark (14). The stock solution was diluted to a working solution with absorbance of 1.50±0.05 at 414 nm. A 150 μL of ABTS+ radical working solution was mixed with and without 50 μL of EAF, following incubation for 10 min, and the absorbance was measured at 414 nm. The ABTS+ radical scavenging activity was calculated by the following equation.
The IC50 value was defined as the concentration required for scavenging 50% of ABTS+ radical.
Determination of reducing power
The reducing power was determined using the method described by Oyaizu (15). Briefly, EAF (100 μg/mL) was mixed with 0.5 mL phosphate buffer (0.2 M, pH 6.6) and 0.5 mL potassium ferricyanide (1%, w/v). The mixture was incubated at 50°C for 20 min. Next, 0.5 mL trichloroacetic acid (TCA) (10%, w/v) was added to the mixture, which was centrifuged at 1,036 g for 10 min. Finally, 0.5 mL of the supernatant was mixed with 0.5 mL distilled water and 0.1 mL FeCl3 (0.1%, w/v), and the absorbance was measured at 700 nm.
Protection effect against hydroxyl radical-induced DNA damage
To evaluate the protection effect against hydroxyl radical-induced DNA damage, a reaction was induced by placing the following reagents (total volume, 12 μL) in an Eppendorf tube: 0.5 μg of pBR322 DNA, 2 mM FeSO4, and various concentrations of EAF. The mixture was then incubated at 37°C for 30 min, followed by the addition of 4 μL of 10 mM H2O2 (16). Next, the mixture was subjected to 0.8% agarose gel electrophoresis, after which the DNA bands (supercoiled and open circular) were stained with ethidium bromide.
Measurement of lipid peroxidation inhibition activity
Lipid peroxidation inhibitory activity was measured by determination of thiobarbituric acid reactive substances (TBARS) in a linoleic acid emulsion. For that, the emulsion was prepared by mixing of 200 μL aliquot of 2.5% linoleic acid in ethanol, 200 μL of EAF, 200 μL distilled water, and 400 μL phosphate buffer (50 mM, pH 7.0). The mixture was incubated in the dark at 40°C for 4 days. After 4 days, 100 μL of the mixture was mixed with 1 mL of TBA-TCA solution (20 mM TBA in 15% TCA) and heated in a 100°C water bath for 15 min then cooled. After 1 mL of chloroform was added, the mixture was mixed and centrifuged at 3,000 rpm for 15 min. The chloroform layer was measured at an absorbance wavelength of 532 nm (17). A malondialdehyde (MDA) standard curve was prepared, and TBARS were expressed as μM of MDA.
Cytoprotective effect of EAF under oxidative stress in Chang liver cells
Cytotoxic and protective effects of EAF against hydrogen peroxide-induced hepatic damage
Human normal Chang liver cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 100 U/mL of penicillin, and 100 μg/mL of streptomycin. The cells were incubated at 37°C in an atmosphere of 5% CO2 and 95% air.
Cytotoxicity of EAF was estimated by the MTT assay. Chang liver cells were grown in 96-well plates at a density of 1×104 cells/well. After 24 h, the cells were treated with EAF at the desired concentrations (in DMEM) and incubated at 37°C for 24 h. After aspiration of the medium, a 100 μL of the MTT solution (1 mg/mL) was added and incubated for 4 h. Then, the supernatant was aspirated, and finally, a 100 μL of DMSO was added to solubilize the formazan crystals. The amount of formazan crystals was determined by measuring the absorbance at 540 nm.
To determine the protection ability against oxidative stress, the cells were seeded into 96-well plate and incubated for 24 h. EAF (0~0.1 μg/mL) was added into the well and incubated for 1 h. After washing three times with phosphate buffered saline (PBS), 650 μM of H2O2 was added to induce oxidative stress, and the cells were incubated for 24 h. After 24 h incubation, the MTT assay was performed as described above.
Inhibition of intracellular ROS formation
Chang liver cells were grown in a 96-well plate and labeled with 20 μM of 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) dye and left in the dark for 20 min, followed by the addition of 0~0.1 μg/mL of EAF and incubation for 1 h. Thereafter, the cells were washed with PBS, followed by the addition of 650 μM H2O2 to each well. Fluorescence due to oxidative formation of 2′,7′-dichlorofluorescein (DCF) by ROS was measured after 30, 60, and 90 min at excitation and emission wavelengths of 485 and 528 nm, respectively (18). The percentage of fluorescence intensity (ROS generation) was compared with that of the control cells without the EAF treatment, which were arbitrarily assigned a value of 100%.
Inhibition of cell membrane lipid peroxidation
Chang liver cells were grown in culture dishes and washed with PBS followed by the addition of 13 μM 1,3-bis(diphenylphosphino)propane (DPPP) in DMSO and incubated for 30 min at 37°C in the dark. The cells were washed with PBS and transferred into a 96-well plate at 4×105 cells/mL using serum-free media. After complete attachment, the cells were treated with 0~0.1 μg/mL of EAF and incubated for 1 h followed by the addition of 3 mM 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) in PBS to initiate cell membrane lipid peroxidation. DPPP oxide fluorescence intensity after 6 h was measured at excitation and emission wavelengths of 361 and 380 nm, respectively (18). The percentage of fluorescence intensity (membrane lipid peroxidation) was compared with that of the control cells without the EAF treatment, which were arbitrarily assigned a value of 100%.
Determination of glutathione (GSH) level
The effect of EAF on the expression of GSH under oxidative stress in Chang liver cells was measured using a thiol-staining agent, monobromobimane (mBBr) (18). The cells were seeded in a 96-well plate at 4×105 cells/mL, after attaining confluence, the cells were treated with 0~0.1 μg/mL of EAF for 1 h. After washing the cells three times with PBS, 650 μM H2O2 was added to each well and incubated for 2 h to induce oxidative stress. The cells were labeled with 40 μM mBBr for 30 min. Fluorescence due to mBBr-GSH was measured at excitation and emission wavelengths of 360 and 465 nm, respectively. The percentage of fluorescence intensity (GSH level) was compared with that of the blank cells, which were arbitrarily assigned a value of 100%.
Statistical analysis
The data are presented as the mean±standard deviation (SD) of at least three independent experiments (n=3). Differences between means of each group were assessed by one-way analysis of variance followed by Duncan’s test using PASW Statistics 19.0 software (SPSS, Chicago, IL, USA). A P-value <0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Determination of TPC, TFC, and phenolic composition of EAF
Foods containing phytochemicals have protective effects against degenerative diseases (8). Prior to evaluating the antioxidant activity of EAF, TPC, TFC, and phenolic compositions were determined and are summarized in Table 1. The EAF exhibited 346.26±15.22 mg GAE/g EAF of TPC and 115.86±1.12 mg QUE/g EAF of TFC. The ratio of TFC/TPC in EAF is 0.33 and this indicated that high flavonoid levels in EAF may play an important antioxidant role. To analyze the phenolic compositions of EAF, fourteen standard phenolic compounds were used and analyzed by an UV detector and phenolic compositions in EAF were identified by retention times compared to the HPLC profiles. As summarized in Table 1, EAF contained plentiful phenolic compounds and fourteen phenolic compounds were detected. The major phenolic compounds in EAF from lotus leaves were rutin (11,331.3±4.5 mg/100 g EAF), catechin (10,853.8±5.8 mg/100 g EAF), sinapic acid (1,961.3±5.6 mg/100 g EAF), chlorogenic acid (631.9±2.3 mg/100 g EAF), syringic acid (512.3±2.5 mg/100 g EAF), and quercetin (415.0±2.1 mg/100 g EAF). Hydroxybenzoic acid (139.1 ±0.1 mg/100 g EAF) and protocatechuic acid (128.3± 0.1 mg/100 g EAF) were also abundant in EAF. Concentrations of the rest of the compounds were between 5.3±0.1 and 71.2±2.1 mg/100 g EAF. The current results on the phenolic composition analysis indicate that lotus leaves extract represents a good source of natural antioxidants.
Table 1.
TPC, TFC, and phenolic compositions of EAF
| Retention time (min) | mg/100 g EAF | |
|---|---|---|
| Gallic acid | 5.1 | 31.6±0.1 |
| Protocatechuic acid | 9.5 | 128.3±0.1 |
| Hydroxybenzoic acid | 16.2 | 139.1±0.1 |
| Catechin | 18.1 | 10,853.8±5.8 |
| Vanillic acid | 25.8 | 27.3±0.3 |
| Caffeic acid | 27.0 | 5.3±0.1 |
| Chlorogenic acid | 29.5 | 631.9±2.3 |
| Syringic acid | 35.0 | 512.3±2.5 |
| p-Coumaric acid | 37.9 | 71.2±2.1 |
| Ferulic acid | 41.1 | 21.0±0.6 |
| Sinapic acid | 42.5 | 1,961.3±5.6 |
| Rutin | 47.7 | 13,311.3±4.5 |
| Cinnamic acid | 54.5 | 30.2±0.5 |
| Quercetin | 56.5 | 415.0±2.1 |
| TPC (mg GAE/g EAF) | 346.26±15.22 | |
| TFC (mg QUE/g EAF) | 115.86±1.12 | |
| Yield (%) | 1.25 |
Antioxidant effects of EAF in non-cellular systems
Three assays including DPPH, ABTS+ radical scavenging, and reducing power were employed to evaluate the anti-oxidant effects of lotus leaves EAF. The DPPH assay is widely used to evaluate antioxidant activity in food components. The reduction of the stable purple free radical DPPH to the yellow hydrazine is achieved by trapping the unpaired electrons, and the degree of discoloration indicates the scavenging activity of samples. The calculated IC50 value of EAF was determined to be 4.28 μg/mL and the IC50 value of ascorbic acid as a positive control was 3.04 μg/mL (Table 2). Several researches have reported the antioxidant activities of lotus leaves extracts. Jung et al. (19) reported that the IC50 values of ethyl acetate and n-butanol fractions were 9.86 μg/mL and 1.74 μg/mL. Lin et al. (8) demonstrated that the IC50 values of ethyl acetate and n-butanol fractions were 15.7 μg/mL and 9.7 μg/mL. Our result showed that the DPPH scavenging activity was higher than that of Lin’s report, but lower than that of n-butanol fraction from Jung’s report. These differences were attributed to the lotus cultivar and cultivation area.
Table 2.
Antioxidant activities of EAF from lotus leaves
| Assays | EAF | Ascorbic acid |
|---|---|---|
| DPPH (IC50, μg/mL)1) | 4.46±0.01a | 3.04±0.01b |
| ABTS+ (IC50, μg/mL) | 5.35±0.02a | 3.45±0.01b |
| Reducing power (A700)2) | 0.224±0.003a | 0.218±0.001b |
Different letters indicated a significant difference at the same assay (P<0.05).
The IC50 value was defined as the concentration required to scavenge 50% of DPPH or ABTS+ radical.
Reducing power was evaluated at 100 μg/mL.
To further characterize the antioxidant ability of EAF, ABTS+ radical scavenging activity and reducing power were determined. These assays are associated with hydrogen and/or electron donating ability of the tested antioxidant compounds. The results of ABTS+ radical scavenging activity and reducing power of EAF are shown in Table 2. The calculated IC50 value of EAF against the ABTS+ radical was determined to be 5.35 μg/mL and the IC50 value of ascorbic acid as a positive control was 3.45 μg/mL. Since the antioxidant activity of a constituent is directly related to its reducing power, this is a commonly used method to evaluate the antioxidant capacities of various compounds. A high absorbance value at 700 nm indicates high reducing power, i.e. antioxidant compounds possess high hydrogen and/or electron donating ability. As shown in Table 2, the optical value of EAF at 700 nm was 0.224 and that of ascorbic acid was 0.218.
Protection ability against hydroxyl radical-induced DNA damage
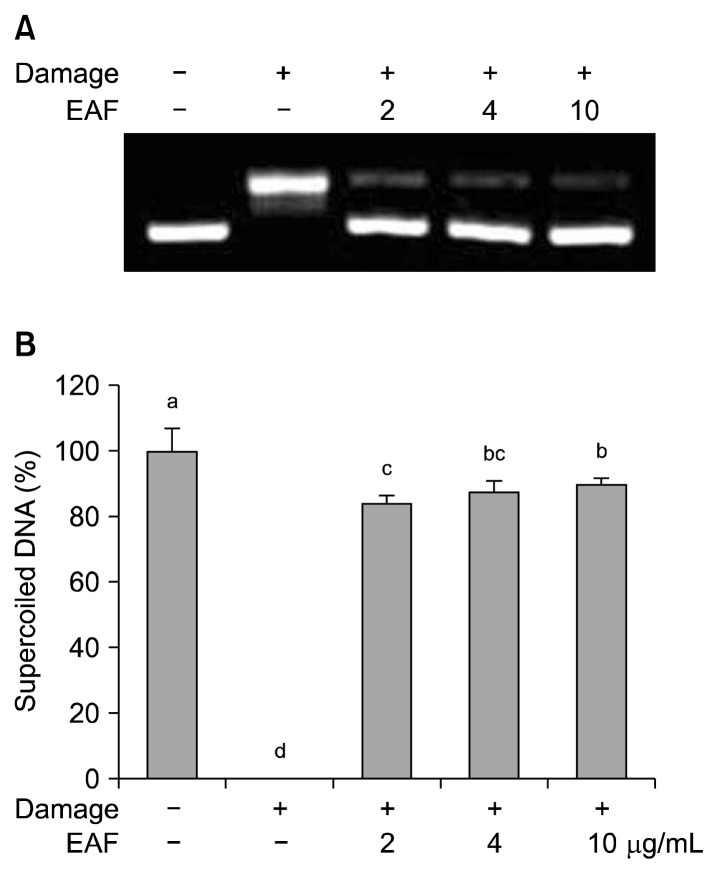
ROS causes oxidative damage in cellular components such as DNA, proteins and membrane lipids. Among them, DNA is most sensitive target and it can be changed to single and double strand breaks, base and sugar molecule damages (20). In this study, plasmid DNA was employed for the protective ability of EAF against the hydroxyl radical-induced supercoied (SC) form of DNA damage to the open circular (OC) form. A clear damage response was seen with respect to a decrease in the SC form to the OC form (Fig. 1A). Co-treatment with EAF at various concentrations prevented the formation of DNA strand breaks, and the SC form DNA was recovered up to 89.67% at 10 μg/mL of EAF (Fig. 1B).
Fig. 1.
Electrophoretic pattern of pBR322 DNA in the presence of EAF (A), and the protection effect of EAF on hydroxyl radical-induced DNA damage (B). The bars with different letters (a–d) represent significant differences (P<0.05). Values are expressed as means±SD (n=3).
Lipid peroxidation inhibition in linoleic acid emulsion
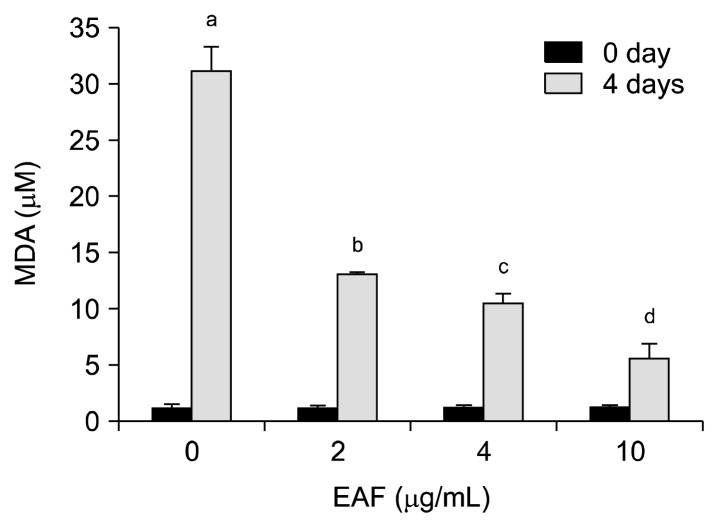
The oxidative deterioration of lipids is of great concern in the shelf life of foods, and oxidative decomposition of unsaturated fatty acids is the main factor causing food deterioration during storage and processing. In addition, lipid peroxidation impairs food safety and nutritional quality by the formation of potentially toxic products and secondary oxidation products, which cause oxidative damage related to numerous health disorders such as diabetes, cancer, neurodegenerative and inflammatory diseases (21). To evaluate the lipid peroxidation inhibitiory activity of EAF, linoleic acid emulsions with and without EAF were prepared and then incubated for 4 days at 40°C, followed by the determination of TBARS. MDA levels in linoleic acid emulsions without EAF were elevated up to 31.14 μM, whereas a dose-dependent decrease of MDA levels in co-incubation of EAF was observed and 5.55 μM MDA was determined at 10 μg/mL of EAF after 4 days (Fig. 2). Plant phenols and flavonoids are known to inhibit lipid peroxidation by quenching lipid peroxy radicals and reducing or chelating iron in lipoxygenase enzymes and thus preventing the initiation of lipid peroxidation reactions (22). Our result also agreed with this, where EAF contains high amounts of phenolic acid and flavonoid.
Fig. 2.
Lipid peroxidation inhibition activity of EAF in a linoleic acid emulsion. The bars with different (a–d) letters represent significant differences (P<0.05). Values are expressed as means±SD (n=3).
Cytoprotective effect of EAF under oxidative stress in cultured human hepatocytes
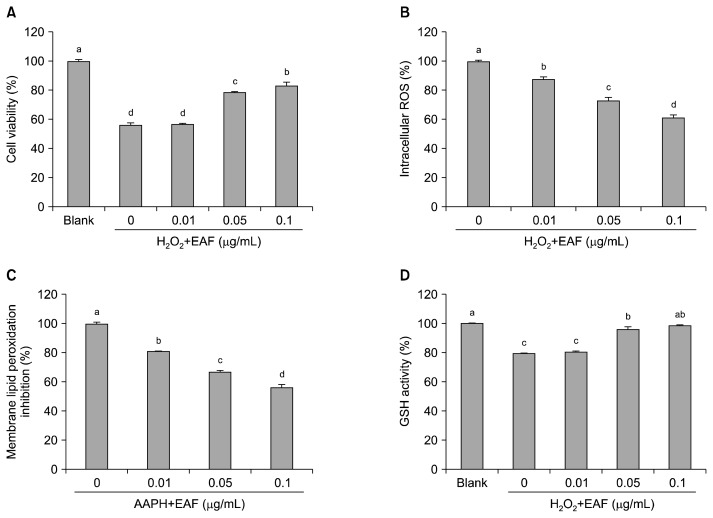
To evaluate the cytoprotective effect of EAF in cultured human hepatocytes, oxidative stress conditions were employed by treatment with 650 μM H2O2 with and without EAF. After 24 h of incubation, the treatment with H2O2 alone resulted in 56.30% cell viability compared to that of the non-treatment group; however, pre-treatment with EAF significantly (P<0.05) increased cell viability by 78.50 and 83.19% in the presence of 0.05 and 0.1 μg/mL of EAF (Fig. 3A). This result could be due to the direct scavenging effect of phenolic compounds in EAF as shown in the non-cellular antioxidant results (Table 2). To determine how EAF exerts a cytoprotective effect under oxidative stress, we further measured the inhibition ability of EAF towards intracellular ROS formation by using a DCFH-DA fluorescence probe in cultured human hepatocytes. As shown in Fig. 3B, pre-treatment with EAF significantly (P<0.05) decreased intracellular ROS formation by 18.19%, 26.95%, and 38.28% in the presence of 0.01, 0.05, and 0.1 μg/mL of EAF, which may explain in part the mechanism behind the extract’s ability to protect cells from oxidative damage. Overproduction of ROS results in oxidative stress, which leads to oxidative damage in cells by altering the structure of biomacromolecules, and this process has been implicated in a number of human diseases as well as in the ageing process. Therefore, the reduction of intracellular ROS may help prevent the onset and progression of diseases via protection of vital molecules. As shown in Fig. 3C, we further evaluated the cell membrane lipid peroxidation inhibitiory ability of EAF because ROS can easily attack and alter the structure of polyunsaturated fatty acids in cell membranes. Membrane lipid peroxidation was induced by the addition of AAPH, an initiator of lipid peroxidation. The retardation of ROS formation by EAF was accompanied by the inhibition of membrane lipid peroxidation in a similar dose-dependent manner, with 18.63%, 32.64%, and 43.33% inhibition at 0.01, 0.05, and 0.1 μg/mL of EAF, respectively. It is well known that oxidative stress typically induces cell death and depletion of cellular GSH levels requiring intervention with exogenous anti-oxidants to either support the endogenous system in protecting the cells from oxidation or in restoring the normal physiological redox state (23). Therefore, enhancement of GSH level in cellular system is a potential strategy to prevent oxidative stress. As shown in Fig. 3D, treatment with H2O2 alone caused the depletion of GSH (79.33%) compared to the non-treatment group; however, pre-treatment with EAF markedly (P<0.05) restored the intracellular GSH levels by 96.06% and 98.56% in the presence of 0.05 and 0.1 μg/mL of EAF, respectively. These results indicate that treatment with EAF exerted the protection ability via inhibiting intracellular ROS formation and membrane lipid peroxidation, and enhancement of intracellular GSH levels in the cultured human hepatocytes under oxidative stress.
Fig. 3.
Effects of EAF on cytoprotection (A), intracellular ROS formation (B), membrane lipid peroxidation (C), and GSH level (D) in the cultured human hepatocytes under oxidative stress. The bars with different (a–d) letters represent significant differences (P<0.05). Values are expressed as means±SD (n=3).
This study demonstrated the antioxidant and cytoprotective effects of phenolic rich ethyl acetate fraction (EAF) from lotus leaves in non-cellular and cellular models. EAF showed similar antioxidant activity compared to ascorbic acid, and also protected hepatic cell damage induced by oxidative stress. Therefore, lotus leaves extract could be used in food systems and/or as an antioxidant ingredient for functional food applications.
ACKNOWLEDGEMENTS
This work was supported by a Research Grant of Pukyong National University (granted in 2014).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Aruoma OI. Nutrition and health aspects of free radicals and antioxidants. Food Chem Toxicol. 2004;32:671–683. doi: 10.1016/0278-6915(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Dong H, Thompson DC, Shertzer HG, Nebert DW, Vasiliou V. Glutathione defense mechanism in liver injury: insights from animal models. Food Chem Toxicol. 2013;60:38–44. doi: 10.1016/j.fct.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yen GC, Duh PD, Su HJ. Antioxidant properties of lotus seed and its effect on DNA damage in human lymphocytes. Food Chem. 2005;89:379–385. doi: 10.1016/j.foodchem.2004.02.045. [DOI] [Google Scholar]
- 4.Du H, You JS, Zhao X, Park JY, Kim SH, Chang KJ. Antiobesity and hypolipidemic effects of lotus leaf hot water extract with taurine supplementation in rats fed a high fat diet. J Biomed Sci. 2010;17:S42. doi: 10.1186/1423-0127-17-S1-S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang B, Ban XQ, He JS, Tong J, Tian J, Wang YW. Hepatoprotective and antioxidant activity of ethanolic extracts of edible lotus (Nelumbo nucifera Gaertn.) leaves. Food Chem. 2010;120:873–878. doi: 10.1016/j.foodchem.2009.11.020. [DOI] [Google Scholar]
- 6.Ha JY, Lee KE, Park JM, Dong AY, Shin HS. Cytoprotective activity of lotus (Nelumbo nucifera Gaertner) leaf extracts on the mouse embryonic fibroblast cell. Food Sci Biotechnol. 2010;19:1171–1176. doi: 10.1007/s10068-010-0167-y. [DOI] [Google Scholar]
- 7.Huang CF, Chen YW, Yang CY, Lin HY, Way TD, Chiang W, Liu SH. Extract of lotus leaf (Nelumbo nucifera) and its active constituent catechin with insulin secretagogue activity. J Agric Food Chem. 2011;59:1087–1094. doi: 10.1021/jf103382h. [DOI] [PubMed] [Google Scholar]
- 8.Lin HY, Kuo YH, Lin YL, Chiang WC. Antioxidative effect and active components from leaves of lotus (Nelumbo nucifera) J Agric Food Chem. 2009;57:6623–6629. doi: 10.1021/jf900950z. [DOI] [PubMed] [Google Scholar]
- 9.Li MY, Xu ZT. Quercetin in a lotus leaves extract may be responsible for antibacterial activity. Arch Pharm Res. 2008;31:640–644. doi: 10.1007/s12272-001-1206-5. [DOI] [PubMed] [Google Scholar]
- 10.Yang DM, Wang QS, Ke LQ, Jiang JM, Ying TJ. Antioxidant activities of various extracts of lotus (Nelumbo nuficera Gaertn) rhizome. Asia Pac J Clin Nutr. 2007;16:158–163. [PubMed] [Google Scholar]
- 11.Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 12.Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- 13.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 14.Park MK, Kim CH. Extraction of polyphenols from apple peel using cellulase and pectinase and estimation of antioxidant activity. J Korean Soc Food Sci Nutr. 2009;38:535–540. doi: 10.3746/jkfn.2009.38.5.535. [DOI] [Google Scholar]
- 15.Oyaizu M. Studies on products of browning reaction: antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr Diet. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- 16.Yeung SY, Lan WH, Huang CS, Lin CP, Chan CP, Chang MC, Jeng JH. Scavenging property of three cresol isomers against H2O2, hypochlorite, superoxide and hydroxyl radicals. Food Chem Toxicol. 2002;40:1403–1413. doi: 10.1016/S0278-6915(02)00102-3. [DOI] [PubMed] [Google Scholar]
- 17.Stoilova I, Krastanov A, Stoyanova A, Denev P, Gargova S. Antioxidant activity of a ginger extract (Zingiber officinale) Food Chem. 2007;102:764–770. doi: 10.1016/j.foodchem.2006.06.023. [DOI] [Google Scholar]
- 18.Lee DS, Cho YS, Je JY. Antioxidant and antibacterial activities of chitosan-phloroglucinol conjugates. Fish Aquat Sci. 2013;16:229–235. [Google Scholar]
- 19.Jung HA, Jung YJ, Yoon NY, Jeong DM, Bae HJ, Kim DW, Na DH, Choi JS. Inhibitory effects of Nelumbo nucifera leaves on rat lens aldose reductase, advanced glycation end-products formation, and oxidative stress. Food Chem Toxicol. 2008;46:3818–3826. doi: 10.1016/j.fct.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Maurya DK, Adhikari S, Nair CK, Devasagayam TP. DNA protective properties of vanillin against γ-radiation under different conditions: possible mechanisms. Mutat Res. 2007;634:69–80. doi: 10.1016/j.mrgentox.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Pryor WA. Free radical biology: xenobiotics, cancer, and aging. Ann NY Acad Sci. 1982;393:1–22. doi: 10.1111/j.1749-6632.1982.tb31228.x. [DOI] [PubMed] [Google Scholar]
- 22.Torel J, Cillard J, Cillard P. Antioxidant activity of flavonoids and reactivity with peroxy radical. Phytochemistry. 1986;25:383–385. doi: 10.1016/S0031-9422(00)85485-0. [DOI] [Google Scholar]
- 23.Reid M, Jahoor F. Glutathione in disease. Curr Opin Clin Nutr Metab Care. 2001;4:65–71. doi: 10.1097/00075197-200101000-00012. [DOI] [PubMed] [Google Scholar]