Abstract
Most of the wheat germ in cereal grains is removed during the milling process. Various physiological effects have been reported for bioactive substances in wheat germ such as phenolic acids and flavonoids. In this study, the anti-oxidant and anti-adipogenic effects of ethanol extracts from wheat germ (WGE) and wheat germ fermented with Aspergillus oryzae (F-WGE) were investigated in HepG2 and 3T3-L1 cells. The anti-oxidant activity of F-WGE was demonstrated by a dose-dependent increase in the enhanced scavenging capacity of hydroxyl radicals and Cu2+-chelating activity compared to WGE. WGE and F-WGE treatment at doses between 10 and 400 μg/mL did not affect the viability of HepG2 and 3T3-L1 cells. Intracellular ROS levels from Cu2+-induced oxidative stress were significantly decreased by F-WGE treatment in HepG2 cells compared to WGE. Lipid accumulation was increased in 3T3-L1 adipocytes by 100 μM Fe2+ treatment, but the accumulation was strongly inhibited by 100 μg/mL of WGE and F-WGE treatment. These results suggest that changes in bioactive substances during the fermentation of wheat germ can potentiate scavenging activities against transition metal-induced oxidative stress and lipid accumulation in 3T3-L1 adipocytes. Therefore, we propose that F-WGE is a novel food materials and provided scientific evidences for its efficacy in the development of functional foods.
Keywords: fermented wheat germ, anti-oxidant, anti-adipogenic, Aspergillus oryzae, transition metal
INTRODUCTION
Many pysiological systems in the human body are damaged by reactive oxygen species (ROS) generated from exogenous (radiation, chemotherapy, smoking, stress, etc.) and endogenous (mitochondria, NADPH oxidase, and xanthine oxidase etc.) sources (1). Excessive ROS leads to oxidative stress which is known to impair various cellular functions. Oxidative stress leads to the development of many pathological conditions related to metabolic syndrome such as, insulin resistance, type 2 diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer (2).
Obesity is a well known public health problem that also causes various diseases (2). Adipose tissue increases as a result of increased size and the number of adipocytes due to an imbalance in energy intake and expenditure, leading to decreased life expectancy and health problems. The process of cell differentiation from preadipocytes to mature adipocytes, known as adipogenesis, is important for intracellular lipid accumulation (3). Furthermore, it has been reported that excessive lipid accumulation is promoted by up-regulation the expression of adipogenic transcription factors as a consequence of ROS production in adipocytes (4). During ROS production, H2O2 and transition metal ions including Cu2+ or Fe2+ in cells react, generating both hydroxyl radicals and higher oxidation states of the transition metals in a process known as the Fenton reaction (5). Transition metals ions (Cu2+ or Fe2+) can also induce weight gain and increase adipose tissue mass in rats (6). H2O2 has been demonstrated as a signaling messengers that induces phosphorylation, oxidation, and dimerization related to the expression of adipogenic genes, such as C/EBPβ and PPARγ (7). Furthermore, anti-oxidant and anti-adipogenic effects of various natural materials are well known to suppress adipogenesis by preventing increased ROS levels. They also have potential roles in the prevention of chronic disease related to oxidative stress and obesity (8). Thus, controlling adipogenesis may be one of the most important strategies in preventing obesity.
Wheat germ comprises of 2~3% of the total weight of wheat and its main contents are α-tocopherol, group B vitamins, dietary fiber, polyunsatured fats, minerals and phytochemicals (9). The constituents of wheat germ have been reported to have various physiological effects. In a previous study, we reported that wheat germ fermented with Aspergillus oryzae exerts anti-oxidant effects by promoting scavenging activity against 2,2′-azobis (2-amidinopropane) dihydrochloride- and H2O2-induced oxidative stress. It also enhanced the induction of phase II enzymes, such as heme oxygenase-1, glutathione S-transferase, and NAD(P)H quinone oxidoreductase 1 by increasing ferulic acid (10). Wheat germ agglutinin (WGA) in wheat germ (up to 0.5 g/kg) has been reported to improve immunity through the induction of interleukin (IL)-2, IL-4, IL-12, and IL-13 while simultaneously inhibiting the proliferation of activated lymphocytes (11). Accorting to Tai et al., fermented wheat germ extract containing chemotherapeutic agents such as cisplatin and 5-fluorouracil exhibited synergistic effects of cytotoxicity in human hepatocellular carcinoma, HepG2, Hep3B, and HepJ5 cells (12). However, wheat germ is almost completely removed during milling because it affects the shelf-life and the processing quality of the flour. Thus, numerous studies have investigated the availability of wheat germ using various methods (13).
In a previous study, fermented wheat germ extract with baker’s yeast was reported to have potential anti-carcinogenic activities through the down-regulation of major histocompatibility complex-1, tumor necrosis factors-α, and various IL in vitro and in vivo (14). Nuruk, which is similar to fermented wheat germ and used as a fermentation starter in traditional Korean recipes, is naturally produced by the proliferation of fungi (Aspergillus sp., Absidia sp., Rhizopus sp., and Mucor sp. etc.) and microorganisms on rice or wheat. It is mostly utilized in the fermention process for making traditional alcoholic drinks and it produces various organic acids or flavor compounds (15). In particular, Aspergillus oryzae is commonly utilized to produce commercially fermented products such as doengang, miso, and gochujang (16). However, the antioxidant and anti-adipogenic activity of F-WGE and its molecular mechanisms have not yet been investigated.
Previous studies have reported that antioxidant compounds derived from natural plants can prevent oxidative stress induced by free radicals and that fermentation with microorganisms increases antioxidative activity against ROS (17,18). Furthermore, inhibition of cellular ROS generation can result in anti-adipogenic activity against excessive lipid accumulation in oxidative stress-induced adipocytes (19). In this study, ethanol extracts from wheat germ (WGE) and wheat germ fermented with Aspergillus oryzae (F-WGE) were prepared. We evaluated their anti-oxidative properties in vitro and in HepG2 cells by assessing their scavenging activities against Cu2+-induced oxidative stress. We also examined the anti-adipogenic effect of F-WGE against Fe2+-enhanced lipid accumulation during adipogenesis in 3T3-L1 cells and discussed their potential as functional food materials against oxidative stress and adipogenesis.
MATERIALS AND METHODS
Materials
Wheat germ was obtained from Young Nam Flour Mills Co. (Busan, Korea). Aspergillus oryzae KCTC 6377 were purchased from the Korean Collection for Type Cultures (Biological Resource Center, Daejeon, Korea). Dulbecco’s modified Eagle’s medium (DMEM), penicillin-streptomycin, phosphate-buffered saline (PBS), and bovine calf serum (BCS) were obtained from WELGENE Inc. (Daegu, Korea). Fetal bovine serum (FBS) was purchased from GE Healthcare Bio-Sciences Co. (Piscataway, NJ, USA). Dimethyl sulfoxide (DMSO) and isopropanol were obtained from Junsei (Tokyo, Japan) and formaldehyde was purchased from Bio Basic Inc. (Markham, ON, Canada). Unless noted, all chemicals were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA).
Preparation of WGE and F-WGE using Aspergillus oryzae
Sixty grams of wheat germ was soaked in 150 mL of distilled water for 4 h and sterilized at 121°C for 30 min. The sterilized wheat germs (WG) were extracted overnight with 80% ethanol in a shaking incubator. Furthermore, to obtain the fermented WG, it was inoculated with Aspergillus oryzae and cultured at 30°C for 72 h in incubator. The inoculated WG was then extracted overnight with 80% ethanol in a shaking incubator. The WGE and F-WGE were lyophilized using a vacuum freeze dryer (IlShinBioBase, Dongducheon, Korea) and kept at −20°C until analyzed.
Oxygen radicals absorbance capacity (ORAC) assay
The scavenging capacities of WGE and F-WGE against hydroxyl radical (HO.) were measured using ORAC assay. The 40 nM fluorescein and 1, 5, 10, 20, and 50 μg/mL of WGE and F-WGE in 75 mM potassium phosphate buffer (pH 7.4) added to triplicate wells in 96-well black microplate. Fluorescein was used as a target of free radical attack and the fluorescein solutions contained acetone (0.01%, v/v). The 100 μL of CuSO4-H2O2 (5 μM CuSO4; H2O2 0.75%) as mainly a hydroxyl radical generator was added to the 96-well black microplate. Before the measurement of fluorescence intensity, 96-well black microplate was shook and settled for 10 and 5 s in Tecan GENios multi-functional plate reader (GENios; Tecan Trading AG, Salazburg, Austria), respectively. The fluorescence intensity at emission wavelength 535 nm was measured with excitation wavelength 485 nm every 2 min for 200 min (100 cycles) at 37°C. ORAC values were expressed as TE (Trolox equivalents, μM). One ORAC unit is equivalent to the net protection area provided by 1 μM of Trolox.
Cu2+-chelating capacity
The metal chelating activities of WGE and F-WGE referred to the method of Mariana et al. (20) with a slight modification. The WGE and F-WGE were mixed at 1, 5, 10, 20, and 50 μg/mL with 200 μL of 0.4 μM CuSO4 solution. Then, the mixed solution was added 100 μL of 0.05 μM calcein solution. The fluorescence intensity of the final mixed solution was measured with the fluorescence reader (GENios; Tecan Trading AG, Salazburg, Austria) at an excitation wavelength of 485 nm and an emission wavelength of 535 nm. The percentage of metal chelating capacity was calculated as a percentage versus control.
HepG2 and 3T3-L1 cells culture and MTT assay
HepG2 cells and 3T3-L1 preadipocytes were purchased from American Type Culture Collection (ATCC, Rockville, MD, USA) and Korean Cell Line Bank (KCLB, Seoul, Korea), respectively. HepG2 cells were cultured in DMEM medium supplemented with 10% FBS and 100 unit/mL penicillin-streptomycin at 37°C in a humidified atmosphere with 5% CO2. HepG2 cells were exposed with 50, 100, and 200 μg/mL of WGE and F-WGE for 3 h and then 0.5 mg/mL 3-(4,5-dimethylthiazol)-2,5-diphenyltetrazolium bromide (MTT) reagent dissolved in PBS was added to each well and the plate was incubated for 1 h. 3T3-L1 preadipocytes were maintained DMEM medium supplemented with 10% BCS and 100 unit/mL penicillin-streptomycin at 37°C in a humidified atmosphere with 5% CO2. Confluent 3T3-L1 preadipocytes were treated with 10, 50, 100, and 200 μg/mL of WGE and F-WGE for 24 h. The 0.2 mg/mL MTT-media, including 10% BCS, 1% penicillic-streptomycin was added at 1 mL/well and the plate was incubated for 1 h. The media was removed and the intracellular insoluble formazan was dissolved in DMSO. The absorbance of each cell was measured at 570 nm using a microplate reader (Molecular Devices, Sunnyvale, CA, USA) and the cell viability was calculated as a percentage versus untreated cells.
Cellular antioxidant capacity (CAC)
Cellular oxidative stress, due to ROS generated by CuSO4, was measured by the 2′,7′-dichlorofluorescin diacetate (DCFH-DA) method. DCFH-DA is commonly used to detect the oxidative stress inside of cells. When used to treat cells, DCFH-DA is taken up and hydrolyzed into dichlorofluorescin (DCFH) by a cellular esterase. In the presence of ROS, DCFH becomes 2′,7′-dichlorofluorescein (DCF) which can be quantified (21). HepG2 cells were seeded into 96-well culture black plates (5×105 cells/mL) for 24 h. The cells were treated with various concentrations of 10, 20, 50, 100, and 200 μg/mL WGE and F-WGE for 30 min. After incubation for 30 min, the medium was discarded. The cells were gently washed twice using Hank’s balanced salt solution (HBSS). Instead of media, HBSS was added in each well which is stable to fluorescence and then the cells were treated with 10 μM CuSO4 for 30 min as an inducer of oxidative stress. Then, cells were treated with 40 μM DCFH-DA for 30 min at 37°C in the darkness. Then DCF fluorescence intensity was measured at 485 nm (excitation wavelength) and 535 nm (emission wavelength) using the Tecan GENios multi-functional plate reader (GENios; Tecan Trading AG).
Observation of ROS levels in HepG2 cells by fluorescence microscopy
HepG2 cells were pre-incubated with 50 and 100 μg/mL of WGE and F-WGE for 30 min. After incubation, the cells were exposed to 10 μM CuSO4 for 30 min and treated with 10 μM DCFH-DA. The cells were fixed with 3.5% (v/v) formaldehyde for 30 min and then washed twice in PBS (pH 7.4). The cells were observed using a fluorescence microscope (Olympus Optical, Tokyo, Japan).
3T3-L1 cells differentiation
3T3-L1 preadipocytes were maintained DMEM medium supplemented with 10% BCS and 100 unit/mL penicillin-streptomycin until confluency. At 2 days after reaching confluency, designated as Day 0, the 3T3-L1 preadipocytes were cultured in DMEM (FBS-medium) supplemented with 10% FBS and 100 unit/mL penicillin-streptomycin containing 500 μM 3-isobutyl-1-methylxanthine, 5.2 μM dexamethasone, and 167 nM insulin (differentiation medium; DM). After 2 days of 3T3-L1 differentiation, the medium was changed to 10% FBS-medium with 167 nM insulin added for another 2 days (post-differentiation medium; Post-DM). On Day 4, the 3T3-L1 adipocytes were cultured in 10% FBS-medium. The 3T3-L1 cells were treated the 50 and 100 μg/mL of WGE and F-WGE with 100 μM Fe2+ during the periods indicated in Fig. 1.
Fig. 1.
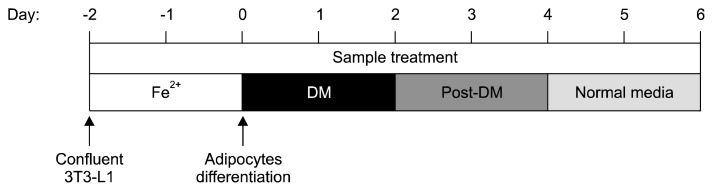
Scheme of WGE and F-WGE treatment with Fe2+ and 3T3-L1 differentiation.
Oil red O (ORO) staining
To examine the effects of intracellular lipid accumulation by WGE and F-WGE treatment with 100 μM Fe2+ in 3T3-L1 adipocytes, the cells were quantified by ORO staining. The cultured medium was removed and washed with PBS. The cells were fixed with 3.7% formaldehyde for 30 min at room temperature. The fixed 3T3-L1 adipocytes were washed three times with tap water. The lipid droplets were stained with 3 mg/mL ORO in isopropanol for 15 min. After staining, the stained cells were washed three times with distilled water. The lipid droplets in ORO stained mature 3T3-L1 adipocytes were observed using inverted microscope (Korealabtech, Seongnam, Korea) and it was then dissolved in DMSO and transferred at 100 μL/well to a 96-well plate. The absorbance of each well was quantified using microplate reader at a wavelength of 510 nm.
Statistical analysis
All experiments were performed at least three times, and data are presented as means±standard deviations (SDs). Statistical analysis was performed using the SPSS software 18.0 (SPSS Inc., Chicago, IL, USA). The significance of differences between groups was assessed with one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test. A P value<0.05 or 0.01 was considered to indicate statistical significance. The significance of each groups was analysed as compared with control group or between WGE and F-WGE groups by Student’s t-test.
RESULTS
The scavenging activities of WGE and F-WGE against hydroxyl radicals
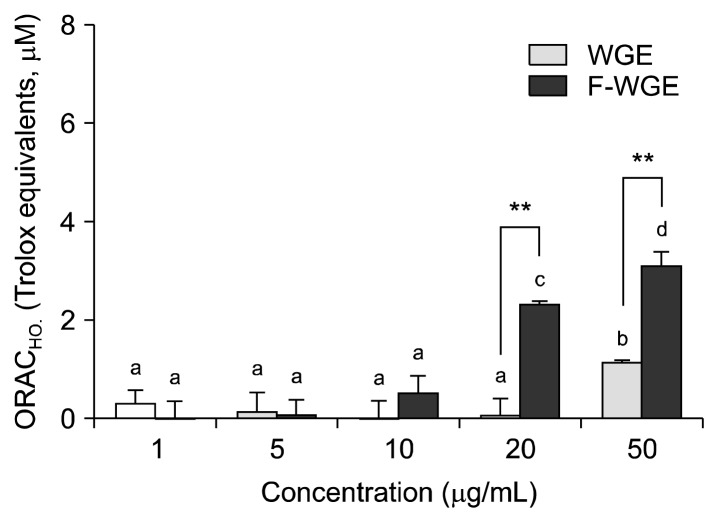
The scavenging activities of WGE and F-WGE against hydroxyl radicals were measured using the ORAC assay. In this assay, CuSO4 and H2O2 is used as a hydroxyl radical generator. The scavenging activity of hydroxyl radicals by WGE treatment increased between 20 and 50 μg/mL whereas the scavenging activity of F-WGE increased dose-dependently to 0.12, 2.31, and 3.18 TE at 10, 20 and 50 μg/mL, respectively (Fig. 2). Furthermore, the scavenging activity of hydroxyl radicals by F-WGE treatment was significantly higher than that of WGE at 20 and 50 μg/mL.
Fig. 2.
The scavenging activities of WGE and F-WGE against hydroxyl radicals produced by Cu2+-H2O2. The WGE and F-WGE were dose-dependently increased in hydroxyl radical scavenging activities at 1, 5, 10, 20, and 50 μg/mL. The ORAC values were calculated by dividing the area under the sample curve by the area under the trolox curve. One ORAC unit was assigned as the net area of protection provided by trolox at a final concentration of 1 μM. The results represent mean±SD of values obtained from each 3 individual measurements. Different corresponding letters indicate significant differences at P<0.05 by Duncan’s test. One-way ANOVA (P<0.05) followed by comparisons using Student’s t-test. **P<0.01 comparing WGE and F-WGE at each concentration.
Cu2+-chelating activities of WGE and F-WGE
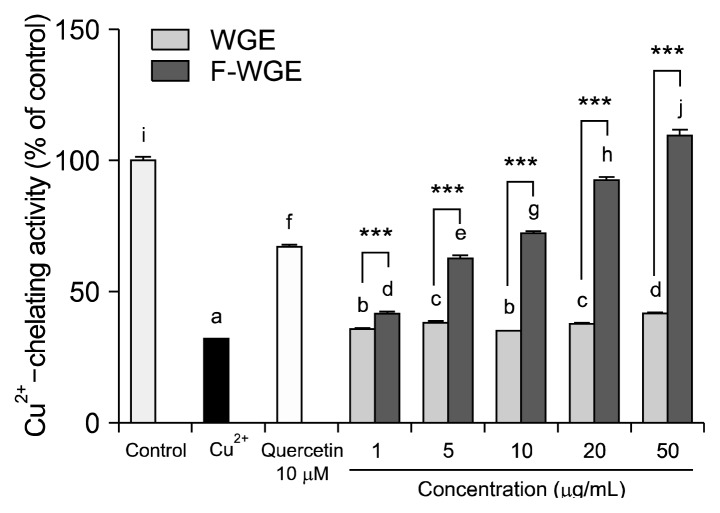
Cu2+-chelating activity was measured as the inhibition percentage of calcein-Cu2+ complex formation by each antioxidant. Calcein is used as metal chelator and emits fluorescence. As shown in Fig. 3, the Cu2+-chelating activity of the positive control, quercetin was 67.2% at 10 μg/mL. The Cu2+-chelating activities of WGE and F-WGE increased dose-dependently at 1, 5, 10, 20, and 50 μg/mL. F-WGE had stronger Cu2+-chelating activity than the positive control at 10, 20, and 50 μg/mL and individual concentrations also had significantly stronger activity than WGE. Thus, these results are consistent with the results observed against hydroxyl radicals. The anti-oxidant effect of F-WGE in vitro is believed to occur through either direct scavenging or occurrence suppression of hydroxyl radicals by metal chelation. WGE was confirmed to act only through direct scavenging of the hydroxyl radicals generated in the Fenton reaction.
Fig. 3.
The Cu2+-chelating activities of WGE and F-WGE. The Cu2+-chelating activity of WGE and F-WGE was measured using calcein. The Cu2+-chelating activity of F-WGE was dose-dependently increased at 1, 5, 10, 20, and 50 μg/mL. The results represent mean±SD of values obtained from each of 3 individual experiments. Different corresponding letters indicate significant differences at P<0.05 by Duncan’s test. One-way ANOVA (P<0.05) followed by comparison Student’s t-test. ***P<0.001 comparing WGE and F-WGE at each concentration.
Cell viability of HepG2 and confluent 3T3-L1 cells by WGE and F-WGE treatment
To evaluate the effect of WGE and F-WGE on cell viability, the HepG2 cells and confluent 3T3-L1 preadipocytes were assessed using the MTT assay. The HepG2 cells were exposed to 50, 100, and 200 μg/mL of WGE and F-WGE for 3 h, while the confluent 3T3-L1 preadipocytes were treated at 10, 50, 100, and 200 μg/mL for 24 h. The cell viability of the HepG2 cells were maintained at 50, 100, and 200 μg/mL over 80% and the cell viability of confluent 3T3-L1 preadipocytes did not significantly differ between 10 and 200 μg/mL with either WGE or F-WGE treatment (Table 1). Thus, the non-toxicity concentration range of WGE and F-WGE concentrations up to 200 μg/mL in HepG2 cells and 3T3-L1 preadipocytes were chosen for subsequent studies.
Table 1.
The cytotoxicity by WGE and F-WGE treatment in HepG2 and 3T3-L1 cells (% of control)
| Samples (μg/mL) | Viability of HepG2 cells1) | Viability of 3T3-L1 cells2) | ||
|---|---|---|---|---|
|
|
|
|||
| WGE | F-WGE | WGE | F-WGE | |
| 10 | – | – | 85.91±7.93 | 91.45±7.41 |
| 50 | 89.59±3.33* | 84.83±4.80* | 102.09±4.97 | 99.03±5.54 |
| 100 | 85.26±3.05* | 86.25±3.04* | 103.74±4.43 | 94.41±5.93 |
| 200 | 87.37±4.68* | 82.49±3.49** | 103.19±7.81 | 92.69±15.01 |
WGE and F-WGE were treated for 3 h in HepG2 cells.
WGE and F-WGE were treated for 24 h in confluent 3T3-L1 cells.
The results represent mean±SD of values obtained from 3 measurements. One-way ANOVA (P<0.05) followed by comparison Student’s t-test.
P<0.05
P<0.01 as compared with control group.
Change in intracellular ROS levels on WGE and F-WGE-treated HepG2 cells
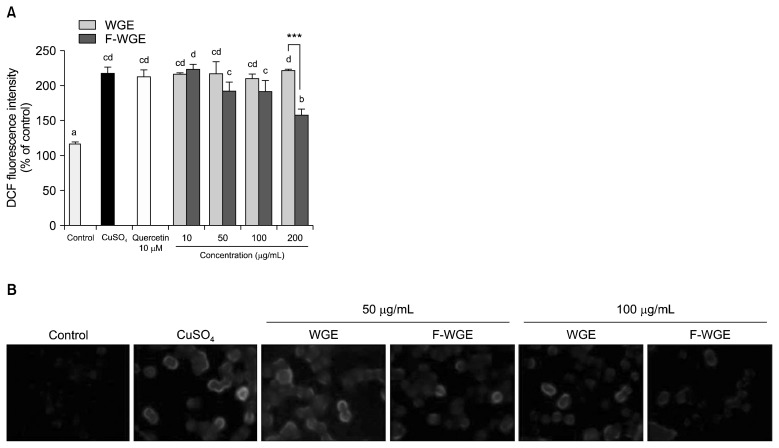
The cellular antioxidant capabilities of WGE and F-WGE were evaluated using a DCFH-DA probe in HepG2 cells. Cellular oxidative stress in HepG2 cells increased by 186.57% in comparison with the control group following treatment with CuSO4 (Fig. 4A). Quercetin (10 μM), used as a positive control, maintained the intracellular oxidative stress from CuSO4 at 182.50%. The intracellular oxidative stress induced by CuSO4 was dose-dependently decreased by F-WGE treatment at 50~200 μg/mL for 30 min. Furthermore, there was significant difference in antioxidant capabilities between 200 μg/mL WGE and F-WGE, with no apparent effect by WGE observed. The cellular antioxidant capability of F-WGE treatment was also observed between 50 and 100 μM in DCFH-DA-stained HepG2 cells using fluorescence microscopy (Fig. 4B). These results were similarly repeated for the Cu2+-chelating activities of WGE and F-WGE in comparison to that of quercetin. Therefore, the anti-oxidant effect of F-WGE through direct or indirect scavenging of hydroxyl radicals by Cu2+-chelation in vitro and in HepG2 cells was confirmed to be stronger than that of WGE, which directly scavenges hydroxyl radicals but does not possess Cu2+-chelating activity.
Fig. 4.
The decreases in intracellular ROS (CuSO4) levels by WGE and F-WGE treatment in HepG2 cells. F-WGE shown to dose-dependently decrease intracellular ROS (CuSO4) levels at between 10 and 200 μg/mL in HepG2 cells (A). The results represent mean±SD of values obtained from each of 3 individual measurements. Different corresponding letters indicate significant differences at P<0.01 by Duncan’s test. One-way ANOVA (P<0.05) followed by comparison Student’s t-test. ***P<0.001 comparing WGE and F-WGE at each concentration. Observation of intracellular ROS levels in HepG2 cells using DCFH-DA (B).
Inhibitory effect of intracellular lipid accumulation by WGE and F-WGE treatment with Fe2+ in 3T3 adipocytes
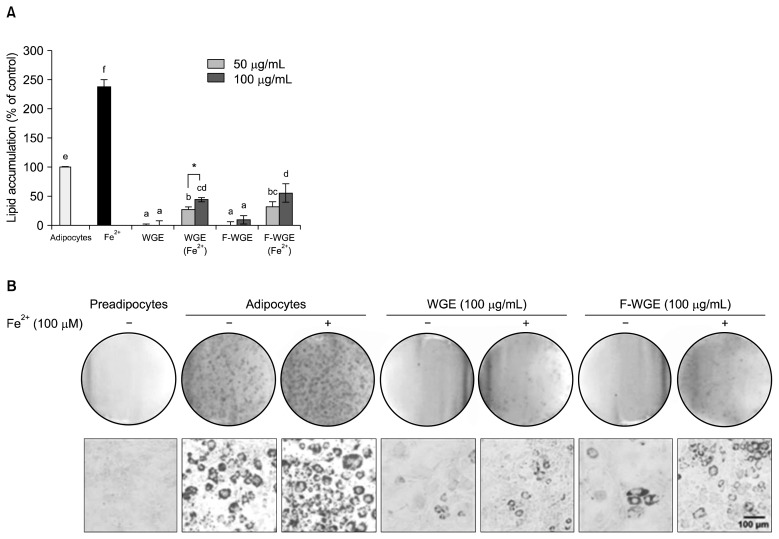
To determine the inhibitory effects of WGE and F-WGE on adipogenesis in 3T3-L1 cells, the cells were treated with adipogenic medium in the presence or absence of various concentrations of WGE and F-WGE with 100 μM Fe2+. The levels of lipid accumulation after 6 days of WGE and F-WGE treatment in differentiated 3T3-L1 adipocytes were quantified using ORO staining. Mature 3T3-L1 adipocytes (control, 100%) accumulated a significant amount of intracellular ORO-stained lipid droplets by Day 6 and the amount increased over 137.60% after treatment with 100 μM Fe2+ (Fig. 5A). Intracellular lipid accumulation was completely suppressed after treatment with 10 and 50 μg/mL WGE and F-WGE compared to control. Furthermore, lipid accumulation in 100 μM Fe2+ treated mature 3T3-L1 adipocytes decreased significantly after treatment with 50 and 100 μg/mL WGE and F-WGE. To examine the effects of WGE and F-WGE on the size and number of lipid droplets in 3T3-L1 adipocytes, the lipid droplets were visualized using microscopy after ORO staining. The mature adipocytes contained a large number of lipid droplets and the amount of droplets in 100 μM Fe2+-treated 3T3-L1 adipocytes was greater than in the control. The size and number of lipid droplets were decreased in mature 3T3-L1 adipocytes with 50 and 100 μg/mL WGE and F-WGE and they also effectively suppressed lipid droplets increased by 100 μM Fe2+ treatment (Fig. 5B). These results indicated that the anti-oxidant activities of WGE and F-WGE, such as metal-chelation, contributed to the inhibition of lipid accumulation in 3T3-L1 cells.
Fig. 5.
Inhibition of lipid accumulation by WGE and F-WGE treatment in 3T3-L1 adipocytes. Inhibitory activities of WGE and F-WGE on 3T3-L1 lipid accumulation measured by ORO staining (A). The results represent mean±SD of values obtained from each 3 individual measurements. Different corresponding letters indicate significant differences at P<0.05 by Duncan’s test. One-way ANOVA (P<0.05) followed by comparison Student’s t-test. *P<0.05 comparing WGE and F-WGE at each concentration. Inhibitory effect of intracellular lipid accumulation by WGE and F-WGE treatment in 3T3-L1 adipocytes observed by microscopy using ORO staining (B).
Taken together, we prepared ethanol extract from wheat germ and wheat germ was fermented with Aspergillus oryzae. The anti-oxidant activities of F-WGE in vitro and in HepG2 cells were shown to occur through direct or indirect scavenging of hydroxyl radicals via Cu2+-chelating and were stronger than the activities of WGE. Intracellular lipid accumulation in 3T3-L1 adipocytes was strongly inhibited by WGE and F-WGE treatment in control cells as well as in cells treated with Fe2+ (Day 6).
DISCUSSION
Numerous studies have reported that changes in the redox state by intracellular ROS levels serve as essential markers for the induction or inhibition of various metabolic processes (22). Furthermore, in adipogenesis, increased ROS levels can cause excessive lipid accumulation during the conversion of preadipocytes into adipocytes and accelerate adipocyte differentiation through the expression of adipogenic genes in response to oxidative stress (23). The decrease in excessive ROS levels and the inhibition of adipocyte differentiation are important strategies for preventing of various chronic diseases related to oxidative stress and obesity. Thus, we investigated whether WGE and F-WGE could prevent oxidative stress against ROS generation and thereby inhibit lipid accumulation in 3T3-L1 cells. Moreover, we evaluated the differences in antioxidant and anti-adipogenic activities between WGE and WGE fermented with Aspergillus oryzae (F-WGE).
Although wheat germ is removed as a by-products during milling, it was recently reported to possess potential anti-carcinogenic properties, but its anti-oxidant and anti-adipogenic effects are still unknown (24). We prepared ethanol extracts of wheat germ and wheat germ fermented using Aspergillus oryzae. Fermentation using Aspergillus oryzae is known to cleave glycosidic linkages from various glycosides into aglycones which is initiated by various enzymes, such as protease, α-galactosidase, amylase, invertase, lignin peroxidase, and tannase. Fermentation is an effective technique for the production or extraction of anti-oxidant active compounds from natural sources (25).
Cell viability can be assessed by the ability of functional mitochondria to catalyze the reduction of MTT to insoluble purple formazan. The cytotoxicity of WGE and F-WGE were measured in both HepG2 cells and 3T3-L1 preadipocytes (Table 1). The cell viability and confluency of 3T3-L1 preadipocytes did not differ significantly between 10 and 200 μg/mL WGE and F-WGE treatment. The cell viability of HepG2 cells was shown to decrease significantly after WGE and F-WGE treatment at 50, 100, and 200 μg/mL. However, the concentration of WGE and F-WGE were chosen to maintain cell viability over 75% because previous studies reported that cytotoxins could cause false-negative results with cell viability below 75% by inducing of DNA migration in the nucleus (26,27). Thus, HepG2 cells and 3T3-L1 preadipocytes were treated with WGE and F-WGE between 10 and 200 μg/mL.
Anti-oxidant activities of WGE and F-WGE were evaluated by the scavenging activity of Cu2+-H2O2-induced hydroxyl radicals and the Cu2+-chelating activity in vitro (Fig. 2 and 3). The scavenging activity of hydroxyl radicals and Cu2+-chelating activity were increased dramatically by F-WGE treatment compared to WGE. Similarly, Cu2+-induced oxidative stress in HepG2 cells was significantly decreased by F-WGE (Fig. 4). Numerous studies have reported that the anti-oxidant activities of fermented plant-based foods are primarily due to increases in the amounts of phenolic compounds and flavonoids during fermentation, which can be attributed to the liberation or synthesis of various antioxidant compounds (28). Zhang et al. (29) reported that the anti-oxidant activities of water, 70% acetone and 70% ethanol extracts from wheat fermented with Cordyceps militaris, measured by scavenging activities against hydroxyl radicals, reducing potential, and Fe2+ chelating activities, were significantly higher in comparison with unfermented wheat extract. The study also reported that the total phenolic, total flavonoids and free phenolic acid content were enhanced in fermented wheat extracts. According to Huang et al. (30), the anti-oxidant activities of methanol extract from tofu fermented by Aspergillus oryzae, measured by 2,2-diphenyl-2-picylhydroxyl radical-scavenging activity, Fe2+-chelating activity, and reduction potential, were stronger than non-fermented tofu extract. Nam et al. (31) reported that soybean fermented with Aspergillus oryzae decreased H2O2-induced oxidative stress in HepG2 cells, which was mainly attributed to the production of free isoflavones during fermentation. Therefore, F-WGE could permeate the cell membrane and suppress hydroxyl radicals and higher oxidation states via the Fenton reaction through rapid combination with Cu2+. Additionally, fermentation with Aspergillus oryzae changes the contents of bioactive substances in wheat germ.
Previous studies have reported that differentiating 3T3-L1 adipocytes already perform lipid synthesis-related metabolic processes to store intracellular glucose (32). The biosynthetic and metabolic pathways of glucose can include glycerol-3-phosphate used in triglyceride and phospholipid synthesis as well as sugar phosphates through hexose monophosphate (HMP). NADPH derived from glucose-6-phosphate dehydrogenase, the limiting enzyme of HMP, is known to increase the levels of cellular ROS production by NADPH oxidase. Furthermore, excessive ROS generation is associated with increased lipid accumulation during adipocyte differentiation (33,34). Previous studies reported excessive differentiation of 3T3-L1 cells induced by transition metals (Cu2+ or Fe2+), due to increased expression of adipogenic transcriptional factors related to the NADPH-dependent H2O2-generating system (35). Thus, we investigated the inhibitory effect of WGE and F-WGE treatment on adipogenesis in 3T3-L1 cells. WGE and F-WGE treatment suppressed lipid accumulation in 3T3-L1 cells, whereas lipid accumulation was increased by Fe2+ treatment compared to mature 3T3-L1 adipocytes. The adipogenesis in Fe2+ treated 3T3-L1 cells was strongly inhibited by WGE and F-WGE treatment (Fig. 5). Gabrielsen et al. (36) showed that adiponectin and insulin sensitivity in FeSO4-treated 3T3-L1 cells were decreased by increased expression of adipogenic regulators such as Akt, which play essential roles in regulating the expression of C/EBPs and PPARγ during 3T3-L1 adipogenesis, as part of the insulin signaling pathway. The improvement in anti-adipogenic effects of plant extracts by fermentation were reported as well as the discovery that the free phenolic content of rice bran fermented with Issatchenkia orientalis MFST1 was higher than that of non-fermented rice bran and strongly suppressed ROS generation induced by H2O2 in 3T3-L1 adipocytes (37). Previous studies reported that an ethanol extract of black soybean koji (BSK) produced by the fermentation of black soybean with Aspergillus awamori was rich in isoflavones such as benzoic acid, daidzin, genistin, and genistein. BSK extract exhibited anti-adipogenic effects such as suppression of lipid accumulation and inhibition of PPARγ protein expression in 3T3-L1 adipocytes. Taken together, our results indicate that the anti-adipogenic activities of WGE and F-WGE on the adipogenesis of 3T3-L1 cells may correlate with antioxidant activity against oxidative stressors such as hydroxyl radical and transition metals.
We determined that the antioxidant activities of F-WGE were stronger than those of WGE and also demonstrated the anti-adipogenic activity of WGE and F-WGE in control and Fe2+-treated 3T3-L1 cells. However, we did not clearly elucidate the bioactive substances derived from wheat germ fermented with Aspergillus oryzae responsible for the anti-adipogenic activity or the direct correlation between anti-oxidant and anti-adipogenic activities. Further studies should focus on whether the ethanol extract from fermented wheat germ should be used to examine the effect of natural compounds derived from wheat germ on anti-oxidant and anti-adipogenic activities. Our results provide new evidence for anti-adipogenic activity of fermented wheat germ extract and suggests its usefulness for the development of value-added functional foods.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Lee YJ, Kim DB, Lee JS, Cho JH, Kim BK, Choi HS, Lee BY, Lee OH. Antioxidant activity and anti-adipogenic effects of wild herbs mainly cultivated in Korea. Molecules. 2013;18:12937–12950. doi: 10.3390/molecules181012937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsuda M, Shimomura I. Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obese Res Clin Pract. 2013;7:e330–e341. doi: 10.1016/j.orcp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Ntambi JM, Kim YC. Adipocyte differentiation and gene expression. J Nutr. 2000;130:3122S–3126S. doi: 10.1093/jn/130.12.3122S. [DOI] [PubMed] [Google Scholar]
- 4.Ristow M, Wolfrum C. A radical opposition in body weight control. EMBO Mol Med. 2013;5:1147–1148. doi: 10.1002/emmm.201303094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winterbourn CC. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol Lett. 1995;82–83:969–974. doi: 10.1016/0378-4274(95)03532-X. [DOI] [PubMed] [Google Scholar]
- 6.Tinkov AA, Polyakova VS, Nikonorov AA. Chronic administration of iron and copper potentiates adipogenic effect of high fat diet in Wistar rats. Biometals. 2013;26:447–463. doi: 10.1007/s10534-013-9630-6. [DOI] [PubMed] [Google Scholar]
- 7.Chen B, Lam KS, Wang Y, Wu D, Lam MC, Shen J, Wong L, Hoo RL, Zhang J, Xu A. Hypoxia dysregulates the production of adiponectin and plasminogen activator inhibitor-1 independent of reactive oxygen species in adipocytes. Biochem Biophys Res Commun. 2006;341:549–556. doi: 10.1016/j.bbrc.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Charradi K, Elkahoui S, Limam F, Aouani E. High-fat diet induced an oxidative stress in white adipose tissue and disturbed plasma transition metals in rat: prevention by grape seed and skin extract. J Physiol Sci. 2013;63:445–455. doi: 10.1007/s12576-013-0283-6. [DOI] [PubMed] [Google Scholar]
- 9.Rizzello CG, Mueller T, Coda R, Reipsch F, Nionelli L, Curiel JA, Gobbetti M. Synthesis of 2-methoxy benzoquinone and 2,6-dimethoxybenzoquinone by selected lactic acid bacteria during sourdough fermentation of wheat germ. Microb Cell Fact. 2013;12:105. doi: 10.1186/1475-2859-12-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song JH, Kim JK, Jang HD. Ferulic acid released by treatment with Aspergillus oryzae contributes to the cellular antioxidant capacity of wheat germ extract. Food Sci Biotechnol. 2014;23:1327–1333. doi: 10.1007/s10068-014-0182-5. [DOI] [Google Scholar]
- 11.de Punder K, Pruimboom L. The dietary intake of wheat and other cereal grains and their role in inflammation. Nutrients. 2013;5:771–787. doi: 10.3390/nu5030771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tai CJ, Wang WC, Wang CK, Wu CH, Yang MD, Chang YJ, Jian JY, Tai CJ. Fermented wheat germ extract induced cell death and enhanced cytotoxicity of cisplatin and 5-fluorouracil on human hepatocellular carcinoma cells. Evid Based Complement Alternat Med. 2013;2013:121725. doi: 10.1155/2013/121725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorado MP, Lin SKC, Koutinas A, Du C, Wang R, Webb C. Cereal-based biorefinery development: utilisation of wheat milling by-products for the production of succinic acid. J Biotechnol. 2009;143:51–59. doi: 10.1016/j.jbiotec.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Mueller T, Voigt W. Fermented wheat germ extract-nutritional supplement or anticancer drug? Nutr J. 2011;10:89. doi: 10.1186/1475-2891-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SJ, Cho SW, Kwon YY, Kwon HS, Shin WC. Inhibitory effects of ethanol extracts from Nuruk on oxidative stress, melanogenesis, and photo-aging. Mycobiology. 2012;40:117–123. doi: 10.5941/MYCO.2012.40.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marui J, Tada S, Fukuoka M, Wagu Y, Shiraishi Y, Kitamoto N, Sugimoto T, Hattori R, Suzuki S, Kusumoto K. Reduction of the degradation activity of umami-enhancing purinic ribonucleotide supplement in miso by the targeted suppression of acid phosphatases in the Aspergillus oryzae starter culture. Int J Food Microbiol. 2013;166:238–243. doi: 10.1016/j.ijfoodmicro.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Lee OH, Kown YI, Hong HD, Park CS, Lee BY, Kim YC. Production of reactive oxygen species and changes in anti-oxidant enzyme activities during differentiation of 3T3-L1 adipocyte. J Korean Soc Appl Biol Chem. 2009;52:70–75. doi: 10.3839/jksabc.2009.012. [DOI] [Google Scholar]
- 18.Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Đorđević TM, Šiler-Marinković SS, Dimitrijević-Branković SI. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010;119:957–963. doi: 10.1016/j.foodchem.2009.07.049. [DOI] [Google Scholar]
- 20.Argirova MD, Ortewerth BJ. Activation of protein-bound copper ions during early glycation: study on two proteins. Arch Biochem Biophys. 2003;420:176–184. doi: 10.1016/j.abb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Lautraite S, Bigot-Lasserre D, Bars R, Carmichael N. Optimisation of cell-based assays for medium throughput screening of oxidative stress. Toxicol In Vitro. 2003;17:207–220. doi: 10.1016/S0887-2333(03)00005-5. [DOI] [PubMed] [Google Scholar]
- 22.Moini H, Packer L, Saris NE. Antioxidant and pro-oxidant activities of α-lipoic acid and dihydrolipoic acid. Toxicol Appl Pharmacol. 2002;182:84–90. doi: 10.1006/taap.2002.9437. [DOI] [PubMed] [Google Scholar]
- 23.Liu GS, Chan EC, Higuchi M, Dusting GJ, Jiang F. Redox mechanisms in regulation of adipocyte differentiation: beyond a general stress response. Cells. 2012;1:976–993. doi: 10.3390/cells1040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mueller T, Jordan K, Voigt W. Promising cytotoxic activity profile of fermented wheat germ extract (Avemar®) in human cancer cell lines. J Exp Clin Cancer Res. 2011;30:42. doi: 10.1186/1756-9966-30-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riou C, Salmon JM, Vallier MJ, Günata Z, Barre P. Purification, characterization, and substrate specificity of a novel highly glucose-tolerant β-glucosidase from Aspergillus oryzae. Appl Environ Microbiol. 1998;64:3607–3614. doi: 10.1128/aem.64.10.3607-3614.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henderson L, Wolfreys A, Fedyk J, Bourner C, Windebank S. The ability of the Comet assay to discriminate between genotoxins and cytotoxins. Mutagenesis. 1998;13:89–94. doi: 10.1093/mutage/13.1.89. [DOI] [PubMed] [Google Scholar]
- 27.Zhu L, Wang P, Qin QL, Zhang H, Wu YJ. Protective effect of polypeptides from larva of housefly (Musca domestica) on hydrogen peroxide-induced oxidative damage in HepG2 cells. Food Chem Toxicol. 2013;60:385–390. doi: 10.1016/j.fct.2013.07.074. [DOI] [PubMed] [Google Scholar]
- 28.Hur SJ, Lee SY, Kim YC, Choi IW, Kim GB. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014;160:346–356. doi: 10.1016/j.foodchem.2014.03.112. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, Lu G, Pan H, Fan L, Soccol CR, Pandey A. Production of powerful antioxidant supplements via solid-state fermentation of wheat (Triticum aestivum Linn.) by Cordyceps militaris. Food Technol Biotechnol. 2012;50:32–39. [Google Scholar]
- 30.Huang YH, Lai YJ, Chou CC. Fermentation temperature affects the antioxidant activity of the enzyme-ripened sufu, an oriental traditional fermented product of soybean. J Biosci Bioeng. 2011;112:49–53. doi: 10.1016/j.jbiosc.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Nam DH, Kim HJ, Lim JS, Kim KH, Park CS, Kim JH, Lim JK, Kwon DY, Kim IH, Kim JS. Simultaneous enhancement of free isoflavone content and antioxidant potential of soybean by fermentation with Aspergillus oryzae. J Food Sci. 2011;76:194–200. doi: 10.1111/j.1750-3841.2011.02350.x. [DOI] [PubMed] [Google Scholar]
- 32.Krieger-Brauer HI, Kather H. Antagonistic effects of different members of the fibroblast and platelet-derived growth factor families on adipose conversion and NADPH-dependent H2O2 generation in 3T3 L1-cells. Biochem J. 1995;307:549–556. doi: 10.1042/bj3070549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim DY, Han GD. Ameliorating effects of fermented rice bran extract on oxidative stress induced by high glucose and hydrogen peroxide in 3T3-L1 adipocytes. Plant Foods Hum Nutr. 2011;66:285–290. doi: 10.1007/s11130-011-0243-3. [DOI] [PubMed] [Google Scholar]
- 34.Huang CC, Huang WC, Hou CW, Chi YW, Huang HY. Effect of black soybean koji extract on glucose utilization and adipocyte differentiation in 3T3-L1 cells. Int J Mol Sci. 2014;15:8280–8292. doi: 10.3390/ijms15058280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee YJ, Han OT, Choi HS, Lee BY, Chung HJ, Lee OH. Antioxidant and anti-adipogenic effects of PineXol®. Korean J Food Sci Technol. 2013;45:97–103. doi: 10.9721/KJFST.2013.45.1.97. [DOI] [Google Scholar]
- 36.Gabrielsen JS, Gao Y, Simcox JA, Huang J, Thorup D, Jones D, Cooksey RC, Gabrielsen D, Adams TD, Hunt SC, Hopkins PN, Cefalu WT, McClain DA. Adipocyte iron regulates adiponectin and insulin sensitivity. J Clin Invest. 2012;122:3529–3540. doi: 10.1172/JCI44421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]