Abstract
This study was to investigate the preventive effect of taemyeongcheong (TMC, a Korean traditional health drink) on acetaminophen (APAP, 800 mg/kg BW)-induced hepatic damage in ICR mice. TMC is prepared from Saururus chinensis, Taraxacum officinale, Zingiber officinale, Cirsium setidens, Salicornia herbacea, and Glycyrrhizae. A high dose of TMC (500 mg/kg BW) was found to decrease APAP-induced increases in serum levels of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and lactate dehydrogenase. TMC pretreatment also increased the hepatic levels of hepatic catalase, superoxide dismutase, glutathione peroxidase, and glutathione, and reduced serum levels of the inflammatory cytokines tumor necrosis factor (TNF)-α and interleukin (IL)-6 in mice administered APAP (P<0.05). TMC (500 mg/kg BW) reduced hepatic mRNA levels of TNF-α, IL-1β, IL-6, COX-2, and iNOS by 87%, 84%, 89%, 85%, and 88%, respectively, in mice treated with APAP (P<0.05). Furthermore, histological observations suggested TMC pretreatment dose-dependently prevented APAP-induced hepatocyte damage. These results suggest that TMC could be used as a functional health drink to prevent hepatic damage.
Keywords: taemyeongcheong, acetaminophen, antioxidant enzymes, hepatic damage
INTRODUCTION
The liver has many physiological functions, such as detoxification, protein synthesis, and production of biochemicals required for digestion. Furthermore, the liver is highly sensitive to some drugs (1). Direct hepatotoxicity can be caused by a drug itself or by its metabolites. Acetaminophen (APAP) is commonly used to relieve headaches and minor aches and pains, and it is a major ingredient in numerous cold remedies. However, acute overdoses of APAP can cause potentially fatal liver damage (1). The hepatotoxicity induced by APAP is believed to be caused by the oxidative metabolism of APAP in liver by the mixed function of the oxidase P450 system to the toxic metabolite N-acetyl-p-benzoquinone imine (NAPQI) (2). The potential toxic effects of NAPQI are ameliorated by its binding to intracellular reduced glutathione (3,4).
Herbal medicines that have been used in Korea for thousands of years are now being manufactured as medicinal agents that contain ingredients of standardized quality and quantity (5). Taemyeongcheong (TMC) is a traditional healthy drink prepared using six types of health-promoting herbs, including Saururus chinensis, Taraxacum officinale, Zingiber officinale, Cirsium setidens, Salicornia herbacea, and Glycyrrhizae, as described in ancient medical books, such as Donguibogam and Sangayorok (6). These herbs were first recognized to have medicinal properties in the Joseon Dynasty and continue to be used as functional foods due to their excellent anti-oxidant, anti-inflammatory and hepatoprotective activities (7–11). Recent studies have shown TMC protects against gastritis and colitis in rodent models (6). However, its hepatoprotective properties have not been previously addressed. Therefore, the present study was conducted to determine the protective effect of TMC against APAP-induced toxicity, and to elucidate the mechanism responsible for its protection in a mouse model.
MATERIALS AND METHODS
TMC preparation
TMC samples were provided by the Taemyeongcheong Company (Yongin, Korea). To prepare TMC, Saururus chinensis (34.6 g), Taraxacum officinale (21.0 g), Zingiber officinale (20.4 g), Cirsium setidens (9.0 g), Salicornia herbacea (9.0 g), and Glycyrrhizae (6.0 g) were extracted with 1,000 mL of boiling water for 3 h. After removing the supernatant, the residue was further extracted with 1,000 mL of water for 1 h by heating gently at 80~100°C. The combined supernatants (2,000 mL) were freeze-dried at −80°C, and stored at −4°C until further analysis.
Animal treatments and the induction of liver damage using APAP
Male ICR mice (25~30 g) were purchased from Samtako Bio Co. (Osan, Korea), and maintained in a controlled facility (20±2°C; relative humidity, 50±10%) under a 12-h light/dark cycle with free access to water and a standard rodent diet. Animals were allocated to six experimental groups, namely; normal control, APAP control, the APAP+TMC [low (L), medium (M), or high (H)] groups, and the APAP+silymarin (positive control) group. TMC was administered at 100, 250, or 500 mg/kg body weight (BW) and silymarin was administered at 100 mg/kg BW. Mice in the normal and APAP control groups were administered distilled water orally for 14 days. In the TMC and silymarin treated groups, TMC or silymarin was dissolved in distilled water and orally administered for 14 days (experimental days 1~14). To induce acute liver damage, mice were given an intra-peritoneal (i.p.) injection of 800 mg/kg BW of APAP (dissolved in a 1% Tween-80 solution) (8). After 16 h, all mice were anaesthetized using carbon dioxide and sacrificed. Animal weights and excised liver weights were recorded. The protocol used in this study was approved by the Institutional Animal Care and Use Committee of Pusan National University (Busan, Korea; PNU-IACUC approval number PNU-2013-0451).
Serum ALT, AST, ALP, and LDH assays
At the end of the experimental days, blood was collected from the abdominal aorta of each mouse using a heparinized syringe and centrifuged at 3,000 g for 15 min at 4°C to obtain serum. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) levels were analyzed using a specific commercially available kit (Asan Pharm Co., Seoul, Korea), and serum levels of lactate dehydrogenase (LDH) were determined using another specific kit (Cayman Chemical Co., Ann Arbor, MI, USA).
Hepatic antioxidant enzymes and GSH assays
Livers were quickly removed, placed on ice, and washed with cooled phosphate buffered saline (PBS, pH 7.4) to remove red blood cells and clots. Hepatic catalase, superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) activities and GSH levels were analyzed using a commercially available kit (Cayman Chemical Co.).
Serum pro-inflammatory cytokine assay
For the serum cytokine assay, blood was collected from the inferior vena cava using a tube and centrifuged at 3,000 g for 15 min at 4°C. Interleukin (IL)-6 and tumor necrosis factor (TNF)-α levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Bio-legend ELISA MAXTM Deluxe kits; Biolegend, Inc., San Diego, CA, USA).
RT-PCR assay of inflammatory gene expressions in liver tissue
The mRNA expressions of TNF-α, IL-1β, IL-6, cyclo-oxygenase (COX)-2, and inducible nitric oxide synthase (iNOS) in liver were measured by RT-PCR. Total RNA was isolated from liver tissues using Trizol reagent (Invitrogen, Carlsbad, CA, USA), chloroform was added, and the mixtures were centrifuged at 13,000 g for 15 min at 4°C. Isopropanol was added to the supernatants at a 1:1 v/v ratio and RNA pellets were obtained by centrifugation (13,000 g, 15 min). After washing the pellets with ethanol, extracted RNAs were solubilized in diethyl pyrocarbonate-treated RNase-free water and quantified by measuring absorbance at 260 nm using spectrophotometer (Shimadzu UV-2401PC, Shimadzu Corp., Tokyo, Japan). Equal amounts of RNA (1 μg) were reverse transcribed in a master mix containing 5× reverse transcriptase buffer, 0.1 M dithiothreitol, 2.5 mM dNTPs, moloney murine leukemia virus reverse transcriptase, and RNase inhibitor (Invitrogen) for 2 h at 37°C. Polymerase chain reactions were carried out using an automatic BIONEER thermocycler (Bioneer, Daejeon, South Korea). Amplified PCR products were electrophoresed on 2% agarose gels and visualized by ethidium bromide (EB) staining. GAPDH was used as the internal control.
Histological examinations
Fresh liver tissues were fixed in 10% neutral buffered formalin for 24 h before routine histological processing. They were then dehydrated using an ethanol series and cleared in xylene before being embedded in paraffin. Cross sections of thickness 4-μm were obtained and stained with hematoxylin and eosin (H&E). Images were acquired using a Zeiss Axioskop 2 Plus microscope (Carl Zeiss MicroImaging Inc., Thornwood, NY, USA) equipped with an AxioCam MRc5 CCD camera (Carl Zeiss MicroImaging Inc.).
Statistical analysis
Data are presented as means±standard deviations. One-way ANOVA and Duncan’s multiple range tests were used to determine the significances of intergroup differences. P values of <0.05 were considered statistically significant, and the analysis was conducted using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Effect of TMC on liver weights in APAP-treated mice
No significant difference in body weight was observed among the six experimental groups (Table 1). However, APAP (800 mg/kg BW) significantly increased relative liver weights to 5.6±0.005 g as compared with normal controls (4.1±0.002 g) (P<0.05). TMC dose-dependently prevented these liver weight increases (TMC-L: 4.9± 0.004 g, TMC-M: 4.5±0.003 g, TMC-H: 4.1±0.003 g), and treatment with TMC-H (500 mg/kg BW) resulted in a mean liver weight similar to that found in normal controls and in silymarin-treated mice.
Table 1.
Effects of taemyeongcheong (TMC) on body weights and relative liver weights in acetaminophen (APAP)-treated mice
| Groups1) | Initial body weight (g) | Final body weight (g) | Relative liver weight (g/100 g) |
|---|---|---|---|
| Nor | 31.7±1.5b | 32.0±1.2ab | 4.1±0.002d |
| Con | 31.5±1.6b | 31.8±1.4b | 5.6±0.005a |
| TMC-L | 31.6±1.0b | 32.0±1.0ab | 4.9±0.004b |
| TMC-M | 32.3±1.6ab | 32.5±1.6ab | 4.5±0.003c |
| TMC-H | 33.0±1.0a | 33.4±1.0a | 4.1±0.003cd |
| S | 31.4±1.0b | 31.6±0.9b | 4.3±0.002cd |
Values are presented as means±SD.
Values with different letters are significantly different (P<0.05) by Duncan’s multiple range test.
Nor, normal mice; Con, mice treated with APAP (800 mg/kg BW); TMC-L, 100 mg/kg of TMC; TMC-M, 250 mg/kg of TMC; TMC-H, 500 mg/kg of TMC; S, 100 mg/kg of silymarin.
Effects of TMC on serum levels of ALT, AST, ALP, and LDH in APAP-treated mice
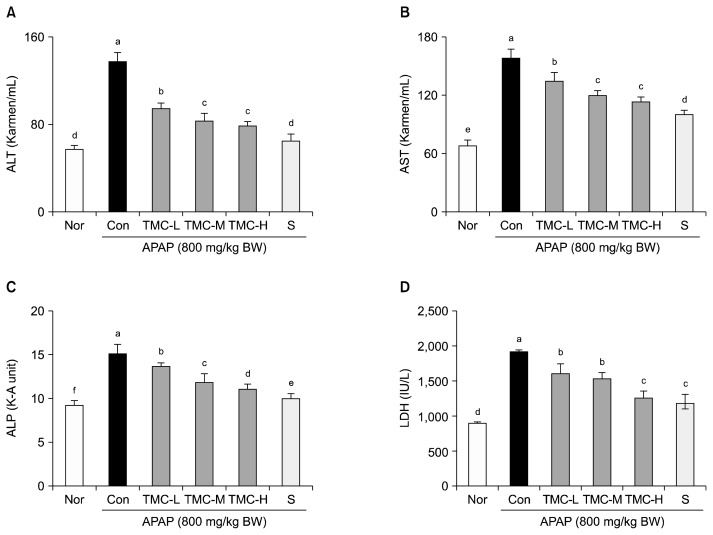
APAP treatment significantly increased serum ALT and AST levels versus normal controls (P<0.05) (Fig. 1). TMC effectively prevented APAP-induced increases in ALT levels and AST levels, especially in the TMC-H group (79.1±4.8 Karmen/mL for ALT, 112.0±9.1 Karmen/mL for AST, respectively). APAP (800 mg/kg BW) significantly increased serum ALP and LDH levels, but these increases were dose-dependently reduced by TMC (P<0.05). In the TMC-H group, mean ALP and LDH levels were 11.1±0.6 KA units and 1279.2±132.3 IU/L, respectively. However, silymarin was found to have more activity in terms of preventing APAP-induced increases in ALT, AST, ALP, and LDH levels than TMC (500 mg/kg BW).
Fig. 1.
Effects of TMC on serum levels of (A) ALT, (B) AST, (C) ALP, and (D) LDH in APAP-treated mice. Values are presented as means±SD (n=10). Different letters above bars (a–f) indicate that the mean values are significantly different (P<0.05) according to Duncan’s multiple range test. Nor, normal mice; Con, mice treated with APAP (800 mg/kg BW); TMC-L, 100 mg/kg of TMC; TMC-M, 250 mg/kg of TMC; TMC-H, 500 mg/kg of TMC; S, 100 mg/kg of silymarin.
Effects of TMC on antioxidant enzyme and GSH levels in the livers of APAP-treated mice
APAP significantly reduced hepatic antioxidant enzyme levels, including catalase, SOD, and GSH-Px, and GSH levels in mice (Table 2) (P<0.05). However, TMC dose-dependently prevented such reductions in APAP-treated mice; and in the TMC-H group, catalase (4.7±0.8 U/mg protein), SOD (2.1±0.3 U/mg protein), and GSH-Px (7.0±1.0 U/mg protein) were similar to those in the silymarin-treated group. Furthermore, TMC dose-dependently prevented APAP-induced decreases in the hepatic levels of GSH, which is required to counteract the hepatotoxicity of APAP in the liver (12).
Table 2.
Effects of taemyeongcheong (TMC) on liver antioxidant enzyme and glutathione (GSH) levels in acetaminophen (APAP)-treated mice
| Groups1) | Catalase (U/mg protein) | SOD2) (U/mg protein) | GSH-Px3) (U/mg protein) | GSH (μmol/g liver weight) |
|---|---|---|---|---|
| Nor | 6.2±1.5a | 2.2±0.2a | 8.6±1.5a | 18.9±0.3a |
| Con | 2.8±0.7c | 1.1±0.2c | 1.7±0.4f | 9.1±0.3f |
| TMC-L | 2.8±0.7c | 1.5±0.2b | 3.8±0.3e | 10.7±0.3e |
| TMC-M | 4.2±0.8b | 1.6±0.4b | 6.0±0.5d | 14.7±0.4d |
| TMC-H | 4.7±0.8b | 2.1±0.3a | 7.0±1.0c | 15.7±0.5c |
| S | 4.2±0.6b | 2.2±0.2a | 7.9±0.6b | 17.8±0.7b |
Values are presented as means±SD (n=10).
Values with different letters are significantly different (P<0.05) by Duncan’s multiple range test.
Nor, normal mice; Con, mice treated with APAP (800 mg/kg BW); TMC-L, 100 mg/kg of TMC; TMC-M, 250 mg/kg of TMC; TMC-H, 500 mg/kg of TMC; S, 100 mg/kg of silymarin.
SOD, superoxide dismutase.
GSH-Px, glutathione peroxidase.
Effects of TMC on serum IL-6 and TNF-α levels in APAP-treated mice
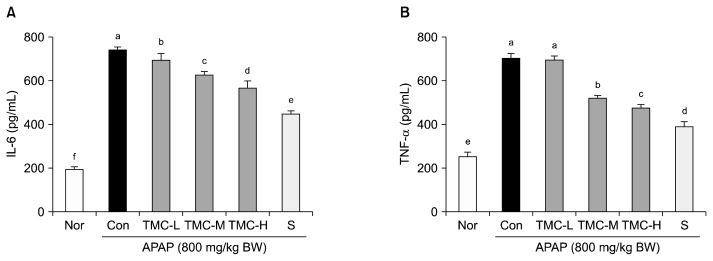
Increased levels of some pro-inflammatory cytokines are associated with APAP-induced acute hepatitis (13). In the present study, APAP caused a significant increase in the serum levels of IL-6 (743.0±15.9 pg/mL) and TNF-α (703.2±20.1 pg/mL) versus normal controls (198.1± 10.0 pg/mL in IL-6 and 250.1±20.0 pg/mL in TNF-α) (P<0.05) (Fig. 2). However, TMC treatment dose-dependently prevented these increases. In the TMC-H group, mean IL-6 and TNF-α levels were 567.1±33.3 pg/mL and 475.4±21.1 pg/mL, respectively.
Fig. 2.
Effects of TMC on serum (A) IL-6 and (B) TNF-α levels in APAP-treated mice. Values are presented as means±SD (n=10). Different letters above bars (a–f) indicate that the mean values are significantly different (P<0.05) according to Duncan’s multiple range test. Nor, normal mice; Con, mice treated with APAP (800 mg/kg BW); TMC-L, 100 mg/kg of TMC; TMC-M, 250 mg/kg of TMC; TMC-H, 500 mg/kg of TMC; S, 100 mg/kg of silymarin.
Effects of TMC on inflammation-related gene expressions in liver tissues
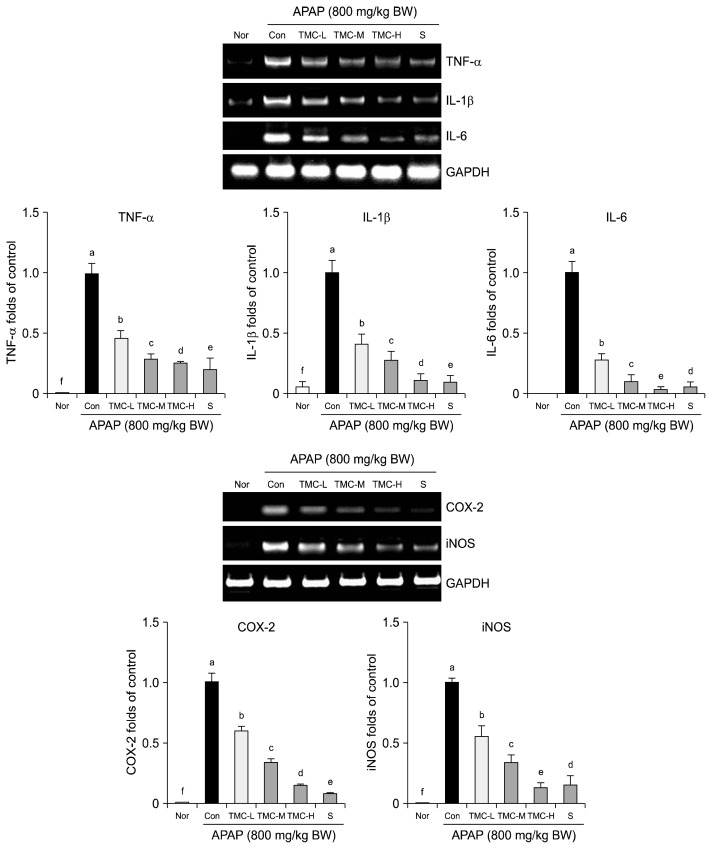
Treatment with APAP significantly increased the mRNA expressions of TNF-α, IL-1β, and IL-6 versus normal controls. However, TMC significantly prevented these increases, and TMC-H effectively reduced the hepatic mRNA expressions of TNF-α, IL-1β, and IL-6 by 87%, 84% and 89%, respectively (P<0.05). Similarly, TMC-H significantly reduced APAP-induced increases in the hepatic mRNA expressions of inflammatory COX-2 and iNOS by 85% and 88%, respectively (P<0.05) (Fig. 3).
Fig. 3.
Effects of TMC on inflammation-related gene expression in liver tissue. Band intensity was measured with a densitometer and expressed as fold of the control. Fold ratio=gene expression/GAPDH×control numerical value (control fold ratio: 1). Values are presented as means±SD (n=10). Different letters above bars (a–f) indicate that the mean values are significantly different (P<0.05) according to Duncan’s multiple range test. Nor, normal mice; Con, mice treated with APAP (800 mg/kg BW); TMC-L, 100 mg/kg of TMC; TMC-M, 250 mg/kg of TMC; TMC-H, 500 mg/kg of TMC; S, 100 mg/kg of silymarin.
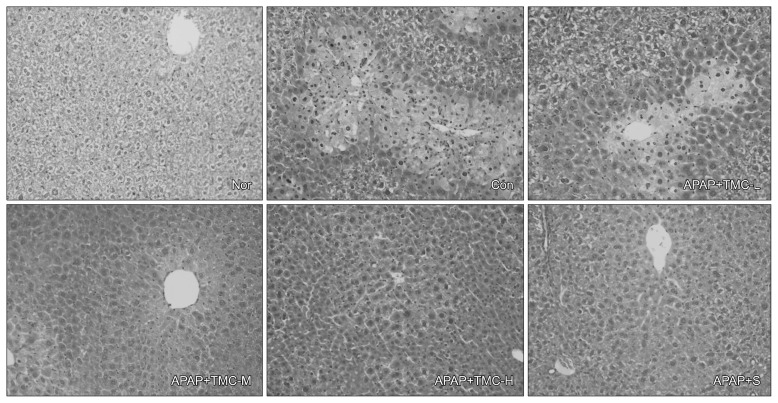
Effects of TMC on histological observations in APAP-treated mice
The histologic changes observed in APAP treated mice were: hepatocyte ballooning, fatty change, and inflammatory cell infiltration in liver tissues; however, TMC attenuated these effects. In the TMC-M group, histological appearances of liver were similar to those in the silymarin group, and in the TMC-H group, livers resembled those of normal controls (Fig. 4).
Fig. 4.
Effects of TMC on histological observation in APAP-treated mice. Nor, normal mice; Con, mice treated with APAP (800 mg/kg BW); TMC-L, 100 mg/kg of TMC; TMC-M, 250 mg/kg of TMC; TMC-H, 500 mg/kg of TMC; S, 100 mg/kg of silymarin. Liver sections were stained with hematoxyline-eosin (H&E, ×500). The hepatocytes were analyzed to determine necrosis, fatty change, hepatocyte ballooning, and inflammatory cells infiltration.
DISCUSSION
The present study shows that TMC dose-dependently protects against APAP-induced acute liver damage in ICR mice. This conclusion is supported by the observed enhancement of endogenous antioxidant system activity and by the reduced APAP-induced acute inflammatory reactions in mice administered TMC.
APAP treatment significantly increased liver weights as compared with normal controls, and TMC dose-dependently reduced these increases. The APAP-induced hepatic damage was evidenced by the elevated serum levels of ALT, AST, ALP, and LDH, which are located in hepatocytes (14), and TMC significantly prevented these increases. These observations suggest TMC is able to maintain hepatocyte health and inhibit enzyme leakage from cellular membranes.
APAP overdose-induced hepatic damage is closely linked to oxidative stress, which is characterized by the diminished activities of endogenous antioxidant enzymes and the depletion of GSH levels (15), and finally causes irreversible membrane injury and cell death (16,17). In a previous study, TMC treatment maintained hepatic levels of catalase, SOD, and GSH-Px in mice exposed to APAP. SOD catalyzes the conversion of super-oxide into H2O2, which is converted to H2O by catalase (18). GSH-Px is the major peroxide detoxification enzyme, which catalyzes the intercellular reduced GSH and is a hydrogen donor to generate oxidized glutathione, and scavenges H2O2 and catalyzes the reduction of peroxides in hepatocytes (17,18). In addition, we found TMC effectively protected hepatic GSH levels in APAP-treated mice. GSH, an important ROS scavenger, can participate in the removal of ROS by reducing hydroperoxides in the presence of GSH-Px (19). In hepatocytes, a high APAP dose (500 mg/kg BW) resulted in the depletion of GSH, and led to binding between toxic NAQPI and essential hepatocellular proteins and acute hepatic damage (20,21). Furthermore, maintaining GSH levels are essential for protecting thiol and other nucleophilic groups in proteins from toxic metabolites in a background of APAP-induced liver damage (2,19).
APAP-induced hepatocyte damage is also associated with serious inflammatory response, which is characterized by the accumulation of inflammatory cells and the over-production of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, which promote liver tissue damage (22–24). Agarwal and Piersco reported GSH depletion enhances the expression of TNF-α and induces cell damage (25). In the present study, TMC dose-dependently prevented elevations in the serum levels of TNF-α and IL-6 in mice treated with APAP. In addition, we observed APAP significantly increased hepatic mRNA levels of TNF-α, IL-1β, and IL-6 in mice, which agrees with the findings of Martin-Murphy et al. (13), whereas TMC significantly prevented these increases. In addition to the pro-inflammatory cytokines, APAP also induces the overexpressions of inflammatory COX-2 and iNOS in the livers of mice (26), and TMC effectively reduced these overexpressions. Furthermore, it has been suggested the suppression of iNOS expression may reduce IL-1β generations during APAP-induced acute liver damage (12). In another study, reduced activation of COX-2 inhibited prostaglandin E2 generation, and subsequently attenuated inflammatory reactions associated with APAP-induced liver injury (26).
Finally, we also found the TMC treatment ameliorated APAP-induced liver tissue damage, such as necrosis of centrilobular hepatocytes, inflammatory infiltration, fatty change, and ballooning degeneration in mice. These results suggest that the suppression of serum transaminase levels and serious inflammatory reactions play important roles in the amelioratory effects of TMC on APAP-induced hepatic damage in ICR mice.
TMC is prepared as a traditional Korean health drink using many health-promoting herbs, such as Saururus chinensis, Taraxacum officinale, Zingiber officinale, Cirsium setidens, Salicornia herbaceax, and Glycyrrhizae. These herbs also possess active bio-functional compounds and have been reported to have many health benefits, which include antioxidant, anti-inflammatory, and hepatoprotective activities. For example, lignans from Saururus chinensis exhibit antioxidant activity (27), and attenuate CCl4-induced toxicity in primary cultures of rat hepatocytes (28). Flavonoids, such as luteolin and luteolin-7-O-glucoside isolated from Taraxacum officinale have been reported to show anti-inflammatory activity by inhibiting the activations of iNOS and COX-2 in LPS-activated RAW264.7 cells (29). Zingiber officinale and gingerol related compounds exhibit antioxidant activities and suppress APAP-induced liver damage (9,30). Furthermore, pectolinarin and pectolinarigenin from Cirsium setidens were found to prevent D-galactosamine induced hepatotoxicity by enhancing the endogenous antioxidant enzyme system in rats (31), and 3-caffeoyl, 4-dihydrocaffeoyl quinic acid from Salicornia herbacea exhibited antioxidative effects by scavenging ROS and upregulating the expressions of antioxidant enzymes (32). Furthermore, glycyrrhizin and glycyrrhizic acid from Glycyrrhizae were found to have hepatoprotective (33), antioxidant (34), and anti-inflammatory activities (35). These findings indicate that various bioactive molecules, such as lignans, gingerol, glycyrrhizin, and glycyrrhizic acid in raw materials of TMC may exhibit great activity to protect the APAP-induced hepatic damage in TMC-treated ICR mice (9,27,30,33).
In conclusion, TMC dose-dependently protected ICR mice from APAP-induced liver damage, as indicated by serum levels of ALT, AST, ALP, and LDH, the activities of antioxidant enzymes in liver tissues (catalase, SOD, and GSH-Px), hepatic GSH levels, and serum pro-inflammatory cytokines (IL-6 and TNF-α). The APAP-induced hepatic expressions of pro-inflammation cytokines (the mRNAs of TNF-α, IL-1β, and IL-6) and of inflammatory COX-2 and iNOS were reduced by TMC treatment. Furthermore, our histologic findings show that TMC attenuated APAP-induced liver damage. These results suggest consumption of TMC in the form of a health drink acts to protect against APAP-induced hepatic damage. In addition, the therapeutic effect of TMC will be investigated in our further study.
ACKNOWLEDGEMENTS
This work was supported by a research grant (BLLR-B13011) from the National Center of Efficacy Evaluation for the Development of Health Products Targeting Digestive Disorders (NCEED) funded by the Ministry of Health and Welfare (Republic of Korea).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther. 1973;187:185–194. [PubMed] [Google Scholar]
- 2.Dahlin DC, Miwa GT, Lu AY, Nelson SD. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci USA. 1984;81:1327–1331. doi: 10.1073/pnas.81.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin Liver Dis. 1990;10:267–278. doi: 10.1055/s-2008-1040482. [DOI] [PubMed] [Google Scholar]
- 4.Lores Arnaiz S, Llesuy S, Cutrin JC, Boveris A. Oxidative stress by acute acetaminophen administration in mouse liver. Free Radic Biol Med. 1995;19:303–310. doi: 10.1016/0891-5849(95)00023-Q. [DOI] [PubMed] [Google Scholar]
- 5.Fujiwara K, Ohta Y, Ogata I. New Trends in Peptic Ulcer and Chronic Hepatitis: Chronic Hepatitis. Excerpta Medica; Tokyo, Japan: 1987. Treatment trial of traditional oriental medicine in chronic viral hepatitis; pp. 141–146. [Google Scholar]
- 6.Kim SJ. MS Thesis. Pusan National University; Busan, Korea: 2012. Preventive effects of beopje ginger and taemyeongcheong on in vivo gastritis and colitis. [Google Scholar]
- 7.Wang L, Cheng D, Wang H, Di L, Zhou X, Xu T, Yang X, Liu Y. The hepatoprotective and antifibrotic effects of Saururus chinensis against carbon tetrachloride induced hepatic fibrosis in rats. J Ethnopharmacol. 2009;126:487–491. doi: 10.1016/j.jep.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Ajith TA, Hema U, Aswathy MS. Zingiber officinale Roscoe prevents acetaminophen-induced acute hepatotoxicity by enhancing hepatic antioxidant status. Food Chem Toxicol. 2007;45:2267–2272. doi: 10.1016/j.fct.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Yemitan OK, Izegbu MC. Protective effects of Zingiber officinale (Zingiberaceae) against carbon tetrachloride and acetaminophen-induced hepatotoxicity in rats. Phytother Res. 2006;11:997–1002. doi: 10.1002/ptr.1957. [DOI] [PubMed] [Google Scholar]
- 10.Yoon YH, Yoo SN, Yoon HG, Park JJ, Lee YH, Kim SO, Oh KT, Lee JM, Cho HY, Jun WJ. In vitro and in vivo hepatoprotective effects of the aqueous extract from Taraxacum officinale (dandelion) root against alcohol-induced oxidative stress. Food Chem Toxicol. 2010;48:1632–1637. doi: 10.1016/j.fct.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 11.Yin G, Cao L, Xu P, Jeney G, Nakao M, Lu C. Hepatoprotective and antioxidant effects of Glycyrrhiza glabra extract against carbon tetrachloride (CCl4)-induced hepatocyte damage in common carp (Cyprinus carpio) Fish Physiol Biochem. 2011;37:209–216. doi: 10.1007/s10695-010-9436-1. [DOI] [PubMed] [Google Scholar]
- 12.James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 2003;31:1499–1506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- 13.Martin-Murphy BV, Holt MP, Ju C. The role of damage associated molecular pattern molecules in acetaminophen-induced liver injury in mice. Toxicol Lett. 2010;192:387–394. doi: 10.1016/j.toxlet.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ansari RA, Tripathi SC, Patnaik GK, Dhawan BN. Antihepatotoxic properties of picroliv: an active fraction from rhizomes of Picrorhiza kurrooa. J Ethnopharmacol. 1991;34:61–68. doi: 10.1016/0378-8741(91)90189-K. [DOI] [PubMed] [Google Scholar]
- 15.Bessems JGM, Vermeulen NPE. Paracetamol (acetaminophen)-induced toxicity: molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol. 2001;31:55–138. doi: 10.1080/20014091111677. [DOI] [PubMed] [Google Scholar]
- 16.Kyle ME, Miccadei S, Nakae D, Farber JL. Superoxide dismutase and catalase protect cultured hepatocytes from the cytotoxicity of acetaminophen. Biochem Biophys Res Commun. 1987;149:889–896. doi: 10.1016/0006-291X(87)90491-8. [DOI] [PubMed] [Google Scholar]
- 17.Hochstein P, Atallah AS. The nature of oxidants and antioxidant systems in the inhibition of mutation and cancer. Mutat Res. 1988;202:363–375. doi: 10.1016/0027-5107(88)90198-4. [DOI] [PubMed] [Google Scholar]
- 18.Kaplowitz N, Tsukamoto H. Oxidative stress and liver disease. Prog Liver Dis. 1996;14:131–159. [PubMed] [Google Scholar]
- 19.Halliwell B, Gutteridge JM. Lipid peroxidation, oxygen radicals, cell damage, and antioxidant therapy. Lancet. 1984;1:1396–1397. doi: 10.1016/S0140-6736(84)91886-5. [DOI] [PubMed] [Google Scholar]
- 20.Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr Rev. 2012;70:257–265. doi: 10.1111/j.1753-4887.2012.00476.x. [DOI] [PubMed] [Google Scholar]
- 21.Zakowski JJ, Tappel AL. Purification and properties of rat liver mitochondrial glutathione peroxidase. Biochim Biophys Acta. 1978;526:65–76. doi: 10.1016/0005-2744(78)90290-5. [DOI] [PubMed] [Google Scholar]
- 22.Ross D. Glutathione, free radicals and chemotherapeutic agents: mechanisms of free-radical induced toxicity and glutathione-dependent protection. Pharmacol Ther. 1988;37:231–249. doi: 10.1016/0163-7258(88)90027-7. [DOI] [PubMed] [Google Scholar]
- 23.Blazka ME, Elwell MR, Holladay SD, Wilson RE, Luster MI. Histopathology of acetaminophen-induced liver changes: role of interleukin 1α and tumor necrosis factor α. Toxicol Pathol. 1996;24:181–189. doi: 10.1177/019262339602400206. [DOI] [PubMed] [Google Scholar]
- 24.Ishida Y, Kondo T, Ohshima T, Fujiwara H, Iwakura Y, Mukaida N. A pivotal involvement of IFN-γ in the pathogenesis of acetaminophen-induced acute liver injury. FASEB J. 2002;16:1227–1236. doi: 10.1096/fj.02-0046com. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal S, Piesco NP. Poly ADP-ribosylation of a 90-kDa protein is involved in TNF-α-mediated cytotoxicity. J Immunol. 1994;153:473–481. [PubMed] [Google Scholar]
- 26.Reilly TP, Brady JN, Marchick MR, Bourdi M, George JW, Radonovich MF, Pise-Masison CA, Pohl LR. A protective role for cyclooxygenase-2 in drug-induced liver injury in mice. Chem Res Toxicol. 2001;14:1620–1628. doi: 10.1021/tx0155505. [DOI] [PubMed] [Google Scholar]
- 27.Lee WS, Beak YI, Kim JR, Cho KH, Sok DE, Jeong TS. Antioxidant activities of a new lignan and a neolignan from Saururus chinensis. Bioorg Med Chem Lett. 2004;14:5623–5628. doi: 10.1016/j.bmcl.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 28.Sung SH, Lee EJ, Cho JH, Kim HS, Kim YC. Sauchinone, a lignan from Saururus chinensis, attenuates CCl4-induced toxicity in primary cultures of rat hepatocytes. Biol Pharm Bull. 2000;23:666–668. doi: 10.1248/bpb.23.666. [DOI] [PubMed] [Google Scholar]
- 29.Hu C, Kitts DD. Luteolin and luteolin-7-O-glucoside from dandelion flower suppress iNOS and COX-2 in RAW264.7 cells. Mol Cell Biochem. 2004;265:107–113. doi: 10.1023/B:MCBI.0000044364.73144.fe. [DOI] [PubMed] [Google Scholar]
- 30.Masuda Y, Kikuzaki H, Hisamoto M, Nakatani N. Antioxidant properties of gingerol related compounds from ginger. Biofactors. 2004;21:293–296. doi: 10.1002/biof.552210157. [DOI] [PubMed] [Google Scholar]
- 31.Yoo YM, Nam JH, Kim MY, Choi JW, Park HJ. Pectolinarin and pectolinarigenin of Cirsium setidens prevent the hepatic injury in rats caused by D-galactosamine via an antioxidant mechanism. Biol Pharm Bull. 2008;31:760–764. doi: 10.1248/bpb.31.760. [DOI] [PubMed] [Google Scholar]
- 32.Hwang YP, Yun HJ, Chun HK, Chung YC, Kim HK, Jeong MH, Yoon TR, Jeong HG. Protective mechanisms of 3-caffeoyl, 4-dihydrocaffeoyl quinic acid from Salicornia herbacea against tert-butyl hydroperoxide-induced oxidative damage. Chem Biol Interact. 2009;181:366–367. doi: 10.1016/j.cbi.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Nagai T, Egashira T, Yamanaka Y, Kohno M. The protective effect of glycyrrhizin against injury of the liver caused by ischemia-reperfusion. Arch Environ Contam Toxicol. 1991;20:432–436. doi: 10.1007/BF01064416. [DOI] [PubMed] [Google Scholar]
- 34.Kiso Y, Tohkin M, Hikino H, Hattori M, Sakamoto T, Namba T. Mechanism of antihepatotoxic activity of glycyrrhizin. I: effect on free radical generation and lipid peroxidation. Planta Med. 1984;50:298–302. doi: 10.1055/s-2007-969714. [DOI] [PubMed] [Google Scholar]
- 35.Finney RS, Somers GF. The antiinflammatory activity of glycyrrhetinic acid and derivatives. J Pharm Pharmacol. 1958;10:613–620. doi: 10.1111/j.2042-7158.1958.tb10349.x. [DOI] [PubMed] [Google Scholar]