Abstract
1,4-Dihydroxy-2-naphthoic acid (DHNA) is a bifidogenic growth stimulator (BGS) and could be a functional food ingredient since bifidobacteria are beneficial for human health. For that reason, lactic acid bacteria producing DHNA have been screened. A lactic acid bacterium LP1 strain isolated from a natural cheese was confirmed to produce DHNA, analyzed by a HPLC method. The strain was identified as Lactobacillus casei by 16S rRNA gene sequence analysis. The cell-free supernatant of fermented whey produced by L. casei LP1 presented the BGS activity for three bifidobacterial strains such as Bifidobacterium longum subsp. infantis KCTC 3127, Bifidobacterium bifidum KCTC 3202, and Bifidobacterium breve KCTC 3220 which were human-originated. To the best of our knowledge, a L. casei strain which can produce DHNA was firstly identified in this study.
Keywords: Lactobacillus casei LP1; 1,4-dihydroxy-2-naphthoic acid; bifidogenic growth stimulator
INTRODUCTION
1,4-Dihydroxy-2-naphthoic acid (DHNA) is well known as a bifidogenic growth stimulator (BGS), and it can be commercially used as a prebiotic (1,2). Moreover, it improved survival rate and histological damage in mice that suffered from dextran sodium sulfate (DSS) induced colitis (3). Recently, it also reduced inflammation in IL-10 deficient mice with colitis by controlling pro-inflammatory cytokines derived from macrophages (4). At first, DHNA was identified as a novel bifidogenic growth stimulator from a culture broth produced by Propionibacterium freudenreichii ET-3 strain and approximately 10 mg/L of DHNA was detected by HPLC (high performance liquid chromatography) (1).
DHNA is a precursor of menaquinone (vitamin K2) which functions as an electron transfer agent in the bacterial respiratory chain (5–8). The biosynthesis of menaquinone is processed by two independent syntheses in which DHNA and isoprenoid unit syntheses are included. DHNA is converted into menaquinone by incorporating the isoprenoid unit (5). Theoretically, the amount of DHNA released from bacterial producers can be improved by blocking the synthesis of the isoprenoid unit, which is produced from glyceraldehyde-3-phosphate and pyruvate (9–11).
Bifidobacteria is recognized as beneficial bacteria for human gut homeostasis and has been largely studied (12). The human gut microbiota can be changed negatively by inner or outer conditions, which is deeply correlated with pathogenic physiology such as inflammatory bowel disease, colorectal cancer, irritable bowel syndrome, etc. (12). However, probiotics including bifidobacteria and lactobacilli can modulate human gut microbiota positively; therefore, they can prevent or improve the human gut pathogenesis (13). In this study, a novel Lactobacillus casei strain that produces DHNA was isolated, and the fermented whey with the strain was confirmed to stimulate the growth of bifidobacterial strains.
MATERIALS AND METHODS
Bacterial strains and culture conditions
L. casei LP1 was cultured in MRS (De Man, Rogosa, and Sharpe) broth (Difco Laboratories Inc., Detroit, MI, USA) at 37°C without agitation. Bifidobacterial strains including Bifidobacterium longum subsp. infantis KCTC 3127, Bifidobacterium bifidum KCTC 3202, and Bifidobacterium breve KCTC 3220 were purchased from the Korean Collection for Type Cultures (KCTC) at Korea Research Institute of Bioscience and Biotechnology (KRIBB, Daejeon, Korea). They were cultured in Reinforced Clostridial Medium (RCM) (Difco Laboratories Inc.) broth at 37°C in an anaerobic jar (Oxoid Ltd., Hampshire, UK) with BD GasPak™ EZ Anaerobic Container System (BD, Sparks, MD, USA).
Isolation and identification of LP1
LP1 strain was previously isolated from a natural cheese and stored at −76°C until use. The strain was sent to a biotech company (Macrogen Co., Seoul, Korea) for 16S rRNA gene sequence analysis. The primer set 27F (5′-AGA GTT TGA TCC TGG CTC AG-3′) and 1492R (5′-TAC GGY TAC CTT GTT ACG ACT T-3′) was used for amplification of the 16S rRNA gene and 518F (5′-CCA GCA GCC GCG GTA ATA CG-3′) and 800R (5′-TAC CAG GGT ATC TAA TCC-3′) primer set was used for sequencing. Homology search for the sequence was performed using a Basic Local Alignment Search Tool (BLAST) program from The National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov/).
Detection of 1,4-dihydroxy-2-naphthoic acid from L. casei LP1
L. casei LP1 was inoculated into 50 mL of sterile whey broth (10%, w/v) and incubated anaerobically at 37°C for 48 h. Four mL of the culture was mixed with 2 volumes of methanol and sodium-ascorbate (Sigma-Aldrich, St. Louis, MO, USA) as an antioxidant was added at 0.2%. After extraction, the mixture was centrifuged at 3,600 rpm for 10 min and the supernatant was evaporated at 40°C by a rotary evaporator (N-1000, Eyela, Tokyo, Japan). Four hundred microliters of methanol was added to the concentrate and filtered with a syringe filter (0.45 μm pore size; Millipore, Billerica, MA, USA). The filtrate was injected for HPLC analysis. An Agilent HC-C18 column (4.6×150 mm; Agilent Technologies Inc., Santa Clara, CA, USA) was used and its temperature was maintained at 45°C during the analysis. The flow rate was 1 mL/min, injection volume was 20 μL, and detection wavelength was 254 nm. Reagent grade DHNA (Sigma-Aldrich) was used for the standard material. The mobile phase was composed of acetonitrile : methanol : water : acetic acid (15:25:225:0.1), and the pH was adjusted to be 5.5 with 5% (w/v) ammonium hydroxide.
BGS activity test
As mentioned above, the whey culture of L. casei LP1 was centrifuged at 3,600 rpm for 10 min. The cell-free supernatant was filtered and the filtrate was used for BGS activity test. As a control, whey broth which was adjusted to pH 4.5 with lactic acid was used. One hundred microliters of the filtrate was added to 1/10 RCM broth (Difco Laboratories Inc.) which was previously inoculated with each bifidobacterial strain (0.2%, v/v). These inocula were incubated at 37°C for 12 h in the anaerobic jar (Oxoid Ltd.) supplemented with the GasPak™ EZ Anaerobe Container System (BD) and the optical density (OD) at 600 nm was measured at times (0, 4, 8, and 12 h).
RESULTS AND DISCUSSION
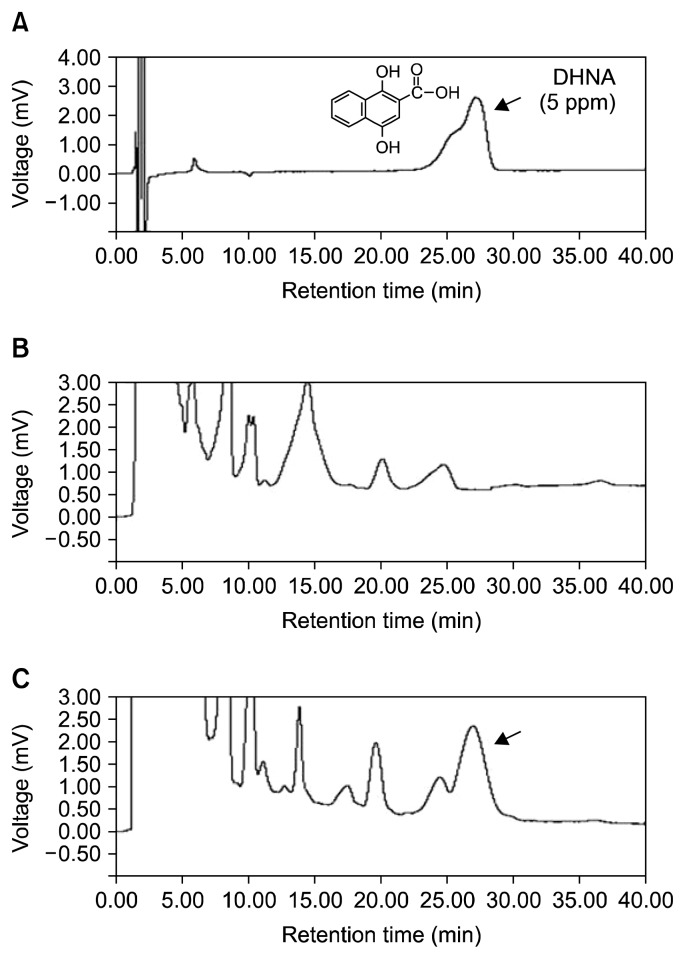
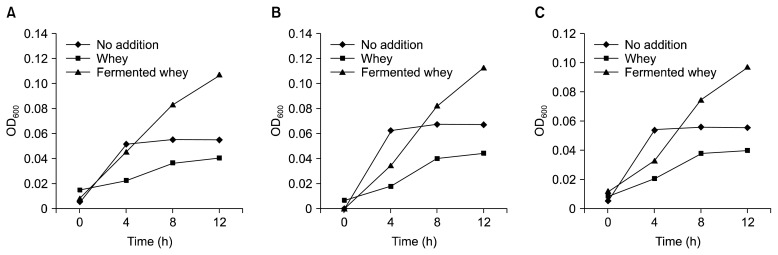
LP1 strain which was previously isolated from a natural cheese was confirmed to produce DHNA which act as a BGS. The strain was identified by 16S rRNA gene sequence analysis. The 16S rRNA gene sequence (GenBank accession no., KP696456) of LP1 was best matched with L. casei strains (99% identity; GenBank accession no. CP002618.1, CP002616.1, CP001084.1, FM177140.1, and CP000423.1), and the LP1 strain was named L. casei LP1. DHNA production from L. casei LP1 strain was confirmed by the HPLC method with the standard material DHNA. A C18 column was used for the analysis. Retention time for the standard DHNA was 27.1 min (Fig. 1A) and a corresponding peak was detected from the concentrated whey culture supernatant of L. casei LP1 (Fig. 1C). Based on a standard curve for quantification (data not shown), the amount of DHNA produced from L. casei LP1 was 0.37 ppm. This amount is not enough for mass purification and much less than that from P. freudenreichii ET-3 which has been studied by a Japanese dairy company (1,14). However, to the best of our knowledge, the L. casei strain which can produce DHNA was firstly identified. Moreover, a tiny amount (1 ng/mL) of DHNA can stimulate the growth of bifidobacteria (1). Therefore, the L. casei LP1 strain can be broadly used as a functional starter culture for various fermented foods including dairy products. For these reasons, BGS activity of the cell-free supernatant of L. casei LP1 was tested in 1/10 RCM broth which aimed at nutrient limitation. The bifidobacterial strains such as B. longum subsp. infantis KCTC 3127, B. bifidum KCTC 3202, and B. breve KCTC 3220 used in this study were human-originated. Because of the nutrient limitation, the bifidobacterial strains grew slowly in all the cases: no addition, addition of whey broth as a control, and addition of fermented whey with L. casei LP1 (Fig. 2). Nevertheless, the growth of bifidobacterial strains with the fermented whey was significantly increased compared with the other cases. Unexpectedly, whey broth, which was adjusted to pH 4.5 inhibited the growth of the bifidobacterial strains. In case of no addition, the growth rate of early stage was similar (Fig. 2A) to or higher (Fig. 2B, 2C) than that of addition of fermented whey, but the growth reached a plateau state after 4 h, which might be due to nutrient exhaustion. On the other hand, the growth of the bifidobacterial strains with fermented whey was steadily increased up to 12 hours, which was the final time for the test. At that time, the growth rate with fermented whey was 1.83, 1.71, and 1.67 times higher than those with no addition for B. longum subsp. infantis KCTC 3127, B. bifidum KCTC 3202, and B. breve KCTC 3220, respectively. From these results, we concluded that the fermented whey with L. casei LP1 producing DHNA could stimulate the growth of bifidobacterial strains, and the functional L. casei LP1 can be used as a probiotic or a prebiotic producer.
Fig. 1.
Chromatogram of 1,4-dihydroxy-2-naphthoic acid in fermented whey with Lactobacillus casei LP1. (A) DHNA standard, (B) whey, and (C) fermented whey. The arrows indicate DHNA peaks. HPLC condition: column, Agilent HC-C18 (4.6×150 mm); column temperature, 45°C; flow rate, 1 mL/min; injection volume, 20 μL; detection wavelength, 254 nm; mobile phase, acetonitrile: methanol: water: acetic acid (15:25:225:0.1; pH 5.5).
Fig. 2.
Bifidogenic growth stimulator activity of fermented whey with Lactobacillus casei LP1. (A) Bifidobacterium longum subsp. infantis KCTC 3127, (B) Bifidobacterium bifidum KCTC 3202, and (C) Bifidobacterium breve KCTC 3220. No addition, only bifidobacterial strain was inoculated into 1/10 RCM broth; Whey, bifidobacterial strain plus whey broth adjusted to pH 4.5 with lactic acid; Fermented whey, bifidobacterial strain plus fermented whey with L. casei LP1.
ACKNOWLEDGEMENT
This research (grant no., 111014-01-1-HD110) was supported by High Value-added Food Technology Development Program, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Isawa K, Hojo K, Yoda N, Kamiyama T, Makino S, Saito M, Sugano H, Mizoguchi C, Kurama S, Shibasaki M, Endo N, Sato Y. Isolation and identification of a new bifidogenic growth stimulator produced by Propionibacterium freudenreichii ET-3. Biosci Biotechnol Biochem. 2002;66:679–681. doi: 10.1271/bbb.66.679. [DOI] [PubMed] [Google Scholar]
- 2.Sanders ME, Lenoir-Wijnkoop I, Salminen S, Merenstein DJ, Gibson GR, Petschow BW, Nieuwdorp M, Tancredi DJ, Cifelli CJ, Jacques P, Pot B. Probiotics and prebiotics: prospects for public health and nutritional recommendations. Ann NY Acad Sci. 2014;1309:19–29. doi: 10.1111/nyas.12377. [DOI] [PubMed] [Google Scholar]
- 3.Okada Y, Tsuzuki Y, Miyazaki J, Matsuzaki K, Hokari R, Komoto S, Kato S, Kawaguchi A, Nagao S, Itoh K, Watanabe T, Miura S. Propionibacterium freudenreichii component 1,4-dihydroxy-2-naphthoic acid (DHNA) attenuates dextran sodium sulphate induced colitis by modulation of bacterial flora and lymphocyte homing. Gut. 2006;55:681–688. doi: 10.1136/gut.2005.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okada Y, Tsuzuki Y, Narimatsu K, Sato H, Ueda T, Hozumi H, Sato S, Hokari R, Kurihara C, Komoto S, Watanabe C, Tomita K, Kawaguchi A, Nagao S, Miura S. 1,4-Dihydroxy-2-naphthoic acid from Propionibacterium freudenreichii reduces inflammation in interleukin-10-deficient mice with colitis by suppressing macrophage-derived pro-inflammatory cytokines. J Leukoc Biol. 2013;94:473–480. doi: 10.1189/jlb.0212104. [DOI] [PubMed] [Google Scholar]
- 5.Bentley R, Meganathan R. Biosynthesis of vitamin K (menaquinone) in bacteria. Microbiol Rev. 1982;46:241–280. doi: 10.1128/mr.46.3.241-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myers CR, Myers JM. Role of menaquinone in the reduction of fumarate, nitrate, iron(III) and manganese(IV) by Shewanella putrefaciens MR-1. FEMS Microbiol Lett. 1993;114:215–222. doi: 10.1111/j.1574-6968.1993.tb06576.x. [DOI] [Google Scholar]
- 7.Newman DK, Kolter R. A role for excreted quinones in extracellular electron transfer. Nature. 2000;405:94–97. doi: 10.1038/35011098. [DOI] [PubMed] [Google Scholar]
- 8.Saffarini DA, Blumerman SL, Mansoorabadi KJ. Role of menaquinone in Fe(III) reduction by membrane fraction of Shewanella putrefaciens. J Bacteriol. 2002;184:846–848. doi: 10.1128/JB.184.3.846-848.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohmer M, Knani M, Simonin P, Sutter B, Sahm H. Isoprenoid biosynthesis in bacteria: a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem J. 1993;295:517–524. doi: 10.1042/bj2950517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohmer M, Seemann M, Horbach S, Bringer-Meyer S, Sahm H. Glyceraldehyde 3-phosphate and pyruvate as precursors of isoprenic units in an alternative non-mevalonate pathway for terpenoid biosynthesis. J Am Chem Soc. 1996;118:2564–2566. doi: 10.1021/ja9538344. [DOI] [Google Scholar]
- 11.Schwender J, Seemann M, Lichtenthaler HK, Rohmer M. Biosynthesis of isoprenoids (carotenoids, sterols, prenyl side-chains of chlorophylls and plastoquinone) via a novel pyruvate/glyceraldehyde 3-phosphate non-mevalonate pathway in the green alga Scenedesmus obliquus. Biochem J. 1996;316:73–80. doi: 10.1042/bj3160073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tojo R, Suárez A, Clemente MG, de los Reyes-Gavilán CG, Margolles A, Gueimonde M, Ruas-Madiedo P. Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World J Gastroenterol. 2014;20:15163–15176. doi: 10.3748/wjg.v20.i41.15163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plaza-Diaz J, Gomez-Llorente C, Fontana L, Gil A. Modulation of immunity and inflammatory gene expression in the gut, in inflammatory diseases of the gut and in the liver by probiotics. World J Gastroenterol. 2014;20:15632–15649. doi: 10.3748/wjg.v20.i42.15632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuichi K, Katakura Y, Ninomiya K, Shioya S. Enhancement of 1,4-dihydroxy-2-naphthoic acid production by Propionibacterium freudenreichii ET-3 fed-batch culture. Appl Environ Microbiol. 2007;73:3137–3143. doi: 10.1128/AEM.01307-06. [DOI] [PMC free article] [PubMed] [Google Scholar]