Abstract
The class I myosin genes are conserved in diverse organisms, and their gene products are involved in actin dynamics, endocytosis, and signal transduction. Drosophila melanogaster has three class I myosin genes, Myosin 31DF (Myo31DF), Myosin 61F (Myo61F), and Myosin 95E (Myo95E). Myo31DF, Myo61F, and Myo95E belong to the Myosin ID, Myosin IC, and Myosin IB families, respectively. Previous loss-of-function analyses of Myo31DF and Myo61F revealed important roles in left–right (LR) asymmetric development and enterocyte maintenance, respectively. However, it was difficult to elucidate their roles in vivo, because of potential redundant activities. Here we generated class I myosin double and triple mutants to address this issue. We found that the triple mutant was viable and fertile, indicating that all three class I myosins were dispensable for survival. A loss-of-function analysis revealed further that Myo31DF and Myo61F, but not Myo95E, had redundant functions in promoting the dextral LR asymmetric development of the male genitalia. Myo61F overexpression is known to antagonize the dextral activity of Myo31DF in various Drosophila organs. Thus, the LR-reversing activity of overexpressed Myo61F may not reflect its physiological function. The endogenous activity of Myo61F in promoting dextral LR asymmetric development was observed in the male genitalia, but not the embryonic gut, another LR asymmetric organ. Thus, Myo61F and Myo31DF, but not Myo95E, play tissue-specific, redundant roles in LR asymmetric development. Our studies also revealed differential colocalization of the class I myosins with filamentous (F)-actin in the brush border of intestinal enterocytes.
Keywords: myosin I, Myosin 31DF, Myosin 61F, Myosin 95E, left-right asymmetry
THE class I myosin genes encode myosin heavy chains, which are conserved in phylogenetically diverse organisms (Sellers 2000; Krendel and Mooseker 2005). The class I myosins are nonfilamentous, actin-based motor proteins and were the first discovered unconventional myosin proteins. These myosins are involved in a variety of cellular processes, such as cell migration, cell adhesion, and cell growth, through their regulation of actin dynamics, endocytosis, and signal transduction (Osherov and May 2000; Krendel and Mooseker 2005; Kim and Flavell 2008; McConnell and Tyska 2010).
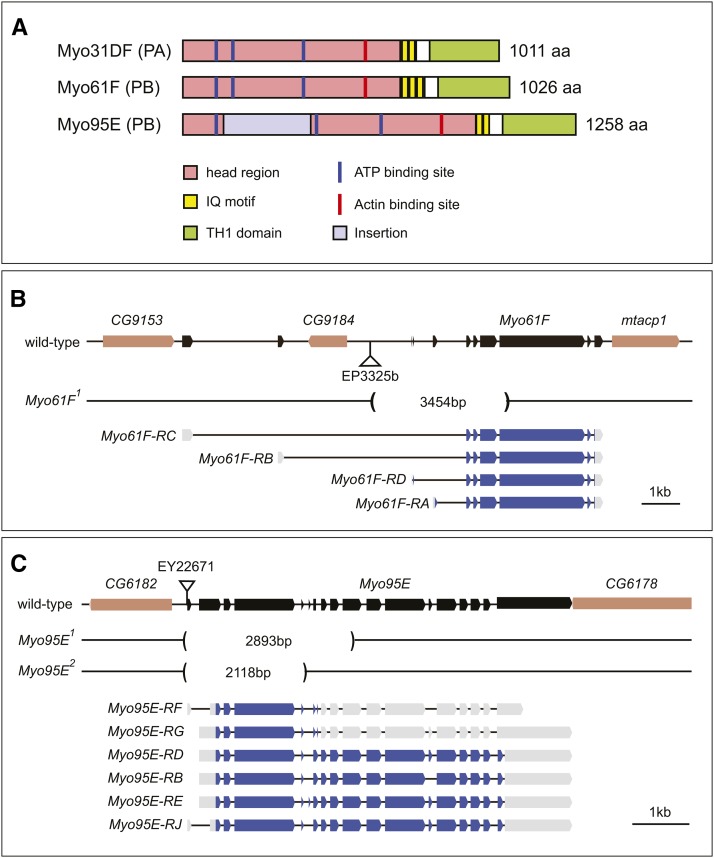
The structure of the myosin I heavy chains is evolutionarily conserved and composed of head (or motor), neck, and tail domains (Figure 1A) (Coluccio 1997; Barylko et al. 2000). The head domain binds to filamentous (F)-actin and adenosine triphosphate (ATP), a common feature of myosin proteins (Figure 1A) (Mermall et al. 1998); the neck domain possesses one or more IQ motifs, which directly interact with calmodulin or calmodulin-related myosin light chains (Coluccio 1997; Barylko et al. 2000), and the tail domains are divided into short and long types. Short tails contain a single tail homology 1 (TH1) domain, which is rich in basic residues and thought to interact with plasma membranes (Coluccio 1997; Barylko et al. 2000); while long tails contain the TH1 domain; a tail homology 2 (TH2) domain, which is proline-rich and binds to F-actin in an ATP-independent manner; and a tail homology 3 (TH3), or Src homology 3 (SH3) domain, at the C terminus (Coluccio 1997; Barylko et al. 2000).
Figure 1.
Deduced structures of the Drosophila myosin I family proteins and the genes and transcripts encoding them. (A) Drosophila Myo31DF-PA, Myo61F-PB, and Myo95E-PB structures, all of which possess characteristic domains/motifs/sites of class I myosins, including ATP- and actin-binding sites, IQ motifs, and TH1 domains, represented by the colors at the bottom. The insertion in Myo95E is shown in light purple. (B and C) Diagrams of the genomic regions of Myo61F (B) and Myo95E (C) loci. Exons of Myo61F and Myo95E are represented by black boxes. Neighboring genes are shown by light brown boxes. Deleted regions of Myo61F1, Myo95E1, and Myo95E2, generated by the imprecise excision of EP3325b or EY22671, are indicated by parentheses, in which the length (in base pairs) of the deletion is indicated. Coding and noncoding regions in the four and six predicted transcripts of Myo61F and Myo95E are represented by blue and gray boxes, respectively.
In single-celled eukaryotes that have multiple myosin I genes, redundant roles of these genes have been reported (Novak et al. 1995; Geli and Riezman 1996; Goodson et al. 1996; Jung et al. 1996). Saccharomyces cerevisiae encodes two class I myosins that function redundantly in growth and endocytosis (Geli and Riezman 1996; Goodson et al. 1996), and Dictyostelium encodes multiple class I myosins I with overlapping functions in macropinocytosis (Novak et al. 1995; Jung et al. 1996). Eight class I myosins are expressed in humans and mice (Berg et al. 2001). Myosin IA is thought to maintain brush border structure and membrane tension and to power the release of vesicles from the tips of microvilli (Tyska et al. 2005; McConnell et al. 2009; Nambiar et al. 2009), while Myosin IB regulates the actin-dependent post-Golgi trafficking of cargo (Almeida et al. 2011). Myosin IC is involved in vesicle transport both in the fertilized egg of Xenopus and in mammalian cells (Bose et al. 2002; Sokac et al. 2006; Fan et al. 2012) and regulates ion channels in the hair cells of the inner ear (Gillespie and Cyr 2004). Interestingly, an isoform of Myosin IC localizes to the nucleus and contributes to transcription (Pestic-Dragovich et al. 2000; Philimonenko et al. 2004), and Myosin IF is involved in neutrophil migration (Kim et al. 2006). In addition, mutations in Myosin IA, Myosin IC, and Myosin IF are associated with hereditary hearing loss (Chen et al. 2001; Donaudy et al. 2003; Zadro et al. 2009). In vertebrates, each class I myosin is expressed in various cell types and has distinct functions that depend on their cellular context (Gillespie 2004; Philimonenko et al. 2004; Sokac et al. 2006). Even so, these multiple class I myosins are predicted to have overlapping functions, as found in yeast and Dictyostelium (Tyska et al. 2005; Nambiar et al. 2009; Chen et al. 2012), complicating the understanding of their in vivo roles (Kim and Flavell 2008). Thus, the knockout and analysis of multiple class I myosin genes in vertebrates would represent a major challenge.
Three class I myosin genes, Myo31DF, Myo61F, and Myo95E have been identified in Drosophila (Figure 1A) (Tzolovsky et al. 2002). Myo31DF and Myo61F are closely related to the mammalian Myosin ID and Myosin IC, respectively (Morgan et al. 1994; Berg et al. 2001). However, the head domain of Myo95E contains an atypical insertion (Figure 1A) (Tzolovsky et al. 2002) (FlyBase, http://flybase.org/reports/FBgn0039157.html). All of these class I myosins possess short tails with characteristic motifs/sites such as the actin- and ATP-binding sites and the IQ motifs (Figure 1A) (Tzolovsky et al. 2002). Myo31DF is involved in the development of left–right (LR) asymmetry (Hozumi et al. 2006; Speder et al. 2006), and its loss leads to the LR inversion of several organs, including the embryonic gut, male genital plate, spermiduct, and testes (Hozumi et al. 2006; Speder et al. 2006). Myo61F is required for maintenance of the enterocyte brush border structure, as determined genetically (Hegan et al. 2007), while its roles in LR asymmetric development are mostly based on overexpression and RNA interference experiments (Hozumi et al. 2006, 2008; Petzoldt et al. 2012). Myo61F overexpression antagonizes Myo31DF’s function, leading to LR inversion of the embryonic gut and the male genitalia (Hozumi et al. 2008; Petzoldt et al. 2012), and a model was proposed suggesting that overexpressed Myo61F prevents the binding of Myo31DF to adherens junction components, leading to impaired LR rotation of the male genitalia (Petzoldt et al. 2012). In contrast, no studies of Myo95E have been reported.
The limited number of class I myosin genes in Drosophila is an advantage for elucidating their functions and interactions in vivo. Here, we studied the roles of the three Drosophila class I myosins, using novel mutant alleles of Myo61F and Myo95E, as well as the previously isolated null mutant allele of Myo31DF.
Materials and Methods
Fly stocks
Flies were cultured on standard medium at 25°. Canton-S was the wild-type strain. The stocks carrying P{EP}Myo61FEP3325a, P{EPgy2}Myo95EEY22671, patched (ptc)16, Df(3L)BSC250 (uncovering the Myo61F locus), Df(3R)Exel6198 (uncovering the Myo95E locus), daughterless (da)-GAL4, Actin5C (Act5C)-GAL4, H{PDelta2-3}HoP8, or P{Delta2-3}99B (stock nos. 17114, 22577, 35500, 23150, 7677, 5460, 4414, 2080, and 1610, respectively) were obtained from the Bloomington Stock Center. white (w)1118, roughoid (ru)1 hairy (h)1 scarlet (st)1 rosy (ry)506 ebony (e)1, and sepia (se)1 spineless (ss)1 kidney (k)1 es rough (ro)1 (stock nos. 150534, 105729, and 105998) were obtained from the Drosophila Genetic Resource Center. The other stocks used in this study were described previously: Myo31DFL152 (Hozumi et al. 2006), Myo31DFK2 (Speder et al. 2006), UAS-Myo31DF-GFP (Speder et al. 2006), UAS-Myo31DF-mEGFP (Taniguchi et al. 2011), UAS-Myo61F (Hozumi et al. 2006), byn-GAL4 (Iwaki and Lengyel 2002), 24B-GAL4 (how24B) (Fyrberg et al. 1997), Abdominal-B (AbdB)-GAL4 (Foronda et al. 2006), hedgehog (hh)-GAL4 (Suzanne et al. 2010), and patched (ptc)-GAL4 (Johnson et al. 1995). Df(3R)Exel6198 was balanced using TM3, P{w[+mC]=sChFP}3 (Bloomington Stock Center). For the germline transformation of UAS-Myo95E-RF, y1 M{vas-int.Dm}ZH-2A w*; M{3xP3-RFP.attP’}ZH-51C (Bloomington stock no. 24482) (Bischof et al. 2007) was used.
Constructs and germline transformation
UAS-Myo95E-RB and UAS-Myo95E-RD produce slightly different alternative splicing products of the Myo95E locus, which are both long isoforms of Myo95E, and UAS-Myo95E-RF produces a short form of Myo95E (Figure 1C) (FlyBase, http://flybase.org/reports/FBgn0039157.html). The entire coding sequence of Myo95E is covered by two complementary DNA (cDNA) fragments, GH25580 and RE40416 (Rubin et al. 2000; Stapleton et al. 2002). RE40416 carries a Myo95E cDNA fragment encompassing its 5′ region, which contains extra insertions. To construct pUAST-Myo95E-RB, we first removed a 17-bp insertion in RE40416. Two fragments were amplified by PCR, using a T7 and T3 primer in conjunction with the gene-specific primers 5′-GGGAGATCCAATGGGAACCCGTATAACGGACCCTATC-3′ and 5′-GATAGGGTCCGTTATACGGGTTCCCATTGGATCTCCC-3′, respectively, with RE40416 as a template. Using these two PCR products as templates, the fragment from which the extra insertion was removed was amplified by PCR with T7 and T3 primers. This fragment (the modified RE40416) was digested by EcoRI and BamHI and subcloned into the pGEM7 vector. The modified RE40416 was digested with EcoRI and SacI, and GH22580 was digested with XhoI and SacI. These fragments were then subcloned into the EcoRI and XhoI sites of pBluescript SK(−) to generate the full-length Myo95E-RB fragment. Myo95E-RD was a variant of Myo95E-RB containing a 60-bp deletion. To obtain the 5′ fragment of the Myo95E-RD cDNA, two fragments were first amplified by PCR with the T7 and pM001 primers (BDGP, http://www.fruitfly.org/about/methods/pOT2vector.html) in conjunction with the gene-specific primers, 5′-TCCACAAATGCCTACGACCAGTGAAGGGAATGCCAAATCGGCATTCCCGCCACGTGCGCCAGGCACTCG-3′ and 5′-TTCCCTTCACTGGTCGTAGGCATTTGTGGAGTTTATATCGTGTCGCCCGCGAGGAGTATCGGTCC-3′, using the cDNA of GH25580 as a template. Using these two PCR products as templates, PCR was again performed with the T7 and pM001 primers. The resulting PCR fragment (the modified GH25580) was subcloned into pBluescript SK(−), as described above. The modified RE40416 and GH25580 fragments were subcloned into the EcoRI and XhoI sites of pBluescript SK(−) to generate the full-length Myo95E-RD fragments, as described above. After sequence confirmation, the Myo95E-RB and Myo95E-RD fragments were subcloned into the EcoRI and XhoI sites of the pUAST vector (Brand and Perrimon 1993).
To construct pUAST-Myo95E-RF, RE40416 (obtained from the Drosophila Genomics Resource Center), which includes the cDNA for the entire coding sequence for Myo95E-RF, was digested by EcoRI and BamHI. The full-length Myo95E-RF cDNA was cloned into the EcoRI and BamHI sites of BluescriptII SK(−). This construct was digested by EcoRI and NotI, and the full-length Myo95E-RF cDNA was cloned into the EcoRI and NotI sites of the pUASTattB vector (Bischof et al. 2007).
UAS-Myo95E-RB-FLAG encodes a full-length Myo95E-PB protein with a FLAG (DYKDDDDK) tag at the C terminus (Hopp et al. 1988). For this construct, a Myo95E-RB cDNA fragment was amplified by PCR with two primers, 5′-CACCGAATTCATGGAGCAGGAAATCGGCA-3′ and 5′-CACCCTCGAGTCACTTATCGTCATCGTCCTTGTAATCCACAATTATCTCCATGCGGTTCG-3′, containing the FLAG coding sequence, using the Myo95E-RD cDNA fragment as a template. The resulting PCR fragment was subcloned into the EcoRI and XhoI sites of pUAST.
UAS-Myo61F-mRFP encodes a full-length Myo61F protein tagged with a monomeric RFP (mRFP) at the C terminus (Campbell et al. 2002). For this construct, a full-length Myo61F cDNA was amplified by PCR, using a full-length Myo61F cDNA as a template (Hozumi et al. 2006) with the primers 5′-GGGGTACCTTGCGTTCCAATGATAACTAGATGG-3′ and 5′-CCGGAATTCCTGACACATCCTCCAGAGAA-3′. An mRFP cDNA fragment was also obtained by PCR, using pRSET-mRFP1 (gift of A. Miyawaki, RIKEN, Wako, Saitama, Japan) as a template with the primers 5′-GGGGTACCATGGCCTCCTCCGAGGACGTCATC-3′ and 5′-TTCGAATTCTTAGGCGCCGG-3′. The two fragments were digested with EcoRI and KpnI and subcloned into the EcoRI site of pBluescript SK(−). After sequence confirmation, these fragments were subcloned into the EcoRI and NotI sites of pUAST.
To generate the pChs-Act5C-Gal4 construct, a fragment of the Actin 5C proximal promoter was amplified from the Canton-S genome by PCR, using the primers 5′-AGAATTCACGCCCTAAAACACCAGATCATCC-3′ and 5′-TGGTACCGCACGGTTTGAAAGGAATGACTGG-3′. The PCR product was subcloned into the EcoRI and KpnI sites of the pChs-Gal4 vector (provided by the Drosophila Genomics Resource Center).
To construct pUAST-mGFP and pUAST-mRFP, the cDNAs for mGFP and mRFP were amplified by PCR from pUAST-Myo31DF-mGFP and pUAST-Myo61F-mRFP, respectively. Primers 5′-GGAATTCAACCAAACATGGTGAGCAAGGGCGAGGAG-3′ and 5′-GGACTAGTTTACTTGTACAGCTCGTCCATGCC-3′ were used to amplify mGFP, and 5′-GGAATTCAACCAAACATGGCCTCCTCCGAGGACGTCATC-3′ and 5′-GGACTAGTTTAGGCGCCGGTGGAGTGGCGG-3′ were used to amplify mRFP. The resulting fragments were subcloned into the EcoRI and SpeI sites of pBluescript SK(−). After sequence confirmation, these fragments were subcloned into the EcoRI and NotI sites of the pUASTattB vector (Bischof et al. 2007).
These constructs were used for germline transformation using standard protocols (Spradling 1986; Bischof et al. 2007) and/or for transfection into Drosophila Schneider 2 (S2) cells as described below (Cherbas and Cherbas 2000).
Generation of novel mutant alleles of Myo61F and Myo95E
To generate a novel mutant allele of Myo61F, we used imprecise excision of the P element inserted in the w1118;; P{EP}Myo61FEP3325a P{EP}Myo61FEP3325b line. First, we removed P{EP}3325b inserted at the cytological position of 86E18 by recombination. w1118;; P{EP}Myo61FEP3325a P{EP}Myo61FEP3325b virgin females were mated with w1118;; ru1 h1 st1 ry506 e1 males. The F1 females (w1118;; P{EP}Myo61FEP3325a P{EP}Myo61FEP3325b/ ru1 h1 st1 ry506 e1) were mated with w1118;; ru1 h1 st1 ry506 e1 males again, and the F2 males (w1118;; P{EP}Myo61FEP3325a ry506 e1/ ru1 h1 st1 ry506 e1) were balanced with TM3, P{ry+t7.2 = ftz-lacZ.ry+}TM3, Sb1 ry* to generate stocks. The presence of P{EP}Myo61FEP3325a and absence of P{EP}Myo61FEP3325b were checked by PCR with the appropriate primers. P{EP}Myo61FEP3325a was inserted 2460 bp upstream of the presumptive initiation codon of Myo61F-PA and 750 bp upstream of the first exon in CG9184. The CG9184 locus is present in the second intron of Myo61F. H{PDelta2-3}HoP8 y1 w* virgin females, providing P transposase, were mated with w1118;; P{EP}Myo61FEP3325a ry506 e1 males. The F1 males (H{PDelta2-3}HoP8 y1 w*/ Y;; P{EP}Myo61FEP3325a ry506 e1/ +) were mated with w1118;; Dr1/TM3, P{ry+t7.2 = ftz-lacZ.ry+}TM3, Sb1 ry* virgin females. The F2 males (w1118/ Y;; P{EP}Myo61FEP3325a ry506 e1/TM3, P{ry+t7.2 = ftz-lacZ.ry+}TM3, Sb1 ry*) were individually balanced to generate stocks. For each stock, genomic DNA was extracted from single flies, and potential deletions in the Myo61F locus were detected by PCR with the primers 5′-GATCGATCAAGCACCGTT-3′ and 5′-CGAAGTACTCCACAGGTATC-3′. We isolated a stock in which a part of the Myo61F locus was deleted and used it as a Myo61F null allele (Myo61F1) (Figure 1B). Myo61F1 has a 3454-bp deletion, which removes the putative initiation codon and the region encoding the ATP-binding site in all the Myo61F isoforms (Myo61F-PA, -PB, -PC, and -PD; Figure 1B). The deletion uncovers from nucleotide 1,323,560 to 1,327,013, by the numbering system used for the Drosophila genome in FlyBase (Drosophila melanogaster chromosome 3L, GenBank no. AE014296.5) (Adams et al. 2000). The Myo61F1 ry506 e1 chromosome was cleaned up by recombination with ru1 h1 st1 ry506 e1 and w1118 flies, and the markers were subsequently removed to establish the Myo61F1 stock used in all the experiments.
Myo95E mutant alleles were also induced by imprecise excision of a P element. P{EPgy2}Myo95EEY22671 carries a P element inserted 450 bp upstream of the putative initiation codon shared by all the alternative splicing products of Myo95E (Myo95E-RB, -RD, -RE, -RF, -RG, and -RJ). w*; Dr1/TMS, P{Delta2-3}99B virgin females, providing P transposase, were mated with P{EPgy2}Myo95EEY22671/TM3, Sb1 Ser1 males. The F1 males (w*/Y;; P{EPgy2}Myo95EEY22671/TMS, P{Delta2-3}99B) were mated with w*; TM3, P{ry+t7.2 = ftz-lacZ.ry+}TM3, Sb1 ry*/TM6B, P{iab-2(1.7)lacZ}6B, Tb1 virgin females. The F2 males (w1118/Y;; P{EPgy2}Myo95EEY22671/TM3, P{ry+t7.2 = ftz-lacZ.ry+}TM3, Sb1 ry* or w1118/Y;; P{EPgy2}Myo95EEY22671/TM6B, P{iab-2(1.7)lacZ}6B, Tb1) were individually balanced to establish stocks. For each stock, genomic DNA was extracted from single flies, and deletions in the Myo95E locus were detected by PCR with the primers 5′-TAGGTTTCCACGTTGTCGTC-3′ and 5′-CTGCGGGAAATGCTTAAGAAG-3′. Two lines in which part of the Myo95E locus was removed were obtained and used as null alleles of Myo95E (Myo95E1 and Myo95E2) (Figure 1C). Myo95E1 and Myo95E2 contain 2893- and 2118-bp deletions in the Myo95E locus, respectively, which remove the putative initiation codon and the region encoding the ATP-binding site in all the Myo95E isoforms (Myo95E-PB, -PD, -PE, -PF, -PG, and -PJ) (Figure 1C). The deletions in Myo95E1 and Myo95E2 uncover the nucleotides from 24,161,480 to 24,158,588 and from 24,161,480 to 24,159,363, respectively, by the numbering system used for the Drosophila genome in FlyBase (D. melanogaster chromosome 3R, GenBank no. AE014297.3) (Adams et al. 2000). The chromosome carrying Myo95E1 was cleaned up by recombination with w1118 and se1 ss1 k1 es ro1.
A chromosome carrying both Myo61F1 and Myo95E1 was generated by recombination. The presence of deletions at these loci was confirmed by PCR as described above.
Whole-mount in situ hybridization
Canton-S embryos were dechorionated with 50% bleach, fixed with 4% paraformaldehyde, and devitellinized by 100% methanol. They were rehydrated and washed with phosphate-buffered saline containing 0.1% Tween 20 (PBStw) and then incubated in PBStw containing 10 μg/ml proteinase K for 3 min at 25°, washed with PBStw, and fixed with 4% paraformaldehyde for 20 min at 25°. The embryos were washed again with PBStw and then hybridized with digoxygenin-labeled RNA probes diluted with hybridization buffer (50% formamide, 5xSSC, 1 mg/ml tRNA, 50 μg/ml heparin, 0.1% tween) at 60° overnight. They were then washed with PBStw and incubated in 0.1% blocking reagent (Roche) for 1 hr at 25°. Anti-digoxygenin antibody labeled with alkaline-phosphatase (Roche, 1:7000) was added, and then the embryos were incubated for 1 hr at 25° and washed with PBStw. The alkaline-phosphatase activity was detected by Nitro blue tetrazolium chloride/5-Bromo-4-chloro-3-indolyl phosphate (NBT/BCIP) (Roche). The embryos were then mounted in 90% glycerol and analyzed with an Axioskop2 plus (Zeiss, Thornwood, NY). Myo31DF, Myo61F, and Myo95E cDNAs were used as templates for RNA probes prepared using a DIG RNA-labeling kit (Roche).
Real-time PCR
Total RNA was isolated from 10 pupae (23–25 hr after pupation) of wild-type or the Myo61F1 homozygote, using Isogen (Nippon Gene, Tokyo), and 1 μg of each RNA sample was used for cDNA synthesis with the PrimeScript RT reagent Kit with genomeDNA Eraser (Takara), according to the product manual. The amount of CG9134 transcript was normalized to that of a housekeeping gene, Glyceraldehyde 3 phosphate dehydrogenase 1 (Gapdh1) (Miyashita et al. 2012). The primers used to amplify CG9134 were 5′-GGAGAGTGACTGCGATTGGTTTG-3′ and 5′-TGTTGCTGCTTTTCGGTCCTG-3′. The primers used to amplify Gapdh1 were 5′-TTCAGCGACACCCATTCGTC-3′ and 5′-TACCACGAGATTAGCTTGACGAAC-3′. Quantitative PCR was performed using SYBR Premix Ex Taq II (Takara) and the Applied Biosystems (Foster City, CA) 7300 Real Time PCR System according to the product manuals.
Immunohistochemistry
Antibody staining of the embryo, larval midgut, and S2 cells was performed as described previously (Ashburner et al. 1989). The primary antibodies used were mouse anti-αSpectrin [Developmental Studies Hybridoma Bank (DSHB), 1:25], mouse anti-Elav (DSHB, 1:100), mouse anti-Fas2 (DSHB, 1:10), rat anti-GFP (Nacalai Tesque, 1:500), rabbit anti-RFP (Medical & Biological Laboratories, Nagoya, Japan, 1:500), and mouse anti-FLAG M2 [Sigma (St. Louis), 1:1000]. The secondary antibodies used were Cy3-conjugated anti-mouse IgG (Jackson ImmunoResearch, 1:500), Alexa488-conjugated anti-mouse IgG [Molecular Probes (Eugene, OR), 1:500], Cy3-conjugated rabbit IgG (Jackson ImmunoResearch, 1:500), and Alexa488-conjugated anti-rat IgG (Molecular Probes, 1:500). We used rhodamine-, Alexa488-, and Alexa633-phalloidin to stain F-actin (Molecular Probes, 1: 35).
Stained embryos and larval gut tissues were mounted in 90% glycerol and analyzed with an Axioskop2 plus (Zeiss), LSM 5 PASCAL (Zeiss), or ECLIPSE Ti (Nikon, Garden City, NY). The images were processed with a Ziess LSM Image Browser, a Nikon EZ-C1 viewer, Adobe Photoshop CS4, and Adobe Illustrator CS6.
Analyses of the LR asymmetry of the embryonic gut and male genital plate
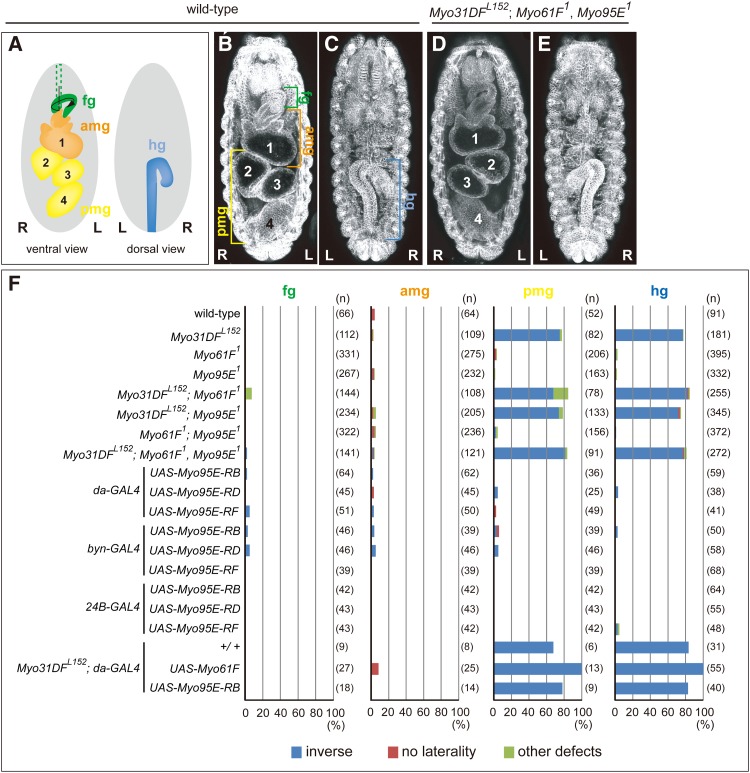
Following embryo fixation with 4% paraformaldehyde, the handedness of each section of the embryonic gut, the foregut (fg), the anterior midgut (amg), the posterior midgut (pmg), and the hindgut (hg) (see Figure 3A) was scored at stages 14–16, 16, 16, and 13–16, respectively, with an Axioskop2 plus. The genotypes of the embryos were determined with blue-balancers CyO, P{en1}wgen11 and TM6B, P{iab-2(1.7)lacZ}6B, Tb1, after β-galactosidase staining according to a standard protocol (O’Kane 1998). The following embryos were analyzed: (1) Canton-S, (2) Myo31DFL152/Myo31DFL152, (3) Myo61F1/Myo61F1, (4) Myo95E1/Myo95E1, (5) Myo31DFL152/Myo31DFL152; Myo61F1/Myo61F1, (6) Myo31DFL152/Myo31DFL152; Myo95E1/Myo95E1, (7) Myo61F1 Myo95E1/Myo61F1 Myo95E1, (8) Myo31DFL152/Myo31DFL152; Myo61F1 Myo95E1/Myo61F1 Myo95E1, (9) UAS-Myo95E-RB/da-GAL4, (10) UAS-Myo95E-RD/+; da-GAL4/+, (11) UAS-Myo95E-RF/+; da-GAL4/+, (12) UAS-Myo95E-RB/byn-GAL4, (13) UAS-Myo95E-RD/+; byn-GAL4/+, (14) UAS-Myo95E-RF/+; byn-GAL4/+, (15) UAS-Myo95E-RB/24B-GAL4, (16) UAS-Myo95E-RD/+; 24B-GAL4/+, (17) UAS-Myo95E-RF/+; 24B-GAL4/+, (18) Myo31DFL152/Myo31DFL152; da-GAL4/+, (19) Myo31DFL152, UAS-Myo61F/Myo31DFL152; da-GAL4/+, and (20) Myo31DFL152, UAS-Myo95E-RB/Myo31DFL152; da-GAL4/+.
Figure 3.
LR phenotypes of the embryonic gut associated with mutations and overexpression of Myo31DF, Myo61F, and Myo95E. (A) Diagram showing the LR asymmetric morphology of the embryonic gut viewed from the dorsal (right) and ventral (left) sides at late stage 16. The gut was divided into four parts, the foregut (fg), anterior midgut (amg), posterior midgut (pmg), and hindgut (hg). (B–E) Dorsal (C and E) and ventral (B and D) views of the wild-type (B and C) and Myo31DFL152; Myo61F1 Myo95E1 homozygous (D and E) embryos at late stage 16, stained with an anti-α-Spectrin antibody. Anterior is up. Numbers indicate each chamber of the midgut. R, right; L, left. (F) Bar graphs showing the frequency of LR defects in each part of the embryonic gut in embryos with various genotypes, indicated at left. Blue, red, and green bars indicate LR inversion, no laterality, and other morphological defects, respectively. The number of embryos examined is indicated in parentheses at right.
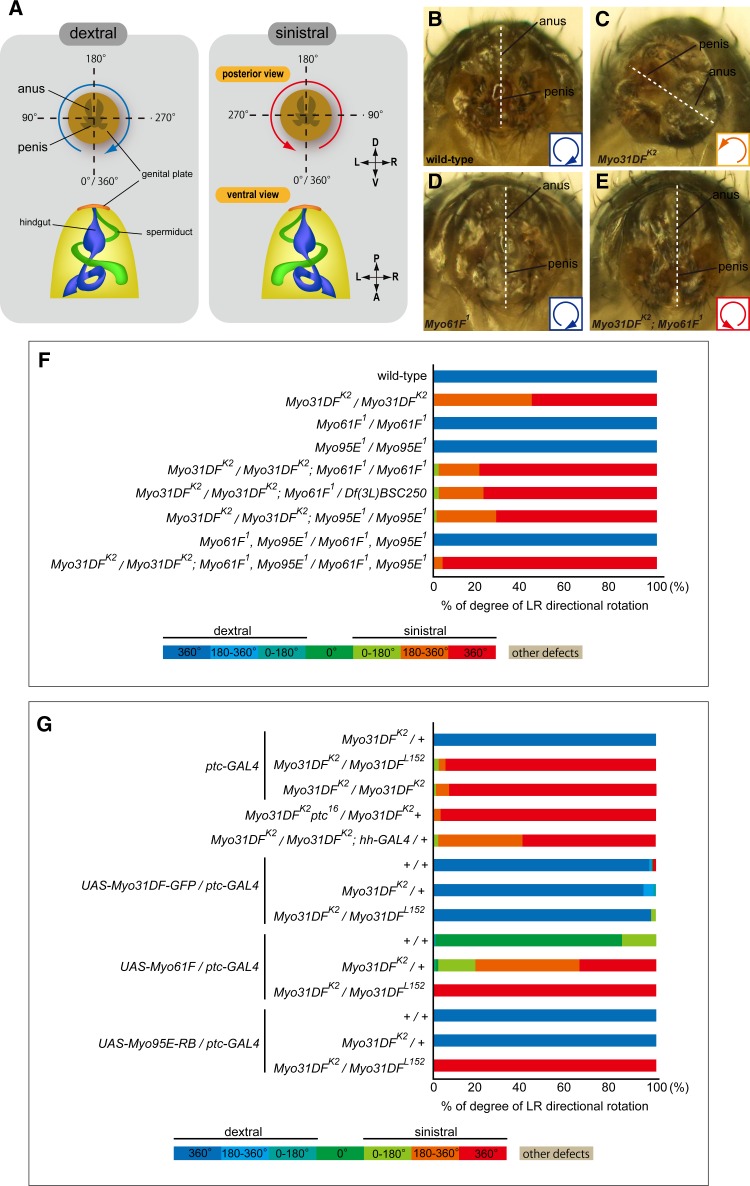
The direction of the male genitalia rotation was determined from the looping direction of the spermiduct, which attaches to the male genital plate and coils around the hindgut (Speder et al. 2006). The wild-type rotational direction was designated as dextral and the reversed direction as sinistral. The rotational extent (0°–360°) was determined by positions of the penis and anus. Flies with each of the genotypes indicated below were obtained from three separate vials, and the genitalia of 30 flies from each vial were scored. The average frequency (percentage) of individuals exhibiting genitalia rotation angles every 180° was calculated. The male genital plate images were captured with a VHX-100 (Keyence). Male flies with the following genotypes were analyzed: (1) Canton-S, (2) Myo31DFK2/Myo31DFK2, (3) Myo61F1/Myo61F1, (4) Myo95E1/Myo95E1, (5) Myo31DFK2/Myo31DFK2; Myo61F1/Myo61F1, (6) Myo31DFK2/Myo31DFK2; Myo61F1/Df(3L)BSC250, (7) Myo31DFK2/Myo31DFK2; Myo95E1/Myo95E1, (8) Myo61F1 Myo95E1/Myo61F1 Myo95E1, (9) Myo31DFK2/Myo31DFK2; Myo61F1 Myo95E1/Myo61F1 Myo95E1, (10) Myo31DFK2 ptc-GAL4/+, (11) Myo31DFK2 ptc-GAL4/Myo31DFL152, (12) Myo31DFK2 ptc-GAL4/Myo31DFK2, (13) Myo31DFK2 ptc16/Myo31DFK2, (14) Myo31DFK2/Myo31DFK2; hh-GAL4/+, (15) ptc-GAL4/UAS-Myo31DF-GFP, (16) Myo31DFK2 ptc-GAL4/UAS-Myo31DF-GFP, (17) Myo31DFK2 ptc-GAL4/Myo31DFL152, UAS-Myo31DF-GFP, (18) ptc-GAL4/UAS-Myo61F, (19) Myo31DFK2 ptc-GAL4/UAS-Myo61F, (20) Myo31DFK2 ptc-GAL4/Myo31DFL152, UAS-Myo61F, (21) ptc-GAL4/UAS-Myo95E-RB, (22) Myo31DFK2 ptc-GAL4/UAS-Myo95E-RB, and (23) Myo31DFK2, ptc-GAL4/Myo31DFL152, UAS-Myo95E-RB.
Hatching and survival rate analysis
The hatching and survival rates of wild-type and class I myosin single, double, or triple mutants were measured as follows. For hatching rates, 50 embryos were collected and maintained at 25° for 24 hr, and the number of hatched larvae was counted. To obtain Myo95E1/Df(3R)Excel6198 flies, Myo95E1 homozygous females were mated with Df(3R)Excel6198/TM3, P{w[+mC]=sChFP}3 males. In other experiments, to remove the maternal contribution, the progenies of females homozygous for the single, double, and triple mutants of Myo31DF, Myo61F, or/and Myo95E1 were obtained.
For survival rates from the first-instar larvae to adult, 50 first-instar larvae were cultured in vials with the standard medium at 25°, and the number of eclosed flies was counted 14 days later. For adult survival rates, 20 adult flies of each genotype were collected 0–8 hr after eclosion. Males and females were distinguished among these flies, and they were transferred separately into standard medium. Surviving flies were counted when the flies were transferred into new culture medium every 3 days. During this experiment, flies were kept at 25°. For both the hatching and the survival analyses, five experiments were performed independently, and the results were statistically analyzed. Embryos and larvae with the following genotypes were analyzed: (1) Canton-S, (2) Myo31DFL152/Myo31DFL152, (3) Myo61F1/Myo61F1, (4) Myo95E1/Myo95E1, (5) Myo31DFL152/Myo31DFL152; Myo61F1/Myo61F1, (6) Myo31DFL152/Myo31DFL152; Myo95E1/Myo95E1, (7) Myo61F1 Myo95E1/Myo61F1 Myo95E1, and (8) Myo31DFL152/Myo31DFL152; Myo61F1 Myo95E1/Myo61F1 Myo95E1.
Subcellular localization analysis using S2 cells
The Drosophila S2 cells were cultured in M3 insect medium (Sigma) with 10% fetal bovine serum (Biowest). S2 cells were cotransfected with pChs-Act5C-Gal4 and pUAST-Myo31DF-mEGFP, pUAST-Myo61F-mRFP, pUAST-Myo95E-RB-FLAG, pUAST-mEGFP, or pUAST-mRFP or combinations of any two, using Cellfectin (Invitrogen, Carlsbad, CA). One to 2 days after transfection, the cells were plated on glass coverslips coated with concanavalin A (Sigma), fixed with 4% paraformaldehyde, stained with antibodies and fluorescently labeled phalloidin as described above, mounted in 90% glycerol, and analyzed with an Axioskop2 plus and LSM 5 PASCAL. The captured images were processed with a Ziess LSM Image Browser and Adobe Photoshop CS4.
Results
Generation of novel mutant alleles of Myo61F and Myo95E
In this study, we generated novel mutant alleles of Myo61F and Myo95E, using a method involving imprecise excision of P elements (Grigliatti 1998). To induce deletions, we mobilized the P-elements EP3325b and EY22671 inserted in the vicinity of the Myo61F and Myo95E loci, respectively (Figure 1, B and C). We isolated one mutant allele of Myo61F, Myo61F1, and two mutant alleles of Myo95E, Myo95E1 and Myo95E2 (Figure 1, B and C).
The Myo61F gene produces four alternative transcripts, Myo61F-RA, -RB, -RC, and -RD (Figure 1B) (FlyBase, http://flybase.org/reports/FBgn0010246.html). These four alternative splicing products encode Myo61F isoforms that have all of the characteristic structures of class I myosins, such as the ATP- and actin-binding sites, IQ motifs, and TH1 domains (Coluccio 1997; Barylko et al. 2000). Our sequencing analysis of the Myo61F1 locus revealed that it lacked the genomic region encompassing the putative initiation codons for all four alternative splicing products as well as exons encoding portions of the motor domains, including the ATP-binding site (Figure 1B). Since the ATP-binding site is necessary for the motor activity of myosin family proteins (Molloy et al. 1995; Fan et al. 2012), this mutant was predicted to function as null alleles. In addition, our real-time PCR analysis revealed that the transcription efficiency of the CG9184 gene, which is located in the second intron of Myo61F, was not affected in the Myo61F1 homozygote (Supporting Information, Figure S1). We examined the amount of CG9184 messenger RNA (mRNA) at the pupal stage, because our analysis described below revealed that Myo61F functions in the rotation of the male genitalia, which occurs at this stage. We found that the mRNA level of CG9184 was not significantly different between wild-type (1.0 ± 0.22) and the Myo61F1 homozygote (1.37 ± 0.31) (Figure S1), indicating that the CG9184 expression was intact in this mutant.
The Myo95E gene produces six alternative transcripts, Myo95E-RB, -RD, -RE, -RF, -RG, and -RJ (Figure 1C) (FlyBase, http://flybase.org/reports/FBgn0039157.html). Among them, Myo95E-RB, -RD, -RE, and -RJ encode long isoforms and Myo95E-RF and -RG encode short forms of Myo95E. The head region of all six Myo95E isoforms has an atypical insertion (Figure 1A) (Tzolovsky et al. 2002). Genome sequencing analyses showed that the Myo95E1 and Myo95E2 loci lacked the genomic region containing the putative initiation codon for all six alternative splicing products from the Myo95E locus (Figure 1C). These six alternative splicing products also lost exons encoding portions of the motor domain, including part of the ATP-binding site (Figure 1C). These findings indicate that Myo95E1 and Myo95E2 are null alleles of Myo95E. We used the Myo95E1 allele in subsequent studies, because it had a larger deletion than Myo95E2 (Figure 1C).
Myo31DF or Myo61F homozygous mutant flies were previously reported to be viable and fertile (Hozumi et al. 2006; Speder et al. 2006; Hegan et al. 2007). Our finding that the Myo61F1 or Myo95E1 homozygotes were also viable and fertile (data not shown) combined with the earlier data suggests that all class I myosins are dispensable for survival in Drosophila, if their functions are disrupted separately. In this study, we further measured the hatching and survival rates of the single, double, and triple mutants. To remove the effect of maternal contributions of these genes, only the progenies of females homozygous for the single, double, and triple mutants were scored. The hatching rates and survival rates from first-instar larvae to adult (L1 to adult) of Myo31DFL152 (null mutant of Myo31DF) (Hozumi et al. 2006), Myo61F1, or Myo95E1 single mutants were similar to those of wild type (Figure S2, A and B). We further characterized the Myo95E1 allele as the first reported mutant of Myo95E. The hatching rate of embryos that were trans-heterozygotes for a deletion mutant uncovering the Myo95E locus [Df(3R)Exel6198] and the Myo95E1 mutant (obtained from Myo95E1 homozygous females) was similar to that of the Myo95E1 homozygote obtained from Myo95E1 homozygous females (not significant by t-test, Figure S3) (Parks et al. 2004). This result supported the idea that Myo95E1 is a null allele of Myo95E. We then analyzed in detail the structures of the embryonic and adult organs of the Myo95E1 homozygotes obtained from Myo95E1 homozygous females, but observed no abnormalities (data not shown).
Furthermore, the double and triple mutants of Myo31DFL152, Myo61F1, and/or Myo95E1 were viable and fertile (data not shown), and their hatching rates and survival rates of L1 to adult were largely comparable to those of wild-type (Figure S2, A and B). However, certain mutant combinations showed some reduction (up to 50%) in their hatching and/or survival rate (Figure S2, A and B). In addition, the survival rates of adults were decreased in the single, double, and triple mutants (Figure S2C). These results demonstrated that class I myosins are not essential for survival itself, but are required for optimal hatching and survival rates, and thus are important mediators in Drosophila, at least under our experimental conditions.
Differential expression of Myo31DF, Myo61F, and Myo95E during embryogenesis
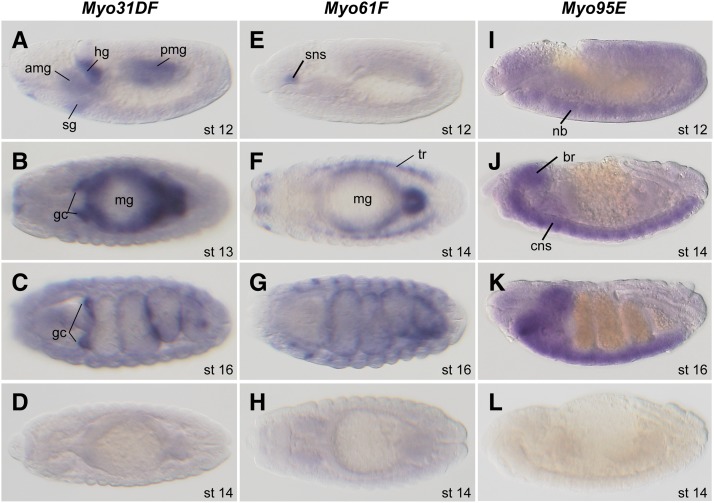
Myo31DF and Myo61F mRNAs and their protein products are detected in the gut epithelium and genital disc (Morgan et al. 1995; Hozumi et al. 2006; Speder et al. 2006; Petzoldt et al. 2012). To gain insight into the tissue-specific roles and functional redundancy of the three class I myosins in the LR asymmetric development of the embryonic gut, we analyzed their expression in embryos, using in situ hybridization. The Drosophila embryonic gut was divided into four sections, the fg, amg, pmg, and hg, as depicted clearly at stage 16 (see Figure 3A). Myo31DF mRNA was detected in the amg, pmg, hg, and salivary gland (sg) primordia (Figure 2, A and B) at stages 12–16 and in the dorsal ectoderm at stage 12 (data not shown). Myo31DF expression was also detected in the visceral mesoderm starting at stage 13 (Figure 2, B and C) and showed a strong signal in the presumptive region of the gastric caeca (gc) and the three constriction sites of the midgut (Figure 2, B and C). Thus, Myo31DF expression underwent dynamic changes during embryogenesis. The Myo31DF signal was not detected by the sense probe (Figure 2D), indicating that it was specific.
Figure 2.
Differential expression of Myo31DF, Myo61F, and Myo95E transcripts during embryogenesis. (A–L) Whole-mount in situ hybridization of embryos stained with antisense (A–C) and sense (D) probes for Myo31DF mRNA, antisense (E–G) and sense (H) probes for Myo61F mRNA, and antisense (I–K) and sense (L) probes for Myo95E mRNA. Lateral (A, E, and I–L), dorsal (B, D, F, and H), and ventral (C and G) views of embryos are oriented with anterior to the left. amg, anterior midgut; br, brain; cns, central nervous system; gc, gastric caeca; hg, hindgut; mg, midgut; nb, neuroblast; pmg, posterior midgut; sg, salivary gland; sns, stomatogastric nervous system; tr, trachea. The embryonic stages are indicated at the bottom right.
Myo61F expression was first detected at stage 12 in the amg, pmg, and stomatogastric nervous system (sns) (Figure 2E) and then in the trachea from stage 14 onward (Figure 2F). Myo61F expression continued until stage 16 (Figure 2G) and was not detected with the sense probe (Figure 2H).
Myo95E mRNA was specifically detected in the neuroblast (nb) at stage 12 (Figure 2I) and continued to be expressed in the central nervous system (CNS) throughout embryogenesis (Figure 2, J and K). This signal was also undetectable with the sense probe (Figure 2L).
In summary, except for the expression of Myo31DF and Myo61F in the amg and pmg, which form the prospective midgut epithelium, the three myosin I genes were expressed in different tissues during embryogenesis. Since Myo95E was specifically expressed in the embryonic CNS, we further examined the CNS and third-instar larval brain in the Myo95E1 homozygotes obtained from Myo95E1 homozygous females, by immunostaining with anti-Elav and anti-Fas2 antibodies. We did not find any obvious morphological defects in these organs (data not shown), suggesting that Myo95E was not required for the overall formation of the CNS; however, we cannot rule out the possibility that a redundant mechanism exists.
LR asymmetric development of the embryonic gut does not involve Myo61F or Myo95E
Our results showed that both Myo31DF and Myo61F were expressed in the amg and pmg, suggesting potential functional redundancy during LR asymmetric development of the embryonic gut. Although the expression of Myo95E was predominantly detected in the CNS in embryos, it was possible that low levels of Myo95E contributed to the LR asymmetric development of the embryonic gut as well. Therefore, to determine the involvement of the class I myosins in this process, we examined the morphology of the embryonic gut in double and triple myosin I mutants.
Each section of the wild-type gut exhibits a directional LR asymmetric morphology, as shown previously and in Figure 3, A–C, which is genetically determined (Figure 3F) (Hayashi and Murakami 2001; Ligoxygakis et al. 2001). The pmg and hg exhibit an LR-reversed phenotype in ∼80% of the Myo31DFL152 homozygotes (Figure 3F) (Hozumi et al. 2006). However, the Myo61F and Myo95E single- and double-homozygous mutant embryos (Myo61F1/Myo61F1, Myo95E1/Myo95E1, and Myo61F1 Myo95E1/Myo61F1 Myo95E1) showed normal LR asymmetric development in all sections of the embryonic gut (Figure 3F). In addition, double- and triple-homozygous mutant flies carrying the Myo31DF mutant (Myo31DFL152/Myo31DFL152; Myo61F1/Myo61F1, Myo31DFL152/Myo31DFL152; Myo95E1/Myo95E1, and Myo31DFL152/Myo31DFL152; Myo61F1 Myo95E1/Myo61F1 Myo95E1) showed LR inversion in the pmg and hg with ∼80% frequency, equivalent to the frequency of LR inversion in the Myo31DFL152 homozygote (Figure 3, D–F). These results suggest that Myo61F and Myo95E do not play redundant roles with Myo31DF in LR asymmetric development in the embryonic gut and that Myo61F and Myo95E are not involved in the LR asymmetric development of this organ.
The overexpression of Myo61F in wild-type embryos results in LR inversion of the embryonic pmg and hg and the male genitalia (Hozumi et al. 2006, 2008; Petzoldt et al. 2012). We also reported that the ubiquitous overexpression of Myo31DF reverses the LR asymmetry of the fg and amg, whereas the Myo31DF loss-of-function mutant does not show LR inversion in these organs (Hozumi et al. 2006). In this study, to examine the potential activity of Myo95E in LR asymmetric development, we overexpressed UAS-Myo95E-RB, UAS-Myo95E-RD, and UAS-Myo95E-RF (Figure 1C). The first two produce long isoforms and the last one produces a short isoform of Myo95E (Figure 1C). These three transgenes were misexpressed under the control of da-GAL4 (ubiquitous expression), byn-GAL4 (gut specific), 24B-GAL4 (mesoderm), ptc-GAL4 (male genitalia), and Abd-B-GAL4 (male genitalia) (Brand and Perrimon 1993; Foronda et al. 2006; Suzanne et al. 2010). However, we did not observe LR asymmetry defects in any part of the embryonic gut or in genital disc rotation (Figure 3F, Figure 4G, and Figure S4). These results suggest that the misexpression of Myo95E, encoding the long or short isoforms, does not influence the LR asymmetric development of Drosophila.
Figure 4.
LR phenotypes of the male genitalia associated with mutations and overexpression of Myo31DF, Myo61F, and Myo95E. (A) Male genital plate of the wild-type (top left) and Myo31F homozygous mutant (top right) showing dextral (blue arrow) and sinistral (red arrow) rotations, respectively, viewed from the posterior during pupal stages. The spermiduct attached to the genital plate also showed dextral and sinistral looping around the hindgut in wild-type (bottom left) and Myo31F mutant homozygous flies (bottom right), respectively. A, anterior; D, dorsal; L, left; P, posterior; R, right; V, ventral. (B–E) Posterior views of the male genital plate of wild-type (B), Myo31DFK2 homozygote (C), Myo61F1 homozygote (D), and Myo31DFK2; Myo61F1 double-homozygote (E) flies. Dorsal is up. Insets at bottom right indicate the 360° dextral (blue), 180°–360° sinistral (orange), and 360° sinistral (red) rotations. White dotted lines indicate the midline of the genital plate. (F and G) Bar graphs showing the frequency (percentage) of dextral and sinistral rotation phenotypes observed in the male genital plate, color coded according to the categories shown at the bottom. Genotypes are indicated at left.
We also overexpressed Myo61F and Myo95E in Myo31DFL152 homozygous embryos, 70–80% of which exhibited LR inversion of the pmg and hg, as reported previously (Figure 3F). Myo95E-RB or Myo95E-RD overexpression did not affect the frequency of LR defects in the Myo31DFL152 homozygous embryos (Figure 3F). However, unexpectedly, Myo61F overexpression increased the frequency of LR defects to 100% in Myo31DFL152 homozygous embryos (Figure 3F). This result suggests that the sinistral activity of Myo61F was independent of Myo31DF. This finding prompted us to reconsider the previously proposed model suggesting that Myo61F does not have intrinsic sinistral activity, but rather acts as a negative regulator of Myo31DF (Hozumi et al. 2006; Petzoldt et al. 2012). However, despite the strong sinistral activity of overexpressed Myo61F, there was no detectable effect of the Myo61F loss-of-function mutation, even in combination with Myo31DF and/or Myo95E mutations, on the LR asymmetric development of the embryonic gut (Figure 3F).
Functional redundancy of Myo61F and Myo31DF in establishing LR asymmetry of the male genitalia
The male genital disc is another organ in which LR asymmetric development is studied extensively (Speder et al. 2006; Suzanne et al. 2010; Kuranaga et al. 2011; Petzoldt et al. 2012; Coutelis et al. 2013). The male genitalia normally undergo a dextral 360° rotation during wild-type pupal development (Figure 4, A and B) (Suzanne et al. 2010). The 360° rotation results from two stepwise 180° rotations involving two ring-shaped domains of the A8 segment (Suzanne et al. 2010; Kuranaga et al. 2011). In contrast, Myo31DF mutant flies exhibit a sinistral (LR-inversed) rotation of this organ (Figure 4, A and C) (Speder et al. 2006; Suzanne et al. 2010). Myo61F overexpression in the A8 segment also causes LR inversion of the male genital disc rotation (Petzoldt et al. 2012). This observation is consistent with our previous finding that Myo61F overexpression antagonizes dextral LR asymmetric development in the pmg and hg (Hozumi et al. 2006), although Myo31DF is not a target of Myo61F in this process, as found above.
To analyze the rotational defects of male genitalia quantitatively, we first defined the dorsal and the ventral axis from the positions of the penis (ventral side) and anus (dorsal side) (Figure 4A). In wild-type, the ventral and dorsal sides were then defined as 0°/360° and 180°, respectively (Figure 4A). The LR direction of rotation was determined from the coiling orientation of the spermiduct, observed when the male abdomen was dissected (Figure 4A) (Speder et al. 2006). In dextral (right-handed) rotation, the left and right sides of the male genitalia were defined as 90° and 270°, respectively (Figure 4A). Conversely, in the sinistral (left-handed) rotation, the left and right sides of the male genitalia were defined as 270° and 90°, respectively (Figure 4A). In the genitalia of each male, a straight line connecting the penis and anus was defined, and the rotation angle required to position the penis at 0°/360° by either dextral or sinistral rotation was determined (Figure 4A). Based on this rotation angle and its direction, adult males were classified into seven categories, which were color coded (Figure 4, F and G, and Figure S4).
As reported previously, wild-type males showed the dextral 360° rotation with 100% frequency, while Myo31DFK2 homozygotes showed either the sinistral 180°–360° or the sinistral 360° rotation (Speder et al. 2006) (Figure 4F). Here, we showed that the Myo61F1 or Myo95E1 homozygote and the corresponding double homozygote showed the dextral 360° rotation with 100% frequency, as found in the wild-type (Figure 4, D and F), indicating that these mutations alone did not disrupt the LR asymmetric rotation. However, we found that Myo31DFK2/Myo31DFK2; Myo61F1/Myo61F1 and Myo31DFK2/Myo31DFK2; Myo61F1/Df(3L)BSC250 males exhibited stronger sinistral rotation phenotypes, compared with that of the Myo31DF mutant homozygote (P < 0.01, Z-test; Figure 4F). This enhancing effect was also observed in the triple-homozygous, Myo31DFK2; Myo61F1; Myo95E1 males (P < 0.01, Z-test; Figure 4F). These results suggest that the Myo61F mutation enhanced the sinistral rotation phenotype associated with the Myo31DF mutation. In contrast, the sinistral rotation phenotype was not modified in the Myo31DFK2; Myo95E1 homozygous males (no statistical significance; Figure 4F). This functional redundancy of Myo31DF and Myo61F was tissue specific, as it was not observed in the LR asymmetric development of the embryonic pmg and hg (Figure 3F).
Next, we examined whether the overexpression of Myo31DF, Myo61F, or Myo95E affects the LR rotation of the male genitalia in the wild-type or Myo31DF mutant background. Their expression was driven by ptc-GAL4, which expresses GAL4 in the entire A8 and the anterior part of the A9 and A10 segments (Suzanne et al. 2010). As a control, ptc-GAL4 was introduced into the wild-type (+/+), Myo31DFK2/+, Myo31DFK2/Myo31DFK2, or Myo31DFK2/Myo31DFL152 backgrounds without any UAS transgene. Myo31DFK2 ptc-GAL4/+ flies (Myo31DF heterozygotes) showed the complete dextral rotation, while Myo31DFK2 ptc-GAL4/Myo31DFL152 or Myo31DFK2 ptc-GAL4/Myo31DFK2 flies (Myo31DF homozygotes) primarily showed the complete sinistral rotation (Figure 4G). We noted that the penetrance of the sinistral rotation phenotype was higher in these flies than in the Myo31DFK2/Myo31DFK2 flies, which did not carry ptc-GAL4 (Figure 4, F and G). This enhancement of the sinistral rotation phenotype was probably due to ptc-GAL4, which is known to be a hypomorphic allele of ptc (Shyamala and Bhat 2002). On the other hand, the hh-GAL4 line, which drives GAL4 expression in the male genitalia, alone did not affect the sinistral rotation phenotype, suggesting that the influence of ptc-GAL4 on this phenotype was specific (Figure 4G). This idea was further supported by our observation that Myo31DFK2 ptc16/Myo31DFK2 flies showed a more severe sinistral rotation phenotype than Myo31DFK2/Myo31DFK2 flies. ptc16 is a loss-of-function allele of ptc (Strutt et al. 2001). Therefore, in the following experiments, the baseline of the rotation phenotype was highly penetrant (>95%) complete sinistral rotation (Figure 4G).
We then overexpressed Myo31DF-GFP, Myo61F, and Myo95E-RB under the control of ptc-GAL4 in wild-type (+/+), Myo31DFK2/+, or Myo31DFK2/Myo31DFL152 flies (Figure 4G). The overexpression of Myo31DF-GFP completely suppressed the sinistral rotation phenotype in Myo31DFK2/Myo31DFL152 flies (Figure 4G). On the other hand, the overexpression of Myo61F resulted in no rotation and partial sinistral rotational phenotypes in wild-type and Myo31DFK2/+ flies, respectively (Figure 4G). This result suggested that Myo31DF antagonizes the sinistral activity of Myo61F overexpression, as reported previously (Taniguchi et al. 2007). However, our loss-of-function analysis of Myo31DF and Myo61F showed that these two myosins function redundantly in dextral rotation of the male genital disc. Therefore, as found in the hindgut (Figure 3), it is likely that the sinistral activity of Myo61F is a neomorphic function associated with its overexpression.
In contrast, overexpression of Myo95E-RB did not affect the LR asymmetric rotation of the male genitalia in wild-type (+/+), Myo31DFK2/+, or Myo31DFK2/Myo31DFL152 flies (Figure 4G). Therefore, in our loss-of-function and overexpression analyses, we did not detect an effect of Myo95E in the LR asymmetric rotation of the embryonic gut or male genitalia.
Myo31DF, Myo61F, and Myo95E colocalize with F-actin in S2 cells and in larval midgut enterocytes
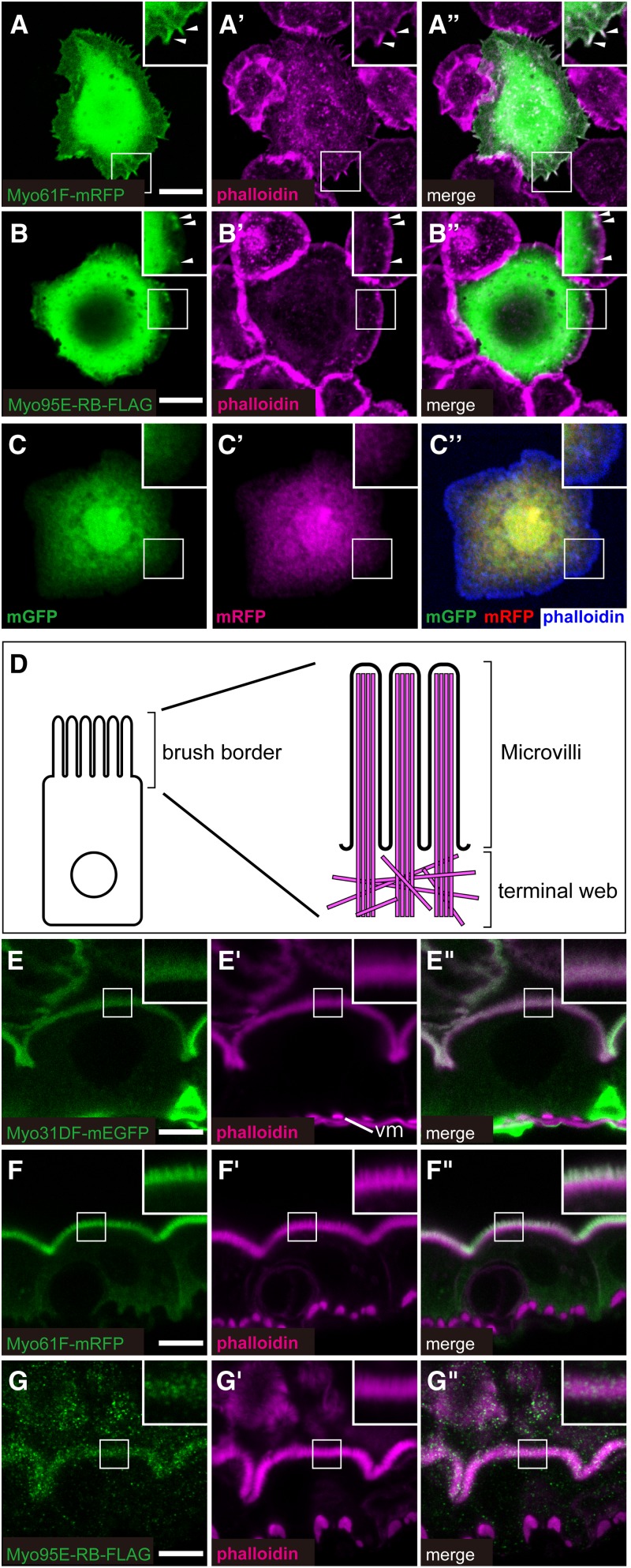
The head region of Myo95E has an atypical insertion (Figure 1A) (Tzolovsky et al. 2002). Therefore, we speculated that Myo95E may exhibit a different subcellular distribution from that of Myo31DF and Myo61F. The colocalization of Myo31DF-GFP and F-actin was previously reported in Drosophila S2 cells (Hozumi et al. 2006). Thus, we analyzed the subcellular localization of Myo61F-mRFP and Myo95E-PB-FLAG in S2 cells, in which F-actin was detected by phalloidin staining (Figure 5, A–A′′ and B–B′′). Myo61F-mRFP and Myo95E-PB-FLAG colocalized with F-actin in most of the cells overexpressing these proteins (Figure 5, A–A′′ and B–B′′). This colocalization pattern was similar to that of Myo31DF-GFP described previously (Hozumi et al. 2006). On the other hand, the mGFP and mRFP proteins, used as negative controls, did not show such colocalization with F-actin in any case examined, supporting the idea that the colocalization of these class I myosins with F-actin was specific (Figure 5, C–C′′). In a small population of cells (<20%) in which F-actin did not form aggregates, Myo61F-mRFP and Myo95E-RB-FLAG were evenly distributed in the cytoplasm (data not shown). Thus, in those cells, it was difficult to analyze the colocalization of F-actin with Myo61F and Myo95E. However, overall, our results were consistent with previously described interactions between class I myosins and F-actin.
Figure 5.
Subcellular localization of Drosophila class I myosin proteins. (A–C) Subcellular localization of Myo61F-mRFP (green in A and A′′), Myo95E-RB-FLAG (green in B and B′′), and control proteins mGFP (green in C and C′′) and mRFP (magenta in C′ and C′′) in Drosophila S2 cells. F-actin (magenta in A′, A′′, B′, and B′′ and blue in C′′) was stained by fluorescently labeled phalloidin. Insets in A–C′′ are high magnifications of the areas shown by white squares, and arrowheads in the insets (A–B′′) indicate the colocalization of Myo61F-mRFP or Myo95E-RB-FLAG with F-actin. A′′, B′′, and C′′ are merged images of A and A′, B and B′, and C and C′, respectively. (D) Schematic diagram of an enterocyte (left) and its brush border F-actin structure (right, magenta), showing the microvilli and terminal web. (E–G′′) Subcellular localization of Myo31DF-mEGFP (green in E and E′′), Myo61F-mRFP (green in F and F′′), and Myo95E-RB-FLAG (green in G and G′′), which were overexpressed in the larval midgut enterocytes. The F-actin-enriched apical domain was stained by fluorescently labeled phalloidin (magenta in E′, E′′, F′, F′′, G′, and G′′). Insets in E–G′′ are high magnifications of the areas shown by white squares. In E′, vm indicates visceral muscle. E′′, F′′, and G′′ are merged images of E and E′, F and F′, and G and G′, respectively. Bars, 10 μm.
We next studied the subcellular distribution of the three class I myosin proteins in vivo. Myo31DF and Myo61F are both known to be present in the apical brush border of the larval midgut enterocyte (Morgan et al. 1995). The apical brush border is composed of microvilli supported by F-actin bundles that protrude into the terminal web domain (Fath et al. 1993; Phillips and Thomas 2006) (Figure 5D). Protein delivery and recovery by endocytosis and exocytosis actively occur in the microvilli. The terminal web domain is a filamentous structure found in the most apical cytoplasmic region, in which F-actin bundles associated with the brush border are cross-linked by Spectrin and nonmuscle myosin II (Phillips and Thomas 2006). In the apical brush border, Myo31DF is enriched in the terminal web domain, and Myo61F is predominantly detected in the microvilli, and these expression regions overlap (Morgan et al. 1995). In this study, we compared the distribution of Myo95E-PB-FLAG with those of Myo31DF-mEGFP and Myo61F-mRFP in the enterocytes of the larval midgut overexpressing one of these three types of myosin I. F-actin in the apical brush border was detected by phalloidin staining (Figure 5, E′–G′ and E′′–G′′, magenta).
In our experiments, Myo31DF-mEGFP, Myo61F-mRFP, and Myo95E-RB-FLAG were all detected in the apical brush border, where F-actin was enriched (Figure 5, D and E–G′′). Although the three class I myosin proteins were detected in both regions of the brush border, Myo31DF-mEGFP was enriched in the terminal web domain, and Myo61F-mRFP was enriched in the microvilli (Figure 5, D, E–E′′, and F–F′′), consistent with previous findings in which endogenous Myo31DF and Myo61F proteins were detected using specific antibodies (Morgan et al. 1995). We found that the distribution of Myo95E-RB-FLAG was punctate, distinct from that of Myo31DF-mEGFP and Myo61F-mRFP, although the puncta were detected primarily in both the microvilli and the terminal web domain (Figure 5, G–G′′). Therefore, although the head region of Myo95E has an atypical insertion, our results suggest that Myo95E, like Myo31DF and Myo61F, colocalizes with F-actin in a similar yet distinct subcellular location.
Myo31DF, Myo61DF, and Myo95E show overlapping but distinct subcellular distributions in the apical brush border of larval midgut enterocytes
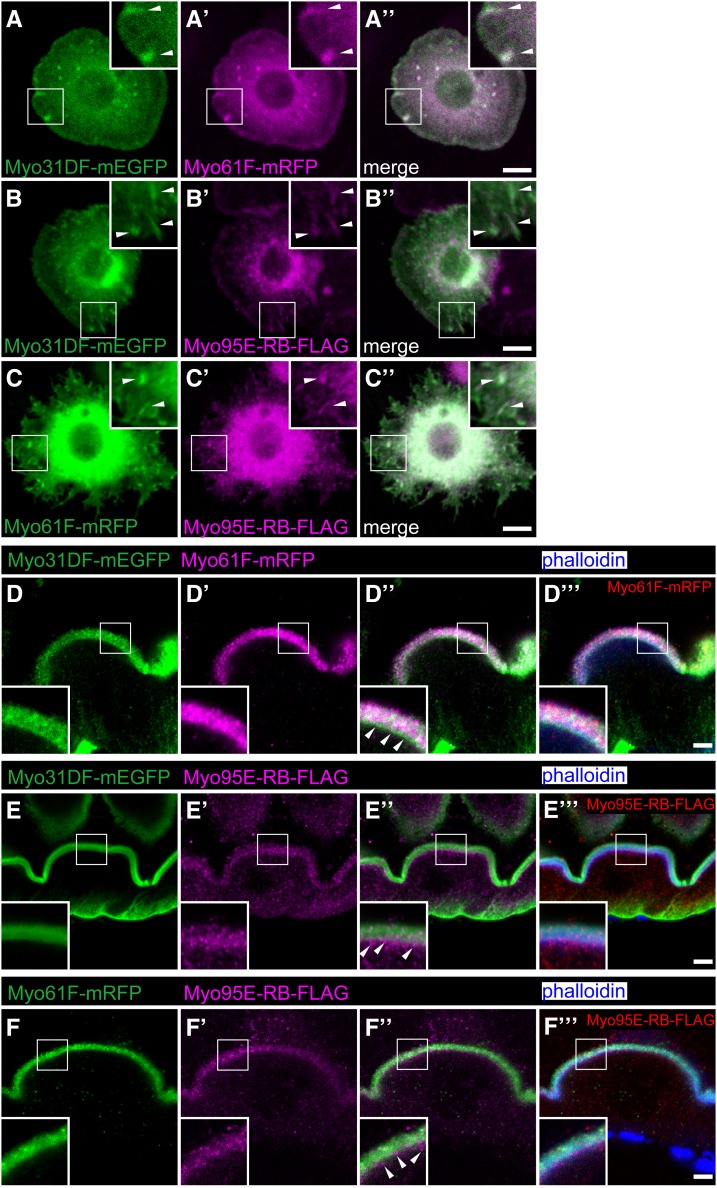
It was previously reported that the endogenous Myo31DF and Myo61F are distinctly localized in the cells of the genital disc and that both the RNA knockdown and overexpression of Myo61F lead to a decreased Myo31DF protein signal detected by immunostaining (Petzoldt et al. 2012). In addition, our analysis above showed that the three class I myosin proteins colocalized with F-actin, but exhibited distinct subcellular distributions in the apical brush border of the larval midgut enterocytes (Figure 5, E–G′′). To further analyze the subcellular localization of these proteins, we next coexpressed the tagged myosin I proteins (Myo31DF-mEGFP, Myo61F-mRFP, and Myo95E-RB-FLAG) two at time and observed their localization.
In S2 cells, the coexpression of any two of the tagged myosins resulted in their colocalization in cytoplasmic aggregates (Figure 6, A–C). These observations were consistent with the above findings that all three class I myosin proteins similarly colocalized with F-actin in S2 cells (Figure 5, A and B). We then coexpressed these genes (two at a time) in the larval midgut enterocytes, where endogenous Myo31DF and Myo61F are enriched in the terminal web domain and the microvilli, respectively (Morgan et al. 1995). Here we found that Myo31DF-mEGFP was localized to the terminal web domain and the microvilli, whereas Myo61F-mRFP was enriched in the microvilli but mostly excluded from the basal region (Figure 6, D–D′′′) corresponding to the terminal web domain (Figure 5D); these findings are largely consistent with a previous report (Morgan et al. 1995). In addition, as shown in the inset of Figure 6D′′, Myo31DF-mEGFP (in green), but not Myo61F-mRFP (in magenta), was detected along the terminal web domain (white arrowheads). When Myo95E-RB-FLAG was coexpressed with either Myo31DF-mEGFP or Myo61F-mRFP, the Myo95E-RB-FLAG was distributed more basally than the Myo31DF-mEGFP or Myo61F-mRFP (Figure 6, E–E′′′ and F–F′′′ and arrowheads in Figure 6, E′′ and F′′). We also found that the overexpression of these three myosin I genes did not affect the overall distribution of F-actin in the enterocytes, detected by phalloidin staining (Figure 6, D′′′–F′′′).
Figure 6.
Colocalization of Drosophila class I myosin proteins. (A–C′′) S2 cells coexpressing Myo31DF-mEGFP (green) and Myo61F-mRFP (magenta) (A–A′′), Myo31DF-mEGFP (green) and Myo95E-RB-FLAG (magenta) (B–B′′), and Myo61F-mRFP (green) and Myo95E-RB-FLAG (magenta) (C–C′′). Insets in A–C′′ are high magnifications of the areas shown by white squares, and arrowheads in the insets indicate colocalization of the two coexpressed class I myosin proteins. A′′, B′′, and C′′ are merged images of A and A′, B and B′, and C and C′, respectively. (D–F′′′) The larval anterior midgut epithelial cells coexpressing Myo31DF-mEGFP (green) and Myo61F-mRFP (magenta) (D–D′′′), Myo31DF-mEGFP (green) and Myo95E-RB-FLAG (magenta) (E–E′′′), and Myo61F-mRFP (green) and Myo95E-RB-FLAG (magenta) (F–F′′′) were stained with fluorescently labeled phalloidin (blue). Insets in D–F′′′ are high magnifications of the areas shown by white squares. D′′, E′′, and F′′ are merged images of D and D′, E and E′, and F and F′, respectively. D′′′, E′′′, and F′′′ are merged images of fluorescently labeled phalloidin (blue) and D′′, E′′, and F′′, respectively. Bars, 5 μm.
Discussion
Roles of class I myosins in Drosophila
In various organisms, the class I myosins often have overlapping functions, but can have distinct roles in certain cases (Osherov and May 2000; Kim and Flavell 2008). In simple organisms, such as yeast and Dictyostelium, the class I myosins have overlapping functions in various cellular processes, in which the actin cytoskeleton plays crucial roles (Osherov and May 2000; Kim and Flavell 2008). Knock-out or knock-in mice for several class I myosin genes (myosins IA, IC, IE, and IF) have been generated (Stauffer et al. 2005; Tyska et al. 2005; Kim et al. 2006; Krendel et al. 2009; Venit et al. 2013) and studies of these mice indicated that these genes are dispensable for survival under normal conditions, when disrupted separately (Tyska et al. 2005; Kim et al. 2006; Krendel et al. 2009). However, the results of deleting all class I myosin genes have not been reported in any metazoan.
D. melanogaster has three class I myosin genes, Myo31DF, Myo61F, and Myo95E (Tzolovsky et al. 2002). Taking advantage of the fact that Drosophila has a relatively small number of class I myosin genes, we obtained or generated mutants of each myosin gene, which were defined as null mutations based on their molecular lesions. We then generated various combinations of the mutants and analyzed their phenotypes.
We found that flies containing mutations in all three type I myosin genes were viable and fertile. Schizosaccharomyces pombe has a single class I myosin gene, and a loss-of-function mutation in this gene is not lethal under normal conditions (Lee et al. 2000; Toya et al. 2001). S. cerevisiae has two class I myosin genes, and cells lacking both of these genes are also viable (Geli and Riezman 1996; Goodson et al. 1996). Similarly, our results demonstrated that class I myosin genes are not essential for survival in Drosophila. However, we found that the hatching and survival rates were decreased in these mutants and their double and triple mutants. The decreased survival rate might be explained by the previous finding that a Drosophila Myo61F homozygous mutant exhibits midgut brush border defects, which are unlikely to be attributed to Myo61F’s role in LR asymmetric development (Hegan et al. 2007). This brush border defect causes an increased susceptibility to lethal infection caused by bacterial pathogens (Hegan et al. 2007). However, due to the broad variability of this defect in the structure of the brush border, we could not analyze whether this phenotype was enhanced in the double and triple mutants (data not shown). We also observed a 50% reduction in the hatching rate of the triple-mutant embryos, which may not be explained by the defect of the brush border. However, we did not find specific defects in their embryonic development, other than the LR inversion of the embryonic gut (shown above).
Redundant roles of Myo31DF and Myo61F in LR asymmetric development of the male genitalia
In the Myo31DF homozygous mutant, the LR asymmetry of various, but not all, organs assumes the mirror image of the wild type (Hozumi et al. 2006; Speder et al. 2006). Thus, it was previously proposed that the sinistral state of LR asymmetry may be a default state in organ development that is reversed by the wild-type function of Myo31DF (Hozumi et al. 2006; Speder et al. 2006). The overexpression of Myo61F also induces sinistral LR asymmetry in various organs, including the embryonic gut and male genitalia (Hozumi et al. 2006; Speder et al. 2006; Petzoldt et al. 2012). This activity was thought to involve the inhibition of Myo31DF’s dextral activity (Hozumi et al. 2006; Speder et al. 2006; Petzoldt et al. 2012). Myo31DF physically interacts with β-catenin, and this interaction is required for the LR activity of Myo31DF (Petzoldt et al. 2012). In the male genitalia, overexpressed Myo61F antagonizes Myo31DF’s activity by preventing Myo31DF’s interaction with β-catenin (Petzoldt et al. 2012). However, in this study, we found that Myo61F overexpression in the Myo31DF null mutant embryos, 80% of which showed sinistral phenotypes, increased this percentage to 100% (Figure 3F). This finding suggested that Myo61F exerts its sinistral activity even in the absence of Myo31DF. The target of Myo61F overexpression responsible for this activity is currently unknown.
Although Myo61F overexpression reverses the dextral LR asymmetry in the embryonic gut and male genitalia (Hozumi et al. 2006; Petzoldt et al. 2012), our loss-of-function analysis revealed that Myo61F shared an overlapping dextral activity with Myo31DF in the LR asymmetric development of the male genitalia. The Myo31DF/Myo61F double mutant showed a significant increase (P < 0.01) in the sinistral rotation phenotype compared with that of the single Myo31DF mutant, although the Myo61F mutant alone did not show LR defects. A similar increase in sinistral activity was observed in the Myo31DF/Myo61F/Myo95E triple mutant, with the same statistical significance (P < 0.01). The Myo61F activity deduced from our loss-of-function analysis (dextral activity) was inconsistent with the activity associated with its overexpression (sinistral activity). Thus, even though Myo61F overexpression induces LR inversion, it is likely that Myo61F has dextral activity in the LR asymmetric development of the male genitalia under physiological conditions. Notably, the Myo61F mutation did not affect LR asymmetric development of the embryonic gut, even in combination with Myo31DF and Myo95E mutations, suggesting that the role of Myo61F in LR asymmetric development is tissue specific. This tissue-specific function may be due to the tissue specificity of Myo61F gene expression, given that Myo61F was not detected in the hg by in situ hybridization at stage 12, when the expression of Myo31DF is required for the normal LR asymmetric development of this organ (Hozumi et al. 2008).
Drosophila Myo95E is a Myosin IB isoform
The Myo95E protein has a large insertion in the head region. Except for this insertion, Myo95E is most similar to Myosin IB (MyoIB), which is conserved from Drosophila to mammals (Tzolovsky et al. 2002). This insertion is found in the MyoIB of Diptera, including flies and mosquitoes [Ceratitis capitata, NCBI reference sequence XP_004533658; Aedes aegypti, XP_001663112.1 (Nene et al. 2007)], but not in the MyoIB of beetles and lice (Tribolium Genome Sequencing Consortium et al. 2008; Kirkness et al. 2010; Keeling et al. 2013). No obvious MyoIB ortholog is found in bees, ants, butterflies, or moths, whose genomes have been completely sequenced (Honeybee Genome Sequencing Consortium 2006; Xia et al. 2008; Bonasio et al. 2010; Werren et al. 2010; Nygaard et al. 2011; Zhan et al. 2011). Thus, we speculated that the insertion was acquired by MyoIB relatively recently during insect evolution.
A role for Myo95E was not observed in the present study. However, the expression of Myo95E was detected in the embryonic nervous system, in contrast to Myo31DF and Myo61F, which were barely detected there. Therefore, we cannot exclude the possibility that Myo95E plays a role in the physiology of neural cells, which may not have been detected by our analysis.
Supplementary Material
Acknowledgments
We are grateful to S. Noselli for providing the Myo31DFK2 and UAS-Myo31DF-GFP lines. We also thank the Drosophila Genetic Resource Center at the Kyoto Institute of Technology, the Bloomington Drosophila Stock Center at Indiana University, and the Development Studies Hybridoma Bank at the University of Iowa for providing flies and antibodies. We thank members of the Matsuno laboratory for critical comments and discussions. This work was supported by The Ministry of Education, Culture, Sports, Science and Technology (MEXT) Grants-in-Aid for Scientific Research (KAKENHI) grant 22127004 and the National Institute of Genetics (NIG) Cooperative Research Program (2007-A51 and 2009-A79).
Footnotes
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.174698/-/DC1.
Communicating editor: W. T. Sullivan
Literature Cited
- Adams M. D., Celniker S. E., Holt R. A., Evans C. A., Gocayne J. D., et al. , 2000. The genome sequence of Drosophila melanogaster. Science 287: 2185–2195. [DOI] [PubMed] [Google Scholar]
- Almeida C. G., Yamada A., Tenza D., Louvard D., Raposo G., et al. , 2011. Myosin 1b promotes the formation of post-Golgi carriers by regulating actin assembly and membrane remodelling at the trans-Golgi network. Nat. Cell Biol. 13: 779–789. [DOI] [PubMed] [Google Scholar]
- Ashburner M., Golic K. G., Hawley R. S., 1989. Drosophila: A Laboratory Handbook, Ed. 2 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Barylko B., Binns D. D., Albanesi J. P., 2000. Regulation of the enzymatic and motor activities of myosin I. Biochim. Biophys. Acta 1496: 23–35. [DOI] [PubMed] [Google Scholar]
- Berg J. S., Powell B. C., Cheney R. E., 2001. A millennial myosin census. Mol. Biol. Cell 12: 780–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof J., Maeda R. K., Hediger M., Karch F., Basler K., 2007. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA 104: 3312–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonasio R., Zhang G., Ye C., Mutti N. S., Fang X., et al. , 2010. Genomic comparison of the ants Camponotus floridanus and Harpegnathos saltator. Science 329: 1068–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose A., Guilherme A., Robida S. I., Nicoloro S. M., Zhou Q. L., et al. , 2002. Glucose transporter recycling in response to insulin is facilitated by myosin Myo1c. Nature 420: 821–824. [DOI] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N., 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- Campbell R. E., Tour O., Palmer A. E., Steinbach P. A., Baird G. S., et al. , 2002. A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA 99: 7877–7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A. H., Stephan D. A., Hasson T., Fukushima K., Nelissen C. M., et al. , 2001. MYO1F as a candidate gene for nonsyndromic deafness, DFNB15. Arch. Otolaryngol. Head Neck Surg. 127: 921–925. [DOI] [PubMed] [Google Scholar]
- Chen C. L., Wang Y., Sesaki H., Iijima M., 2012. Myosin I links PIP3 signaling to remodeling of the actin cytoskeleton in chemotaxis. Sci. Signal. 5: ra10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbas L., Cherbas P., 2000. Drosophila cell culture and transformation, pp. 373–387 in Drosophila Protocols, edited by Sullivan W., Ashburner M., Hawley R. S. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Coluccio L. M., 1997. Myosin I. Am. J. Physiol. 273: C347–C359. [DOI] [PubMed] [Google Scholar]
- Coutelis J. B., Géminard C., Spéder P., Suzanne M., Petzoldt A. G., et al. , 2013. Drosophila left/right asymmetry establishment is controlled by the Hox gene Abdominal-B. Dev. Cell 24: 89–97. [DOI] [PubMed] [Google Scholar]
- Donaudy F., Ferrara A., Esposito L., Hertzano R., Ben-David O., et al. , 2003. Multiple mutations of MYO1A, a cochlear-expressed gene, in sensorineural hearing loss. Am. J. Hum. Genet. 72: 1571–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Eswarappa S. M., Hitomi M., Fox P. L., 2012. Myo1c facilitates G-actin transport to the leading edge of migrating endothelial cells. J. Cell Biol. 198: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath K. R., Mamajiwalla S. N., Burgess D. R., 1993. The cytoskeleton in development of epithelial cell polarity. J. Cell Sci. Suppl. 17: 65–73. [DOI] [PubMed] [Google Scholar]
- Foronda D., Estrada B., de Navas L., Sánchez-Herrero E., 2006. Requirement of Abdominal-A and Abdominal-B in the developing genitalia of Drosophila breaks the posterior downregulation rule. Development 133: 117–127. [DOI] [PubMed] [Google Scholar]
- Fyrberg C., Becker J., Barthmaier P., Mahaffey J., Fyrberg E., 1997. A Drosophila muscle-specific gene related to the mouse quaking locus. Gene 197: 315–323. [DOI] [PubMed] [Google Scholar]
- Geli M. I., Riezman H., 1996. Role of type I myosins in receptor-mediated endocytosis in yeast. Science 272: 533–535. [DOI] [PubMed] [Google Scholar]
- Gillespie P. G., 2004. Myosin I and adaptation of mechanical transduction by the inner ear. Philos. Trans. R. Soc. Lond. B Biol. Sci. 359: 1945–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie P. G., Cyr J. L., 2004. Myosin-1c, the hair cell’s adaptation motor. Annu. Rev. Physiol. 66: 521–545. [DOI] [PubMed] [Google Scholar]
- Goodson H. V., Anderson B. L., Warrick H. M., Pon L. A., Spudich J. A., 1996. Synthetic lethality screen identifies a novel yeast myosin gene (MYO5): myosin I proteins are required for polarization of the actin cytoskeleton. J. Cell Biol. 133: 1277–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigliatti T. A., 1998. Transposons—gene tagging and mutagenesis, pp. 85–107 in Drosophila: A Practical Approach, Ed. 2, edited by Roberts D. B. IRL Press, Oxford. [Google Scholar]
- Hayashi T., Murakami R., 2001. Left-right asymmetry in Drosophila melanogaster gut development. Dev. Growth Differ. 43: 239–246. [DOI] [PubMed] [Google Scholar]
- Hegan P. S., Mermall V., Tilney L. G., Mooseker M. S., 2007. Roles for Drosophila melanogaster myosin IB in maintenance of enterocyte brush-border structure and resistance to the bacterial pathogen Pseudomonas entomophila. Mol. Biol. Cell 18: 4625–4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeybee Genome Sequencing Consortium , 2006. Insights into social insects from the genome of the honeybee Apis mellifera. Nature 443: 931–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Prickett K. S., Price V. L., Libby R. T., March C. J., et al. , 1988. A short polypeptide marker sequence useful for recombinant protein identification and purification. Nat. Biotechnol. 6: 1204–1210. [Google Scholar]
- Hozumi S., Maeda R., Taniguchi K., Kanai M., Shirakabe S., et al. , 2006. An unconventional myosin in Drosophila reverses the default handedness in visceral organs. Nature 440: 798–802. [DOI] [PubMed] [Google Scholar]
- Hozumi S., Maeda R., Taniguchi-Kanai M., Okumura T., Taniguchi K., et al. , 2008. Head region of unconventional myosin I family members is responsible for the organ-specificity of their roles in left-right polarity in Drosophila. Dev. Dyn. 237: 3528–3537. [DOI] [PubMed] [Google Scholar]
- Iwaki D. D., Lengyel J. A., 2002. A Delta-Notch signaling border regulated by Engrailed/Invected repression specifies boundary cells in the Drosophila hindgut. Mech. Dev. 114: 71–84. [DOI] [PubMed] [Google Scholar]
- Johnson R. L., Grenier J. K., Scott M. P., 1995. patched overexpression alters wing disc size and pattern: transcriptional and post-transcriptional effects on hedgehog targets. Development 121: 4161–4170. [DOI] [PubMed] [Google Scholar]
- Jung G., Wu X., Hammer J. A., 3rd, 1996. Dictyostelium mutants lacking multiple classic myosin I isoforms reveal combinations of shared and distinct functions. J. Cell Biol. 133: 305–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling C. I., Yuen M. M., Liao N. Y., Roderick Docking T., Chan S. K., et al. , 2013. Draft genome of the mountain pine beetle, Dendroctonus ponderosae Hopkins, a major forest pest. Genome Biol. 14: R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. V., Flavell R. A., 2008. Myosin I: from yeast to human. Cell. Mol. Life Sci. 65: 2128–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. V., Mehal W. Z., Dong X., Heinrich V., Pypaert M., et al. , 2006. Modulation of cell adhesion and motility in the immune system by Myo1f. Science 314: 136–139. [DOI] [PubMed] [Google Scholar]
- Kirkness E. F., Haas B. J., Sun W., Braig H. R., Perotti M. A., et al. , 2010. Genome sequences of the human body louse and its primary endosymbiont provide insights into the permanent parasitic lifestyle. Proc. Natl. Acad. Sci. USA 107: 12168–12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krendel M., Mooseker M. S., 2005. Myosins: tails (and heads) of functional diversity. Physiology 20: 239–251. [DOI] [PubMed] [Google Scholar]
- Krendel M., Kim S. V., Willinger T., Wang T., Kashgarian M., et al. , 2009. Disruption of Myosin 1e promotes podocyte injury. J. Am. Soc. Nephrol. 20: 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuranaga E., Matsunuma T., Kanuka H., Takemoto K., Koto A., et al. , 2011. Apoptosis controls the speed of looping morphogenesis in Drosophila male terminalia. Development 138: 1493–1499. [DOI] [PubMed] [Google Scholar]
- Lee W. L., Bezanilla M., Pollard T. D., 2000. Fission yeast myosin-I, Myo1p, stimulates actin assembly by Arp2/3 complex and shares functions with WASp. J. Cell Biol. 151: 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligoxygakis P., Strigini M., Averof M., 2001. Specification of left-right asymmetry in the embryonic gut of Drosophila. Development 128: 1171–1174. [DOI] [PubMed] [Google Scholar]
- McConnell R. E., Tyska M. J., 2010. Leveraging the membrane - cytoskeleton interface with myosin-1. Trends Cell Biol. 20: 418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R. E., Higginbotham J. N., Shifrin D. A., Jr, Tabb D. L., Coffey R. J., et al. , 2009. The enterocyte microvillus is a vesicle-generating organelle. J. Cell Biol. 185: 1285–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermall V., Post P. L., Mooseker M. S., 1998. Unconventional myosins in cell movement, membrane traffic, and signal transduction. Science 279: 527–533. [DOI] [PubMed] [Google Scholar]
- Miyashita T., Oda Y., Horiuchi J., Yin J. C., Morimoto T., et al. , 2012. Mg(2+) block of Drosophila NMDA receptors is required for long-term memory formation and CREB-dependent gene expression. Neuron 74: 887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy J. E., Burns J. E., Kendrick-Jones J., Tregear R. T., White D. C., 1995. Movement and force produced by a single myosin head. Nature 378: 209–212. [DOI] [PubMed] [Google Scholar]
- Morgan N. S., Skovronsky D. M., Artavanis-Tsakonas S., Mooseker M. S., 1994. The molecular cloning and characterization of Drosophila melanogaster myosin-IA and myosin-IB. J. Mol. Biol. 239: 347–356. [DOI] [PubMed] [Google Scholar]
- Morgan N. S., Heintzelman M. B., Mooseker M. S., 1995. Characterization of myosin-IA and myosin-IB, two unconventional myosins associated with the Drosophila brush border cytoskeleton. Dev. Biol. 172: 51–71. [DOI] [PubMed] [Google Scholar]
- Nambiar R., McConnell R. E., Tyska M. J., 2009. Control of cell membrane tension by myosin-I. Proc. Natl. Acad. Sci. USA 106: 11972–11977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nene V., Wortman J. R., Lawson D., Haas B., Kodira C., et al. , 2007. Genome sequence of Aedes aegypti, a major arbovirus vector. Science 316: 1718–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak K. D., Peterson M. D., Reedy M. C., Titus M. A., 1995. Dictyostelium myosin I double mutants exhibit conditional defects in pinocytosis. J. Cell Biol. 131: 1205–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard S., Zhang G., Schiøtt M., Li C., Wurm Y., et al. , 2011. The genome of the leaf-cutting ant Acromyrmex echinatior suggests key adaptations to advanced social life and fungus farming. Genome Res. 21: 1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Kane C. J., 1998. Enhancer traps, pp. 131–178 in Drosophila: A Practical Approach, Ed. 2, edited by Roberts D. B. IRL Press, Oxford. [Google Scholar]
- Osherov N., May G. S., 2000. In vivo function of class I myosins. Cell Motil. Cytoskeleton 47: 163–173. [DOI] [PubMed] [Google Scholar]
- Parks A. L., Cook K. R., Belvin M., Dompe N. A., Fawcett R., et al. , 2004. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat. Genet. 36: 288–292. [DOI] [PubMed] [Google Scholar]
- Pestic-Dragovich L., Stojiljkovic L., Philimonenko A. A., Nowak G., Ke Y., et al. , 2000. A myosin I isoform in the nucleus. Science 290: 337–341. [DOI] [PubMed] [Google Scholar]
- Petzoldt A. G., Coutelis J. B., Géminard C., Spéder P., Suzanne M., et al. , 2012. DE-Cadherin regulates unconventional Myosin ID and Myosin IC in Drosophila left-right asymmetry establishment. Development 139: 1874–1884. [DOI] [PubMed] [Google Scholar]
- Philimonenko V. V., Zhao J., Iben S., Dingová H., Kyselá K., et al. , 2004. Nuclear actin and myosin I are required for RNA polymerase I transcription. Nat. Cell Biol. 6: 1165–1172. [DOI] [PubMed] [Google Scholar]
- Phillips M. D., Thomas G. H., 2006. Brush border spectrin is required for early endosome recycling in Drosophila. J. Cell Sci. 119: 1361–1370. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Hong L., Brokstein P., Evans-Holm M., Frise E., et al. , 2000. A Drosophila complementary DNA resource. Science 287: 2222–2224. [DOI] [PubMed] [Google Scholar]
- Sellers J. R., 2000. Myosins: a diverse superfamily. Biochim. Biophys. Acta 1496: 3–22. [DOI] [PubMed] [Google Scholar]
- Shyamala B. V., Bhat K. M., 2002. A positive role for Patched-Smoothened signaling in promoting cell proliferation during normal head development in Drosophila. Development 129: 1839–1847. [DOI] [PubMed] [Google Scholar]
- Sokac A. M., Schietroma C., Gundersen C. B., Bement W. M., 2006. Myosin-1c couples assembling actin to membranes to drive compensatory endocytosis. Dev. Cell 11: 629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speder P., Adam G., Noselli S., 2006. Type ID unconventional myosin controls left-right asymmetry in Drosophila. Nature 440: 803–807. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., 1986. P element-mediated transformation, pp. 175–197 in Drosophila: A Practical Approach, edited by Roberts D. B. IRL Press, Oxford. [Google Scholar]
- Stapleton M., Liao G., Brokstein P., Hong L., Carninci P., et al. , 2002. The Drosophila gene collection: identification of putative full-length cDNAs for 70% of D. melanogaster genes. Genome Res. 12: 1294–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer E. A., Scarborough J. D., Hirono M., Miller E. D., Shah K., et al. , 2005. Fast adaptation in vestibular hair cells requires myosin-1c activity. Neuron 47: 541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H., Thomas C., Nakano Y., Stark D., Neave B., et al. , 2001. Mutations in the sterol-sensing domain of Patched suggest a role for vesicular trafficking in Smoothened regulation. Curr. Biol. 11: 608–613. [DOI] [PubMed] [Google Scholar]
- Suzanne M., Petzoldt A. G., Speder P., Coutelis J. B., Steller H. et al, 2010. Coupling of apoptosis and L/R patterning controls stepwise organ looping. Curr. Biol. 20: 1773–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Hozumi S., Maeda R., Okumura T., Matsuno K., 2007. Roles of type I myosins in Drosophila handedness. Fly 1: 287–290. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Maeda R., Ando T., Okumura T., Nakazawa N., et al. , 2011. Chirality in planar cell shape contributes to left-right asymmetric epithelial morphogenesis. Science 333: 339–341. [DOI] [PubMed] [Google Scholar]
- Toya M., Motegi F., Nakano K., Mabuchi I., Yamamoto M., 2001. Identification and functional analysis of the gene for type I myosin in fission yeast. Genes Cells 6: 187–199. [DOI] [PubMed] [Google Scholar]
- Tribolium Genome Sequencing Consortium , S. Richards, R. A. Gibbs, G. M. Weinstock, S. J. Brown et al, 2008. The genome of the model beetle and pest Tribolium castaneum. Nature 452: 949–955. [DOI] [PubMed] [Google Scholar]
- Tyska M. J., Mackey A. T., Huang J. D., Copeland N. G., Jenkins N. A., et al. , 2005. Myosin-1a is critical for normal brush border structure and composition. Mol. Biol. Cell 16: 2443–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzolovsky G., Millo H., Pathirana S., Wood T., Bownes M., 2002. Identification and phylogenetic analysis of Drosophila melanogaster myosins. Mol. Biol. Evol. 19: 1041–1052. [DOI] [PubMed] [Google Scholar]
- Venit T., Dzijak R., Kalendová A., Kahle M., Rohožková J., et al. , 2013. Mouse nuclear myosin I knock-out shows interchangeability and redundancy of myosin isoforms in the cell nucleus. PLoS ONE 8: e61406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren J. H., Richards S., Desjardins C. A., Niehuis O., Gadau J., et al. , 2010. Functional and evolutionary insights from the genomes of three parasitoid Nasonia species. Science 327: 343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Q., Wang J., Zhou Z., Li R., Fan W., et al. , 2008. The genome of a lepidopteran model insect, the silkworm Bombyx mori. Insect Biochem. Mol. Biol. 38: 1036–1045. [DOI] [PubMed] [Google Scholar]
- Zadro C., Alemanno M. S., Bellacchio E., Ficarella R., Donaudy F., et al. , 2009. Are MYO1C and MYO1F associated with hearing loss? Biochim. Biophys. Acta 1792: 27–32. [DOI] [PubMed] [Google Scholar]
- Zhan S., Merlin C., Boore J. L., Reppert S. M., 2011. The monarch butterfly genome yields insights into long-distance migration. Cell 147: 1171–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.