Abstract
The neurotransmitter gamma-aminobutyric acid (GABA) is depolarizing in the developing vertebrate brain, but in older animals switches to hyperpolarizing and becomes the major inhibitory neurotransmitter in adults. We discovered a similar developmental switch in GABA response in Caenorhabditis elegans and have genetically analyzed its mechanism and function in a well-defined circuit. Worm GABA neurons innervate body wall muscles to control locomotion. Activation of GABAA receptors with their agonist muscimol in newly hatched first larval (L1) stage animals excites muscle contraction and thus is depolarizing. At the mid-L1 stage, as the GABAergic neurons rewire onto their mature muscle targets, muscimol shifts to relaxing muscles and thus has switched to hyperpolarizing. This muscimol response switch depends on chloride transporters in the muscles analogous to those that control GABA response in mammalian neurons: the chloride accumulator sodium-potassium-chloride-cotransporter-1 (NKCC-1) is required for the early depolarizing muscimol response, while the two chloride extruders potassium-chloride-cotransporter-2 (KCC-2) and anion-bicarbonate-transporter-1 (ABTS-1) are required for the later hyperpolarizing response. Using mutations that disrupt GABA signaling, we found that neural circuit development still proceeds to completion but with an ∼6-hr delay. Using optogenetic activation of GABAergic neurons, we found that endogenous GABAA signaling in early L1 animals, although presumably depolarizing, does not cause an excitatory response. Thus a developmental depolarizing-to-hyperpolarizing shift is an ancient conserved feature of GABA signaling, but existing theories for why this shift occurs appear inadequate to explain its function upon rigorous genetic analysis of a well-defined neural circuit.
Keywords: GABA response, switch, inhibitory, signaling
NEURONS alter expression and activity of Cl− transporters to alter the driving force for Cl− ions through the GABA-gated Cl− channel known as the GABAA receptor (Rivera et al. 1999; Yamada et al. 2004; Blaesse et al. 2009). Mature neurons generally have a low intracellular Cl− concentration ([Cl−]i) so that Cl− influx occurs through the GABAA receptor to hyperpolarize and inhibit the cells. However, across many vertebrate species and brain regions, immature neurons have relatively high [Cl−]i so that Cl− efflux occurs through the GABAA receptor to depolarize these neurons. While strong depolarization excites action potentials, weakly depolarizing GABA can cause an opposite effect, “shunting inhibition,” which holds membrane potential below the threshold required to fire action potentials (Staley and Mody 1992). The biological purpose of early depolarizing GABA in circuit formation and maturation remains unclear. GABA signaling in the vertebrate brain generally develops prior to glutamate signaling and, if excitatory, potentially provides the initial activity in developing circuits (Saint-Amant and Drapeau 2000; Gao and Van Den Pol 2001; Hennou et al. 2002; Gozlan and Ben-Ari 2003; Johnson et al. 2003). Genetically manipulating Cl− transporters to eliminate early depolarizing effects of GABA leads to defects in dendrite and synapse development (Chudotvorova et al. 2005; Akerman and Cline 2006; Ge et al. 2006; Cancedda et al. 2007; Young et al. 2012). However, elucidating the precise linkage between the role of early GABA signaling in specific neurons and a manifested behavior has been difficult due to the complexity of the vertebrate brain.
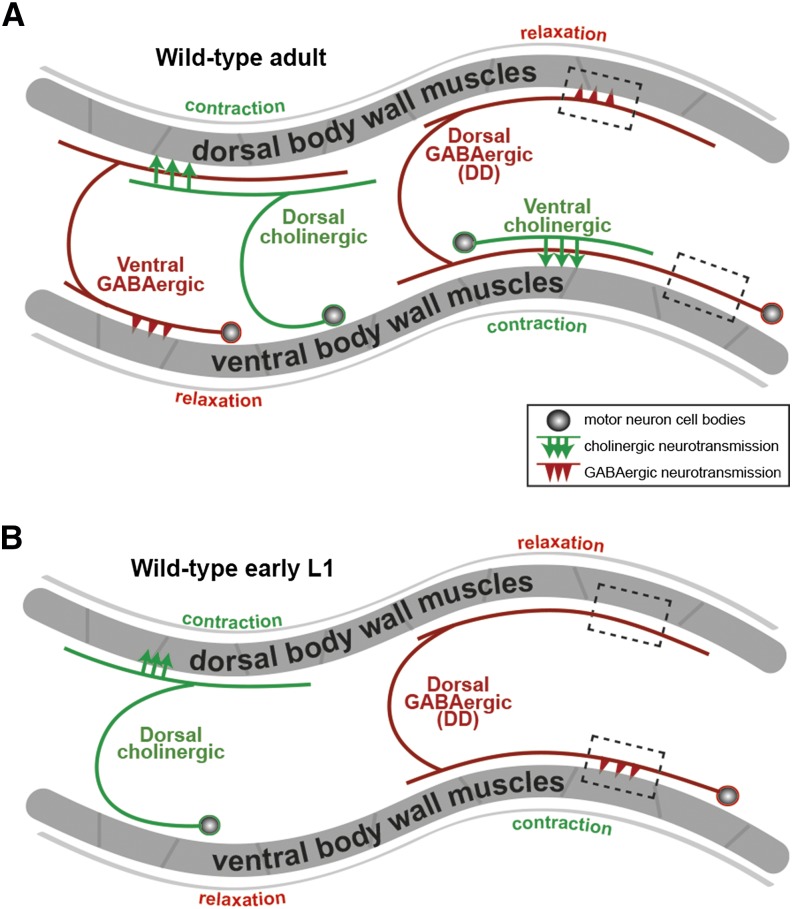
Caenorhabditis elegans provides the potential to study developmental changes in GABA response within the well-studied locomotor circuit. In adult worms, cholinergic motor neurons excite body wall muscles to generate body bends. They also excite GABAergic neurons that synapse onto the opposing body wall muscles so that when acetylcholine excites and contracts one set of muscles, GABA is released onto the opposing muscles to inhibit and relax them (White et al. 1976) (Figure 1A). Thus inhibitory GABA helps adults coordinate body bends (McIntire et al. 1993b; Schuske et al. 2004). However, in newly hatched, first-stage larvae (L1s), cholinergic neurons that will later excite the ventral muscles have not yet developed (Figure 1B). Instead, six GABAergic DD neurons temporarily synapse onto the ventral muscles (White et al. 1992; Jin et al. 1994). Later in the L1 stage, new cholinergic neurons develop and make synapses onto the ventral muscles, while the existing DD neurons eliminate their ventral synapses and form new synapses onto the dorsal muscles. Thus in both the mammalian brain and in the C. elegans L1 ventral locomotor circuit, GABA signaling precedes the development of mature excitatory synapses. The analysis we present here shows that a depolarizing-to-hyperpolarizing GABA response switch appears to occur in the C. elegans locomotor circuit. However, we show that synapse formation in the locomotor circuit proceeds relatively normally when the switch in the polarity of GABA response is disrupted genetically, and that early depolarizing GABA is not the initial source of excitation during development of the locomotor circuit. Thus the developmental GABA response switch is conserved across evolution, but genetic analysis of this switch in a well-defined neural circuit suggests the switch has functions other than providing excitation or supporting synapse development.
Figure 1.
The anatomy and development of C. elegans locomotor circuit. Diagrams of neuronal wiring in the locomotor circuit of C. elegans adults (A) or newly hatched first-stage larvae (L1s) (B). The anterior of the animals is to the left. Circles, motor neuron cell bodies; lines extending from circles, neural processes; arrows, acetylcholine release sites; arrowheads, GABA release sites; black dashed boxes, representative regions of DD (dorsal GABAergic) neuron processes adjacent to dorsal and ventral body wall muscles. Each neuron diagrammed represents a class of motor neurons that repeats along the length of the animal. In adults (A), ventral cholinergic neurons release acetylcholine onto the ventral body wall muscles to excite them and produce ventral body bends. Dorsal cholinergic neurons excite dorsal muscles to produce dorsal bends. GABAergic neurons assist body bends by releasing GABA to relax muscles opposite a contraction. Type DD motor neurons relax dorsal muscles, and ventral GABAergic motor neurons relax ventral muscles. In newly hatched L1s (B), however, neuronal processes from neither the ventral cholinergic nor ventral GABAergic motor neurons have developed yet. Instead, the dorsal GABAergic DD neurons make temporary synapses onto the ventral body wall muscles. As ventral motor neurons develop, DD neurons rewire to eliminate ventral synapses and form new dorsal synapses. Diagram adapted from WormAtlas.org.
Materials and Methods
C. elegans strains
C. elegans strains were maintained at 20° on nematode growth medium (NGM) agar plates with Escherichia coli OP50 as a source of food, as described previously (Brenner 1974). All strains are derived from the Bristol N2 wild-type strain. The C. elegans strains used in this work were as follows:
Figure 4: nkcc-1(ok1621), kcc-2(vs132), abts-1(ok1566), kcc-2(vs132) nkcc-1(ok1621), abts-1(ok1566); nkcc-1(ok1621), vsEx743[pmyo-3::gfp, pmyo-3::gfp-LacZ(nuclear localization signal, NLS)], nkcc-1(ok1621); vsEx744[pmyo-3::nkcc-1c, pmyo-3::gfp-LacZ(NLS)], and LX1941 nkcc-1(ok1621); vsEx745[pmyo-3::gfp, pmyo-3::gfp-LacZ(NLS)]
Figure 5: wyIs202[pflp-13::gfp::rab-3, pflp-13::mCherry, podr-1::dsRED], wyIs222[punc-4::gfp::rab-3; pmig-13::mCherry::rab-3, podr-1::dsRED]
Figure 7: zxIs3[punc-47::chokp-2(H134R)::yfp; lin-15+], gbb-1(tm1406); zxIs3, gbb-2(tm1165); zxIs3, unc-49(e407); zxIs3, unc-49(e407); gbb-1(tm1406); zxIs3, unc-49(e407); gbb-2(tm1165); zxIs3
Figure S1: nkcc-1(ok1621); wyIs202[pflp-13::gfp::rab-3, pflp-13::mCherry, podr-1::dsRED], kcc-2(vs132); wyIs202[pflp-13::gfp::rab-3, pflp-13::mCherry, podr-1::dsRED]
Figure 2.
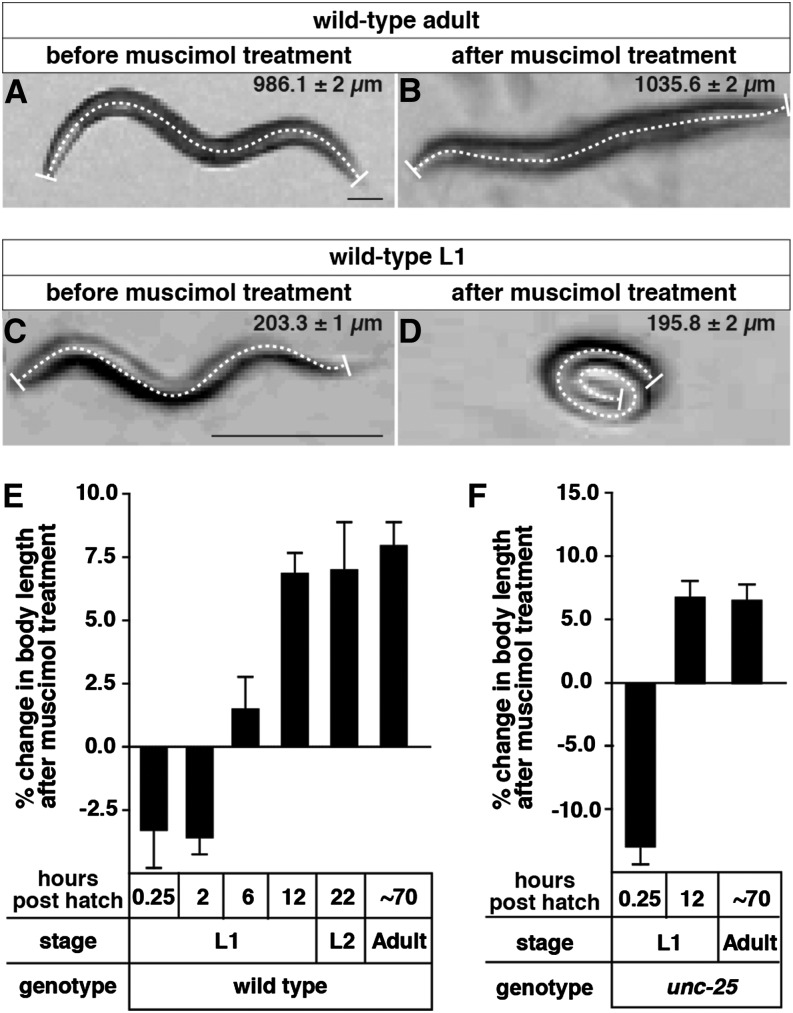
Muscle response to a GABAA receptor agonist switches from excitatory to inhibitory during C. elegans development. (A and B) Images of a representative wild-type adult in the absence (A) or presence (B) of 1 mM muscimol. (C and D) Images of a representative wild-type L1 at 0.25 hr posthatch in the absence (C) or presence (D) of 1 mM muscimol. A dashed line was drawn down the midline of the worm in each image to measure its body length. The average lengths and standard deviations from 30 animals thus measured are shown at upper right of each image. Bar, 100 µm. (E) Percent changes in body length of wild-type animals in response to 1 mM muscimol throughout development. Muscimol response switches from excitatory to inhibitory at ∼6 hr posthatch. (F) The muscimol response switch was still observed in mutant animals lacking the GABA biosynthetic enzyme UNC-25. n = 30 animals per genotype per time point. Error bars represent 95% confidence intervals calculated using a paired t-test.
Figure 3.
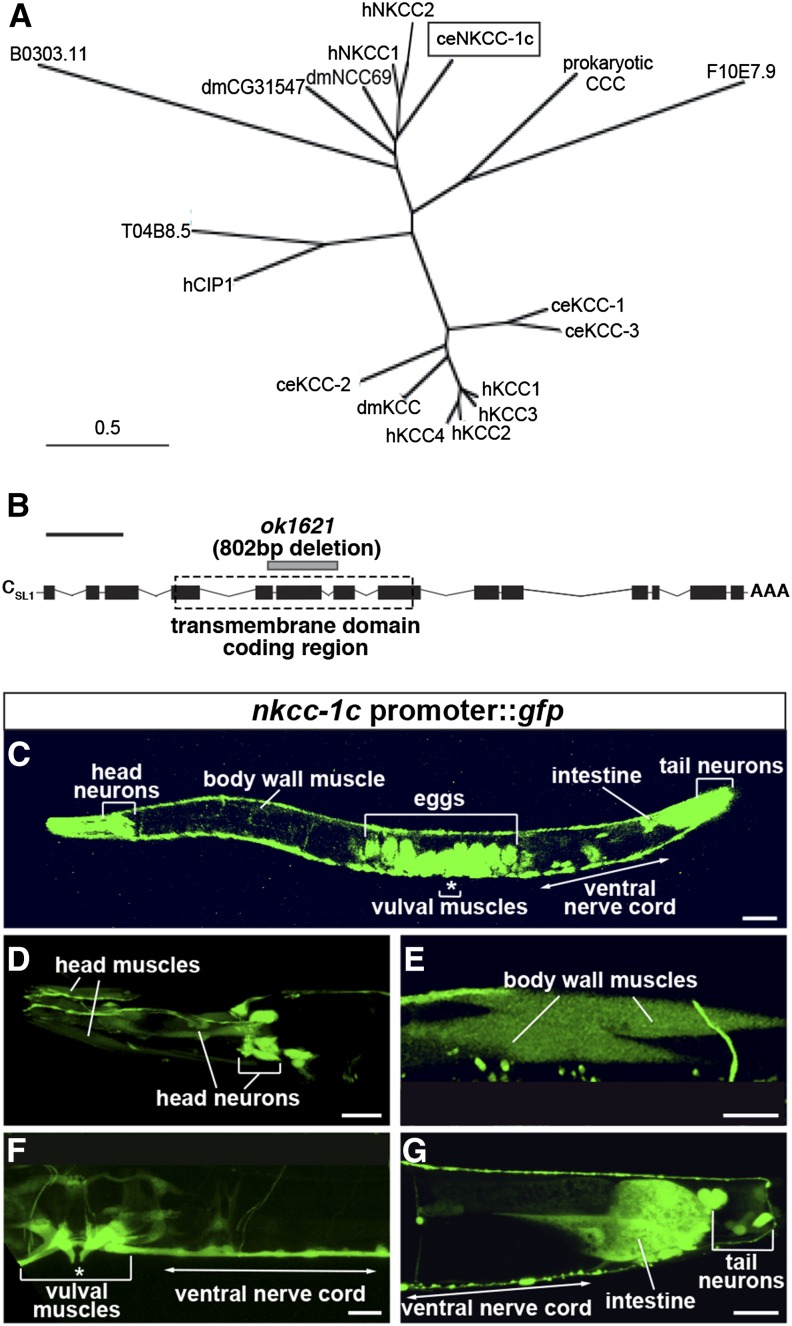
NKCC-1 is expressed predominantly in muscles and neurons. (A) Phylogenetic comparison of the C. elegans sodium potassium chloride cotransporter (ceNKCC-1; boxed) to the two human NKCCs (hNKCC-1 and hNKCC-2), two predicted Drosophila melanogaster NKCCs (dmCG31547 and dmNCC69), four C. elegans potassium chloride cotransporters (KCCs; ceKCC-1–ceKCC-4), four human KCCs (hKCC-1–hKCC-4), a human chloride transporter interacting protein (hCIP1), and a predicted C. elegans chloride transporter interacting protein (T04B8.5). A prokaryotic cation chloride cotransporter (CCC) is used to root the comparison. Bar, 0.5 substitutions per 100 amino acids. (B) Structure of the nkcc-1c transcript. Solid black boxes, exons; connecting lines, introns; AAA, the polyadenylation site; dashed box, the predicted transmembrane domain coding region; gray bar, the ok1621 deletion mutation. Bar (black line above the schematic), 1 kb. (C–G) GFP fluorescence in an animal carrying a nkcc-1c promoter::gfp::nkcc-1c 3′ UTR reporter transgene. This reporter transgene was expressed primarily in the body wall muscles (E) and neurons, including the head and tail neurons (D), and the ventral cord neurons (F and G). It was also expressed in the vulval muscles (F) and the posterior intestine (G). Unlaid eggs inside the adults showed high expression (C). Bar, 50 μm. Eggs visible in C are out of the focal plane and thus not visible in F. Body wall muscles are poorly visualized in C but become apparent at the higher magnification in E.
Figure 4.
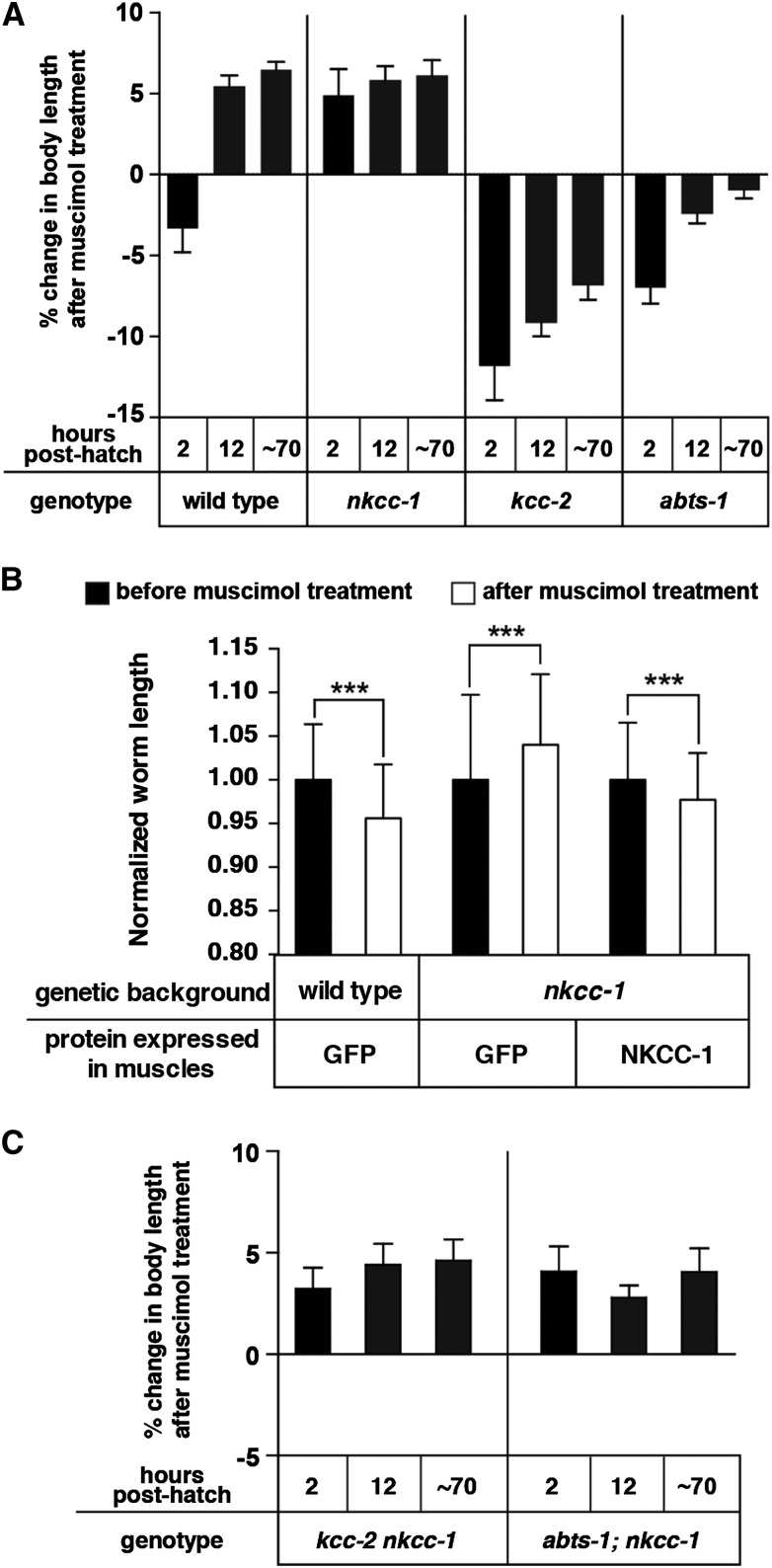
NKCC-1 promotes excitatory signaling by the GABAA agonist muscimol onto body wall muscles. (A) Percent change in body length of the wild type and of Cl− transporter mutants after 1 mM muscimol exposure. Pairwise measurements were normalized to the body length of each animal prior to muscimol exposure. n = 30 animals. (B) Rescue of the muscimol response defect of early L1 nkcc-1 mutants by reexpression of nkcc-1 in body wall muscles. Average normalized worm length of transgenic newly hatched L1 animals before and after 1 mM muscimol exposure are shown. A muscle-specific promoter was used to transgenically express cDNAs encoding the control protein GFP or NKCC-1 isoform C. n = 50 animals, ***P = 0.0005. (C) nkcc-1 is epistatic to kcc-2 and abts-1 in the muscimol response assay. Each measurement was normalized to body length of the same animal prior to muscimol exposure. n = 30 animals. Error bars represent 95% confidence intervals.
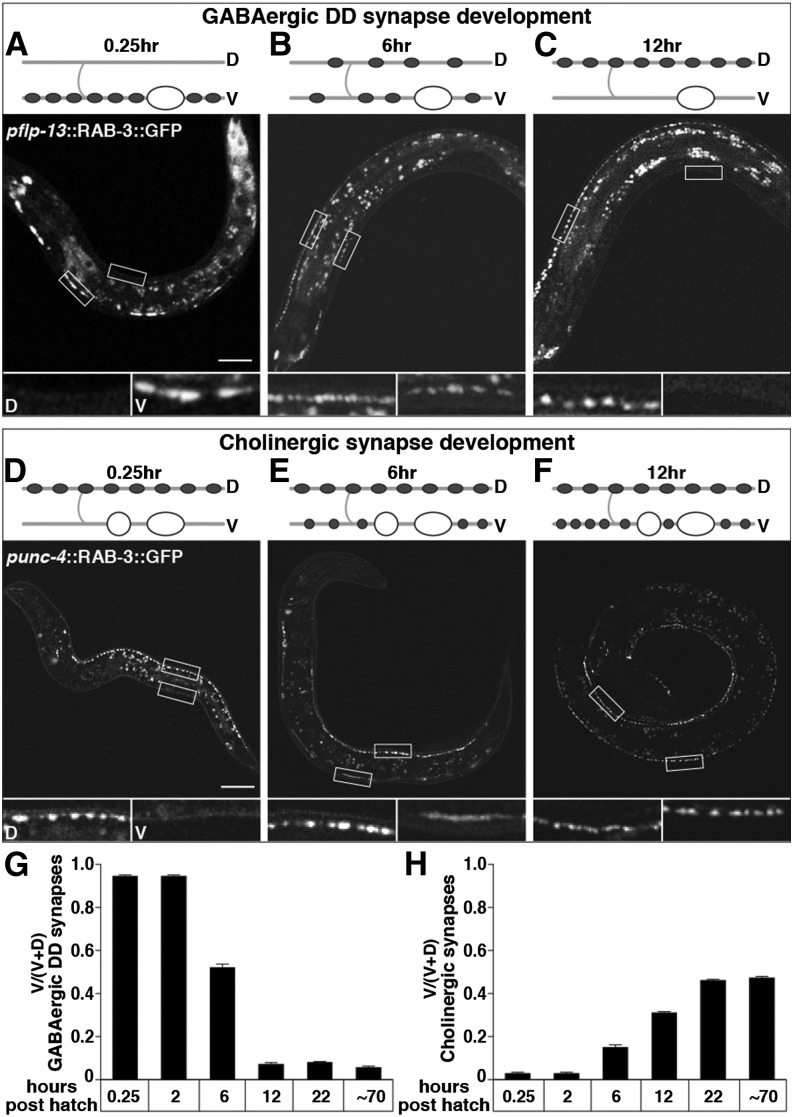
Figure 5.
Presynaptic motor neuron development coincides with the postsynaptic switch in muscle response to muscimol. (A–C) Rewiring of DD presynaptic termini from ventral to dorsal in L1 animals. Wild-type animals expressing GFP::RAB-3 in GABAergic DD motor neuron presynaptic termini under the flp-13 promoter at 0.25 (A), 6 (B), and 12 (C) hr posthatch. (D–F) Development of ventral cholinergic presynaptic termini in L1 animals. Wild-type animals expressing GFP::RAB-3 in cholinergic motor neuron presynaptic termini under the unc-4 promoter at 0.25 (D), 6 (E), and 12 (F) hr posthatch. Cartoons depict a representative DD motor neuron (A–C) or ventral cholinergic motor neuron (D–F) and where their synapses are at each time point. Shaded lines, nerve cords and commissures; “D” and “V”, dorsal, ventral; open ovals, DD motor neuron cell bodies; solid ovals, synapses made from DDs; open circles, ventral cholinergic motor neuron cell bodies; solid circles, synapses made from ventral cholinergic motor neurons. All animals are oriented with head to the left and ventral down. Open boxes outline representative regions of the dorsal and ventral nerve cord that are shown in magnified versions below each image and labeled “D” and “V” for dorsal and ventral in A and D. Magnified images in B, C, E, and F similarly show the dorsal box on the left and the ventral box on the right. Bar, 20 µm. (G and H) Quantification of the proportion of ventral GFP::RAB-3 in DD presynaptic termini (G) or in cholinergic presynaptic termini (H). n = 20 animals.
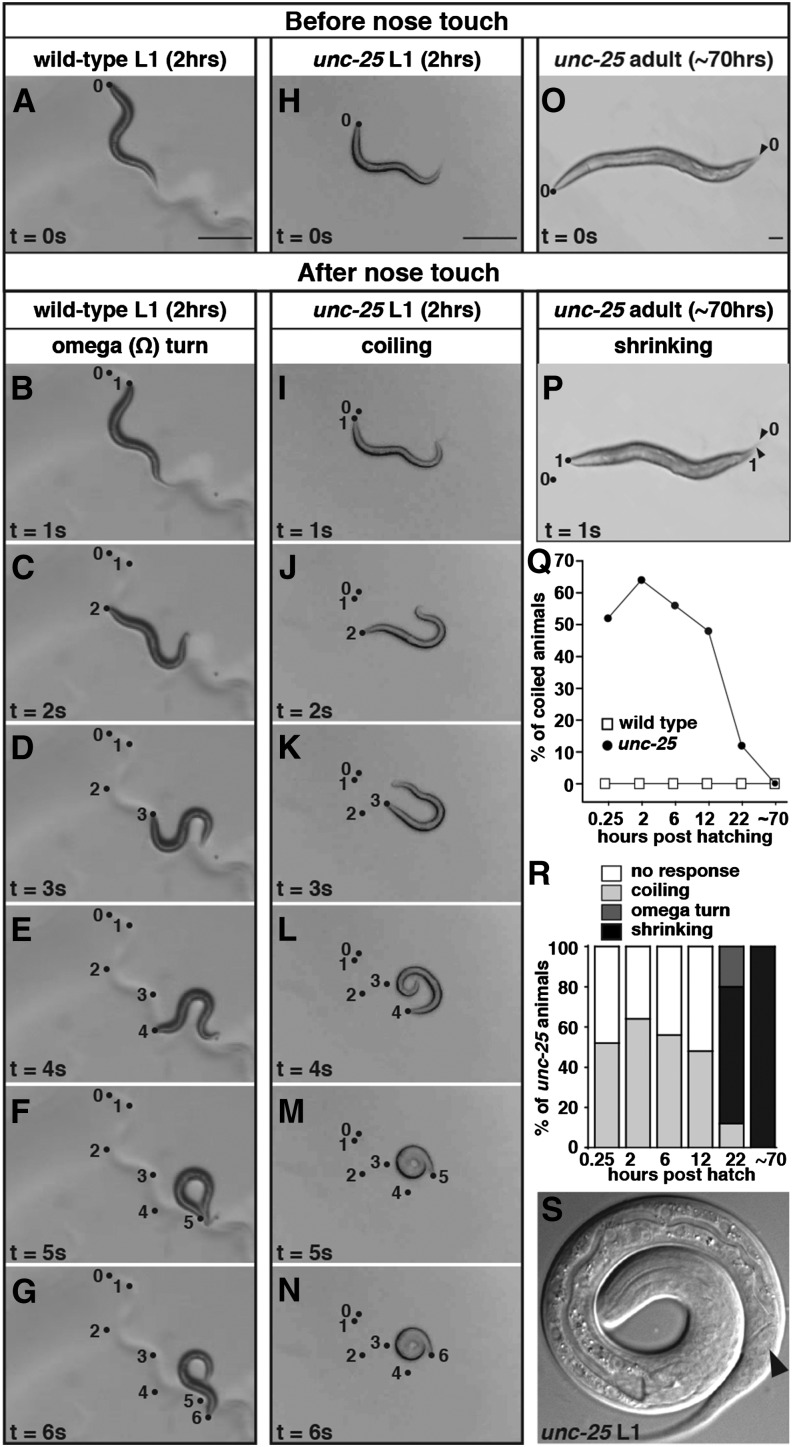
Figure 6.
Endogenous GABA and GABAA receptors relax ventral muscles of early L1 larvae during backing. (A–N) Snapshots of a representative wild type (A–G) or an unc-25 mutant (H–N) L1 at 2 hr posthatch on unseeded NGM plates taken prior to nose touch (t = 0) and every second for 6 sec after nose touch (t = 1 to t = 6). The wild-type L1 responded with backing (B–E), an omega turn (F), and then moving forward again in the direction opposite to that prior to touch (G). The unc-25 L1 curled its tail toward its body (I–K) and eventually coiled its entire body up into a spiral (L and M). This spiral state lasted longer than 1 sec (N). Dots, positions of the tip of the animal’s nose at the corresponding time following nose touch, in seconds, specified by the numbers next to them. The dot labeled 0 represents the nose position one frame before touch. (O and P) Snapshots of an unc-25 adult on an unseeded NGM plate either before (O) or after (P) nose touch. Dots and arrowheads, positions of the tips of the nose and tail, respectively, before nose touch (“0”) and 1 sec after touch (“1”). Contraction of body wall muscles caused the animal to shorten in this response known as “shrinking.” (Q) Quantification of the percentage of animals that coil in response to nose touch as wild-type or unc-25 animals develop. (R) The percentage of unc-25 animals in each category of touch-response behavior during development. The criteria for categorization are described in Materials and Methods. (S) A representative magnified image of a coiled-up unc-25 L1 at 2 hr posthatch. The solid arrowhead points to the ventrally directed opening of the anus, which shows that the animal is coiled with its ventral side in.
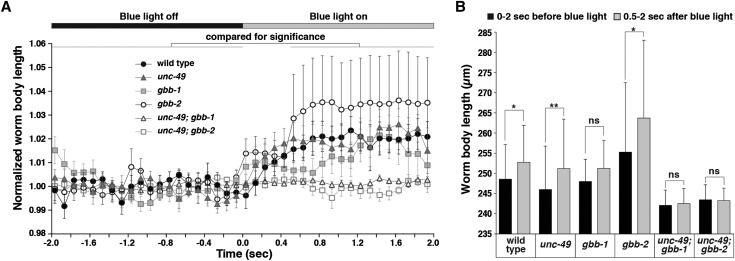
Figure 7.
Optogenetic activation of GABA release confirms that endogenous GABAA signaling is inhibitory in early L1s. (A) Blue-light-induced change in body length of early L1s expressing channel rhodopsin 2 in GABAergic motor neurons. WormLab software was used to track each animal’s body length in every frame of a 30-frame per second movie recorded for 2 sec before and 2 sec after blue light was applied. For each animal, measurements were normalized to the average body length before blue light was on. Data points plotted represent the average of these normalized length measurements collected for each of 10 animals per genotype over each 0.1-sec interval. Solid and open bars across the top of the graph represent the time periods before and after blue light was on, respectively. Error bars represent SEM. (B) Quantification of the average body lengths measured for animals of each genotype both over intervals 2 sec before and 0.5–2 sec after blue light was on. Statistical results of paired t-tests are as follows: wild type, *P = 0.0137; unc-49, **P = 0.0098; gbb-1, P = 0.1055 (ns, not significant); gbb-2, *P = 0.0195; unc-49; gbb-1, P = 0.0840 (ns); and unc-49; gbb-2, P > 0.9999 (ns). Error bars represent SEM. All animals assayed in this experiment were 2 hr posthatch.
Molecular biology and transgenes
Transgenic strains were constructed by injection of plasmid DNA or PCR products into the germline (Mello et al. 1991). To determine the expression patterns of nkcc-1, we amplified an ∼5.6-kb DNA fragment upstream of the nkcc-1 translation start codon that is shared by all nkcc-1 isoforms from genomic DNA with the following primers: 5′-GAC TGG ATC CGT TTC CTG ATG GCT TCT CCC AAG AG-3′ and 5′-GAC TGG TAC CCT GGA CGA CTC CAG CCG CCT GCA AAA TTG-3′. We also amplified an 800-bp 3′ UTR region of the longest isoform nkcc-1c with the following primers: 5′-GAC TGA GCT CGC CCG CAT AAT CTC CGG TTG TTC TAC TC-3′ and 5′-GAC TAC TAG TAG TGT ATC GTG TCC GTG TGG AGT ACA CAC AC-3′. These two sequences, along with coding sequences for GFP, were inserted into pPD49.26 (Fire et al. 1990) to generate pBH3, which was injected at 80 ng/µl with the lin-15 rescuing construct pL15EK at 50 ng/µl into MT8189 lin-15(n765ts) animals to generate the transgene vsIs182.
The body wall muscle cell-specific rescue plasmid (pBH1) was obtained by amplifying the nkcc-1c cDNA from total C. elegans cDNA and inserting it into the pPD96.52 vector (1999 Fire Lab Vector kit) that drives muscle expression under the muscle-specific myo-3 promoter (Reynolds et al. 2005). A myo-3::gfp construct (pAB25A) (Bellemer et al. 2011) was used as a negative control. A construct expressing a nuclear-localized GFP–LacZ fusion in the muscles (pSAK2) was used as a fluorescent injection marker (Fire et al. 1998). Wild-type and LX1668 nkcc-1(ok1621) animals were co-injected with pAB25A (15 ng/µl) and pSAK2 (15 ng/µl) to generate the control strains LX1939 and LX1941, respectively. LX1668 animals were co-injected with pBH1 (15 ng/µl) and pSAK2 (15 ng/µl) to generate the muscle-rescued strain LX1940.
Muscimol assays and analysis
To assay the body wall muscle response of L1 hermaphrodites to 1 mM muscimol at specific time points posthatch, 50–60 eggs at the threefold stage (a late stage of egg development with moving larvae visible inside the egg shell) were placed on a seeded NGM plate around the lawn of food. The number of eggs was checked every 10 min to ensure that any L1 larvae collected had hatched in the previous 10 min. The effect of muscimol on body length was determined by transferring a single L1 onto a no-drug NGM plate, taking a picture, transferring it to an NGM plate containing 1 mM muscimol, monitoring every 10 min, and then taking another picture as soon as it was paralyzed. The time to reach initial paralysis on 1 mM muscimol varied from 30 to 60 min. L1s rapidly desensitized to muscimol and would start moving again typically within 15 min of initial paralysis. Thus our procedure of monitoring every 10 min was required to catch L1 larvae during their initial response to muscimol. L1 larvae were scored as paralyzed if pharyngeal pumping was paused and there was no spontaneous movement of their entire body. Adult animals were scored as paralyzed if they showed the “rubber-band” phenotype (McIntire et al. 1993a), a quick contraction and relaxation of all body wall muscles following a tap on the nose. Animals assayed at the later time points during the L1 stage were obtained by incubating L1s picked within 10 min of hatching at 20° for the corresponding amount of time prior to taking photos for their “before muscimol” body lengths, and then immediately transferring them onto muscimol plates. Photos taken were analyzed using ImageJ software (National Institutes of Health) by drawing a freehand midline down the center of each worm from the tip of the nose to the tip of the tail. For the experiment testing the effect of muscimol on worm body length during development (Figure 2), every animal was individually measured for body length before and after muscimol treatment. The length of each animal after muscimol treatment was normalized to that measured before muscimol treatment to calculate its percentage of change in length. The resulting pairwise measurements were analyzed for a total of 30 animals at each time point. A paired t-test was used for analyzing differences between genotypes at each time point. The average of percent changes in length at each chosen developmental time point was graphed in Figure 2. For the experiment testing cell-specific rescue of the nkcc-1 muscimol sensitivity defect (Figure 4B), 10 animals from each of the five transgenic lines were analyzed. Muscimol plates were prepared the day before an experiment by adding a 100 mM muscimol (Biomol International) stock in water to NGM agar at 50° to a final concentration of 1 mM. Plates were allowed to harden, seeded with OP50, and then left to dry overnight at room temperature. The average of normalized body lengths after muscimol treatment was compared to that before muscimol using two-way ANOVA.
Fluorescence microscopy
To directly visualize the development of GABAergic DD motor neurons and ventral cholinergic motor neurons (Figure 5), L1 and L2 hermaphrodites carrying the fluorescent reporter transgenes of interest were obtained by picking freshly hatched L1s individually and incubating them at 20° for the amount of time specified in each experiment. Adult hermaphrodites were assayed at 24 hr post-L4 stage. Staged animals were immobilized with 10 mM levamisole in water on 4% agarose pads and covered with no. 1 coverslips. A Zeiss LSM 710 confocal microscope was used to obtain Z-stack images with a ×63 Water C-Apochromat objective. The 3D images were compiled into 2D (Figure 3 and Figure 5) using Volocity software (Improvision). To quantify the average GFP::RAB-3 fluorescence intensity expressed in presynaptic termini, a series of boxes with defined dimensions was drawn in ImageJ to include either the dorsal or ventral processes and exclude any motor neuronal cell body or gut autofluorescence. The intensity of the background, which was determined by placing boxes of the same dimension at regions immediately next to the nerve cords, was subtracted using ImageJ. Since animals increase in size during development and the same magnification was used throughout the experiment, the entire bodies of older animals did not fit in the field of view. Therefore, only one-third of each animal’s body starting just posterior to the second pharyngeal bulb was used in the analysis. The ratio of (average intensity of ventral GFP::RAB-3)/[average intensity of (ventral + dorsal GFP::RAB-3)] for each animal within the same age group was averaged and used for comparison across the timeline.
Touch-response behavioral assays and analysis
Adult hermaphrodites were assayed at 24 hr post-L4. L1 and L2 larvae were staged as described above. Prior to the experiment, a single animal was picked onto an unseeded NGM plate and allowed to move freely for 10 min. Then the animal was touched gently at the tip of its nose with a paintbrush hair (Jack Richeson Pure Kolinsky Sable 1 Flat Watercolor Brush; ASIN: B0009I865E) attached to one end of a worm pick. Representative behavioral responses were video recorded for detailed analyses (Figure 6). Responses of additional animals were analyzed without video recording. Four categories of response during the 6 sec after stimulus were observed and defined as follows: (1) “Omega turn”—the animal backs, then moves forward touching its body near the tail with the tip of its nose in a form resembling the symbol Ω, and then proceeds forward in a direction opposite that prior to touch; (2) “coiling”—the animal curls up by wrapping its head or tail completely within its body; (3) “shrinking”—the animal shortens without curling any part of its body; and (4) “no response”—the animal keeps moving forward following the stimulus and does not show any of the aforementioned behaviors. At each developmental time point, 10 animals were assayed by touching each five times in succession for a total of 50 trials per genotype.
Optogenetic assays and data analysis
Optogenetic assays and automated video analysis for the extraction of worm body lengths as shown in Figure 7 were adapted from previous studies (Liewald et al. 2008; Schultheis et al. 2011). All-trans retinal plates were made by adding 4 μl of 100 mM stock all-trans retinal (Spectrum Chemical, no. R3041) in 100% ethanol to 1 ml of 37° OP50 bacteria cultured in B broth. Standard NGM plates were then seeded with 150 μl of this mixture and let dry overnight at room temperature in a light-tight environment. Five late L4s of each genotype were picked onto all-trans retinal plates and maintained in the dark. At least two generations later, L1s of each genotype were staged for experiments. All animals were imaged under a Zeiss M2Bio fluorescence dissecting scope and recorded with a Point Gray camera (FL3-FW-03S3M-C) at 30 frames per second for 2 sec before and 2 sec after blue light illumination. A 480-nm blue light LED (2 mW/mm2, as measured with a Solar Meter model 9.4 blue light radiometer) (Solartech) was used for illumination. The body length in each frame was then determined using automated worm-tracking software (WormLab, MBF Bioscience). n = 10 animals per genotype. For each recording, four parameters in WormLab, namely “threshold level,” “gradient correction,” “smoothing (Gaussian filtering),” and “fill holes,” were adjusted manually to ensure detection of the entire animal by the software in every frame without erroneously including foreign objects. A fine fluorescent powder (Sirchie GREENescent 2 oz Fluorescent Latent Print Powder, cat. no. LL703) was added onto unseeded NGM plates so that the frame during video playback when the blue light came on could be determined. To apply the powder such that only a few grains were present per field of view of the microscope, a small amount of powder was added onto a Kimwipe tissue (Kimtech), which was then tapped to leave only a trace adhering to it. A nylon flat fan-shaped brush (Connoisseur Mini Brush Set, 9-piece, fan no. 2, item model number: 519VP. ASIN: B0041MXGL8) was used to dust the powder traces onto an unseeded NGM plate by one gentle sweep.
Results
Muscle response to a GABAA receptor agonist switches from excitatory to inhibitory during C. elegans development
GABAergic synapses in both the vertebrate brain and in the ventral C. elegans body wall muscles are present prior to development of excitatory synapses, and given this parallel, we hypothesized that GABA response might undergo depolarizing to hyperpolarizing shift in C. elegans similar to the shift previously observed in vertebrates. To test this, we assayed muscle response to the GABAA receptor agonist muscimol at four time points during the L1 stage. We isolated precisely staged larvae and transferred them to plates containing muscimol. We measured the resulting changes in their body lengths as soon as the muscimol paralyzed the animals, thus avoiding the effects of desensitization to muscimol that ensue later. Previous work has shown that in wild-type adults, muscimol inhibits contraction of all body wall muscles via the C. elegans GABAA receptor homolog UNC-49. As a result, the entire worm elongates (Schaeffer and Bergstrom 1988). We reproduced this phenotype in our experiments and observed an 8.0 ± 0.9% increase in wild-type adult body length (Figure 2, A, B, and E). In young L1 larvae at 2 hr posthatch, however, we found that muscimol excited muscle contraction and decreased average body length by 3.3 ± 1.5% (Figure 2, C–E). At 6 hr posthatch, we observed no significant effect of muscimol on body length. By 12 hr posthatch, in the late L1 stage, muscimol assumed its mature effect of relaxing muscles and caused a 6.9 ± 0.8% increase in body length (Figure 2E). Mutants for UNC-49 showed no response to muscimol at any developmental stage (data not shown).
Our results show that the GABAA receptor agonist muscimol switches from contracting to relaxing muscles at ∼6 hr posthatch during the first larval stage of C. elegans development. We interpret the switch in response to muscimol from muscle contraction to muscle relaxation as indicating that the muscle response to GABAA switches from depolarizing to hyperpolarizing, and we will subsequently use these latter terms to describe our results. The high concentration of muscimol used in these experiments likely activates all GABAA receptors, provoking a strong muscle response so that measuring contraction or relaxation gives a sensitive readout of the polarity of GABAA receptor activity. We note that weakly depolarizing GABAA receptor activation, as may occur with endogenous GABA signaling, can be inhibitory rather than excitatory through the electrophysiological phenomenon of shunting inhibition (Ben-Ari et al. 2007; Blaesse et al. 2009) Thus our results with the muscimol assay reveal that GABAA signaling appears to be depolarizing in early L1s, but do not reveal whether the muscle depolarization caused by endogenous GABA signaling in early L1s is strong enough to be excitatory. Experiments presented below will explore the effects of endogenous GABA signaling in early L1s.
The muscimol response switch does not depend on endogenous GABA, as we still observed the switch in mutant animals lacking the GABA biosynthetic enzyme UNC-25 (Figure 2F). Indeed, the excitatory effect of muscimol in early L1s was particularly strong in unc-25 mutants, causing an 18.2 ± 0.1% decrease in average body length.
We found that 56% of the muscimol-treated wild-type L1s at 2 hr posthatch curled dorsally as they became paralyzed and shortened in length (Figure 2D and data not shown). This suggests that the dorsal muscles, which are not yet innervated by GABAergic neurons in early L1s, clearly shortened, while the effect of muscimol on the ventral muscles is less clear. Although GABA is absent from the normally GABAergic DD and VD motor neurons in unc-25 mutants, these neurons are still able to form synapses onto the corresponding body wall muscles (Jin et al. 1999). Moreover, the UNC-49 GABAA receptors in the muscles still cluster at the postsynaptic sites apposing to the presynaptic termini formed by the DD and VD neurons in unc-25 adults (Gally and Bessereau 2003). The dramatic muscimol-induced shortening of unc-25 L1s was not accompanied by the coiling seen in the wild-type L1 response to muscimol. Therefore, it appears that muscimol can excite contraction of both the dorsal and ventral muscles of early L1 animals, and this early excitatory effect is strongest for muscles that have not yet experienced endogenous GABA signaling.
NKCC-1 is a chloride accumulator homolog widely expressed in C. elegans muscles and neurons
To understand the developmental regulation of GABA signaling, we aimed to identify the Cl− transporters that regulate intracellular [Cl−] to alter GABA response. Our laboratory previously used genetic screens to identify two Cl− extruders, KCC-2 and ABTS-1, which promote the hyperpolarizing effect of GABA on adult body wall muscles (Tanis et al. 2009; Bellemer et al. 2011). Here we describe the identification of a Cl− accumulator homolog that has an opposing effect.
The mammalian NKCC-1 transporter accumulates Cl− into neurons and has been shown to promote depolarizing GABA signaling in immature neurons (Sung et al. 2000; Yamada et al. 2004). A phylogenetic analysis of the cation chloride cotransporters in C. elegans and other species shows that NKCC-1 is the sole C. elegans ortholog of the two human sodium potassium chloride cotransporters (NKCCs) (Figure 3A), with 43% sequence identity to NKCC-1 and 41% sequence identity to NKCC-2. There are eight alternatively spliced isoforms predicted for nkcc-1, and they share a common promoter region. To examine the nkcc-1 expression pattern, we constructed a reporter transgene that drove GFP expression from the common nkcc-1 promoter and the 3′ untranslated region of nkcc-1c, the longest nkcc-1 isoform (Figure 3B). We found that this transgene was expressed both in the dorsal and the ventral body wall muscles, a subset of head and tail neurons, the posterior intestine, and the vulval muscles in adults (Figure 3, C–G).
NKCC-1 promotes depolarizing signaling by the GABAA agonist muscimol onto body wall muscles
We obtained a putative nkcc-1 null mutation, ok1621, which has an 802-bp deletion spanning exons 5–7 of nkcc-1c (Figure 3B). This deletion results in a predicted frame shift in the region coding the 3rd of the 11 predicted transmembrane domains of NKCC-1. The nkcc-1 mutant animals appear healthy and grossly wild type.
We found that the GABAA agonist muscimol relaxes body wall muscles of newly hatched nkcc-1 mutant L1s, causing these animals to lengthen by 4.9 ± 1.1%, which is opposite to the muscle contraction evoked by muscimol in wild-type early L1s (Figure 4A). Thus NKCC-1 may function just like its mammalian homolog NKCC-1 to accumulate Cl− into excitable cells to increase [Cl−]i and promote depolarizing GABAA response. Freshly hatched L1s mutant for the Cl− extruders KCC-2 or ABTS-1 showed enhanced muscle contraction in response to muscimol with decreases in body length of 11.8 ± 1.2% and 6.4 ± 0.9%, respectively (Figure 4A). Unlike the wild type, however, the muscimol response of kcc-2 or abts-1 mutants failed to switch muscle relaxation in late L1 stage and thus remained depolarizing through development (Figure 4A).
We note that none of the mutant animals coiled in response to muscimol at any stage during development, suggesting that both their dorsal and ventral body wall muscles were affected similarly by muscimol. Thus wild-type early L1 larvae at stages before the locomotor circuit matures are the only animals we have tested that showed coiling upon muscimol treatment (Figure 2D).
Because NKCC-1 is expressed in the body wall muscles and affects their response to muscimol, we hypothesized that NKCC-1 functions in the muscles themselves to direct their muscimol response. To test this, we reexpressed NKCC-1 specifically in the body wall muscles of nkcc-1 mutants. As a control, we expressed GFP in the muscles and found it did not alter the muscle response to muscimol of early L1 animals (Figure 4B). We found that reexpression of nkcc-1 in the muscles of the nkcc-1 mutant rescued muscimol response in L1s, the only developmental stage that nkcc-1 showed a muscimol response defect, causing them to lengthen (Figure 4B). Therefore NKCC-1 expression in muscles is apparently sufficient to accumulate Cl− into these cells, allowing a depolarizing response to the GABAA agonist muscimol in early L1s.
Since loss of the NKCC-1 Cl− accumulator had an effect on muscimol response opposite to that caused by loss of the KCC-2 or ABTS-1 Cl− extruders, we investigated double mutants lacking the accumulator plus one of the extruders. We found that similar to nkcc-1 single mutants, both the kcc-2 nkcc-1 and abts-1; nkcc-1 double mutant animals showed hyperpolarizing responses to muscimol throughout development (Figure 4C). This result is consistent with the idea that NKCC-1 is the only Cl− accumulator in body wall muscle cells, while KCC-2 and ABTS-1 extruders act partially redundantly (Bellemer et al. 2011) to extrude Cl− and counterbalance the effect of NKCC-1. In double mutants lacking NKCC-1 and one Cl− extruder, the remaining Cl− extruder may still function to lower intracellular Cl− without opposition from NKCC-1 so that muscimol has a hyperpolarizing effect. To examine this idea further, we constructed triple-mutant animals lacking all three Cl− transporters. However, these animals, like the abts-1; kcc-2 double mutants (Bellemer et al. 2011), were too sick for any meaningful analysis.
Hypotheses for the function of depolarizing GABA in the developing locomotor circuit
Studies in vertebrates have suggested two functions for the depolarizing-to-hyperpolarizing switch in GABA response during development. First, early depolarizing GABA is essential for proper circuit development (Chudotvorova et al. 2005; Akerman and Cline 2006; Ge et al. 2006; Cancedda et al. 2007). Second, early depolarizing GABA provides the initial source of excitation in the developing nervous system prior to the time when excitatory neurons are present (Saint-Amant and Drapeau 2000; Gao and Van Den Pol 2001; Hennou et al. 2002; Gozlan and Ben-Ari 2003; Johnson et al. 2003).
Extending these ideas to C. elegans suggests the following two specific hypotheses. First, genetically perturbing the GABA response switch would perturb the development of synapses in the worm locomotor circuit. Second, GABA released from DD neurons might initially excite ventral muscle bends during the early L1 stage, prior to development of the cholinergic neurons that will ultimately function to excite these muscles in later stages. This would explain how ventral body bends are generated during L1 larval movement. Once DD neurons rewire to synapse onto dorsal muscles (Figure 1), GABA response would switch to inhibitory, and DD-released GABA would thenceforth help relax dorsal muscles during the ventrally directed body bends excited by the newly developed ventral cholinergic motor neurons. In the remainder of this work, we systematically investigate the two specific hypotheses outlined above. Our results below, however, demonstrate that early depolarizing GABA is neither required for synaptic development nor for early excitation in the C. elegans locomotor circuit.
Presynaptic motor neuron development is coincident with the postsynaptic muscle response switch to muscimol
Testing the above hypotheses requires knowing when the postsynaptic muscimol response switch occurs relative to presynaptic DD rewiring and ventral cholinergic neuron development in the locomotor circuit. We measured the timing of DD rewiring, as had been done previously (White et al. 1986; Hallam and Jin 1998; Park et al. 2011), but using the same developmental staging procedure we used to measure the timing of the muscimol response switch, and we also monitored the timing of ventral cholinergic neuron development. Thus we could determine the relative timing of these three events.
To determine the timing of DD motor neuron rewiring, we used a chromosomally integrated transgene that expresses the presynaptic protein RAB-3 tagged with GFP from the DD-specific promoter flp-13 (Nelson et al. 1998; Park et al. 2011) and monitored the development of these synapses in living animals at 0.25, 2, 6, 12, 22, and ∼70 hr posthatch (Figure 5, A–C and data not shown). We found that the rewiring process, which involved dissolving DD synapses on the ventral side and reforming them dorsally, reached its midpoint 6 hr posthatch (Figure 5B) and was completed by 12 hr in the late L1 stage (Figure 5C). This timing precisely coincided with the muscimol response switch in the postsynaptic muscles (Figure 2). We note that under our culture and staging conditions, the timing of DD rewiring appears slightly earlier than has been reported (Hallam and Jin 1998; Park et al. 2011; Thompson-Peer et al. 2012). This is most likely due to the different staging methods used: We obtain freshly hatched larvae by simply picking the larvae within 15 min after they hatch, whereas previous studies have obtained synchronized populations of early L1s by lysing gravid adults (Hallam and Jin 1998), letting gravid adults lay eggs for an hour (Park et al. 2011) or picking newly hatched L1s 30 min after incubating a plate of isolated embryos (Thompson-Peer et al. 2012).
We then examined the development of ventral cholinergic synapses using a chromosomally integrated transgene that expresses GFP::RAB-3 from the cholinergic neuron-specific promoter unc-4 (Spilker et al. 2012). We found that cholinergic synapses were present dorsally throughout development. Ventral cholinergic synapses first appeared between 2 and 6 hr posthatch (Figure 5, D and E). The intensity of ventral GFP::RAB-3 was approximately half of that on the dorsal side at 6 hr, and then became comparable by 12 hr posthatch. Thus the timing of ventral cholinergic synapse formation again corresponded with both the postsynaptic muscimol response switch and the rewiring of DD neurons.
Genetically disrupting the depolarizing-to-hyperpolarizing GABA response switch delays but does not disrupt development of the locomotor circuit
We used the GABAergic DD-specific fluorescent marker described above to examine the development of DD synapses in mutants for the Cl− transporters NKCC-1 and KCC-2. Since the depolarizing-to-hyperpolarizing muscimol response switch happens prematurely in nkcc-1 mutants, we were interested to see whether presynaptic DD neuron rewiring would happen prematurely in these animals. Similarly, since the muscimol response switch never happens in kcc-2 mutants, we were interested in whether this would prevent presynaptic DD rewiring. We found that in both Cl− transporter mutant backgrounds synapse development in the locomotor circuit was delayed for ∼6 hr compared to that of wild-type animals, and thus synapse development reached completion at the early L2 stage (Supporting Information, Figure S1). This delay in Cl− transporter mutants was similar to delays in DD remodeling previously observed in other C. elegans mutants (Hallam and Jin 1998; Park et al. 2011; Thompson-Peer et al. 2012). Despite showing delays in locomotor circuit development, both nkcc-1 and kcc-2 mutant animals were able to move forward and backward similarly to the wild type by making both dorsal and ventral body bends (Tanis et al. 2009 and data not shown). We note that previous studies (Jin et al. 1999) as well as our data not shown also show that complete lack of GABA signaling (in mutants for the UNC-25 GABA biosynthetic enzyme) are able to rewire the GABAergic DD neurons correctly. In contrast, disruption of Cl− gradients in vertebrate nervous systems causes severe developmental defects in the nervous system, not just delays in developmental timing that ultimately result in normal adult structures and normal circuit function (Chudotvorova et al. 2005; Akerman and Cline 2006; Ge et al. 2006; Cancedda et al. 2007; Young et al. 2012).
To investigate whether nkcc-1 mutants might show changes in muscimol response due to defects in postsynaptic clustering of GABA receptors, we used a transgene that tags the UNC-49 GABAA receptor with GFP (Bamber et al. 1999). We found that loss of nkcc-1 did not have any effect on the localization of UNC-49 at any point in development (data not shown).
Thus the C. elegans locomotor circuit is able to compensate for perturbations in GABA signaling to develop normally but with delays in the timing of development. The hypothesis based on studies in vertebrate brain that the GABA response switch is essential for synapse and circuit development (Chudotvorova et al. 2005; Akerman and Cline 2006; Ge et al. 2006; Cancedda et al. 2007) does not appear to apply to the C. elegans locomotor circuit.
Endogenous GABA and GABAA receptors relax ventral muscles of early L1 larvae during backing
An alternative hypothesis for the function of the GABA response switch based on vertebrate studies is that during early development, depolarizing GABA provides the initial source of excitation prior to the development of the excitatory neurons that will provide this function in the mature nervous system. This hypothesis is appealing in the case of the C. elegans locomotor circuit, since in early L1 larvae, ventral muscles are innervated only by GABAergic neurons, as the cholinergic neurons that will excite the muscles in adult are not yet present (Figure 1). Thus the GABAergic synapses temporarily made by DD neurons in early L1s could excite ventral body bends in these animals.
The hypothesis described above predicts that early L1 animals lacking GABA signaling will not be able to generate ventral body bends. We first tested this prediction by examining forward locomotion of early L1 larvae in mutants for the GABA biosynthetic enzyme UNC-25, the GABA vesicular transporter UNC-47, or the GABAA receptor UNC-49. Like wild-type early L1s (File S1), all these mutant animals were able to move forward by making both dorsal and ventral body bends, demonstrating that ventral body bends, at least in the forward direction, do not require GABA (File S2). Further, we found that early L1s mutant for any of the Cl− transporters that affect muscimol response (kcc-2, abts-1, and nkcc-1) also move forward by making both dorsal and ventral body bends (data not shown).
Previous studies of adult animals showed that GABA has relatively little effect on forward locomotion, but is essential for the body bends that direct backward locomotion (McIntire et al. 1993b). Thus we next examined backward locomotion to reveal whether endogenous GABA might have an excitatory effect in early L1 larvae. When wild-type C. elegans is touched gently on the nose, it responds with an omega turn by backing up several body bends and then moving forward again with a very deep body bend that causes the head to typically touch the body near the tail, forming a posture that resembles the symbol Ω. Its continuing forward movement is then in a new direction away from the object it initially touched (Croll 1975). Adult animals lacking GABA or the GABAA receptor fail at backing and respond to nose touch with a shrinker phenotype, in which all body wall muscles simultaneously contract, so that the entire animal briefly shortens (McIntire et al. 1993b). This response results from simultaneous excitatory cholinergic signaling onto both the ventral and dorsal muscles. In contrast to adults, early L1 larvae have excitatory acetylcholine synapses only on the dorsal muscles, and the ventral muscles receive synapses only from the DD GABAergic neurons (Figure 1) (White et al. 1992; Jin et al. 1994). According to the hypothesis that endogenous GABA excites ventral muscles early in development, newly hatched L1s mutant for GABA signaling should fail to contract ventrally during backward movement and should bend only dorsally. If instead GABA inhibits ventral muscles early in development, L1 GABA mutants should show increased or prolonged ventral contraction as they would lack an inhibitory input to relax the ventral muscles.
We examined nose-touch response in both the wild-type and mutant animals lacking GABA at six developmental stages, starting with four time points within the L1 stage that span the period of locomotor circuit development. We found that wild-type L1s respond to nose touch just as do wild-type adults (Croll 1975) by backing, making an omega turn, and continuing forward in a new direction (Figure 6, A–G). However, early unc-25 L1 animals, which lack a biosynthetic enzyme necessary to make GABA, failed at the initial backing step. Instead of making the deep bends on both sides of their body necessary for backing, they coiled up (Figure 6, B–N). The unc-25 mutants only displayed this phenotype as L1 larvae. By the early L2 stage (22 hr posthatch), after the locomotor circuit has matured, these mutants instead displayed the shrinking phenotype seen in unc-25 adults (Figure 6, O and P) and no longer curled up during backing [Figure 6, Q (solid circles) and R]. We also observed the coiling phenotype in mutant L1s lacking the GABAA receptor UNC-49 or the GABA vesicular transporter UNC-47 (data not shown). Coiling during backing was always toward the ventral side (Figure 6S) in all these L1 larvae lacking GABA signaling. We never observed the coiling response in wild-type animals (Figure 6Q, open squares). We also tested adults and L1 larvae for each of the Cl− transporter mutants (kcc-2, abts-1, and nkcc-1): all were able to back normally by making both dorsal and ventral body bends without coiling (data not shown).
Our results show the function of endogenous GABA and the GABAA receptors in early L1 animals is to relax their ventral muscles so that they can bend dorsally during backing. Our earlier observations using the GABAA agonist muscimol show that GABAA activity depolarizes muscles in early L1 animals. While the massive GABAA activation caused by application of exogenous muscimol depolarizes muscles enough to excite contraction, it appears that the more modest activation of GABAA receptors by release of endogenous GABA results in shunting inhibition, a well-characterized effect of modest depolarization (Ben-Ari et al. 2007; Blaesse et al. 2009).
Optogenetic activation of GABA release confirms that endogenous GABAA signaling is inhibitory in early L1s
As an alternative approach to determine whether endogenous GABAA signaling is excitatory or inhibitory early in development, we optogenetically activated the GABAergic DD neurons of early L1 larvae and assayed whether this results in contraction or relaxation of the body wall muscles. By knocking out GABAA or GABAB receptors in this assay, we also were able to isolate the components of the muscle response due to each receptor type. The GABAA receptor UNC-49 is expressed on body wall muscles and directly mediates muscle relaxation in adults (McIntire et al. 1993a). Pevious studies have shown that a GABAB receptor, consisting of two obligate subunits GBB-1 and GBB-2, is expressed on the cholinergic motor neurons that excite the body wall muscles, and by inhibiting these excitatory neurons the GABAB receptor indirectly relaxes the muscles (Dittman and Kaplan 2008; Schultheis et al. 2011). Thus GABA leads to body wall muscle relaxation in adult animals via both the GABAA and GABAB receptors.
We simply repeated the optogenetic assays of Schultheis et al. (2011), except using early L1 larvae instead of adult animals, to determine whether GABAA and GABAB signaling inhibit or excite muscles in early L1s. We obtained strains of C. elegans carrying a chromosomally integrated transgene that expresses channel rhodopsin 2 (ChR2), a blue-light-activated cation channel, specifically in GABAergic motor neurons, and that additionally contain various combinations of mutations for the GABAA receptor UNC-49 and the GABAB receptor subunits GBB-1 and GBB-2 (Schultheis et al. 2011). We used the automated worm-tracking system WormLab (MBF Bioscience) to measure the body length of each animal before and after blue light illumination.
We found that upon activation of ChR2 by blue light, which in turn triggers GABAergic neuron depolarization and endogenous GABA release, wild-type L1 animals became paralyzed and lengthened due to relaxation of all body wall muscles [Figure 7, A (solid circles) and B], just as had been observed in wild-type adults by Schultheis et al. (2011). Blue light activation of ChR2 also paralyzed and lengthened unc-49 L1s, in which GABA can only signal via GABAB receptors [Figure 7, A (solid triangles) and B]. Similarly, we found that activation of ChR2 paralyzed and lengthened gbb-1 and gbb-2 L1s, in which GABA signals only via GABAA receptors [Figure 7, A (solid squares and open circles) and B]. While the average lengthening in gbb-2 mutants appeared even larger than that of the wild type, this was not statistically significant due to the large error bars in the gbb-2 measurement that may have resulted from ∼5% variation in the body lengths of the gbb-2 animals prior to blue light illumination. We observed no response upon blue light exposure in mutant L1s lacking the GABA biosynthetic enzyme UNC-25 (data not shown) or in double-mutant animals lacking both the UNC-49 GABAA receptor and the GBB GABAB receptor [Figure 7, A (open triangles and open squares) and B]. We also observed no response to blue light exposure in animals grown on plates without all-trans retinal, a cofactor required for ChR2 activity (data not shown). Thus the effects of ChR2 activation of the GABAergic neurons were entirely due to GABA signaling through GABAA and GABAB receptors.
Our results showed that in early L1 larvae, just as in wild-type adults, endogenous GABA inhibits body wall muscle contraction through both the GABAA and GABAB receptors. Therefore, endogenous GABA signaling in C. elegans is inhibitory throughout development, despite evidence from our muscimol experiments that GABAA response is depolarizing in early L1 animals.
Discussion
A conserved developmental shift in the polarity of GABAA signaling
Previous work had established that a Cl− transporter-dependent depolarizing-to-hyperpolarizing shift in GABAA response during development is evolutionarily conserved across a range of vertebrates, including chickens, amphibians, fish, and mammals (Obata et al. 1978; Ben-Ari et al. 1989, 2007; Blaesse et al. 2009; Zhang et al. 2010). Our work shows for the first time that such a switch also occurs during development of an invertebrate, suggesting the GABA polarity shift is fundamental to nervous system development in all animals and not a specific feature of vertebrates. The deep conservation of the GABA response polarity shift in development suggests that there should be conserved function for the shift that could be revealed using the rigorous genetics and highly characterized wiring of the C. elegans nervous system.
The evidence for a GABA response polarity shift in C. elegans rests on our observation that the GABAA receptor agonist muscimol excites body wall muscles in early L1s. Muscimol then shifts to inhibiting these muscles at the mid-L1 stage as the locomotor circuit matures, coincident with the rewiring of GABAergic DD neurons and the development of ventral cholinergic synapses. We interpret this shift in response to muscimol from muscle contraction to muscle relaxation to indicate that the massive activation of all GABAA receptors from depolarizing to hyperpolarizing during L1 development. The GABA response shift in vertebrate development depends on specific Cl− transporters (Blaesse et al. 2009), and we found that the C. elegans muscimol response shift depends on the worm orthologs of these same Cl− transporters. In mutants for the nkcc-1 Cl− accumulator, the muscimol response shift occurs prematurely, but in kcc-2 or abts-1 Cl− extruder mutants, the switch never occurs. These observations are consistent with the model that changes in the expression or activity level of C. elegans Cl− transporters contribute to a developmental decrease in [Cl−]i, just as has been shown to occur in the developing vertebrate brain (Plotkin et al. 1997; Rivera et al. 1999; Sun and Murali 1999; Yamada et al. 2004). Indeed, transcriptional upregulation of both kcc-2 and abts-1 during C. elegans development has been previously described (Tanis et al. 2009; Bellemer et al. 2011). We note, however, that a recent report suggests that Cl− transporters merely provide assistance to a more dominant role of local impermeant anions in setting [Cl−]i (Glykys et al. 2014), so other factors may also contribute to the muscimol response shift.
A developmental decrease in [Cl−]i has been well established in vertebrate neurons using both perforated patch electrophysiological recordings and genetically encoded fluorescent Cl− reporter proteins (Duebel et al. 2006; Markova et al. 2008). C. elegans membranes are not amenable to the perforated patch technique (Bellemer et al. 2011), and our unpublished studies using fluorescent Cl− reporter proteins in C. elegans muscles have so far shown results too variable to make conclusions. Nevertheless, our results showing a Cl− transporter-dependent shift in muscimol response provides convincing, albeit indirect, evidence of a developmental decrease in [Cl−]i that shifts the polarity of GABAA response.
We found that although muscimol excites muscle contractions in early L1, endogenous GABAA signaling inhibits muscle contraction. This difference could be due to the fact that exogenous muscimol bathes the entire animal and activates all GABAA receptors on the muscle cells, while endogenous GABA is released at synapses and may affect only postsynaptically localized GABAA receptors. If [Cl−]i is higher in the soma of the muscle cells than in the dendrite-like muscle arms where postsynaptic GABAA receptors cluster (Gally and Bessereau 2003), this might account in part for the discrepancy between responses to muscimol vs. synaptic GABA, and also for the fact that the muscimol response switch coincides with the formation of GABA synapses. Although GABAA receptor clusters are observed only on ventral muscles of early L1s (Gally and Bessereau 2003), our finding that unc-49-dependent body wall muscle contraction occurs on both dorsal and ventral sides of L1s implies UNC-49 GABAA receptors are present on dorsal muscles in early L1s, but only form observable clusters on dorsal muscles at a later stage. Consistent with the idea that GABAA receptor clustering controls the response of muscles to activating these receptors, we note that the muscimol response switch still occurs in unc-25 mutants that lack GABA, and that synapse formation and clustering of GABAA receptors also occur normally in these mutants (Gally and Bessereau 2003).
The discrepancy in the responses to muscimol vs. endogenous GABA in early L1 animals could also be due to differences in the kinetics or number of GABAA receptors activated that result in excitation by muscimol but shunting inhibition by synaptic GABA. Depolarizing GABA in the developing vertebrate nervous system is thought to often result in shunting inhibition rather than excitation (Ben-Ari et al. 2007; Blaesse et al. 2009).
What is the biological purpose of the GABA response shift?
Our goal is to exploit the power of the simple C. elegans locomotor circuit as an experimental system to generate a meaningful understanding of the biological purpose of the GABA response shift. This could provide the first detailed example in any organism of how a change in GABAA response helps a specific circuit function as it develops.
C. elegans L1s move by alternating dorsal and ventral body bends, just as do older animals. However, the ventral muscles of early L1s have not yet received any excitatory cholinergic synapses. Instead, they temporarily receive GABAergic DD synapses that are later removed during circuit rewiring at 6 hr posthatch (White et al. 1992; Jin et al. 1994), exactly when we found the depolarizing to hyperpolarizing GABAA signaling switch occurs, and when cholinergic synapses start to form onto the ventral muscles. An appealing model to explain how early L1s move would be that early endogenous GABA is excitatory. If this was true, it would explain (1) how early L1s make ventral body bends; (2) the purpose of presynaptic DD rewiring at the mid-L1 stage; and (3) the biological function of the depolarizing to hyperpolarizing GABA response switch.
Our data show that this model for early excitatory GABA signaling, however appealing, is not valid, and that GABAA signaling appears to be inhibitory throughout postembryonic development. Using nose touch to force early L1s to make deep body bends as they back up, we find that endogenous GABA and GABAA signaling are both required to relax the ventral muscles. In an optogenetic assay to force release of endogenous GABA, we have knocked out GABAB signaling to isolate only GABAA responses and find that it is inhibitory, causing early L1s to elongate. Furthermore, although knocking out Cl− transporters can disrupt the muscimol response switch, all of the transporter mutants we tested are able to move like the wild type throughout development. We note, however, that knocking out the ABTS-1 extruder causes a compensatory change in the partially redundant KCC-2 extruder (Bellemer et al. 2011). This suggests that the locomotor circuit may in general be sophisticated enough to induce compensatory changes and continue generating apparently normal behavior even after we alter GABA responses. Nevertheless, our results are inconsistent with endogenous GABA being excitatory at any point during postembryonic development. The only remaining scenario in which early GABA might be excitatory would be in generating movement of late-stage embryos inside the eggshells. We note that there is one proven example of excitatory GABA signaling in C. elegans, which, however, does not involve a developmental shift or even the GABAA receptor, but rather GABA signaling through a GABA-gated cation channel that excites a muscle in the excretory system (Beg and Jorgensen 2003).
The idea of early excitatory GABA in vertebrates has been the subject of a large body of research. GABA signaling in the developing C. elegans locomotor circuit and in the developing mammalian brain show striking parallels. In C. elegans early L1s, the ventral muscles receive temporary GABAergic synapses prior to the formation of excitatory cholinergic synapses. Similarly, in vertebrate brain, GABA signaling is established prior to the development of excitatory glutamatergic synapses (Liu et al. 1996; Tyzio et al. 1999; Akerman and Cline 2006) A body of work supports the hypothesis that early network activity, and in particular giant depolarizing potentials seen in the developing brain, are largely dependent on excitatory GABAA signaling (Ben-Ari et al. 2007). An argument has been made that GABA is not actually excitatory during vertebrate development (Bregestovski and Bernard 2012), but this argument has been repudiated by a large group of other GABA researchers (Ben-Ari et al. 2012). There is complete agreement, however, that depolarizing GABAA signaling in early development need not always be excitatory, and that it often results in shunting inhibition (Staley and Mody 1992).
Even though C. elegans L1s apparently do show a developmental shift of depolarizing to hyperpolarizing in GABAA signaling, there is no evidence that the early depolarizing GABAA signaling is ever excitatory at any point in this organism. It appears that the shift, rather than being excitatory to inhibitory, is from early shunting inhibition to later hyperpolarizing inhibition. A shift in GABAA response is deeply conserved across species, but because GABA signaling is never excitatory in C. elegans, it is hard to argue that the shift arose in evolution to supply excitation in developing nervous systems.
We also explored the hypothesis that the GABA response shift functions to support neural circuit development, based on studies in vertebrates showing that disrupting the GABA response shift leads to defects in synapse and dendrite development (Chudotvorova et al. 2005; Akerman and Cline 2006; Ge et al. 2006; Cancedda et al. 2007). We found that in the C. elegans locomotor circuit, development of GABAergic synapses proceeded to completion but with a 6-hr delay when we disrupted the GABA response shift with chloride transporter mutations. Thus the GABA response shift is not essential for development of neural circuits, as had been suggested by studies in vertebrates. However, the delay in GABA synapse rewiring observed in C. elegans provides an attractive model for further exploring the role of GABA signaling in neural circuit development.
Our work shows that the depolarizing-to-hyperpolarizing shift in GABAA response during development previously known to occur only in vertebrates is deeply evolutionarily conserved, as it also appears to occur in C. elegans. The two functions previously ascribed to the GABA response shift, to provide excitation early in nervous system development, and to allow the development of neural circuits, do not seem to apply to the C. elegans locomotor circuit. The shift in GABA response from shunting inhibition in early development to hyperpolarizing inhibition in mature circuits must have some conserved functional value to justify its evolutionary conservation, although as yet, even in the simple locomotor circuit of C. elegans, the details of this value remain elusive.
Supplementary Material
Acknowledgments
Heather Holmes and Michael Romero (Mayo Clinic) helped construct the nkcc-1 cDNA. This work was funded by National Institutes of Health grant NS036918 (to M.R.K.). Confocal instrumentation was supported by a grant to the Yale Liver Center (DK34989). Some strains used were obtained from the Caenorhabditis Genetics Center.
Footnotes
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.114.173963/-/DC1.
Communicating editor: P. Sengupta
Literature Cited
- Akerman C. J., Cline H. T., 2006. Depolarizing GABAergic conductances regulate the balance of excitation to inhibition in the developing retinotectal circuit in vivo. J. Neurosci. 26: 5117–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamber B. A., Beg A. A., Twyman R. E., Jorgensen E. M., 1999. The Caenorhabditis elegans unc-49 locus encodes multiple subunits of a heteromultimeric GABA receptor. J. Neurosci. 19: 5348–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg A. A., Jorgensen E. M., 2003. EXP-1 is an excitatory GABA-gated cation channel. Nat. Neurosci. 6: 1145–1152. [DOI] [PubMed] [Google Scholar]
- Bellemer A., Hirata T., Romero M. F., Koelle M. R., 2011. Two types of chloride transporters are required for GABA(A) receptor-mediated inhibition in C. elegans. EMBO J. 30: 1852–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y., Cherubini E., Corradetti R., Gaiarsa J. L., 1989. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J. Physiol. 416: 303–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y., Gaiarsa J. L., Tyzio R., Khazipov R., 2007. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol. Rev. 87: 1215–1284. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y., Woodin M. A., Sernagor E., Cancedda L., Vinay L., et al. , 2012. Refuting the challenges of the developmental shift of polarity of GABA actions: GABA more exciting than ever! Front. Cell. Neurosci. 6: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaesse P., Airaksinen M. S., Rivera C., Kaila K., 2009. Cation-chloride cotransporters and neuronal function. Neuron 61: 820–838. [DOI] [PubMed] [Google Scholar]
- Bregestovski P, Bernard C, 2012 Excitatory GABA: how a correct observation may turn out to be an experimental artifact. Front. Pharmacol. 3: 65. [DOI] [PMC free article] [PubMed]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancedda L., Fiumelli H., Chen K., Poo M. M., 2007. Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo. J. Neurosci. 27: 5224–5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudotvorova I., Ivanov A., Rama S., Hubner C. A., Pellegrino C., et al. , 2005. Early expression of KCC2 in rat hippocampal cultures augments expression of functional GABA synapses. J. Physiol. 566: 671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll N. A., 1975. Behavioural analysis of nematode movement. Adv. Parasitol. 13: 71–122. [DOI] [PubMed] [Google Scholar]
- Dittman J. S., Kaplan J. M., 2008. Behavioral impact of neurotransmitter-activated G-protein-coupled receptors: muscarinic and GABAB receptors regulate Caenorhabditis elegans locomotion. J. Neurosci. 28: 7104–7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duebel J., Haverkamp S., Schleich W., Feng G., Augustine G. J., et al. , 2006. Two-photon imaging reveals somatodendritic chloride gradient in retinal ON-type bipolar cells expressing the biosensor Clomeleon. Neuron 49: 81–94. [DOI] [PubMed] [Google Scholar]
- Fire A., Harrison S. W., Dixon D., 1990. A modular set of lacZ fusion vectors for studying gene expression in Caenorhabditis elegans. Gene 93: 189–198. [DOI] [PubMed] [Google Scholar]
- Fire A., Xu S., Montgomery M. K., Kostas S. A., Driver S. E., et al. , 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391: 806–811. [DOI] [PubMed] [Google Scholar]
- Gally C., Bessereau J. L., 2003. GABA is dispensable for the formation of junctional GABA receptor clusters in Caenorhabditis elegans. J. Neurosci. 23: 2591–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X. B., van den Pol A. N., 2001. GABA, not glutamate, a primary transmitter driving action potentials in developing hypothalamic neurons. J. Neurophysiol. 85: 425–434. [DOI] [PubMed] [Google Scholar]
- Ge S., Goh E. L., Sailor K. A., Kitabatake Y., Ming G. L., et al. , 2006. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439: 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J., Dzhala V., Egawa K., Balena T., Saponjian Y., et al. , 2014. Local impermeant anions establish the neuronal chloride concentration. Science 343: 670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozlan H., Ben-Ari Y., 2003. Interneurons are the source and the targets of the first synapses formed in the rat developing hippocampal circuit. Cereb. Cortex 13: 684–692. [DOI] [PubMed] [Google Scholar]
- Hallam S. J., Jin Y., 1998. lin-14 regulates the timing of synaptic remodelling in Caenorhabditis elegans. Nature 395: 78–82. [DOI] [PubMed] [Google Scholar]
- Hennou S., Khalilov I., Diabira D., Ben-Ari Y., Gozlan H., 2002. Early sequential formation of functional GABA(A) and glutamatergic synapses on CA1 interneurons of the rat foetal hippocampus. Eur. J. Neurosci. 16: 197–208. [DOI] [PubMed] [Google Scholar]
- Jin Y., Hoskins R., Horvitz H. R., 1994. Control of type-D GABAergic neuron differentiation by C. elegans UNC-30 homeodomain protein. Nature 372: 780–783. [DOI] [PubMed] [Google Scholar]
- Jin Y., Jorgensen E., Hartwieg E., Horvitz H. R., 1999. The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. J. Neurosci. 19: 539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J., Tian N., Caywood M. S., Reimer R. J., Edwards R. H., et al. , 2003. Vesicular neurotransmitter transporter expression in developing postnatal rodent retina: GABA and glycine precede glutamate. J. Neurosci. 23: 518–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liewald J. F., Brauner M., Stephens G. J., Bouhours M., Schultheis C., et al. , 2008. Optogenetic analysis of synaptic function. Nat. Methods 5: 895–902. [DOI] [PubMed] [Google Scholar]
- Liu Y. B., Lio P. A., Pasternak J. F., Trommer B. L., 1996. Developmental changes in membrane properties and postsynaptic currents of granule cells in rat dentate gyrus. J. Neurophysiol. 76: 1074–1088. [DOI] [PubMed] [Google Scholar]
- Markova O., Mukhtarov M., Real E., Jacob Y., Bregestovski P., 2008. Genetically encoded chloride indicator with improved sensitivity. J. Neurosci. Methods 170: 67–76. [DOI] [PubMed] [Google Scholar]
- McIntire S. L., Jorgensen E., Horvitz H. R., 1993a Genes required for GABA function in Caenorhabditis elegans. Nature 364: 334–337. [DOI] [PubMed] [Google Scholar]
- McIntire S. L., Jorgensen E., Kaplan J., Horvitz H. R., 1993b The GABAergic nervous system of Caenorhabditis elegans. Nature 364: 337–341. [DOI] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V., 1991. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10: 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson L. S., Kim K., Memmott J. E., Li C., 1998. FMRFamide-related gene family in the nematode, Caenorhabditis elegans. Brain Res. Mol. Brain Res. 58: 103–111. [DOI] [PubMed] [Google Scholar]
- Obata K., Oide M., Tanaka H., 1978. Excitatory and inhibitory actions of GABA and glycine on embryonic chick spinal neurons in culture. Brain Res. 144: 179–184. [DOI] [PubMed] [Google Scholar]
- Park M., Watanabe S., Poon V. Y., Ou C. Y., Jorgensen E. M., et al. , 2011. CYY-1/cyclin Y and CDK-5 differentially regulate synapse elimination and formation for rewiring neural circuits. Neuron 70: 742–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin M. D., Snyder E. Y., Hebert S. C., Delpire E., 1997. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA’s excitatory role in immature brain. J. Neurobiol. 33: 781–795. [DOI] [PubMed] [Google Scholar]
- Reynolds N. K., Schade M. A., Miller K. G., 2005. Convergent, RIC-8-dependent Galpha signaling pathways in the Caenorhabditis elegans synaptic signaling network. Genetics 169: 651–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C., Voipio J., Payne J. A., Ruusuvuori E., Lahtinen H., et al. , 1999. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397: 251–255. [DOI] [PubMed] [Google Scholar]
- Saint-Amant L., Drapeau P., 2000. Motoneuron activity patterns related to the earliest behavior of the zebrafish embryo. J. Neurosci. 20: 3964–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer J. M., Bergstrom A. R., 1988. Identification of gamma-aminobutyric acid and its binding sites in Caenorhabditis elegans. Life Sci. 43: 1701–1706. [DOI] [PubMed] [Google Scholar]
- Schultheis C., Brauner M., Liewald J. F., Gottschalk A., 2011. Optogenetic analysis of GABAB receptor signaling in Caenorhabditis elegans motor neurons. J. Neurophysiol. 106: 817–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuske K., Beg A. A., Jorgensen E. M., 2004. The GABA nervous system in C. elegans. Trends Neurosci. 27: 407–414. [DOI] [PubMed] [Google Scholar]
- Spilker K. A., Wang G. J., Tugizova M. S., Shen K., 2012. Caenorhabditis elegans Muscleblind homolog mbl-1 functions in neurons to regulate synapse formation. Neural Dev. 7: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley K. J., Mody I., 1992. Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABAA receptor-mediated postsynaptic conductance. J. Neurophysiol. 68: 197–212. [DOI] [PubMed] [Google Scholar]
- Sun D., Murali S. G., 1999. Na+-K+-2Cl- cotransporter in immature cortical neurons: a role in intracellular Cl- regulation. J. Neurophysiol. 81: 1939–1948. [DOI] [PubMed] [Google Scholar]
- Sung K. W., Kirby M., McDonald M. P., Lovinger D. M., Delpire E., 2000. Abnormal GABAA receptor-mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J. Neurosci. 20: 7531–7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanis J. E., Bellemer A., Moresco J. J., Forbush B., Koelle M. R., 2009. The potassium chloride cotransporter KCC-2 coordinates development of inhibitory neurotransmission and synapse structure in Caenorhabditis elegans. J. Neurosci. 29: 9943–9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Peer K. L., Bai J., Hu Z., Kaplan J. M., 2012. HBL-1 patterns synaptic remodeling in C. elegans. Neuron 73: 453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzio R., Represa A., Jorquera I., Ben-Ari Y., Gozlan H., et al. , 1999. The establishment of GABAergic and glutamatergic synapses on CA1 pyramidal neurons is sequential and correlates with the development of the apical dendrite. J. Neurosci. 19: 10372–10382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. G., Southgate E., Thomson J. N., Brenner S., 1976. The structure of the ventral nerve cord of Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 275: 327–348. [DOI] [PubMed] [Google Scholar]
- White J. G., Southgate E., Thomson J. N., Brenner S., 1986. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 314: 1–340. [DOI] [PubMed] [Google Scholar]
- White J. G., Southgate E., Thomson J. N., 1992. Mutations in the Caenorhabditis elegans unc-4 gene alter the synaptic input to ventral cord motor neurons. Nature 355: 838–841. [DOI] [PubMed] [Google Scholar]
- Yamada J., Okabe A., Toyoda H., Kilb W., Luhmann H. J., et al. , 2004. Cl- uptake promoting depolarizing GABA actions in immature rat neocortical neurones is mediated by NKCC1. J. Physiol. 557: 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. Z., Taylor M. M., Wu S., Ikeda-Matsuo Y., Kubera C., et al. , 2012. NKCC1 knockdown decreases neuron production through GABA(A)-regulated neural progenitor proliferation and delays dendrite development. J. Neurosci. 32: 13630–13638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R. W., Wei H. P., Xia Y. M., Du J. L., 2010. Development of light response and GABAergic excitation-to-inhibition switch in zebrafish retinal ganglion cells. J. Physiol. 588: 2557–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.