Abstract
An antibody against the posttranslational modification AMPylation was produced using a peptide corresponding to human Rac1 switch I region with AMPylated threonine-35 residue as an antigen. The resulting rabbit antiserum was tested for its abilities to recognize AMPylated proteins by western blot and immunoprecipitation. The antiserum is highly specific for threonine-AMPylated proteins and weakly recognizes tyrosine-AMPylated proteins. Depletion of serum with modified protein abolished its activity against tyrosine-AMPylated proteins. The antiserum also recognized native proteins with modification in an immunoprecipitation experiment. Interactions of the antiserum could be inhibited by competition with AMP but not with GMP or UMP. This antiserum had potential utility for the identification of unknown AMPylated proteins.
Keywords: AMP, AMPylation, Antibody, Western blot, Immunoprecipitation
Protein posttranslational modifications (PTMs) are important cellular events that regulate processes such as gene expression, signal transduction, and cell cycle regulation. However, studies of PTMs, particularly novel PTMs, are hindered by the lack of efficient molecular characterization tools. Identification of potential modifications heavily relies on mass spectrometry, which requires purified materials and is not an easily accessible method. Specific antibodies against PTMs have proven to be invaluable tools for analyzing the dynamics of these modifications. For example, tyrosine phosphorylation (Glenney et al., 1988; Ward et al., 1992) and lysine acetylation (Liu et al., 1999; Zhao et al., 2010) have been identified by specific antibodies, and these tools have been useful in revealing the role of these modifications in many biological processes.
The PTM AMPylation, previously known as adenylylation, is the covalent addition of an Adenosine MonoPhosphate group to a hydroxyl side chain of threonine, tyrosine, and potentially serine residues. Although first discovered in the 1970s (Brown et al., 1971), AMPylation has recently attracted great attention with the discoveries that bacterial effectors utilize this modification during infections of mammalian cells (reviewed in Broberg and Orth, 2010). Initially, Vibrio effector VopS was discovered to modify the threonine residue of human small GTPases and inhibit its interaction with downstream effectors (Yarbrough et al., 2009). Subsequently, Histophilus IbpA and Leginella DrrA were found to modify a tyrosine residue on Rho GTPase resulting in alteration in the signaling molecules activities (Muller et al., 2010; Worby et al., 2009). Proteins catalyzing this modification, known as AMPylators, have been identified from both prokaryotic and eukaryotic species (Brown et al., 1971; Muller et al., 2010; Worby et al., 2009; Yarbrough et al., 2009), which indicates that AMPylation is a conserved mechanism in the regulation of cellular functions.
To further study AMPylation and its significance, tools that can efficiently identify AMPylated proteins are required. In the present study, we report the generation of a specific antibody against threonine-AMPylation. This antibody has many potential applications for the identification, confirmation, and characterization of threonine-AMPylated proteins.
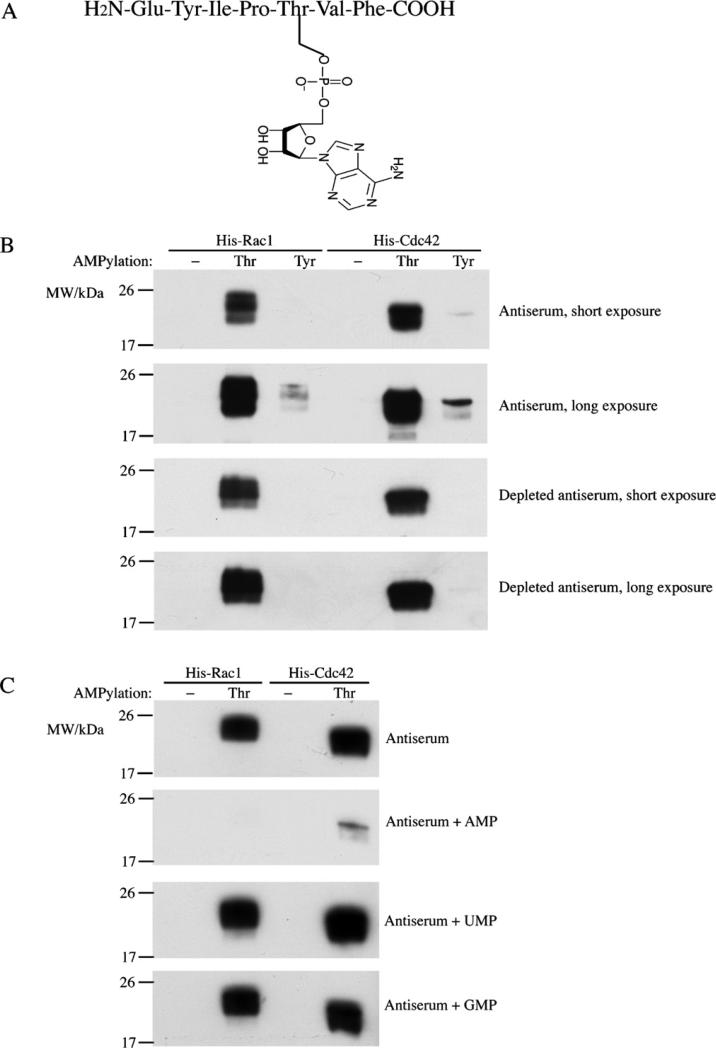
Initially, an AMPylated peptide of Rac1 switch I region EYIPT(AMP)VF (Fig. 1A) was generated using optimized Fmoc chemistry on an Applied Biosystems 433 automated peptide synthesizer (Foster City, CA) (Al-Eryani et al., 2010). Four rabbits were injected with 10 mg of this peptide and were boosted three times. Antisera from different time points were collected and pooled. To eliminate nonspecific activity, pooled sera were depleted with recombinant Rac1 ampylated on a tyrosine residue.
Fig. 1.
Western blot analysis of AMPylated recombinant GTPases. (A) Diagram of the antigenic AMPylated peptide. (B) 50 ng of each unmodified or AMPylated His-Rac1 or His-Cdc42 was subjected to SDS-PAGE, transferred to PVDF membranes, and immunoblotted with antiserum or depleted antiserum at 1:1000 dilution. (C) 5 mM AMP, UMP, or GMP were added directly during incubation with antiserum.
To test the activity of the antibody, AMPylated His6-tagged Rac1 and Cdc42 were generated by coexpression of VopS (for threonine AMPylation) or the Fic2 domain of IbpA (for tyrosine AMPylation) in Escherichia coli, and purified as previously described (Luong et al., 2010). The modifications were confirmed by mass spectrometry. Equivalent amounts of each unmodified and modified proteins were subjected to SDS-PAGE, transferred to PVDF membranes, and immunoblotted with antiserum or depleted antiserum (1:1000) for 1 h at room temperature. After the membranes were incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibody (1:5000), the recognized bands were visualized using ECL Plus (Amersham).
As shown in Fig. 1B, the antiserum recognizes threonine-AMPylated Rac1 and Cdc42, but not the unmodified proteins. Longer exposure reveals that it also weakly recognizes tyrosine-AMPylated Rac1 and Cdc42. After depletion, the antiserum could not recognize tyrosine-AMPylated proteins, while its activity to threonine-AMPylated proteins did not change. These western blot data confirm that the antiserum is a specific antibody to threonine-AMPylation with weak cross-reactivity to tyrosine-AMPylation, which is eliminated by depletion. Results from only one rabbit antiserum are presented in this study unless stated otherwise; however, it should be noted that all antisera have shown consistent results.
To test the specificity of the antiserum, we repeated the western blot experiment with unmodified and threonine-AMPylated proteins, and added 5 mM AMP, UMP, or GMP to compete with the AMPylated proteins during the incubation of PVDF membranes with the antiserum. As shown in Fig. 1C, in the presence of AMP, the association of the antiserum with AMPylated proteins was inhibited, while UMP and GMP had no effect on the activity of antiserum to the AMPylated proteins. This competition of binding by AMP, but not by UMP or GMP, indicates that the antiserum is specific to AMPylation.
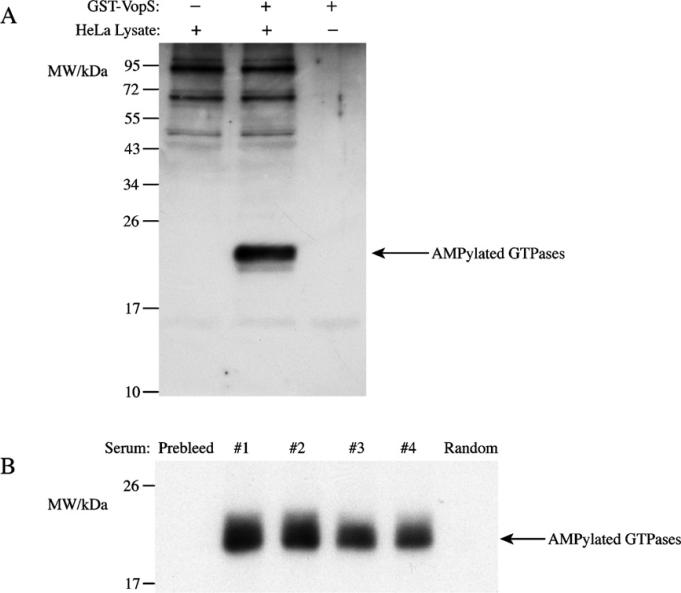
After testing the antiserum against recombinant proteins, we investigated its ability to recognize AMPylated endogenous proteins. Using 32P-α-ATP as a substrate and autoradiography, we have previously demonstrated that VopS can AMPylate threonine of Rho family GTPases in HeLa cell lysate (Yarbrough et al., 2009). We repeated this experiment without isotopic ATP, and used the anti threonine-AMPylation serum to visualize the AMPylated proteins. HeLa cells were lysed in HNMEK buffer (20 mM HEPES 8.0, 150 mM NaCl, 2 mM MgCl2, 1 mM EDTA, and 10 mM KCl) with protease inhibitors and fractionated by differential centrifugation. The fraction corresponding to subcellular organelles (pellet of 10,000 × g centrifugation) was incubated with cold ATP in the presence and absence of VopS at 30 °C for 30 min, and the samples were subjected to western blot as described above. In the presence of VopS, a band at the expected molecular weight of Rho GTPases was detected by the antiserum (Fig. 2A), indicating that the antiserum is sufficiently activated to detect endogenous AMPylated proteins by a supplemented AMPylator. We also noticed that other proteins were recognized by the antiserum in the HeLa lysate, which may represent endogenous threonine-AMPylated proteins and are under further investigation.
Fig. 2.
Anti-AMPylation serum recognizes and pulls down endogenous GTPases AMPylated by VopS. (A) 20 μg HeLa cell lysate was incubated with 10 ng GST-VopS in the presence of 0.5 mM ATP at 30 °C for 30 min and subjected to western blot analysis. (B) 100 μg HeLa cell lysate was incubated with 100 ng GST-VopS in the presence of 32P-α-ATP at 30 °C for 30 min. The reaction mixture was divided evenly among six tubes and 5 μl of antiserum from each rabbit, prebleed serum, or a random serum were added to each tube. Samples were incubated for 1 h at 4 °C and pulled down by Protein-A Sepharose beads. After washing, bound proteins were dissociated with SDS sample buffer and subjected to SDS-PAGE for autoradiography.
The western blot experiments indicate that the antiserum has a very specific activity against denatured AMPylated proteins. An immunoprecipitation experiment was thus performed to test the activity of the antiserum against native proteins. HeLa subcellular organelles fraction prepared as described above was incubated with recombinant VopS and 32P-α-ATP at 30 °C for 30 min. Five microliters of prebleed serum, antiserum from each rabbit, or a random antiserum against an unrelated protein were incubated with the reaction solution for 1 h at 4 °C, and the antibody complexes were pulled down by Protein-A Sepharose beads. After washing away the non-specific binding proteins, bound proteins were dissociated with SDS sample buffer, subjected to SDS-PAGE, and the modified proteins were visualized by autoradiography. As shown in Fig. 2B, antisera from all four immunized rabbits could pull down the VopS-AMPylated Rho GTPases, but not prebleed or unrelated serum, confirming that the antiserum can recognize the AMPylated proteins in their native form.
As a re-emerged protein modification, the understanding of the significance for protein AMPylation is in its infancy, particularly in eukaryotes. Although AMPylators have been identified in most species by bioinformatics, their substrates have been difficult to identify due to the lack of necessary tools. The specificity of the antibody presented here should make it an efficient tool in AMPylation studies. For example, for its immunoprecipitation activity, the antibody could be used to pull down unknown AMPylated proteins from cell lysates for further identification.
Considering the antiserum's weak activity against tyrosine-AMPylation, it is not appropriate for use in studies of tyrosine-AMPylation. The generation of a tyrosine-AMPylation antibody is in progress.
Acknowledgements
We thank members of the Orth lab for insightful discussions and critical reading. K.O., Y.-H.H., and P.L. are supported by grants from NIH-Allergy and Infectious Disease (R01-AI056404 and R01-AI087808) and the Welch Foundation (I-1561). K.O. is a Burroughs Wellcome Investigator in Pathogenesis of Infectious Disease and a W.W. Caruth, Jr. Biomedical Scholar.
Footnotes
Competing interest
The authors declare no competing interests.
References
- Al-Eryani RA, Li Y, Ball HL. Chemical synthesis of Ser/Thr AMPylated peptides. Tetrahedron Lett. 2010;51:1730–1731. [Google Scholar]
- Broberg CA, Orth K. Tipping the balance by manipulating post-translational modifications. Curr. Opin. Microbiol. 2010;13:34–40. doi: 10.1016/j.mib.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Segal A, Stadtman ER. Modulation of glutamine synthetase adenylylation and deadenylylation is mediated by metabolic transformation of the P II – regulatory protein. Proc. Natl. Acad. Sci. U.S.A. 1971;68:2949–2953. doi: 10.1073/pnas.68.12.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney JR, Jr., Zokas L, Kamps MP. Monoclonal antibodies to phosphotyrosine. J. Immunol. Methods. 1988;109:277–285. doi: 10.1016/0022-1759(88)90253-0. [DOI] [PubMed] [Google Scholar]
- Liu L, Scolnick DM, Trievel RC, Zhang HB, Marmorstein R, Halazonetis TD, Berger SL. p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol. Cell. Biol. 1999;19:1202–1209. doi: 10.1128/mcb.19.2.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luong P, Kinch LN, Brautigam CA, Grishin NV, Tomchick DR, Orth K. Kinetic and structural insights into the mechanism of AMPylation by VopS Fic domain. J. Biol. Chem. 2010;285:20155–20163. doi: 10.1074/jbc.M110.114884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MP, Peters H, Blumer J, Blankenfeldt W, Goody RS, Itzen A. The Legionella effector protein DrrA AMPylates the membrane traffic regulator Rab1b. Science. 2010;329:946–949. doi: 10.1126/science.1192276. [DOI] [PubMed] [Google Scholar]
- Ward SG, Reif K, Ley S, Fry MJ, Waterfield MD, Cantrell DA. Regulation of phosphoinositide kinases in T cells. Evidence that phosphatidylinositol 3-kinase is not a substrate for T cell antigen receptor-regulated tyrosine kinases. J. Biol. Chem. 1992;267:23862–23869. [PubMed] [Google Scholar]
- Worby CA, Mattoo S, Kruger RP, Corbeil LB, Koller A, Mendez JC, Zekarias B, Lazar C, Dixon JE. The fic domain: regulation of cell signaling by adenylylation. Mol. Cell. 2009;34:93–103. doi: 10.1016/j.molcel.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarbrough ML, Li Y, Kinch LN, Grishin NV, Ball HL, Orth K. AMPylation of Rho GTPases by Vibrio VopS disrupts effector binding and downstream signaling. Science. 2009;323:269–272. doi: 10.1126/science.1166382. [DOI] [PubMed] [Google Scholar]
- Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, Li Y, Shi J, An W, Hancock SM, He F, Qin L, Chin J, Yang P, Chen X, Lei Q, Xiong Y, Guan KL. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]