SUMMARY
The c ellular surveillance-activated detoxification and defenses (cSADD) theory postulates the presence of host surveillance mechanisms that monitor the integrity of common cellular processes and components targeted by pathogen effectors. Being organelles essential for multiple cellular processes, including innate immune responses, mitochondria represent an attractive target for pathogens. We describe a Vibrio cholerae Type 3 secretion system effector VopE that localizes to mitochondria during infection and interferes with the function of mitochondrial Rho GTPases Miro1 and Miro2 by acting as a specific GTPase-activating protein. Miro GTPases modulate mitochondrial dynamics and interference with this functionality effectively blocks innate immune responses that presumably require mitochondria as signaling platforms. Our data indicate that interference with mitochondrial dynamics may be an unappreciated strategy that pathogens use to block host innate immune responses that would otherwise control these bacterial infections. VopE might represent a bacterial effector that targets the cSADD surveillance response.
Keywords: T3SS, mitochondria, Miro, innate immunity, Vibrio, MAVS
INTRODUCTION
The mammalian innate immune system plays a crucial role in defending against invading pathogens, however at the same time it needs to be tolerant towards commensal microbiota beneficial for the host. Currently, the basis for this discrimination is not well understood. Two prominent theories that attempt to explain bacterial pathogen recognition are: (i) the recognition of pathogen-associated molecular patterns (PAMPs) by pattern-recognition receptors (PRR), and (ii) the recognition of damage-associated molecular patterns (DAMPs) that are released from dying pathogen-infected host cells. While PAMP theory explains a broad recognition of invading microorganisms based on the presence of conserved structures such as lipid A, flagellin, lipoproteins, nucleic acids, and peptidoglycan-related molecules, this model provides no explanation for how the host discriminates between pathogenic and beneficial bacteria, both of which have PAMPs (Vance et al., 2009). The DAMP theory is more attractive in this regard in that it postulates that only pathogenic bacteria induce death of host cells. However, the weakness of this model is the timeline of the defense, as it can only be launched after the death of host cells. Therefore, it may operate more as a ‘damage-control’ strategy than an active antibacterial defense strategy (Kono and Rock, 2008).
An alternative theory known as a ‘guard hypothesis’ originally was suggested in the plant field (Chisholm et al., 2006; Jones and Dangl, 2006), and recently was shown to be applicable to a worm model of infection, namely cellular surveillance-activated detoxification and defenses (cSADD) (Liu et al., 2014; Melo and Ruvkun, 2012). This theory postulates a presence of host surveillance proteins (disease resistance (R) proteins in plants) or pathways that monitor integrity of normal cellular processes and common cellular components that bacterial and viral effectors target. Instead of detecting highly variable bacterial and viral effectors, a host cell can monitor highly conserved cellular systems that these effectors commonly target, such as the actin cytoskeleton and innate immune signaling pathways (Broberg and Orth, 2010). The main advantage of this theory is that it provides an explanation for the ability of the host to specifically identify cell-damaging pathogenic bacteria and to respond to them in a timely manner before the host cell death.
Mitochondria are cellular organelles essential for multiple metabolic processes including production of energy, oxygen and calcium sensing, control of programmed cell death (apoptosis), and induction of innate immune responses (Cloonan and Choi, 2013). Based on cSADD theory, monitoring the normal functionality of mitochondria is extremely important for the host as a large number of known bacterial effectors target various mitochondrial processes (Arnoult et al., 2009). Indeed, recently, Ruvkun and colleagues identified 45 genes of Caenorhabditis elegans that are required for mitochondrial surveillance, the absence of which diminishes detoxification, pathogen-response and mitochondria-repair pathways (Liu et al., 2014). While it remains to be confirmed that similar pathways are functional in mammalian cells, it is highly likely to be the case because the genes identified in this screen, including ceramide and mevalonate synthesis, are highly conserved between worms and mammals.
In this work, we used Vibrio cholerae AM-19226 as a model organism and found that a Type 3 Secretion System (T3SS)-delivered effector VopE is required to prevent mitochondrial perinuclear clustering and to suppress innate immune responses during V. cholerae infection of cultured mammalian cells. We showed that in the absence of VopE, mitochondria were clustered in the perinuclear space, which resulted in aggregation of the mitochondrial outer membrane protein MAVS and induction of inflammatory signaling. However, in the presence of VopE mitochondrial and MAVS localization and inflammatory signaling were indistinguishable from mock-treated cells. We further showed that VopE binds to mitochondrial Rho GTPases Miro1 and Miro2 and activates their GTPase activity, effectively interfering with their ability to modulate mitochondrial trafficking. Because our experiments indicate that Miro-dependent mitochondrial dynamics may be essential for activating innate immune signaling during V. cholerae infection, we conclude that VopE might be an example of a bacterial effector that targets the cSADD surveillance response.
RESULTS
VopE is localized to mitochondria
VopE has been previously identified as an important T3SS effector in V. cholerae. Its translocation into host cells has been demonstrated using a β-lactamase as a reporter assay, and it has been shown to be essential for full pathogenicity in a rabbit model of enteric infection (Alam et al., 2011; Shin et al., 2011; Tam et al., 2007; Tam et al., 2010). A BLAST homology search revealed that sequences homologous to vopE are found in the genomes of virulent non-O1, non-O139 V. cholerae strains and related bacterial species Grimontia hollisae (Fig. S1A). The C-terminus of VopE possesses the ToxGAP (toxic GTPase-activating protein) domain that is found in bacterial T3SS effectors, such as YopE, SptP, and ExoS, known to disrupt the actin cytoskeleton in mammalian cells (Figs. 1A, S1B) (Aktories et al., 2000; Sun et al., 2004). Sequence-based protein structure prediction by the PHYRE2 (Kelley and Sternberg, 2009) predicted that VopE has a similar structure to that of Yersinia YopE (Fig. S1C), however, unlike Yersinia YopE-GFP, ectopic protein expression of VopE-GFP in CHO cells had no effect on the actin cytoskeleton (Fig. S1D), suggesting that VopE plays distinct roles in cellular responses from other conventional ToxGAP-containing T3SS effectors.
Fig. 1.
VopE is targeted to mitochondria. A. V. cholerae AM-19226 VopE and its derivatives: R125K has a loss-of-function mutation in the ToxGAP domain, ΔMTS construct lacks first 23 amino acids that are predicted to be required for mitochondrial targeting, L4E has a single point mutation introducing the positive charged amino acid into predicted MTS. B. Immunostaining of CHO cells transfected for 20 h with the pAcGFP-N1 plasmids expressing indicated proteins. Mitochondrial localization shown with percent of the cells and asterisks. Bar=10 μm. See also Fig. S1
The N-terminus of VopE had a positively charged amphiphilic structure that is a typical mitochondrial targeting sequence (MTS). Plasmids encoding GFP fusions to the wild-type (wt) VopE or to a R125K mutant of VopE lacking ToxGAP activity were transfected into CHO cells. Immunostaining revealed that both wt and R125K mutant are localized to mitochondria in more than 90% of transfected cells (Figs. 1A, B). As a positive control we used a GFP fusion to mitochondrial-associated protein (Map) from enteropathogenic Escherichia coli (EPEC) as it is one of the best-characterized mitochondria-targeting effectors (Arnoult et al., 2009) (Fig. 1B). Disrupting the VopE-GFP MTS either by complete deletion of the domain (ΔMTS) or by introducing a negatively charged amino acid into the MTS (L4E) resulted in VopE failing to localize to mitochondria (Fig. 1B). Additionally, ectopic expression of VopE R125K, but not the wt VopE, often induced net-like mitochondrial structures with the VopE R125K localizing to branching or polar sites of mitochondria (Fig. S2A), suggesting that the putative GAP activity of VopE regulates mitochondrial morphology.
Localization of VopE to mitochondria is dependent on membrane potential
Transfection experiments showed that the protein expression level of VopE in mammalian cells was low likely due to codon usage bias between bacterial and eukaryotic cells (Figs. S2B, D). We therefore synthesized a codon-optimized version of vopE for expression in mammalian cells, which dramatically improved protein expression level compared to the original sequence (Figs. S2C, D). We hereafter used the codon-optimized vopE as the wt for transfection experiments. Cell fractionation analysis of 293T cells expressing FLAG-tagged VopE showed that VopE-FLAG and R125K-FLAG, but not L4E-FLAG, were contained in the mitochondrial fraction (Fig. 2A). Decreased expression of L4E-FLAG may be due to low stability of VopE in the cytoplasm since it is not targeted to the mitochondria. Mitochondrial heat shock protein 70 kDa (mtHSP70) and dynamin-related protein 1 (Drp1) were used as mitochondrial markers (Fig. 2A). Because localization of proteins into mitochondrial organelles is dependent on mitochondrial inner membrane potential (Martin et al., 1991), we tested localization of VopE-FLAG in the presence of mitochondrial potential uncoupler carbonyl cyanide m-chlorophenyl hydrazine (CCCP) and F1-ATPase inhibitor oligomycin A (OLM) (Fig. 2B). Co-treatment with these compounds inhibited mitochondrial localization of VopE-FLAG, suggesting that mitochondrial localization of VopE is dependent on transport processes of host cells that rely on mitochondrial membrane potential.
Fig. 2.

VopE is targeted to mitochondria during V. cholerae infection. A. Immunoblotting of lysates from 293T cells transfected with the indicated VopE-FLAG expression plasmids, and then fractionated as indicated (Cyto, cytosol; Mito, mitochondria). B. Immunostaining of CHO cells transfected for 20 h with VopE-FLAG expression plasmid, and then co-treated for 4 h with CCCP and OLM. Mitochondrial localization is shown with percent of the cells and asterisks. Bar=10 μm. C. Immunostaining of HeLa cells infected for 2 h with the indicated AM-19226 ΔvopE strains harboring the indicated VopE-Myc expression plasmids. Bar=10 μm. See also Fig. S2
VopE is localized to mitochondria during Vibrio infection
To determine localization of VopE in host cells during V. cholerae infection, we used V. cholerae AM-19226 deficient in accessory toxins HapA, HlyA and RtxA that has a low level of cytotoxicity towards eukaryotic cells (Tam et al., 2007; Tam et al., 2010). Immunostaining of HeLa cells infected with AM-19226 ΔvopE strains trans-complemented with Myc-tagged wt vopE, R125K, and L4E mutated vopE genes revealed that translocated VopE-Myc and R125K-Myc, but not L4E-Myc, were localized to mitochondria (Fig. 2C). These data suggest that the presence of an intact MTS is required for mitochondrial targeting of VopE during infection of host eukaryotic cells.
VopE does not mediate death of host cells
Mitochondrial-targeted bacterial effectors often induce programmed cell death of host cells (Arnoult et al., 2009). Therefore, we analyzed cytotoxicity, cytochrome c release, and mitochondrial membrane potential, all of which commonly precede cell death. Lactate dehydrogenase (LDH) cytotoxicity assays using HeLa cells infected with accessory toxin-deficient AM-19226 ΔhapA ΔhlyA ΔrtxA and T3SS-deficient derivative strain AM-19226 ΔvcsN demonstrated that a functional T3SS has no effect on host cell death, whereas the absence of HapA, HlyA, and RtxA toxins drastically reduced cytotoxicity (Fig. 3A). Consistent with this result, we did not detect any difference in number of dead host cells upon infection with ΔvopE strain in ΔhapA ΔhlyA ΔrtxA background using ethidium homodimer-2 staining (Fig. S3A). Furthermore, we detected no change in mitochondrial membrane potential using rhodamine 123 staining (Fig. S3A). As controls we induced cell death using staurosporine (STS), and disrupted mitochondrial membrane potential depolarization by CCCP/OLM treatment (Figs. S3A, B). HeLa cells infected with ΔhapA ΔhlyA ΔrtxA strain did not induce cytochrome c release from mitochondria into the cytosol unlike STS-treated cells (Fig. S3B), and the ectopic protein expression of VopE and the GAP mutant in HeLa cells did not influence mitochondrial localization of cytochrome c (Fig. S3C). These data indicate that localization of VopE to mitochondria, unlike that of many other bacterial effectors, does not correlate with induction of host cell death. Also given that the accessory toxins contribute to host cell death, all further AM-19226 mutant stains are made in a ΔhapA ΔhlyA ΔrtxA background, which we hereafter will refer to as wt.
Fig. 3.
VopE inhibits perinuclear mitochondrial clustering during infection. A. LDH-based cytotoxicity assays on the culture supernatants from HeLa cells infected for up to 8 h with the indicated V. cholerae AM-19226 strains (hap, haemagglutinin protease; hly, hemolysin; rtx, repeats in structural toxin). Percent of cell death was calculated as follows: experimental LDH activity/total LDH activity from Triton-X100-treated cells x 100%. B. Perinuclear mitochondrial clustering in CHO cells infected for 6 h with the indicated V. cholerae strains (AM192, AM-19226; WT, Δhap Δhly Δrtx strain as a wild-type background; ΔE, ΔvopE; V52, O37 serogroup strain V52) (*p<0.01). C. Immunostaining of CHO cells in B. Perinuclear mitochondrial clustering shown with asterisks. Bar=20 μm. See also Fig. S3
VopE inhibits mitochondrial clustering
Mitochondrial morphology of V. cholerae-infected CHO cells revealed that mitochondrial clustering was dramatically induced in the perinuclear space of ΔvopE-infected cells (66.3±4.7% of the cells), compared to cells infected with the wt strain (11.7±2.0% of the cells) (Figs. 3B, C). A ΔvopE strain producing wt VopE, but not loss-of-function mutants, could restore regular peripheral mitochondrial localization (Figs. 3B, C). Perinuclear mitochondria are observed in response to some viral and bacterial infections and are believed to be a more synthetically active form than peripheral organelles (West et al., 2011). In particular, Citrobacter rodentium, a mouse-adapted bacterium closely related to EPEC, causes perinuclear mitochondrial clustering in a T3SS-dependent manner during infection (Ma et al., 2006). Of note, T3SS-deficient AM-19226 ΔvcsN strain and T3SS-negative V. cholerae O37 serogroup strain V52 did not induce perinuclear mitochondrial clustering (Figs. 3B, C). Thus, perinuclear mitochondrial clustering during T3SS-positive V. cholerae infection might be induced by T3SS effector(s) or other components of the T3SS such as its needle complex (Auerbuch et al., 2009). It is noteworthy that regardless of which T3SS component or cellular effect caused by such component is induces mitochondrial clustering, it is clearly suppressed by VopE activity.
VopE interacts with Miro GTPases
Mitochondrial dynamics are regulated by several mitochondrial membrane-bound GTPases, such as mitochondrial Rho GTPases (Miro1 and Miro2), mitofusins (Mfn1 and Mfn2), Drp1, and optic atrophy type 1 (Opa1) (Frederick and Shaw, 2007; Westermann, 2010), any of which could be a potential target of the VopE GAP activity. Consistent with this hypothesis, glutatione S-transferase (GST) pull-down assays demonstrated that VopE-GST, but not Map-GST, specifically interacted with Miro1 and Miro2, both of which are known to regulate mitochondrial morphogenesis and trafficking (Fig. 4A) (Fransson et al., 2003; Reis et al., 2009). Given their close amino acid sequence homology (75.4% amino acid similarity between the human paralogs), we hereafter refer to Miro1 and Miro2 as Miro for simplicity.
Fig. 4.
VopE interacts with Miro GTPases. A. Immunoblotting of lysates from 293T cells transfected for 24 h with the indicated pcDNA3.1 plasmids (input) and pulled-down proteins (PD) by the indicated GST-fused proteins (top). Coomassie brilliant blue (CBB) stains of the purified GST-proteins (bottom). B. Immunostaining of CHO cells co-transfected for 20 h with VopE-GFP and HA-Miro expression plasmids. Bar=10 μm. Pearson’s coefficients for VopE-Miro1 and VopE-Miro2 co-localization were 0.85 and 0.90, respectively. C. Immunoblotting of lysates from HeLa cells infected for 6 h with the indicated V. cholerae AM-19226 strains expressing VopE-Myc following transfection with mock (CTL) or HA-Miro expression plasmids, and then subjected to immunoprecipitation (IP) using anti-HA antibody. See also Fig. S4
Consistent with our in vitro pull-down analysis, we observed significant co-localization of VopE with Miro in mitochondria present in transfected CHO cells (Figs. 4B, S4A, B). Furthermore, immunoprecipitation assays revealed that Myc-epitope-tagged VopE interacted with Miro during V. cholerae infection (Fig. 4C). Mfn1 and Mfn2 coordinately regulate mitochondrial fusion, and Drp1 and Opa1 regulate mitochondrial fission (Westermann, 2010), but little is known about the function of Miro GTPases in these mitochondrial processes. Interestingly, VopE dramatically prevented Mfn1-induced mitochondrial fusion (Fig. S4C), and colocalized with Drp1-mediated mitochondrial fission sites in transfected CHO cells (Fig. S4D), suggesting that VopE modulates mitochondrial dynamics including fusion and fission by interacting with the Miro GTPases.
VopE binds to the GTPase-I domain of Miro
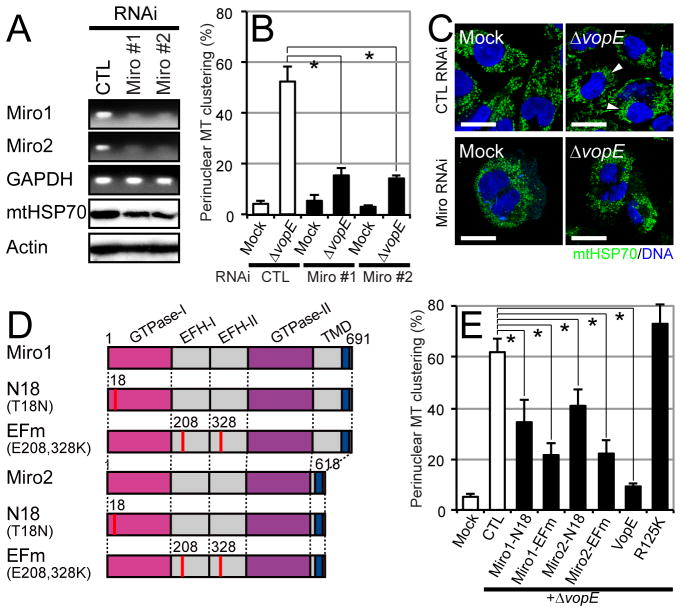
Next, we sought to determine which region of the Miro protein interacts with VopE. Miro GTPases consist of two GTPase domains (GTPase-I and GTPase-II), two EF-hand Ca2+ binding domains (EFH-I and EFH-II), and the transmembrane domain that is located in the C-terminal region and is important for anchoring to mitochondrial membranes (Fig. 5A) (Fransson et al., 2003; Reis et al., 2009). Maltose-binding protein (MBP) pull-down assays using MBP-fused Miro1 truncation derivatives revealed that the GTPase-I domain in Miro was the region responsible for VopE binding (Fig. 5B). To examine the GAP catalytic effect of VopE on Miro GTPase activity we performed a GAP assay that is based on measuring GTP hydrolysis by Miro in the presence or absence of VopE. Our results indicated that GTP hydrolysis by Miro GTPase-I domain is enhanced roughly 5-fold compared to control reactions in which Miro or VopE were absent, or in the presence of R125K mutant. Miro GTPase activity was increased in a VopE dose-dependent manner in these in vitro experiments (Figs. 5C, D). Thus, VopE likely increases GTPase activity of Miro by binding to its GTPase-I domain. By analogy to other small GTPase proteins, this binding likely shifts Miro GTPase-I into an inactive GDP-bound conformation (Bos et al., 2007). Consistent with this conclusion, V. cholerae ΔvopE-induced perinuclear mitochondrial clustering in cells that have been knocked down for Miro expression using siRNAs was ~70% lower than that mock-treated CHO cells (Figs. 6B, C). Thus, VopE likely inhibits perinuclear mitochondrial clustering by inhibiting Miro activity.
Fig. 5.
VopE activates GTP hydrolysis of Miro GTPases. A. Miro1 derivatives (EFH; TMD, transmembrane domain). B. Immunoblotting of lysates from AM-19226 ΔvopE strain producing VopE-Myc (input) and pulled-down proteins (PD) by the indicated MBP-Miro1 constructs (top). CBB stains of the purified MBP-proteins (bottom). C. GAP assays for the indicated Miro GTPase-I domain (5 μg) and VopE [2.5 (+) or 5 μg (++)] in the presence of GTP. GTP hydrolysis was measured from the amount of inorganic phosphate (Pi) produced as a result of a G-protein dependent hydrolysis of GTP to GDP+Pi after 20 min of incubation.
Fig. 6.
Miro is responsible for perinuclear mitochondrial clustering during infection. A. Semi-quantitative RT-PCR (top 3 panels) and immunoblotting (bottom 2 panels) from total RNA and lysates from CHO cells transfected for 48 h with the indicated siRNAs [CTL, control; Miro#1, mix of miro1-1 and miro2-1; Miro#2, mix of miro1-2 and miro2-2]. B. Quantification of perinuclear mitochondrial clustering in CHO cells transfected for 48 h with the indicated siRNAs and then mock-infected or infected for 6 h with AM-19226 ΔvopE (*p<0.01). C. Immunostaining of CHO cells (CTL and Miro#1). Perinuclear mitochondrial clustering is shown with asterisks. Bar=10 μm. D. Miro derivatives and sites of point mutation changes (EFm, E208K and E328K double mutations in the EF-hand domain; N18, T18N mutation in the GTPase-I domain). E. Perinuclear mitochondrial clustering in CHO cells transfected with plasmids and overexpressing the indicated proteins, and then mock-infected or infected for 6 h with AM-19226 ΔvopE (*p<0.01). See also Fig. S5
Miro-dependent Ca2+ sensing is essential for mitochondrial clustering
Miro GTPases are the major Ca2+ sensors mediating Ca2+-induced inhibition of kinesin-mediated mitochondrial motility (Wang and Schwarz, 2009). Ca2+ binding to EFH domains of Miro results in conformational change and functional inhibition (Wang and Schwarz, 2009). RNAi-mediated knockdown of both Miro1 and Miro2 reduced the amount of mtHSP70, namely mitochondrial mass, in CHO cells (Fig. 6A), supporting a critical role for Miro in mitochondrial homeostasis. As noted earlier, V. cholerae ΔvopE-induced perinuclear mitochondrial clustering in Miro knockdown cells was ~70% lower than in mock-treated CHO cells (Figs. 6B, C). Furthermore, ΔvopE-induced perinuclear mitochondrial clustering was also significantly inhibited in the presence of the Ca2+ chelator EDTA or tubulin polymerization inhibitor nocodazole (Figs. S5A–C). In addition, dominant-negative mutants N18 and EFm of Miro that have a loss-of-function mutation in the GTPase-I domain (T18N), or two Ca2+-binging EFH domains (E208K and E328K), respectively (Fig. 6D), also dramatically inhibited ΔvopE-induced perinuclear mitochondrial clustering in CHO cells (Figs. 6E, S5D, F). Taken together, these results suggest that infection with V. cholerae ΔvopE induces Ca2+ influx in the host cells, followed by Ca2+-bound Miro activating mitochondrial trafficking along microtubules. VopE apparently inhibits Ca2+-dependent mitochondrial trafficking by inhibiting Miro function through activation of its GTPase activity.
VopE reduces NF-κB signaling in MAVS-dependent manner
Recent studies have demonstrated that mitochondrial dynamics regulates innate immune signaling in response to pathogens and their components (West et al., 2011). For instance, mitofusins, mediators of mitochondrial fusion, regulate the antiviral signaling protein MAVS, a mitochondrial outer membrane protein that plays an important role in nuclear factor-κB (NF-κB) and type I interferon (IFN) signaling (Arnoult et al., 2009; Seth et al., 2005). Intriguingly, V. cholerae ΔvopE infection activated the IL-8 promoter containing the NF-κB-binding site approximately 8-fold compared to the wt and vopE-complemented V. cholerae ΔvopE-infected HeLa cells (Fig. 7A), whereas infection with a ΔvopE strain did not sufficiently activate the IFN-β promoter containing the interferon regulatory factor-binding sites (data not shown). Ectopic VopE expression reduced V. cholerae ΔvopE infection-induced NF-κB activation in CHO cells by 46.3% and MAVS transfection-induced NF-κB activation in 293T cells by 40.1% compared to of control cells (Fig. 7B). Overexpression of a loss-of-function GTPase mutant of Mfn1 (Mfn1-T109A) or EFm mutants of Miro also significantly blocked NF-κB activation (Fig. 7B), suggesting that the perinuclear mitochondrial clustering phenotype during infection contributes to mitochondria-mediated innate immune responses.
Fig. 7.
VopE-induced mitochondrial dynamics inhibits host inflammatory responses to infection. A. Luciferase assays of lysates from HeLa cells transfected for 20 h with IL-8 reporter (human IL-8 promoter, −1498/+44), and then infected for up to 6 h with the indicated V. cholerae AM-19226 strains. B. Luciferase assays of lysates from CHO cells co-transfected for 20 h with the indicated pcDNA3.1 plasmids and NF-κB reporter, and then infected for 4 h with AM-19226 ΔvopE (left, *p<0.01). Luciferase assays of lysates from 293T cells co-transfected for 24 h with MAVS expression plasmid (100 ng) as a stimulator, the indicated pcDNA3.1 plasmids, and NF-κB reporter (right, *p<0.01). C. Immunostaining of CHO cells transfected for 20 h with FLAG-MAVS expression plasmid, and then mock-infected or infected for 6 h with the indicated AM-19226 strains, or treated for 6 h with synthetic double-stranded RNA Poly(I:C). Bar=10 μm. D. 293T cells were co-transfected with VopE-GFP, FLAG-MAVS, and HA-Miro1 expression plasmids, then subjected to immunoblotting and immunoprecipitation (IP) as indicated. For in vitro kinase assay, IKK complex was immunoprecipitated using anti-Nemo antibody. Purified GST-IκB proteins were co-incubated, and the phosphorylated proteins were detected using anti-p-IκB (Ser32) antibody.
MAVS aggregation is induced in the absence of VopE
MAVS is an essential adaptor for retinoic acid-inducible gene 1 (RIG-I), an intracellular receptor for RNA and DNA. RIG-I signaling depends on the MAVS function and leads to transcriptional activation of the NF-κB and IFN responses (Seth et al., 2005). In response to viral infection, MAVS forms aggregates and this conformational switch activates and propagates the innate immune signaling cascade (Hou et al., 2011). Likewise, V. cholerae ΔvopE infection of CHO cells induced the MAVS aggregates in a similar manner as in cells transfected by polyinosinic-polycytidylic acid [poly(I:C)], a synthetic analog of double stranded RNA, whereas infection with the wt strain did not induce MAVS aggregation (Fig. 7C). Although VopE inhibited MAVS-mediated IκB kinase (IKK) activation in 293T cells (Fig. 7D), VopE-Miro1 complex was not co-immunoprecipitated with MAVS (Fig. 7D), implying that VopE indirectly inhibits MAVS-mediated NF-κB signaling by alteration of Miro-dependent mitochondrial dynamics.
DISCUSSION
Pathogenic bacteria utilize diverse strategies to manipulate the host innate immune response (Reddick and Alto, 2014). Some organisms do this to avoid detection by the innate immune system, while others deliberately hyper-activate the response to induce inflammation which can benefit their growth in the infected host (Winter et al., 2010a; Winter et al., 2010b). In particular, many Gram-negative bacteria use T3SS to deliver effectors targeting eukaryotic cells during infections (Hicks and Galan, 2013). T3SS effectors can alter the activity of Ras superfamily small GTPases of host cells, highly conserved regulators of cell division, differentiation (Ras subfamily), actin cytoskeleton rearrangement (Rho subfamily), and intracellular membrane trafficking (Arf and Rab subfamily) (Alto, 2008; Hall, 2012). By alternating GTPase signaling, T3SS-delivered effectors modify normal defense responses of eukaryotic cells including host immune responses, internalization and transport of bacteria in the cell and targeting them for degradation in lysosomes (Stein et al., 2012). Small GTPases typically cycle between biologically inactive GDP-bound conformation and an active GTP-bound form. These states are under tight regulatory control by two main groups of proteins: guanine nucleotide exchange factors (GEFs) which increase the rate of release of the bound GDP (resulting in its replacement by GTP), and GTPase-activating proteins (GAPs) which donate an essential catalytic group (Arginine) for GTP hydrolysis (Bos et al., 2007). Bacterial GEFs mimic the three-dimensional architecture of host GEFs that allow them to disrupt GTPase signaling through a complimentary lock-and-key pairing with a specific host GTPase (Huang et al., 2009). The well-characterized bacterial GEFs include IpgB1 and IpgB2 of Shigella species and Map of EPEC which activate Rac1, RhoA and Cdc42, respectively. Bacterial GAPs, such as YopE and YopT of Yersinia, are distinct from host GAPs in both sequence and structural organization, but nonetheless engage Rho GTPases in a manner similar to host GAPs by employing a conserved arginine residue (Stebbins and Galan, 2000).
Our results suggest that T3SS effector VopE of V. cholerae AM-19226 functions as a GAP that inactivates Miro by binding and increasing its rate of GTP hydrolysis. Miro belongs to a separate family of Ras superfamily as its structural organization and functional properties are distinct from those of classical small GTPase (Reis et al., 2009). Miro contains two GTPase domains separated by two canonical and two non-canonical EF-hand motifs that are required for calcium binding, and a C-terminal transmembrane domain anchoring Miro to a mitochondrial outer membrane (Fransson et al., 2003; Klosowiak et al., 2013). The N-terminal GTPase domain (GTPase-I, Fig. 6D) of Miro is similar to Rho GTPases, while the C-terminal GTPase domain (GTPase-II) is structurally more similar to the Ras homologue Rheb (Klosowiak et al., 2013). Miro also diverges from the Rho-conserved DxxG Switch II motif, lacks the insert domain and a membrane-targeting CAAX-box (Reis et al., 2009). Our data shows that VopE exerts its activity through binding to GTPase-I of Miro, thus it is likely that the molecular mechanism of the VopE-Miro interaction is similar to that of Rho GTPase inhibition by Yersinia YopE, Pseudomonas aeruginosa ExoS, and Salmonella enterica SptP effectors (Stebbins and Galan, 2000). However, a crystal structure of VopE is required to confirm this hypothesis as the unique tertiary structures and catalytic mechanisms of some T3SS effectors such as LepB of Legionella, VirA of Shigella and EspG of EPEC were not apparent from their primary or predicted secondary structures (Dong et al., 2012; Yu et al., 2013).
Miro is a calcium-sensitive adaptor protein that attaches the mitochondria to KIF5 (kinesin superfamily protein 5) motor proteins through the cargo-adaptor protein Milton and in combination with accessory proteins TRAK1 (trafficking kinesin protein 1), TRAK2 and Myo19 (myosin-XIX) moves mitochondria along microtubules in the cells (Brickley and Stephenson, 2011; Chang et al., 2011; Glater et al., 2006; Quintero et al., 2009). It has also been suggested that Miro interacts with dynein/dynactin complex possibly to promote anterograde mitochondrial trafficking (Morlino et al., 2014). The EF-hand motif allows Miro to acts as a Ca2+-sensor and move along Ca2+ gradient from areas of low Ca2+- and stopping mitochondria in areas of high Ca2+ (Wang and Schwarz, 2009). Here, we show that Ca2+ is also essential for Miro-mediated perinuclear mitochondrial clustering as Ca2+ chelators and mutations in EF-hand motifs abrogated this clustering. Collectively our results suggest that infection with T3SS-positive V. cholerae induces Ca2+ influx in the host cells that would ordinarily cause GTP-bound Miro to bind Ca2+ and activate mitochondrial transport along microtubules. Our data suggest that the T3SS effector VopE apparently inhibits this Ca2+-dependent mitochondrial trafficking by inhibiting Miro function through its GAP activity.
Our results also showed that in the absence of VopE, T3SS-positive V. cholerae are detected by host cells and this results in a Miro-dependent reorganization of the mitochondrial network, an event that causes dramatic perinulear clustering of these organelles. Interestingly, perinulear clustering of mitochondria was previously shown to be induced in the presence of a constitutive mutant of Miro, and was associated with an increase in apoptosis 48 h after transfection with Miro expressing construct (Fransson et al., 2003). In our hands we did not see an increase in the rate of apoptosis, however, our experiments were performed during a shorter time frame. Additionally, Miro is an integral regulatory component of ERMES, an ER-mitochondria encounter structure that tethers components that play roles in phospholipid exchange between organelles, coordination of mitochondrial protein import, mitochondrial DNA replication, and mitochondrial dynamics (Kornmann et al., 2011). The GTPase-I domain of Miro is required for its association with ERMES complex and to break ER-mithochondria contacts during mitochondrial division (Murley et al., 2013). Thus, it is possible that binding of VopE to GTPase-I of Miro leads to changes in ERMES activity (Kornmann et al., 2011; Murley et al., 2013). Thus, suppression of perinuclear mitochondrial clustering by VopE might to some degree reflect an alteration in ERMES activity that, in turn, alters the rates of Miro-dependent ER-mithochondria separation.
Mitochondrial dynamics plays an essential role in mounting of the immune response since mitochondria functions as a signaling platform, and are required for generation of reactive oxygen species (Cloonan and Choi, 2013). This report describes the importance of Miro for the induction of immune responses. Previous studies have focused on the role of Miro in cell processes that occur in neurons and epithelial stem cells (see below). Our data show that perinuclear mitochondrial clustering in the absence of Miro-VopE interaction during V. cholerae infection resulted in an increase of MAVS aggregation and induction of NF-κB signaling. However, because we did not detect a direct VopE-MAVS interaction, the MAVS aggregation we observed is most likely due to Miro-dependent changes in mitochondrial dynamics. Currently a link between Miro-dependent mitochondrial trafficking and MAVS aggregation/activation has not been reported. However, it is possible that Miro indirectly influences MAVS aggregation/activation through modulation by MAVS-regulating proteins (Jacobs and Coyne, 2013). Of a particular interest in this case would be Mfn1 and Drp1 as we showed that VopE prevented Mfn1-induced mitochondrial fusion and was localized with Drp1-mediated mitochondrial fission sites (Figs. S4C, D). Interestingly, the bacterial toxins Helicobacter pylori vacuolating cytotoxin A (VacA) and Listeria monocytogenes listeriolysin O (LLO) are known to activate the mitochondrial fission machinery. VacA causes mitochondrial fragmentation and subsequent apoptosis induction by Drp1 activation (Jain et al., 2011) and LLO mediates Drp1- and Opa1-independent mitochondrial fission (Stavru et al., 2013). Although these changes in mitochondrial dynamics alter some mitochondria-dependent events (e.g., cell death), these toxins have not been implicated in modulating innate immune responses. In contrast, our data suggest that by altering Miro-dependent mitochondrial trafficking, VopE can suppress innate immune responses specifically in the perinuclear region where the endoplasmic reticulum (ER) and MAVS signaling complexes are localized. ER-associated components such as STING (stimulator of interferon genes) are critical components of the MAVS signaling complex and interact with other cytosolic nucleic sensors such as RIG-I and cGAS (cyclic GMP-AMP synthase) (Biacchesi et al., 2012; Hou et al., 2011; Seth et al., 2005; Sun et al., 2013; Takeuchi and Akira, 2010). RIG-I and STING are central mediators of innate immune responses leading to activation of NF-κB and production of type I IFNs (Burdette et al., 2011; Gack, 2014; Loo and Gale, 2011). RIG-I plays a key role in sensing of non-self RNA in the cell cytosol, while STING is required for sensing of cyclic di-nucleotides that are produced by cGAS in response to its detection of cytosolic DNA (Ablasser et al., 2013; Danilchanka and Mekalanos, 2013; Diner et al., 2013; Gao et al., 2013; Schlee, 2013; Sun et al., 2013; Zhang et al., 2013). Future experiments might address whether VopE can interfere with RIG-I and STING signaling since both of these innate immune receptors are dependent on MAVS activation.
Besides innate immune responses, mitochondrial dynamics is also essential for other central cellular processes including energy balance and oxidative stress responses. Thus far, our knowledge of how Miro regulation affects these mitochondrial functions is limited. It has been hypothesized that Miro is regulated by endogenous GAPs and GEFs due to a low rate of GTP hydrolysis of both GTPase-I and GTPase-II (Kornmann et al., 2011; Koshiba et al., 2011), but to our knowledge such host-derived regulatory Miro regulatory proteins have not yet been described. The fact that VopE regulates Miro thorough its GAP activity as shown by our data strongly indicates that Miro is likely under control of undefined host GAPs that VopE is able to mimic. Additionally Miro is regulated at the posttranslational level by the Ser/Thr kinase PINK1 (PTEN-induced kinase 1) and the E3 ubiquitin ligase Parkin, two key proteins whose alteration by mutations has been linked to the early onset of Parkinson’s disease (Sarraf et al., 2013; Wang et al., 2011). How phosphorylation of Miro relates to functions that are protective against this neurological disease is currently not fully understood (Birsa et al., 2014; Liu et al., 2012; Wang et al., 2011).
Additionally, recent reports suggest that Miro1 regulates intercellular mitochondrial trafficking from mesenchymal stem cells (MSC) to epithelial cells and is protective against acute lung injury (Ahmad et al., 2014; Islam et al., 2012). While mitochondrial transfer and a role of MSC in epithelial recovery in the context of gut diseases have not been investigated, it is tempting to speculate that VopE might play a role in gut epithelial injury recovery and disease progression during V. cholerae infection. Interestingly, another T3SS effector VopF is known to reorganize the actin cytoskeleton, depolarize epithelial monolayers and disrupt the tight junctions between epithelial cells (Tam et al., 2010). Thus, T3SS-delivery of VopE to MSCs located under a layer of epithelial cells (Powell et al., 2011) might cause the inhibition of Miro-dependent mitochondrial movement and thus interfere with the recovery of the epithelium during V. cholerae infection. The combination of disruption of tight junctions and inhibition of epithelial repair might explain why both VopE and VopF contribute to the diarrhea response triggered by V. cholerae infection (Tam et al., 2010).
In conclusion, our data suggest that the activity of Miro GTPases can be modulated. They, therefore, should be considered as targets for drug discovery. Compounds that inhibit or activate these proteins might be broadly useful in the treatment of inflammatory conditions of the epithelium (e.g., Crohn’s disease) as well as neurological conditions (e.g., Parkinson’s disease) where alterations of Miro-dependent mitochondrial dynamics may play a protective role.
EXPERIMENTAL PROCEDURES
Cell culture infection experiments
V. cholerae wild-type strains AM-19226 and V52, and their isogenic Δhap Δhly Δrtx, ΔvcsN, and/or ΔvopE mutants were previously described (Tam et al., 2007; Tam et al., 2010). AM-19226 derivatives harboring C-terminal Myc-tagged vopE (wt, R125K, or L4E) genes cloned into pRSFDuet-1 were constructed for this study. V. cholerae strains were cultured according to standard procedures (Tam et al., 2007; Tam et al., 2010). CHO, HeLa, and 293T cells lines were grown in Dulbecco’s modified Eagle’s medium (Sigma–Aldrich) containing 10% fetal bovine serum. Cultured cells were infected with V. cholerae at a multiplicity of infection (MOI) of 20–100. To measure host cell death during infection, LDH assay was performed using CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega) according to the manufacturer’s instructions.
Perinuclear MT clustering
V. cholerae infection of cell lines induced mitochondrial fusion following mitochondrial translocation to the perinuclear region. The series of cellular processes are termed “perinuclear mitochondrial (MT) clustering”. We defined the perinuclear MT clustering phenotype induced by V. cholerae infection as the abnormal perinuclear aggregation of mitochondria. Representative images showing cells with perinuclear MT clustering are shown in Fig. 3C. To obtain quantitative data on MT clustering, more than 100 cells were observed in each experiment, and each experiment was repeated at least three times. Data were analyzed using a Student’s t-test for unpaired groups and expressed as the means ± SE of triplicate experiments.
GST and MBP pull-down assay
Assays were performed as previously described (Suzuki et al., 2011; Suzuki et al., 2009). N-terminal GST- and MBP-fused proteins were purified from E. coli BL21(DE3) by glutathione sepharose (GE Healthcare Life Sciences) and amylose resin (New England Biolabs), respectively using manufacturer’s recommendations.
GAP assay
The GAP activity of VopE on Miro GTPases was measured as GTP hydrolysis using CytoPhos Endpoint Phosphate Assay (Cytoskeleton) according to the manufacturer’s instructions. Purified GST-VopE (2.5 or 5 μg) and the GTPase-I domain of MBP-Miro proteins (5 μg) were incubated at 37°C for 20 min with 200 μM GTP in the reaction buffer [20 mM Tris (pH 7.5), 50 mM NaCl, 10 mM MgCl2, 1 mM DTT]. Phosphate generated by hydrolysis of GTP was measured by the addition of CytoPhos reagent and reading of absorbance at 650 nm.
Luciferase assay
Luciferase assays were performed using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s instructions. The cells were co-transfected for 20 h with the appropriate firefly luciferase reporter plasmid and Renilla luciferase control reporter plasmid phRL-TK. The firefly luciferase levels were measured and normalized to the activity of phRL-TK–derived Renilla luciferase. Data were analyzed using a Student’s t-test for unpaired groups and expressed as the means ± SE of triplicate experiments.
In vitro Kinase Assay
IKK kinase assays using purified GST-IκB protein were performed as described (Suzuki et al., 2011). Briefly, 293T cells (5×107 cells) were transfected for 24 h with VopE-GFP, FLAG-MAVS, and/or HA-Miro1 expression plasmids (1 μg each), and IKK complex was immunoprecipitated using anti-Nemo antibody. Immunoprecipitates and 1 μg of GST-IκB proteins were incubated at 30°C for 5 min. The phosphorylated proteins were detected by immunostaining using anti-p-IκB (Ser32) antibody.
See the Extended Experimental Procedures for detailed methods.
Supplementary Material
Acknowledgments
We thank Brian T. Ho for critically reading the manuscript and the Nikon Imaging Center at Harvard Medical School for help with confocal microscopy. This work was supported by grants AI-0181045 and GM-068851 from the National Institute of Allergy and Infectious Disease to J.J.M.
Footnotes
AUTHOR CONTRIBUTIONS
M.S., J.J.M conceived the work and planned experiments, M.S., O.D. performed experiments, M.S., O.D., J.J.M. analyzed data and wrote the paper.
The authors declare no conflict of interest.
References
- Ablasser A, Goldeck M, Cavlar T, Deimling T, Witte G, Rohl I, Hopfner KP, Ludwig J, Hornung V. cGAS produces a 2′–5′-linked cyclic dinucleotide second messenger that activates STING. Nature. 2013;498:380–384. doi: 10.1038/nature12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Rehman R, Tiwari BK, Jha KA, Barhanpurkar AP, Wani MR, et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. The EMBO journal. 2014;33:994–1010. doi: 10.1002/embj.201386030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktories K, Schmidt G, Just I. Rho GTPases as targets of bacterial protein toxins. Biol Chem. 2000;381:421–426. doi: 10.1515/BC.2000.054. [DOI] [PubMed] [Google Scholar]
- Alam A, Miller KA, Chaand M, Butler JS, Dziejman M. Identification of Vibrio cholerae type III secretion system effector proteins. Infect Immun. 2011;79:1728–1740. doi: 10.1128/IAI.01194-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto NM. Mimicking small G-proteins: an emerging theme from the bacterial virulence arsenal. Cellular microbiology. 2008;10:566–575. doi: 10.1111/j.1462-5822.2007.01110.x. [DOI] [PubMed] [Google Scholar]
- Arnoult D, Carneiro L, Tattoli I, Girardin SE. The role of mitochondria in cellular defense against microbial infection. Seminars in immunology. 2009;21:223–232. doi: 10.1016/j.smim.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Auerbuch V, Golenbock DT, Isberg RR. Innate immune recognition of Yersinia pseudotuberculosis type III secretion. PLoS pathogens. 2009;5:e1000686. doi: 10.1371/journal.ppat.1000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biacchesi S, Merour E, Lamoureux A, Bernard J, Bremont M. Both STING and MAVS fish orthologs contribute to the induction of interferon mediated by RIG-I. PLoS ONE. 2012;7:e47737. doi: 10.1371/journal.pone.0047737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birsa N, Norkett R, Wauer T, Mevissen TE, Wu HC, Foltynie T, Bhatia K, Hirst WD, Komander D, Plun-Favreau H, et al. K27 ubiquitination of the mitochondrial transport protein Miro is dependent on serine 65 of the Parkin ubiquitin ligase. The Journal of biological chemistry. 2014 doi: 10.1074/jbc.M114.563031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Brickley K, Stephenson FA. Trafficking kinesin protein (TRAK)-mediated transport of mitochondria in axons of hippocampal neurons. The Journal of biological chemistry. 2011;286:18079–18092. doi: 10.1074/jbc.M111.236018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberg CA, Orth K. Tipping the balance by manipulating post-translational modifications. Current opinion in microbiology. 2010;13:34–40. doi: 10.1016/j.mib.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KT, Niescier RF, Min KT. Mitochondrial matrix Ca2+ as an intrinsic signal regulating mitochondrial motility in axons. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15456–15461. doi: 10.1073/pnas.1106862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: shaping the evolution of the plant immune response. Cell. 2006;124:803–814. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Cloonan SM, Choi AM. Mitochondria: sensors and mediators of innate immune receptor signaling. Current opinion in microbiology. 2013;16:327–338. doi: 10.1016/j.mib.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilchanka O, Mekalanos JJ. Cyclic dinucleotides and the innate immune response. Cell. 2013;154:962–970. doi: 10.1016/j.cell.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diner EJ, Burdette DL, Wilson SC, Monroe KM, Kellenberger CA, Hyodo M, Hayakawa Y, Hammond MC, Vance RE. The Innate Immune DNA Sensor cGAS Produces a Noncanonical Cyclic Dinucleotide that Activates Human STING. Cell Rep. 2013;3:1355–1361. doi: 10.1016/j.celrep.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N, Zhu Y, Lu Q, Hu L, Zheng Y, Shao F. Structurally distinct bacterial TBC-like GAPs link Arf GTPase to Rab1 inactivation to counteract host defenses. Cell. 2012;150:1029–1041. doi: 10.1016/j.cell.2012.06.050. [DOI] [PubMed] [Google Scholar]
- Fransson A, Ruusala A, Aspenstrom P. Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. The Journal of biological chemistry. 2003;278:6495–6502. doi: 10.1074/jbc.M208609200. [DOI] [PubMed] [Google Scholar]
- Frederick RL, Shaw JM. Moving mitochondria: establishing distribution of an essential organelle. Traffic. 2007;8:1668–1675. doi: 10.1111/j.1600-0854.2007.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack MU. Mechanisms of RIG-I-like receptor activation and manipulation by viral pathogens. Journal of virology. 2014;88:5213–5216. doi: 10.1128/JVI.03370-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Ascano M, Wu Y, Barchet W, Gaffney BL, Zillinger T, Serganov AA, Liu Y, Jones RA, Hartmann G, et al. Cyclic [G(2′,5′)pA(3′,5′)p] Is the Metazoan Second Messenger Produced by DNA-Activated Cyclic GMP-AMP Synthase. Cell. 2013;153:1094–1107. doi: 10.1016/j.cell.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Rho family GTPases. Biochemical Society transactions. 2012;40:1378–1382. doi: 10.1042/BST20120103. [DOI] [PubMed] [Google Scholar]
- Hicks SW, Galan JE. Exploitation of eukaryotic subcellular targeting mechanisms by bacterial effectors. Nature reviews Microbiology. 2013;11:316–326. doi: 10.1038/nrmicro3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou F, Sun L, Zheng H, Skaug B, Jiang QX, Chen ZJ. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell. 2011;146:448–461. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Sutton SE, Wallenfang AJ, Orchard RC, Wu X, Feng Y, Chai J, Alto NM. Structural insights into host GTPase isoform selection by a family of bacterial GEF mimics. Nature structural & molecular biology. 2009;16:853–860. doi: 10.1038/nsmb.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nature medicine. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JL, Coyne CB. Mechanisms of MAVS regulation at the mitochondrial membrane. Journal of molecular biology. 2013;425:5009–5019. doi: 10.1016/j.jmb.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain P, Luo ZQ, Blanke SR. Helicobacter pylori vacuolating cytotoxin A (VacA) engages the mitochondrial fission machinery to induce host cell death. Proc Natl Acad Sci U S A. 2011;108:16032–16037. doi: 10.1073/pnas.1105175108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- Klosowiak JL, Focia PJ, Chakravarthy S, Landahl EC, Freymann DM, Rice SE. Structural coupling of the EF hand and C-terminal GTPase domains in the mitochondrial protein Miro. EMBO reports. 2013;14:968–974. doi: 10.1038/embor.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Rock KL. How dying cells alert the immune system to danger. Nature reviews Immunology. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornmann B, Osman C, Walter P. The conserved GTPase Gem1 regulates endoplasmic reticulum-mitochondria connections. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14151–14156. doi: 10.1073/pnas.1111314108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba T, Holman HA, Kubara K, Yasukawa K, Kawabata S, Okamoto K, MacFarlane J, Shaw JM. Structure-function analysis of the yeast mitochondrial Rho GTPase, Gem1p: implications for mitochondrial inheritance. The Journal of biological chemistry. 2011;286:354–362. doi: 10.1074/jbc.M110.180034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Sawada T, Lee S, Yu W, Silverio G, Alapatt P, Millan I, Shen A, Saxton W, Kanao T, et al. Parkinson’s disease-associated kinase PINK1 regulates Miro protein level and axonal transport of mitochondria. PLoS Genet. 2012;8:e1002537. doi: 10.1371/journal.pgen.1002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Samuel BS, Breen PC, Ruvkun G. Caenorhabditis elegans pathways that surveil and defend mitochondria. Nature. 2014;508:406–410. doi: 10.1038/nature13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo YM, Gale M., Jr Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Wickham ME, Guttman JA, Deng W, Walker J, Madsen KL, Jacobson K, Vogl WA, Finlay BB, Vallance BA. Citrobacter rodentium infection causes both mitochondrial dysfunction and intestinal epithelial barrier disruption in vivo: role of mitochondrial associated protein (Map) Cellular microbiology. 2006;8:1669–1686. doi: 10.1111/j.1462-5822.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- Martin J, Mahlke K, Pfanner N. Role of an energized inner membrane in mitochondrial protein import. Delta psi drives the movement of presequences. The Journal of biological chemistry. 1991;266:18051–18057. [PubMed] [Google Scholar]
- Melo JA, Ruvkun G. Inactivation of conserved C. elegans genes engages pathogen- and xenobiotic-associated defenses. Cell. 2012;149:452–466. doi: 10.1016/j.cell.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlino G, Barreiro O, Baixauli F, Robles-Valero J, Gonzalez-Granado JM, Villa-Bellosta R, Cuenca J, Sanchez-Sorzano CO, Veiga E, Martin-Cofreces NB, et al. Miro-1 links mitochondria and microtubule Dynein motors to control lymphocyte migration and polarity. Mol Cell Biol. 2014;34:1412–1426. doi: 10.1128/MCB.01177-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murley A, Lackner LL, Osman C, West M, Voeltz GK, Walter P, Nunnari J. ER-associated mitochondrial division links the distribution of mitochondria and mitochondrial DNA in yeast. Elife. 2013;2:e00422. doi: 10.7554/eLife.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DW, Pinchuk IV, Saada JI, Chen X, Mifflin RC. Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol. 2011;73:213–237. doi: 10.1146/annurev.physiol.70.113006.100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero OA, DiVito MM, Adikes RC, Kortan MB, Case LB, Lier AJ, Panaretos NS, Slater SQ, Rengarajan M, Feliu M, et al. Human Myo19 is a novel myosin that associates with mitochondria. Curr Biol. 2009;19:2008–2013. doi: 10.1016/j.cub.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddick LE, Alto NM. Bacteria fighting back: how pathogens target and subvert the host innate immune system. Molecular cell. 2014;54:321–328. doi: 10.1016/j.molcel.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis K, Fransson A, Aspenstrom P. The Miro GTPases: at the heart of the mitochondrial transport machinery. FEBS letters. 2009;583:1391–1398. doi: 10.1016/j.febslet.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Sarraf SA, Raman M, Guarani-Pereira V, Sowa ME, Huttlin EL, Gygi SP, Harper JW. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature. 2013;496:372–376. doi: 10.1038/nature12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlee M. Master sensors of pathogenic RNA - RIG-I like receptors. Immunobiology. 2013;218:1322–1335. doi: 10.1016/j.imbio.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Shin OS, Tam VC, Suzuki M, Ritchie JM, Bronson RT, Waldor MK, Mekalanos JJ. Type III secretion is essential for the rapidly fatal diarrheal disease caused by non-O1, non-O139 Vibrio cholerae. MBio. 2011;2:e00106–00111. doi: 10.1128/mBio.00106-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavru F, Palmer AE, Wang C, Youle RJ, Cossart P. Atypical mitochondrial fission upon bacterial infection. Proc Natl Acad Sci U S A. 2013;110:16003–16008. doi: 10.1073/pnas.1315784110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins CE, Galan JE. Modulation of host signaling by a bacterial mimic: structure of the Salmonella effector SptP bound to Rac1. Molecular cell. 2000;6:1449–1460. doi: 10.1016/s1097-2765(00)00141-6. [DOI] [PubMed] [Google Scholar]
- Stein MP, Muller MP, Wandinger-Ness A. Bacterial pathogens commandeer Rab GTPases to establish intracellular niches. Traffic. 2012;13:1565–1588. doi: 10.1111/tra.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Maresso AW, Kim JJ, Barbieri JT. How bacterial ADP-ribosylating toxins recognize substrates. Nature structural & molecular biology. 2004;11:868–876. doi: 10.1038/nsmb818. [DOI] [PubMed] [Google Scholar]
- Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Kiga K, Kersulyte D, Cok J, Hooper CC, Mimuro H, Sanada T, Suzuki S, Oyama M, Kozuka-Hata H, et al. Attenuated CagA oncoprotein in Helicobacter pylori from Amerindians in Peruvian Amazon. The Journal of biological chemistry. 2011;286:29964–29972. doi: 10.1074/jbc.M111.263715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Mimuro H, Kiga K, Fukumatsu M, Ishijima N, Morikawa H, Nagai S, Koyasu S, Gilman RH, Kersulyte D, et al. Helicobacter pylori CagA phosphorylation-independent function in epithelial proliferation and inflammation. Cell host & microbe. 2009;5:23–34. doi: 10.1016/j.chom.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Tam VC, Serruto D, Dziejman M, Brieher W, Mekalanos JJ. A type III secretion system in Vibrio cholerae translocates a formin/spire hybrid-like actin nucleator to promote intestinal colonization. Cell host & microbe. 2007;1:95–107. doi: 10.1016/j.chom.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Tam VC, Suzuki M, Coughlin M, Saslowsky D, Biswas K, Lencer WI, Faruque SM, Mekalanos JJ. Functional analysis of VopF activity required for colonization in Vibrio cholerae. MBio. 2010:1. doi: 10.1128/mBio.00289-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance RE, Isberg RR, Portnoy DA. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell host & microbe. 2009;6:10–21. doi: 10.1016/j.chom.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Schwarz TL. The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–174. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, LaVoie MJ, Schwarz TL. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 2011;147:893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nature reviews Immunology. 2011;11:389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann B. Mitochondrial fusion and fission in cell life and death. Nature reviews Molecular cell biology. 2010;11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- Winter SE, Keestra AM, Tsolis RM, Baumler AJ. The blessings and curses of intestinal inflammation. Cell host & microbe. 2010a;8:36–43. doi: 10.1016/j.chom.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, Russell JM, Bevins CL, Adams LG, Tsolis RM, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010b;467:426–429. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Hu L, Yao Q, Zhu Y, Dong N, Wang DC, Shao F. Structural analyses of Legionella LepB reveal a new GAP fold that catalytically mimics eukaryotic RasGAP. Cell Res. 2013;23:775–787. doi: 10.1038/cr.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Shi H, Wu J, Sun L, Chen C, Chen ZJ. Cyclic GMP-AMP Containing Mixed Phosphodiester Linkages Is An Endogenous High-Affinity Ligand for STING. Molecular Cell. 2013;51:226–235. doi: 10.1016/j.molcel.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.