Abstract
Purpose
Pancreatic ductal adenocarcinoma (PDA) is the fourth leading cause of cancer death in the United States and its incidence is on the rise. Advanced disease is nearly uniformly lethal, emphasizing the need to identify PDA at its earliest stages. To discover early biomarkers of PDA, we evaluated the circulating proteome in murine preinvasive and invasive plasma samples and human pre-diagnostic and diagnostic samples.
Experimental Design
Using a customized antibody microarray platform containing >4000 features, we interrogated plasma samples spanning preinvasive and invasive disease from a highly faithful mouse model of PDA. In parallel, we mined pre-diagnostic plasma from women in the Women’s Health Initiative (WHI) who would later succumb to PDA together with matched, cancer-free control samples. Samples collected after an establishing diagnosis of PDA were also interrogated to further validate markers.
Results
We identified ERBB2 and TNC in our cross-species analyses and multiple antibodies identified ESR1 in pre-diagnostic plasma from people that succumb to PDA. This 3-marker panel had an AUC of 0.86 (0.76–0.96, 95% confidence interval (CI)) for the diagnostic cohort that increased to 0.97 (0.92–1.0, 95% CI) with CA19-9 included. The 3-marker panel also had an AUC of 0.68 (0.58–0.77, 95% CI) for the pre-diagnostic cohort.
Conclusions
We identified potential disease detection markers in plasma up to 4 years prior to death from PDA with superior performance to CA19-9. These markers might be especially useful in high-risk cohorts to diagnose early, resectable disease, particularly in patients that do not produce CA19-9.
INTRODUCTION
Survival rates for many cancers including breast, colon and prostate have improved significantly in the past two decades, but the prognosis for pancreatic ductal adenocarcinoma (PDA), or pancreas cancer, has remained dismal. Five-year survival rates remained unchanged at ~6% from 2002–2008 (1), which is of additional concern given the 1.2% annual increase in incidence from 1999–2010 (SEER Incidence, seer.cancer.gov/faststats/selections). Surgical resection remains the only curative option, but the majority (>80%) of patients present with unresectable disease at diagnosis, highlighting the need for improved early detection strategies (2). Patients diagnosed with localized, resectable disease have 5-year survival rates that improve to a modest 20% (3), with a median survival of ~20 months (4). These outcomes reflect the micrometastatic capability of PDA early in disease progression and the challenges in detecting occult disseminated disease.
The retroperitoneal location of the pancreas together with its cargo of digestive enzymes impede safe and efficient biopsy of the organ, making a diagnostic test on readily accessible biological fluids an attractive alternative. The only FDA-approved blood-based marker for pancreatic cancer is CA19-9, but with sensitivities and specificities ranging from 60–70% and 70–85%, respectively (5), it is not recommended for screening, as a diagnostic, or to determine operability. CA19-9 is instead typically used to assess response to treatment and/or disease recurrence in people that express elevated levels at diagnosis (6, 7). Numerous studies have focused on identifying serum, tissue, ascites and cyst fluid markers for early detection, although the majority of samples in these studies were obtained at diagnosis, at which point most patients are incurable. For markers to be clinically meaningful for disease detection of PDA, they should ideally be present and measurable at subclinical stages. Biological fluids collected in large, prospective, longitudinal cohort studies provide a unique resource for specimens drawn prior to clinical diagnosis of disease. Such specimens are especially invaluable for PDA, which has a relatively low incidence and is frequently asymptomatic at early stages.
In the present study, we used our high density antibody microarray platform (8–10) customized for pancreas cancer (11), to interrogate: 1) plasma drawn at distinct time points from a highly faithful genetically engineered mouse model of pancreas cancer (12); 2) pre-diagnostic plasma from women who later succumbed to PDA; and 3) diagnostic plasma from patients. By further focusing on identified plasma membrane and secreted proteins, we identified two markers that overlapped between mouse and pre-diagnostic human datasets and that have individually been previously implicated in PDA; a third novel marker, ESR1, was identified by multiple distinct antibodies in pre-diagnostic human plasma samples. In a subsequent set of array experiments on a separate cohort of 24 diagnostic PDA samples, all 3 markers were again up-regulated in PDA compared to an equal number of controls, collectively providing preliminary confirmation across multiple sample sets. The implications of these findings and the potential applicability of this 3-marker panel to early diagnosis of pancreas cancer are further discussed.
MATERIALS AND METHODS
Patient samples
Pre-diagnostic samples
Eighty-seven pre-diagnostic PDA and 87 matched control plasma samples collected in EDTA were obtained from the Women’s Health Initiative’s (WHI) observational study. Controls were matched 1:1 to PDA cases based on the following criteria: age at screening; year of WHI enrollment; alcohol consumption at baseline; race/ethnicity; smoking status (never, past, current); diabetes history (yes or no); prior hormone replacement therapy (none, estrogen only, estrogen and progesterone); blood draw visit (baseline only, baseline and year 3, year 3 only); and duration of follow-up. A reference pool of EDTA-collected plasma was created by pooling plasma drawn from a group of seven female volunteers from the Fred Hutchinson Cancer Research Center, aged 27–45. All samples were de-identified and the study was approved by the FHCRC Institutional Review Board.
Diagnostic samples
Twenty-four diagnostic EDTA-collected plasma samples were provided by the Center for Accelerated Translation in Pancreas Cancer (CATPAC) at the Seattle Cancer Care Alliance, Seattle, Washington. Twenty-four unmatched control (not diagnosed with any cancer) plasma samples were collected and processed at the same clinic using the same methods. All patient information provided was done so in accordance with the Institutional Review Board at the Fred Hutchinson Cancer Research Center.
Mouse plasma collection
Murine plasma samples were acquired from seventeen KrasLSL-G12D/+;Trp53R172H/+;Pdx-1-Cre or p48Cre/+ (KPC) and age-matched KP and Cre control mice on a mixed C57BL/6/SV129 mixed background following brief anesthesia with isoflurane (12). Either Pdx-1 or p48 promoters were used to drive Cre expression and target pancreas-specific expression of the point mutant alleles (13). Blood (up to 200 µl) was collected every two weeks from KPC and age-matched control mice into tubes containing 10 µl 0.5M EDTA. Samples were centrifuged (4000 g × 10 minutes), the supernatant collected and re-centrifuged (16,000 g × 10 minutes), and aliquots stored at −80°C until use in array experiments.
Mouse tissue collection and induction of chronic pancreatitis
Tissue from the head of the pancreas of 2-month old (n = 5), 4-month old (n = 5) and end-stage disease KPC (n = 8) and age-matched KP and Cre control animals (n = 4 each per time point) was collected at necropsy and flash frozen until use in antibody microarray experiments. Tissues were similarly harvested from a cohort of six 2-month old wild type (WT) mice injected intraperitoneally with 100 µl cerulein (50 µg/ml) for 23 consecutive days to induce chronic pancreatitis and necropsies were performed within 6 hours of the final injection. All mouse husbandry and procedures were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee at the Fred Hutchinson Cancer Research Center.
Sample preparation for antibody microarray analysis
Plasma samples were depleted of IgG and serum albumin using the Proteoprep Immunoaffinity Albumin and IgG Depletion Kit per the manufacturer’s instructions (Sigma Aldrich, St. Louis, MO). Protein lysates from depleted case and control plasma samples (250 µg total protein) and murine tissue samples (200 µg total protein) were labeled with N-hyroxysuccinimide (NHS)-Cy5 (GE Health Biosciences, Pittsburgh, PA); reference pools of plasma and tissue lysates collected from WT mice were labeled with NHS-Cy3. Murine tissues were incubated in lysis buffer (10:1 volume-to-weight) containing 1% NP-40, 0.25% deoxycholate, 0.25% octyl-β-d-glucopyranoside and 0.25% amidosulfobetaine-14 supplemented with phosphatase inhibitors, Roche protease inhibitor cocktail (Roche USA, Indianapolis, IN) and 1mM PMSF.
Antibody microarray interrogation
Antibody microarrays were printed and incubated with labeled plasma and tissue samples on Nexterion H slides (Schott, Mainz, Germany) essentially as previously described (9). Briefly, antibodies were printed in triplicate in a 16 × 16 block format with 48 blocks per array for a total of 3 × 4096 unique features. Antibodies were printed at a final concentration of 175–350 µg/ml. Following a “case/control versus reference” procedure, individual Cy5-labeled case and control samples were pooled with an equal amount of Cy3-labeled reference to remove dye bias from the analyses. Labeled lysates were incubated on arrays for 1.5 hours, washed serially to remove excess dye, and the arrays scanned and analyzed using an Axon Genepix 4200A scanner (Molecular Devices, LLC, Sunnyvale, CA).
Array analyses and statistics
For each antibody, the difference in case/control signal (red channel) compared to reference (green channel), known as the M value, was calculated as log2(Rc/Gc), where Rc is red corrected, and Gc is green corrected (using the normexp background correction method developed by Smyth (14)). Saturated array spots were flagged and triplicate antibodies with coefficients of variation (CV) >10% were removed prior to array normalization. Following localized background correction, print tip loess intra-array normalization was performed. Interarray green channel quantile adjustment was applied to normalize the reference (green) signal. The median intensity of triplicate spots for each antibody feature was calculated and control signal was subsequently standardized to have a mean signal of zero with a standard deviation of 1. For the WHI pre-diagnostic plasma dataset using the 87 cases and 87 matched controls, linear regression (which considers covariate effects such as incubation day, print day and body mass index (BMI)) was used. Paired t-tests were conducted for the adjusted measures. For the diagnostic plasma dataset, linear regression was fitted factoring in the incubation day; for the mouse plasma and tissue data, we used logistic regression after removing the incubation day effect. Candidate protein markers were then ranked based on their p-values and effect size/odds ratios (WHI and CATPAC) or logistic regression odds ratio (mouse plasma and tissue). A positive odds ratio (OR) means the candidate protein is greater in cancer than controls; a negative value means the converse. All normalization procedures and analyses were conducted using R statistical computing software program. Markers were combined as previously described (15). CA19-9 was analyzed using a Luminex bead based immunoassay as previously described (16). The statistical analyses presented in Fig. 2 used a paired 2-tailed t-test for the WHI samples and unpaired 2-tailed t-tests for the murine and CATPAC diagnostic sample sets, respectively, and were computed using GraphPad Prism 5.0. Receiver operator characteristic (ROC) curves and Area Under the Curves (AUC) were calculated as previously described (17).
Figure 2.
Cross-species identification of elevated TNC and ERBB2 in KPC pancreas cancer plasma and human pre-diagnostic plasma and, for TNC, in diagnostic plasma samples. M-value plots for TNC and ERBB2 show elevated plasma levels in human pre-diagnostic (A) and mouse KPC plasma (B). TNC is also elevated in KPC tumor tissue (B) and diagnostic human plasma (C). Normalized M-values (red/green ratios) for case and control samples are plotted along with the mean and standard deviations for each dataset. CA19-9 levels (U/ml) in pre-diagnostic (A) and diagnostic human samples (C) were also measured. Paired 2-tailed t-tests were used to determine statistical significance for human pre-diagnostic plasma and unpaired 2-tailed t-tests for mouse plasma and tissue and human diagnostic plasma datasets. All statistical analyses were conducted in GraphPad Prism 5.0. *, p< 0.05; **, p<0.01.
Immunohistochemistry
Four-micron sections of formalin-fixed paraffin-embedded tissues were deparaffinized with xylene and rehydrated sequentially. Antigen retrieval was performed in a pressure cooker in Trilogy pH 8.0 Buffer (Cell Marque, Rocklin, CA) and subsequent incubations performed on a Dako Autostainer Plus (Agilent Technologies, Santa Clara, CA). Slides were treated with 3% hydrogen peroxide, blocked with TCT Buffer (0.05M Tris, 0.15M NaCl, 0.25% Casein, 0.1% Tween 20, pH 7.6) and incubated with anti-TNC antibody (1:75) (Novus Biologicals, Littleton, CO) or a matched concentration of rabbit IgG. Poly-HRP anti-Rabbit IgG Polymer (Leica Microsystems, Buffalo Grove, IL) was then applied followed by DAB+ substrate-chromagen (Agilent Technologies, Santa Clara, CA). Slides were counterstained with hematoxylin (Agilent). Serial sections were stained with hematoxylin and eosin for histological analyses. All images were captured with a Nikon DS-Vi1 brightfield camera using NIS Elements 3.2 Basic Research Image software (Nikon Instruments Inc., Melville, NY).
RESULTS
Antibody microarray interrogation of murine preinvasive and invasive plasma
To discover and develop potential diagnostic biomarkers of early stage pancreas cancer, we began with plasma collected serially from the highly faithful KPC mouse model of PDA (12). These mice develop invasive PDA with high penetrance and recapitulate the clinical, histological and molecular characteristics of the cognate human disease. As such, they have been used extensively in preclinical treatment studies (18–20). Plasma samples from two specific time points, namely 6–8 weeks, representing preinvasive disease (time point #1, or TP1); and midway through the lifespan of each individual KPC mouse (time point #2, or TP2), representing early invasive adenocarcinoma, were compared to controls on an antibody array platform containing 4096 unique features (8–10). No palpable masses or clinical symptoms were evident in the cohorts at either of the two time points. The array platform was tailored for interrogation of pancreas cancer tissue and plasma samples by including approximately 130 antibodies based on their relevance to PDA. These included antibodies against proteins identified as markers of PDA in a published compendium (21), with further enrichment for putative early detection markers of PDA, i.e. markers identified in high-grade precursor pancreatic intraepithelial neoplasia 3 (PanIN-3) lesions. Logistic regression analyses identified fifty-four proteins at TP1 (21 proteins that were up-regulated and 33 down-regulated in KPC plasma versus controls) and 25 proteins at TP2 (12 up, 13 down) (Fig. 1A,B and Supplementary Table S1) that differentiated KPC from control plasma with statistical significance (p<0.05). (We note that none are significant if adjusted for multiple comparison testing given the very high dimensionality of the array.) Among the up-regulated markers were the plasma membrane proteins IL12RB2, AQP2, PCDH15, ICAM5, OPN3, CD27 (TP1), and RAB7L1, EZR, ERBB2, CCR2 and CSF3R (TP2); and the extracellular or secreted markers TNC, HBA1, PTHLH (TP1), and B2M and SERPING1 (TP2).
Figure 1.
Antibody microarray interrogation of murine preinvasive (A) and invasive (B) pancreas cancer plasma and human pre-diagnostic (WHI) plasma samples (C, D). Plasma samples were drawn up to four years prior to death from PDA in patients diagnosed across all stages of disease (C). Statistical significance was computed using an unpaired 2-tailed t-test in GraphPad Prism 5.0. *, p<0.05 for stage IA and IIA vs. IV; **, p<0.01 for stage IB and IIB vs. IV; ***, p<0.001 for stage III vs IV. Samples from patients where stage at diagnosis could not be determined were not included in these comparisons. Volcano plots depict the p-values and accompanying odds ratios (OR) for each antibody feature on the array platform. Candidate markers with p<0.05 are found above the line (estimated) and the number of significant candidates—both up regulated and down regulated in disease versus control plasma—are indicated in the top left corner for each dataset.
Antibody microarray interrogation of pre-diagnostic human plasma samples
To identify putative protein markers that could be used to detect PDA in asymptomatic individuals, we interrogated pre-diagnostic plasma samples drawn from a large cohort of subjects who succumbed to PDA within 4 years after the blood draw and matched controls (see Materials and Methods for matching criteria and Table 1 for sample characteristics). The time from blood draw to diagnosis for this sample set ranged from 33 days to just under 4 years, and the time from diagnosis to death ranged from 0 to just under 700 days. The samples were derived from patients diagnosed at different stages of disease and the population reflected the expected heterogeneity of PDA based on the range in time to death following diagnosis (Fig. 1C). A paired t-test identified a total of 88 candidate markers differentiating pre-diagnostic plasma samples from controls with statistical significance (p < 0.05, although none are significant when adjusting for multiple comparison testing). Twenty-three of these markers were up-regulated and 65 were down-regulated (Fig. 1D). The complete list of up-regulated candidate early detection markers is provided in Table 2 (down-regulated proteins are listed in Supplementary Table S2). The median inter-array variation across 27 arrays incubated with replicate samples in a blinded manner was 0.043 (range 0.0009 – 0.29), showing strong reproducibility across individual arrays.
Table 1. Patient characteristics for WHI pre-diagnostic cases and controls and the CATPAC diagnostic cases.
Characteristics of cohort plasma from the Women's Health Initiative's (WHI) observational study and the Center for Accelerated Translation of Pancreas Cancer (CATPAC) diagnostic plasma samples used in our antibody microarray analysis. WHI cases and controls were matched on an extensive list of criteria (see Materials and Methods) including age, ethnicity, smoking status, HRT status and BMI, whose breakdown are shown here. Stage at diagnosis was determined using the T, N, and M classification system for pancreas cancer, with information provided by the WHI and patient information provided with the CATPAC samples.
| WHI | CATPAC | |||
| Age | Cases | Controls | Age at diagnosis | Cases |
| 50–59 | 14 | 14 | 30–49 | 4 |
| 60–69 | 32 | 33 | 50–59 | 7 |
| 70–80 | 41 | 40 | 60–69 | 7 |
| 70–80 | 6 | |||
| Ethnicity | ||||
| Asian | 4 | 5 | Sex | |
| Black | 4 | 3 | Male | 17 |
| White | 76 | 76 | Female | 7 |
| Other | 3 | 3 | ||
| Smoking status | ||||
| Smoking status | Never | 7 | ||
| Never | 40 | 40 | Current | 4 |
| Current | 8 | 7 | Past | 12 |
| Past | 39 | 40 | N.A. | 1 |
| HRT status | BMI | |||
| Estrogen alone | 14 | 14 | Normal | 9 |
| Estrogen + progesterone |
16 | 16 | Overweight | 9 |
| Obese | 6 | |||
| None | 56 | 54 | ||
| BMI | Stage at diagnosis for CATPAC cases | |||
| Normal | 29 | 38 | Stage | Cases |
| Overweight | 35 | 27 | 1A | 1 |
| Obese | 22 | 21 | IB | 2 |
| N.A. | 1 | 1 | IIA | 4 |
| IIB | 6 | |||
| III | 7 | |||
| Stage at diagnosis for WHI cases | IV | 2 | ||
| Stage | Cases | Unknown | 2 | |
| 1A | 3 | 24 | ||
| IB | 5 | |||
| IIA | 19 | |||
| IIB | 12 | |||
| III | 17 | |||
| IV | 26 | |||
| Unknown | 5 | |||
| Total | 87 | |||
Table 2. Upregulated candidate plasma biomarkers from analysis of WHI pre-diagnostic plasma antibody microarray interrogation.
Candidate up-regulated pre-diagnostic plasma biomarkers for human pancreas cancer. Antibody microarray interrogation and paired T test analyses were used to identify candidate plasma proteins differentiating pre-diagnostic pancreas cancer plasma from age-matched control samples with statistical significance. M values were normalized using linear regression to remove the systematic bias due to experimental or characteristic factors such as printing, incubation day and BMI. We then computed the paired T test p-value and the effect size (the mean difference of case and control in the scale of the control standard deviance). Candidates are listed basedon ascending p-values.
| Gene name | Protein name | Effect size |
p-value |
|---|---|---|---|
| SEPT5 | septin 5 | 0.42 | 0.0083 |
| IL2RA | interleukin 2 receptor, alpha | 0.42 | 0.0085 |
| ERBB2 | v-erb-b2 erythroblastic leukemia viral oncogene homolog 2 | 0.59 | 0.0122 |
| KRT16 | keratin 16 | 0.34 | 0.0129 |
| ESR1 | estrogen receptor 1 | 0.63 | 0.0132 |
| GATA3 | GATA binding protein 3 | 0.28 | 0.0181 |
| TLX3 | T-cell leukemia homeobox 3 | 0.36 | 0.0190 |
| CDK2AP1 | cyclin-dependent kinase 2 associated protein 1 | 0.38 | 0.0205 |
| STAT3 (pY705) | signal transducer and activator of transcription 3 | 0.38 | 0.0224 |
| CLU | clusterin | 0.37 | 0.0226 |
| SERPINH1 | serpin peptidase inhibitor, clade H | 0.45 | 0.0227 |
| HOXD13 | homeobox D13 | 0.33 | 0.0229 |
| BCL2 | B-cell CLL/lymphoma 2 | 0.40 | 0.0276 |
| IL1A | interleukin 1, alpha | 0.30 | 0.0326 |
| MLLT10 | myeloid/lymphoid or mixed-lineage leukemia, 10 | 0.33 | 0.0346 |
| DDB2 | damage-specific DNA binding protein 2, 48kDa | 0.26 | 0.0368 |
| CD20 (MS4A1) | membrane-spanning 4-domains, subfamily A, member 1 | 0.21 | 0.0371 |
| BRAF | v-raf murine sarcoma viral oncogene homolog B1 | 0.27 | 0.0379 |
| TNC | tenascin C | 0.35 | 0.0402 |
| STEAP2 | STEAP family member 2, metalloreductase | 0.29 | 0.0421 |
| PKM2 | pyruvate kinase, muscle | 0.39 | 0.0478 |
| NDRG1 | N-myc downstream regulated 1 | 0.45 | 0.0484 |
| ESR1 | estrogen receptor 1 | 0.43 | 0.0498 |
As the goal was to identify early disease plasma markers that could be used in a non-invasive blood test, we focused our attention on the up-regulated proteins. We cross-referenced our list to that at pancreaticcancerdatabase.org and the compendium developed by Harsha et al. (21), and found that 15 of these 23 putative markers have been previously associated with pancreatic neoplasms, confirming the fidelity of the array platform and methodology. Eight of these 23 markers have also been previously reported in the plasma: ERBB2, KRT16, ESR1 (2 antibodies were in the top list), STAT3 (pY705), CLU, SERPINH1, TNC, and PKM (a third antibody to ESR1 showed increased levels with p<0.06, not shown).
Cross-species biomarker identification
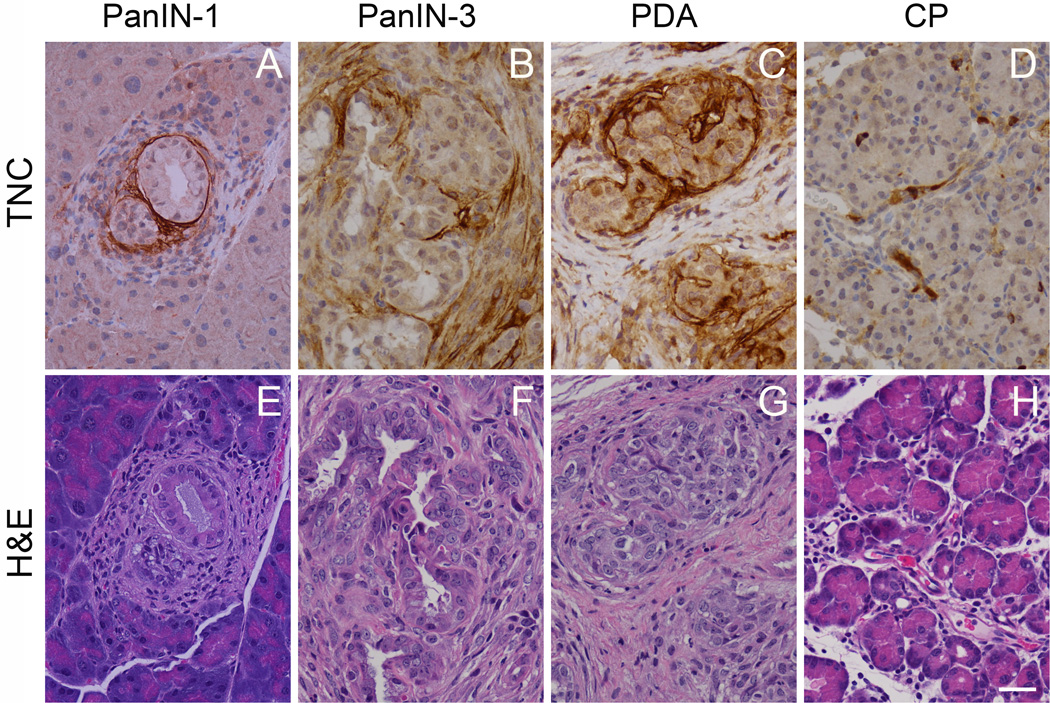
Plotting the M-values (the normalized red/green ratio) of case and control samples for both pre-diagnostic human and preinvasive and invasive KPC plasma shows elevated levels of TNC and ERBB2 up-regulated across species in cases vs. controls with statistical significance (Fig. 2A, B). Increased tissue Erbb2 expression has been demonstrated previously by IHC in preinvasive and invasive PDA in KPC mice (12), corroborating our findings here. To determine whether tissues also expressed increased TNC, we interrogated tissue lysates from KPC mice at 2- and 4-months of age, representing preinvasive and pre-clinical invasive stages of disease, respectively. Array analyses of these tissue lysates showed increased TNC as represented by the M-value plots of cases and controls (Fig. 2B). We also performed IHC on tissues, revealing that TNC levels increased with progression from PanIN to invasive PDA (Fig. 3). Of note, stromal deposition of TNC was seen in regions surrounding even the earliest PanIN-1 lesions (Fig. 3A) and increased along the PanIN-to-PDA progression scheme (Fig. 3B, C). We did not observe appreciable increases in TNC in an experimental mouse model of chronic pancreatitis (CP) (Fig. 3D, H), although others have reported modestly elevated levels in this setting (22). Thus, the finding of elevated plasma ERBB2 and TNC identified through cross-species array analyses is further supported by corresponding increases in pancreatic tissues with preinvasive and invasive disease.
Figure 3.
Immunohistochemistry (IHC) of TNC (A–D) and accompanying serial sections stained with hematoxylin and eosin (E–H) in murine tumor tissue shows the emergence of TNC expression at early preinvasive stages (PanIN-1) (A, E) that increases with progression to invasive PDA. IHC of TNC associated with a PanIN-3 and invasive adenocarcinoma are shown in (B) and (C), respectively. TNC deposition does not increase, however, in a model of chronic pancreatitis (D).
ESR1, ERBB2 and TNC as a marker panel for early diagnosis of pancreas cancer
A receiver operator characteristic (ROC) curve (17) for pre-diagnostic samples was calculated for the 3-marker panel of ERBB2, ESR1 (both of two significant antibodies were included) and TNC, yielding an AUC = 0.68 (0.58–0.77, 95% CI), with ~30% sensitivity at 90% specificity (Fig. 4). Examination of time from blood draw to death in 1-year increments did not show any significant differences between the time points (see Supplementary Fig. S1). CA19-9 alone in these pre-diagnostic samples (Fig. 2A) had an AUC = 0.60 (0.52–0.69, 95% CI). When CA19-9 levels were included with TNC, ERBB2 and ESR1, the AUC did not increase significantly (0.71; 0.62–0.80, 95% CI) (Fig. 4).
Figure 4.
Receiver operator curve (ROC) analysis of a 3-marker panel for the WHI pre-diagnostic plasma sample set. Specificity and sensitivity for ESR1, ERBB2 and TNC as a panel differentiating incipient PDA from controls in pre-diagnostic samples are plotted on x- and y-axes, respectively. The combined AUC for this panel = 0.68 for the pre-diagnostic sample set, 0.86 for the CATPAC diagnostic sample set and 0.71 and 0.97, respectively, when CA19-9 values are included.
Confirmation of ESR1, ERBB2 and TNC in diagnostic PDA plasma
We next interrogated the plasma proteome of diagnostic plasma samples collected through the Center for Accelerated Translation of Pancreas Cancer (CATPAC) (see Table 1 for patient demographics, including age, stage and diagnosis, and smoking status). Twenty-four diagnostic plasma samples (13 from patients with resectable disease) and 24 control samples were interrogated on the array. Linear regression yielded 243 statistically significant (p < 0.05) candidates with 133 candidates increased in case versus control plasma (not shown). When evaluating the 23 up-regulated markers identified from the WHI samples and, more specifically, TNC, ERBB2, and ESR1, in the CATPAC dataset, TNC was again increased with statistical significance (OR = 1.86; p=0.004); and ERBB2 (OR = 1.77; p=0.11) and ESR1 (OR = 1.62; p=0.055) each approached statistical significance in this diagnostic cohort. The AUC for the combined 3-marker panel in diagnostic PDA samples was 0.86 (95% CI 0.76–0.96) (Fig. 4 and see also Supplementary Fig. S2 for dot plots of all composite panel scores). The AUC for CA19-9 for these diagnostic samples was 0.84 (0.73–0.95, 95% CI) (Fig. 2C). The AUC for the 3-marker panel plus CA19-9 increases to 0.97 (0.92–1.0, 95% CI) (Fig. 4), a significant increase over CA19-9 alone (p=0.014) (Supplementary Fig. S2).
DISCUSSION
We applied a cross-species approach to discover and preliminarily validate plasma markers of PDA, using a high-density, pancreas cancer-tailored array platform. Most markers discovered from samples collected at diagnosis understandably do not perform well in pre-diagnostic samples (23). Working from the other direction, we reasoned that array interrogation of plasma collected from the KPC mouse model at preinvasive and focally invasive stages of disease might serve as a useful starting point for initial discovery of markers of resectable disease. Proteins common to both murine and pre-diagnostic human plasma samples may represent true early detection markers for disease stages prior to the development of clinical symptoms. Such markers could potentially be used to more accurately diagnose early stages of pancreas cancer.
PanIN have been identified in pancreata up to 10 years prior to the development of invasive PDA (24) and elevated blood glucose levels have been observed up to 5 years before the clinical diagnosis of PDA (25). That discernible changes in physiology become manifest years prior to diagnosis suggests, in principle, that clinically relevant plasma markers indicative of incipient PDA may exist and can be identified. Array interrogation of pre-diagnostic plasma from the WHI observational study mirrors the challenges of screening asymptomatic individuals. From this set of 87 pre-diagnostic plasma samples, we identified 23 proteins up-regulated in case versus control plasma, 15 of which have been previously implicated in clinically diagnosed PDA.
Comparing the list of plasma membrane and secreted proteins identified in the murine discovery array experiments with the human plasma proteins previously implicated with pancreatic neoplasms revealed that TNC and ERBB2 were common to both. Elevated ERBB2 levels have been reported in PanIN and PDA in the KPC mouse model (12), and increasing levels also correlate with progression from PanIN to invasive PDA in humans (22, 26). Corresponding increases in serum ERBB2 levels have been reported and correlate with metastatic capability and survival (27). That the majority of PDA cases overexpress ERBB2, whereas it is uniformly absent from normal ductal epithelium, makes it even more appealing as part of a candidate early detection marker panel.
We observed increasing levels of TNC with disease progression in KPC mice in both plasma and tissue and also found elevated levels of TNC in diagnostic human plasma samples, corroborating similar observations in human PDA (22) Supplementary Table S3). A previously reported three marker panel of TNC, TFPI and CA19-9 differentiated diagnostic PDA plasma samples from healthy controls with an AUC of 0.99 and 100% specificity at 90% sensitivity (28). We also observed increased levels in diagnostic plasma of ESR1 and ERBB2 that trended towards significance. ESR1 expression has also been reported in invasive PDA (29), and estrogen receptor positivity is a defining characteristic of stromal cells surrounding mucinous cystic neoplasms (MCN), which occur predominantly in women (30, 31). Using ERBB2, ESR1 and TNC as a 3-marker panel yielded an AUC for pre-diagnostic plasma samples of 0.68, and an AUC of 0.86 for diagnostic samples. The AUCs increased to 0.71 and 0.97, respectively, when measured CA19-9 values were included. The 3-marker panel is superior to CA19-9 alone and could be of particular value in patients that are Lewis negative and cannot express CA19-9.
The studies reported here were conducted on plasma drawn up to 4 years prior to patient diagnosis, establishing proof-of-principle that a blood-based assay can identify PDA significantly earlier than current clinical modalities and prior to the onset of symptoms. An AUC approaching 0.7 for predicting incipient PDA that improves to 0.86 (or 0.97 with CA19-9 added) at diagnosis indicates that these markers could potentially help detect disease sufficiently early to be clinically meaningful. Moreover, the further improvement in the performance of the 3-marker panel in diagnostic vs. pre-diagnostic samples suggests that these proteins increase with PDA progression. Our study underscores the difficulty of screening for pancreas cancer in asymptomatic individuals and it must be acknowledged that the performance of this panel is still insufficient for screening in a general population given the low incidence of the disease.
Further analyses of this 3-marker panel may be warranted initially in high-risk cohorts. A variety of at-risk individuals are currently being monitored in pancreas cancer registries including patients with hereditary pancreatitis, those with two or more first-degree relatives with PDA, and those with defined heritable syndromes such as Peutz-Jeghers intestinal polyposis (32). The panel of ERBB2, ESR1 and TNC could also be examined retrospectively with imaging studies, which have high diagnostic potential but are unable to detect microscopic precursor (PanIN) ductal lesions (33). Should imaging modalities identify suspected lesions that are subsequently confirmed at surgery, plasma levels of these markers could be correlated with tumor histology, disease stage, disease latency and survival.
The challenges for pancreas cancer biomarker discovery, validation and implementation are significant. We propose that iterative cross-species analyses such as described here can foster the discovery, assessment and contextualization of early detection markers, with the ultimate goal of use in general screening.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
Pancreatic ductal adenocarcinoma (PDA) is the fourth leading cause of cancer death in the United States. Surgical resection when feasible confers a survival advantage, but PDA is otherwise nearly uniformly lethal. To discover potential biomarkers for improved early diagnosis of PDA, we created a customized, high-dimensional antibody microarray platform and interrogated plasma samples from cohorts of a highly faithful mouse model of PDA representing preinvasive and early invasive stages of disease, respectively. We also tested pre-diagnostic human plasma samples and validated the results in diagnostic samples from patients with PDA. Collectively, these analyses uncovered biologically and potentially clinically relevant markers detectable up to four years prior to death from PDA. These results establish the potential of circulating biomarkers to detect disease prior to the emergence of clinical symptoms and to critically inform clinical decision-making.
Acknowledgments
Financial Support: This work was funded by the National Heart Lung and Blood Institute, National Institutes of Health, under Contract HHSN268200960003C (S.R.H); National Cancer Institute grants U01CA152746 (P.D.L.) as part of the Early Detection Research Network, R21CA149554 (P.D.L.), R01CA108990 (A.E.L.), a Predoctoral Fellowship T32CA080416 (J.E.M); and a grant from the Jeffrey Rosenzweig Foundation (S.R.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: None.
REFERENCES
- 1.American Cancer Society. Cancer Facts & Figures. Atlanta: 2014. [Google Scholar]
- 2.Klein AP, Lindstrom S, Mendelsohn JB, Steplowski E, Arslan AA, Bueno-de-Mesquita HB, et al. An absolute risk model to identify individuals at elevated risk for pancreatic cancer in the general population. PLoS One. 2013;8:e72311. doi: 10.1371/journal.pone.0072311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrone CR, Pieretti-Vanmarcke R, Bloom JP, Zheng H, Szymonifka J, Wargo JA, et al. Pancreatic ductal adenocarcinoma: long-term survival does not equal cure. Surgery. 2012;152:S43–S49. doi: 10.1016/j.surg.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hidalgo M. Pancreatic cancer. The New England journal of medicine. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 5.Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33:266–270. doi: 10.1016/j.ejso.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Winter JM, Yeo CJ, Brody JR. Diagnostic, prognostic, and predictive biomarkers in pancreatic cancer. J Surg Oncol. 2013;107:15–22. doi: 10.1002/jso.23192. [DOI] [PubMed] [Google Scholar]
- 7.Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313–5327. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 8.Li CI, Mirus JE, Zhang Y, Ramirez AB, Ladd JJ, Prentice RL, et al. Discovery and preliminary confirmation of novel early detection biomarkers for triple-negative breast cancer using preclinical plasma samples from the Women's Health Initiative observational study. Breast Cancer Res Treat. 2012;135:611–618. doi: 10.1007/s10549-012-2204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramirez AB, Loch CM, Zhang Y, Liu Y, Wang X, Wayner EA, et al. Use of a single-chain antibody library for ovarian cancer biomarker discovery. Mol Cell Proteomics. 2010;9:1449–1460. doi: 10.1074/mcp.M900496-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loch CM, Ramirez AB, Liu Y, Sather CL, Delrow JJ, Scholler N, et al. Use of high density antibody arrays to validate and discover cancer serum biomarkers. Mol Oncol. 2007;1:313–320. doi: 10.1016/j.molonc.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirus JE, Zhang Y, Hollingsworth MA, Solan JL, Lampe PD, Hingorani SR. Spatiotemporal proteomic analyses during pancreas cancer progression identifies STK4 as a novel candidate biomarker for early stage disease. Mol Cell Proteomics. 2014 Sep 15; doi: 10.1074/mcp.M113.036517. pii: mcp.M113.036517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 13.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 14.Smyth GK, Speed T. Normalization of cDNA microarray data. Methods. 2003;31:265–273. doi: 10.1016/s1046-2023(03)00155-5. [DOI] [PubMed] [Google Scholar]
- 15.Kim YS, Jang MK, Park CY, Song HJ, Kim JD. Exploring Multiple Biomarker Combination by Logistic Regression for Early Screening of Ovarian Cancer. Int J Bio-Sci Bio-Tech. 2013;5:67. [Google Scholar]
- 16.Brand RE, Nolen BM, Zeh HJ, Allen PJ, Eloubeidi MA, Goldberg M, et al. Serum biomarker panels for the detection of pancreatic cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:805–816. doi: 10.1158/1078-0432.CCR-10-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 18.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harsha HC, Kandasamy K, Ranganathan P, Rani S, Ramabadran S, Gollapudi S, et al. A compendium of potential biomarkers of pancreatic cancer. PLoS Med. 2009;6:e1000046. doi: 10.1371/journal.pmed.1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esposito I, Penzel R, Chaib-Harrireche M, Barcena U, Bergmann F, Riedl S, et al. Tenascin C and annexin II expression in the process of pancreatic carcinogenesis. J Pathol. 2006;208:673–685. doi: 10.1002/path.1935. [DOI] [PubMed] [Google Scholar]
- 23.Nolen BM, Brand RE, Prosser D, Velikokhatnaya L, Allen PJ, Zeh HJ, et al. Prediagnostic serum biomarkers as early detection tools for pancreatic cancer in a large prospective cohort study. PLoS One. 2014;9:e94928. doi: 10.1371/journal.pone.0094928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brat DJ, Lillemoe KD, Yeo CJ, Warfield PB, Hruban RH. Progression of pancreatic intraductal neoplasias to infiltrating adenocarcinoma of the pancreas. Am J Surg Pathol. 1998;22:163–169. doi: 10.1097/00000478-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Chari ST, Leibson CL, Rabe KG, Timmons LJ, Ransom J, de Andrade M, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134:95–101. doi: 10.1053/j.gastro.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moriya T, Kimura W, Semba S, Sakurai F, Hirai I, Ma J, et al. Biological similarities and differences between pancreatic intraepithelial neoplasias and intraductal papillary mucinous neoplasms. Int J Gastrointest Cancer. 2005;35:111–119. doi: 10.1385/IJGC:35:2:111. [DOI] [PubMed] [Google Scholar]
- 27.Okada N, Ohshio G, Yamaki K, Imamura T, Imamura M. Elevated serum c-erbB-2 protein levels in patients with pancreatic cancer: correlation to metastasis and shorter survival. Oncology. 1995;52:392–396. doi: 10.1159/000227495. [DOI] [PubMed] [Google Scholar]
- 28.Balasenthil S, Chen N, Lott ST, Chen J, Carter J, Grizzle WE, et al. A migration signature and plasma biomarker panel for pancreatic adenocarcinoma. Cancer Prev Res (Phila) 2011;4:137–149. doi: 10.1158/1940-6207.CAPR-10-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satake M, Sawai H, Go VL, Satake K, Reber HA, Hines OJ, et al. Estrogen receptors in pancreatic tumors. Pancreas. 2006;33:119–127. doi: 10.1097/01.mpa.0000226893.09194.ec. [DOI] [PubMed] [Google Scholar]
- 30.Buerke B, Domagk D, Heindel W, Wessling J. Diagnostic and radiological management of cystic pancreatic lesions: Important features for radiologists. Clin Radiol. 2012;67:727–737. doi: 10.1016/j.crad.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Fukushima N, Sato N, Prasad N, Leach SD, Hruban RH, Goggins M. Characterization of gene expression in mucinous cystic neoplasms of the pancreas using oligonucleotide microarrays. Oncogene. 2004;23:9042–9051. doi: 10.1038/sj.onc.1208117. [DOI] [PubMed] [Google Scholar]
- 32.Canto MI, Harinck F, Hruban RH, Offerhaus GJ, Poley JW, Kamel I, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013;62:339–347. doi: 10.1136/gutjnl-2012-303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796–804. doi: 10.1053/j.gastro.2012.01.005. quiz e14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.