Abstract
Insulin receptor substrates 1 and 2 (IRS1/2) mediate mitogenic and anti-apoptotic signaling from insulin-like growth factor 1 receptor (IGF1R), insulin receptor (IR) and other oncoproteins. IRS1 plays a central role in cancer cell proliferation, its expression is increased in many human malignancies and its up-regulation mediates resistance to anti-cancer drugs. IRS2 is associated with cancer cell motility and metastasis. Currently there are no anti-cancer agents that target IRS1/2. We present new IGF1R/IRS-targeted agents (NT compounds) that promote inhibitory Ser-phosphorylation and degradation of IRS1 and IRS2. Elimination of IRS1/2 results in long-term inhibition of IRS1/2-mediated signaling. The therapeutic significance of this inhibition in cancer cells was demonstrated while unraveling a novel mechanism of resistance to B-RAFV600E/K inhibitors. We found that IRS1 is up-regulated in PLX4032-resistant melanoma cells and in cell lines derived from patients whose tumors developed PLX4032 resistance. In both settings, NT compounds led to elimination of IRS proteins and evoked cell death. Treatment with NT compounds in vivo significantly inhibited the growth of PLX4032-resistant tumors, and displayed potent anti-tumor effects in ovarian and prostate cancers. Our findings offer preclinical proof of concept for IRS1/2 inhibitors as cancer therapeutics including in PLX4032-resistant melanoma. By the elimination of IRS proteins, such agents should prevent acquisition of resistance to mutated-B-RAF inhibitors and possibly restore drug sensitivity in resistant tumors.
Keywords: Insulin-like growth factor 1 receptor, insulin receptor substrates, melanoma, cancer therapy, drug resistance
Introduction
The IGF1R signaling pathway is pivotal in many human malignancies (1–5). Up-regulation of IGF1R signaling in cancer cells results from its overexpression, or from up-regulation of its ligands, IGF1 and IGF2 (6–8). IGF1R signaling is crucial for the establishment and maintenance of transformation, as well as for anchorage-independent growth (9). Moreover, IGF1R-mediated signaling significantly contributes to the emergence of resistance to chemotherapy (10), to radiation (11) and to targeted therapies (12–17). These pro-oncogenic activities of IGF1R are highly dependent on its proximal downstream effectors, IRS1 and IRS2. IRS proteins, once phosphorylated on tyrosine residues by IGF1R, transmit mitogenic, anti-apoptotic and anti-differentiation signals to the cell, mainly through the PI3K–PKB module (18). IRS1/2 also mediate the termination of IGF1R signaling. Ser-phosphorylation of IRS1/2 by various cellular kinases blocks their interaction with the receptor, and targets them for degradation by the proteasome (19). This negative feedback loop is the major cellular pathway that shuts off IGF1R signaling.
The role of IRS proteins in human malignancies has been established: overexpression of IRS1/2 causes cell transformation (20, 21) and IRS1 is constitutively activated in many human tumors, including tumors that display no aberrant activation of IGF1R (22). Down-regulation of IRS1 (by antisense or siRNA procedures) reverses the transformed phenotype (23). While IRS1 is critical for tumor growth, IRS2 is essential for tumor metastasis (2, 18, 24–26). Importantly, IRS proteins integrate signals from multiple kinases other than IGF1R, such as insulin receptor (IR), IR/IGF1R hybrids, epidermal growth factor receptor (EGFR) and Src, all of which are involved in transformation (18, 27–30). Furthermore, IRS1 was found to be a mediator of resistance to EGFR and mTOR inhibitors (16, 17).
The prominent role of IRS proteins in cancer initiation, progression and metastasis, as well as in acquired drug resistance, establishes them as potential targets for novel anti-cancer drugs. Here we present and characterize a unique family of small molecules that lead to Ser-phosphorylation and destruction of IRS1 and IRS2. The elimination of IRS1/2 results in long-term inhibition of IGF1R signaling and powerful inhibition of tumor cell growth.
Materials and Methods
Reagents and antibodies
For details see supplementary.
Cell lines
A375 (human melanoma), HCT116 (colon cancer), HCT15 (colon cancer), SK-ES.1 (Ewing’s sarcoma), NCI-H460 (lung cancer) were cultured in RPMI with 10% fetal calf serum (FCS). HepG2 (hepatocarcinoma) were cultured in DMEM and F12 (1:1) containing 10% FCS. DU145 (prostate cancer) were cultured in RPMI containing 5% FCS and 5mg/L insulin. All cell lines were obtained from the ATCC. YUMAC, YURIF, YUSIK (all human melanoma, kindly provided by Prof. Ruth Halaban, Yale) were cultured in optimem containing 5% FCS. M571, M2068, M560n (all human melanoma), normal melanocytes and normal fibroblasts (kindly provided by Dr. Michal Lotem, Hadassah Hospital) were maintained in RPMI, DMEM and F12 (1:3:1) containing 10% FCS. A375SM (metastatic A375 cells (31)) were maintained in MEM containing 10% FCS. 451Lu (human melanoma) and 451Lu-BR (PLX4032-resistant melanoma (32)) were maintained in RPMI containing 5% FCS (media for resistant lines contained 1 µM PLX4032). All media were supplemented with 100 U/ml penicillin and 100 mg/ml streptomycin, and all cells were grown at 37°C/5% CO2.
Cell proliferation
Cells were grown in complete medium and treated with inhibitors one day following seeding. 72 hrs later the surviving cells were quantified by methylene blue staining or by WST-1 staining for non-adherent cells (Roche).
Anchorage-independent growth assay
Cells were plated in 50µl growth medium containing 0.3% agar on top of a layer of 100µl of medium containing 1% agar. 50µl growth medium containing inhibitors was added on top. A week later, representative images were taken using a microscope eyepiece camera (ANMO Electronics Corporation), and colonies were stained with 0.5% MTT for 4 hrs. The dye was extracted with dissolving buffer (5 gr SDS/8.75 ml DDW/12.5 ml DMF/0.5 ml acetic acid/0.07 ml HCl). Following overnight incubation at 37°C, absorbance was read at 570nm.
Migration assay
Cells were treated with 3µM NT157 for 18 hrs, and wounded at t=0 using a pipette tip. The medium was replaced with medium lacking inhibitor, and images were taken at the indicated times using a microscope eyepiece camera (ANMO Electronics Corporation).
Subcellular fractionation
Assays were performed according to the protocol described by Abcam: http://www.abcam.com/index.html?pageconfig=resource&rid=11473. Cytoplasmic fractions were concentrated using Amicon ultra-4 filters (Millipore).
Immunoblots
Cells were treated as indicated and lysed with boiling sample buffer (10% glycerol, 50 mM Tris-HCl, pH 6.8, 3% SDS, and 5% 2-mercaptoethanol). Western blot analysis was as described previously (33).
Co-immunoprecipitation
Serum-starved cells were treated as specified and lysed on ice. Immunoprecipitation was performed using 0.5–1 mg total protein, 2µg antibody and 50µl protein A–agarose beads at 4°C. Bound proteins were eluted with boiling sample buffer, and analyzed by western blot. A detailed method can be found in the supplementary.
qPCR
Serum-starved cells were treated with NT157 and then RNA was extracted using Trizol reagent (Invitrogen) and subjected to reverse transcription using M-MLV RT (Invitrogen). Quantitative PCR amplification was performed using SYBR Green in a 7900HT Fast Real-Time PCR system (ABI). The relative quantities of gene transcripts were normalized to HuPO (Human acidic ribosomal protein) transcripts. The following primers were used: HuPO: forward 5'-GCTTCCTGGAGGGTGTCC-3', reverse 5'-GGACTCGTTTGTACCCGTTG; IRS1: forward 5'-CTTCTGTCAGGTGTCCATCC-3', reverse 5'-CTCTGCAGCAATGCCTGTTC-3' ; IRS2: forward 5'-ACAATGGTGACTACACCGAG-3', reverse 5'-CTGCTTTTCCTGAGAGAGAC-3'
In vivo tumor growth and survival models
Athymic Nude-nu mice were used for the in vivo studies described in Fig. 6A–C. To study tumor growth, A375 cells were injected subcutaneously (s.c.) into nude mice. Once tumor size reached ~75mm3, mice were treated daily for 12 days by intravenous (i.v.) or intraperitoneal (i.p.) administration of vehicle or NT157 (Fig. 6A,B). For the survival melanoma model (Fig. 6C), A375 cells were injected i.v. into female nude mice (9 mice/group). Administration of vehicle or NT157 (100 mg/kg, 10 ml/kg) i.v. three times a week for 2 weeks (arrows) was initiated 10 days later. Animals that developed tumor signs were sacrificed by an overdose of anesthetic. The internal organs of the sacrificed animals were then excised to verify tumor development. To study the growth of 451Lu-BR tumors (Fig. 7E), cells were injected s.c. into female nude mice (10 mice/group). Administrations of vehicle, PLX4032 (12.5 mg/kg) per os (p.o.), NT157 (70 mg/kg) i.p., or PLX4032 and NT157 were initiated 10 days later. Tumor volume was measured 3 times a week and animals were sacrificed on day 26. For further details see supplementary.
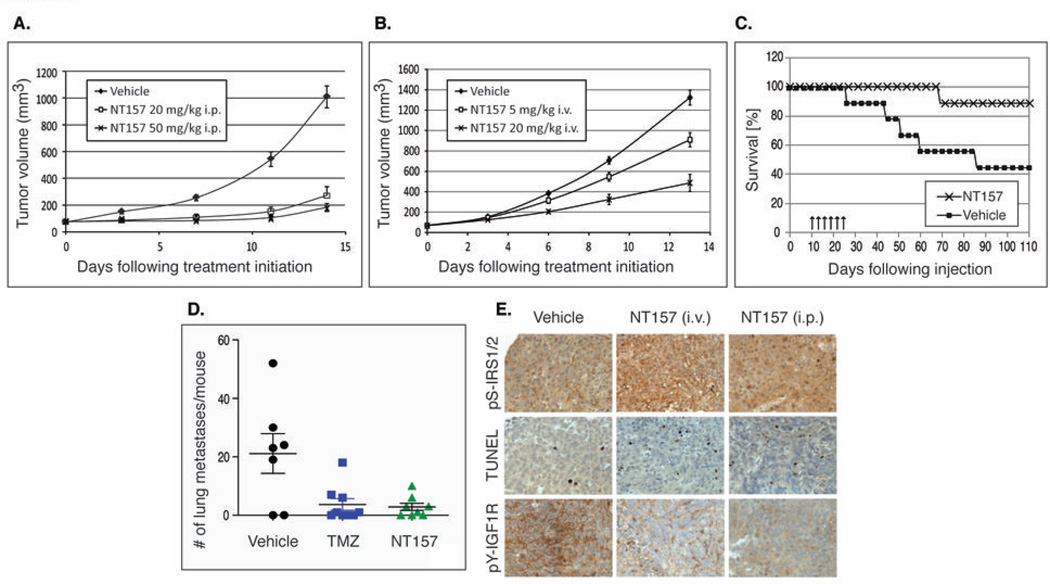
Figure 6. NT157 inhibits tumor growth and metastasis of melanoma in mice.
(A) Growth inhibition of established human melanoma tumors by NT157. A375 cells were injected s.c. into nude mice. Once tumor size reached ~75mm3, mice were treated daily for 12 days by i.p. administration of vehicle or NT157 (20 and 50 mg/kg). (B) Dose-dependent growth inhibition of established A375 tumors by NT157. The experiment was performed as in (A). Vehicle or NT157 (5 and 20 mg/kg) were administered i.v. (C) NT157 increases the survival of mice bearing A375 tumors. Cells were injected i.v. into nude mice. Administration of vehicle or NT157 (100 mg/kg) i.v. three times a week, for 2 weeks (arrows) was initiated 10 days later. (D) NT157 treatment results in a decreased number of pulmonary A375SM metastases in mice. Cells were injected i.v. into nude mice. Treatment was initiated three days later: vehicle or NT157 (70 mg/kg) were administered i.v. three times a week, and Temozolomide (TMZ, 100 mg/kg) was administered i.v. twice a week. Mice were sacrificed after 4 weeks and the number of tumor nodules on the surface of the lungs was counted. (E) Treatment of nude mice harboring A375SM tumors with NT157 results in increased IRS1/2 Ser-phosphorylation, increased apoptosis, and decreased IGF1R phosphorylation. Cells were injected s.c. into nude mice. Administration of NT157 (70 mg/kg) i.v. or i.p. 3 times a week, for 4 weeks, was initiated 10 days later. Samples for immunohistochemistry and TUNEL were taken 48 hrs following the last treatment.
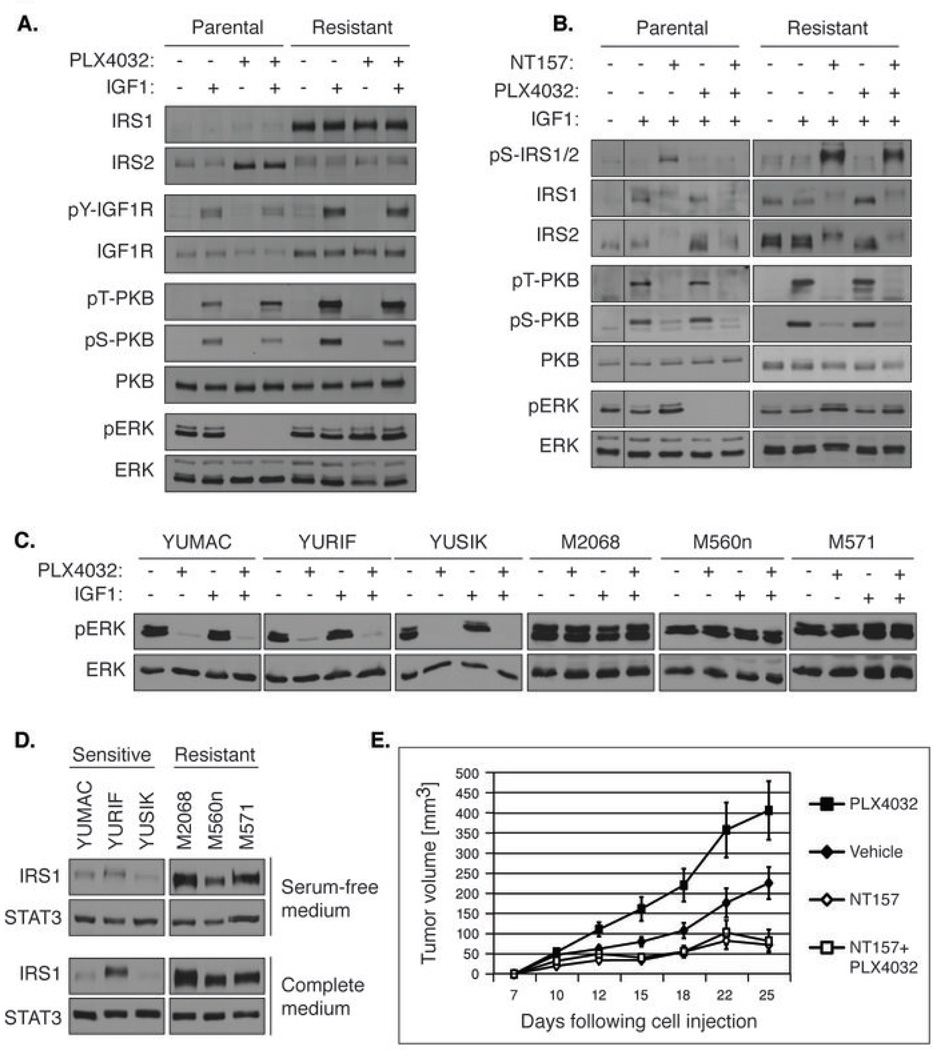
Figure 7.
(A) IRS1 levels and downstream signaling are increased in PLX4032-resistant human melanoma 451-Lu-BR cells, as compared to PLX4032-sensitive human melanoma 451-Lu cells. Serum-starved 451-Lu-BR cells (Resistant) and 451-Lu cells (Parental) were treated with 1 µM PLX4032 for 18 hrs, stimulated with IGF1 for 5 min and lysed. (B) NT157 induces Ser-phosphorylation and degradation of IRS1 in 451-Lu and 451-Lu-BR cells in the presence or absence of PLX4032. Serum-starved cells were treated with 5 µM NT157, and 3 hrs later 1 µM PLX4032 was added for an additional hour. The cells were stimulated with IGF1 for 5 min and lysed. (C) Responsiveness of the ERKMAPK pathway to PLX4032 in patient-derived melanoma cells. B-RAFV600E/K-carrying cells derived from six melanoma patients were serum-starved, treated with 1 µM PLX4032 for 24 hrs and lysed. (D) Cells derived from PLX4032-resistant melanoma patients demonstrate increased levels of IRS1 compared to cells derived from melanoma patients not treated with PLX4032. Patient-derived cells were grown with complete growth medium or under starvation conditions and lysed. IRS1 levels were analysed, and STAT3 levels served as loading controls. (E) NT157 inhibits the growth of PLX4032-resistant tumors in the presence or in the absence of PLX4032. 451-Lu-BR cells were injected s.c. into nude mice (10 mice/group), and administration of vehicle, PLX4032 (12.5 mg/kg; 5 times per week; p.o.), NT157 (70 mg/kg; 3 times per week; i.p.), or PLX4032+NT157 was initiated 10 days later.
In vivo model of lung metastasis of A375SM cells
A375SM cells were injected i.v. (7–9 mice/group) into female athymic BALB/c nude mice, 8–10 weeks. Administration of NT157 (70 mg/kg) i.v. three times a week was initiated 3 days later. Temozolomide (TMZ, 100 mg/kg) was administered i.p. twice a week. A control group was given vehicle i.v. The mice were sacrificed after 4 weeks; the lungs were removed, and fixed in Bouin's solution for 24 hrs. Surface tumor nodules were counted using a dissecting microscope. For further details see supplementary.
Immunohistochemistry (IHC)
5.0×105 A375SM cells were injected s.c. into the right flank of female athymic BALB/c nude mice (10 mice/group). 10 days later, NT157 was administered i.v. or i.p. (70 mg/kg) three times a week for 4 weeks. The mice were sacrificed 48 hrs following the last treatment, and tumors were processed for IHC and TUNEL assay. To detect the levels of pS-IRS1/2 and pY(1131)IGF1R, antibodies were used at 1:50 dilution. Assays were performed as previously described (34).
TUNEL assay
The terminal deoxynucleotidyl transferase-mediated dUTP end labeling (TUNEL) assay was done by using a commercial kit (Promega, Madison, WI) according to the manufacturer's protocol, and as previously described (34).
Results
The NT compounds constitute a novel family of anti-cancer agents that target IRS1/2 to degradation
During our quest for IGF1R kinase inhibitors we discovered a unique subfamily of IGF1R signaling inhibitors. These compounds, represented by NT52, NT75, NT157 and NT205 (Fig. 1A) (35) were developed by rational design based on a structure-activity relationship study of our earlier compounds (Table S1, Fig. S1A). In a cell-free assay, the NT compounds exhibited ATP non-competitive as well as substrate non-competitive inhibition of the full-length IGF1R (Fig. S1B), while they did not inhibit the isolated kinase domain of the receptor (data not shown). These data demonstrate that the NT compounds are mixed-competitive inhibitors (36), namely inhibit IGF1R in an allosteric manner. The new compounds were simultaneously tested for their ability to inhibit IGF1-induced signaling in cells (Fig. 1B) and for their anti-proliferative activity against various cancer cell types (Table S2). Remarkably, the NT compounds induced extensive Ser-phosphorylation of IRS proteins (Fig. 1B,C), in correlation with their ability to inhibit cancer cell growth (see below).
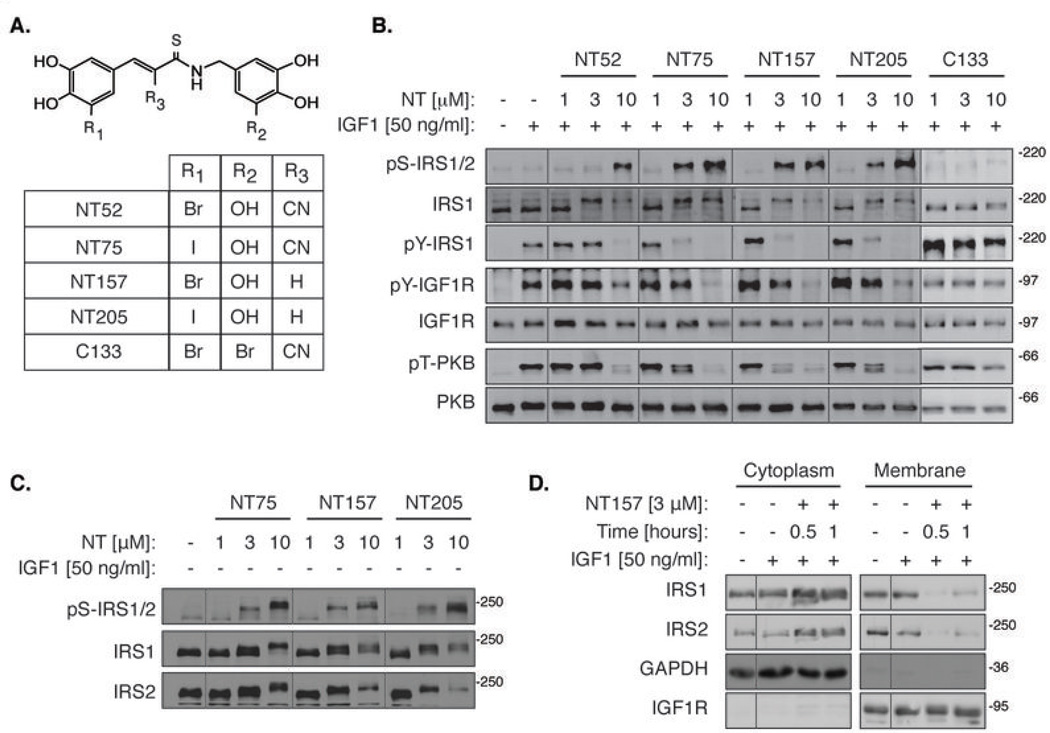
Figure 1. NT inhibitors lead to the dissociation of IRS1/2 from IGF1R, and induce Ser-phosphorylation of IRS1/2.
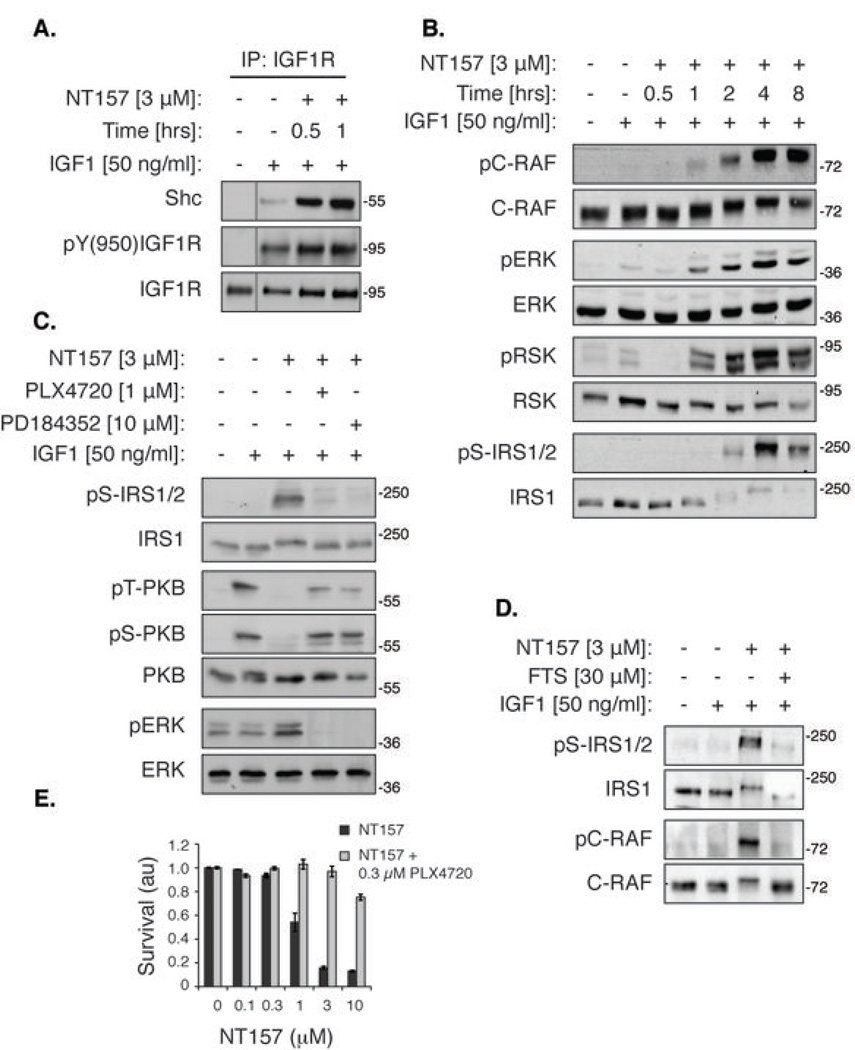
(A) Chemical structures of the NT compounds. The active NT compounds all possess an OH at the 5’ position of the aminobenzyl moiety. The control compound C133 possesses a Br substitution at this position. (B) NT compounds, but not C133, induce IRS1/2 Ser-phosphorylation and strongly inhibit IGF1-induced PKB activation. Serum-starved A375 cells were treated with NT inhibitors for 5 hrs, then stimulated with IGF1 for 5 min and lysed. (C) NT-induced IRS1/2 Ser-phosphorylation is IGF1-independent. Serum-starved A375 cells were treated with NT inhibitors for 5 hrs and lysed without IGF1 stimulation. (D) NT157 induces the translocation of IRS1/2 to the cytosol. Serum-starved A375 cells were treated with NT157 as indicated. Cells were stimulated with IGF1 for 5 min, lysed and fractionated by differential centrifugation.
The inhibitory Ser-phosphorylation of IRS1/2 led to the suppression of signaling to PKB in A375 human melanoma cells (Fig. 1B). We further investigated this phenomenon using NT157 as the lead compound. NT157 induced the dissociation of IRS1/2 from the receptor within 30 min of treatment, before the Ser-phosphorylation of IRS was evident (Fig. 1D and Fig. 2A). This dissociation was probably due to a conformational change in IGF1R induced by the allosteric mode of binding of NT157. The Ser-phosphorylation of IRS1/2 is known to preclude their re-binding to the receptor and transduction of signaling downstream (19).
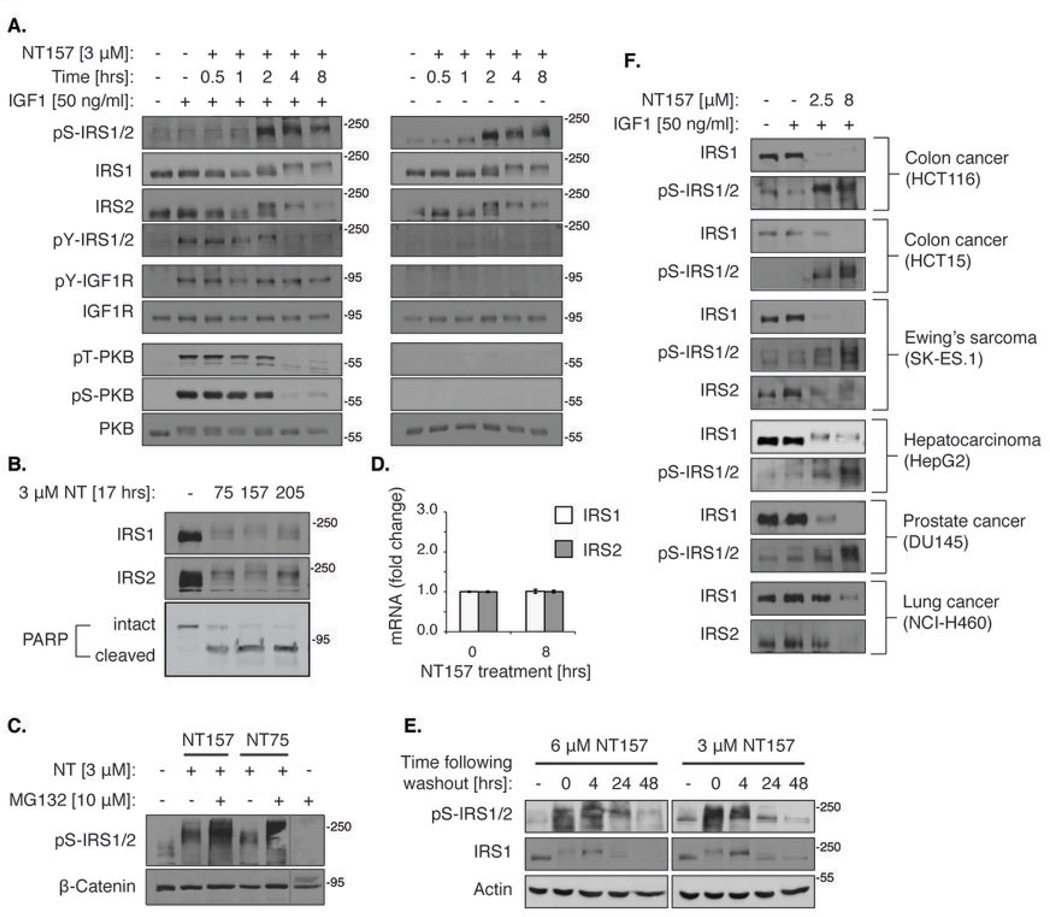
Figure 2. NT inhibitors irreversibly direct Ser-phosphorylated IRS proteins to degradation and induce apoptosis.
(A) NT157 induces IRS1/2 Ser-phosphorylation and PKB inhibition in a time-dependent manner. Serum-starved A375 cells were treated with 3 µM NT157 as indicated, and either stimulated with IGF1 for 5 min (left panel) or not (right panel) before lysis. (B) Treatment with NT inhibitors for 17 hrs leads to IRS1 and IRS2 elimination and to A375 cell apoptosis. Serum-starved A375 cells were treated with 3 µM NT75, NT157 or NT205 for 17 hrs, lysed and analyzed by western blot. Apoptosis was detected by PARP cleavage. (C) The NT-induced elimination of Ser-phosphorylated IRS1/2 is mediated by the proteasome. Serum-starved A375 cells were treated with 3 µM NT157 or NT75 with or without 10 µM MG132 for 17 hrs and lysed. (D) IRS1/2 mRNA levels are not affected by NT157 treatment. Serum-starved A375 cells were treated with 3 µM NT157 and then RNA was extracted and subjected to reverse transcription. PCR amplification was performed and IRS1/2 mRNA levels were normalized to the levels of the ribosomal protein HuPO. (E) The effects of NT inhibitors on the Ser-phosphorylation and degradation of IRS1/2 are maintained long after removal of the inhibitors. Serum-starved A375 cells were treated with NT157 (3 or 6 µM) for 4 hrs. Cells were then washed, grown without serum for 4, 24 or 48 hrs, and lysed. (F) NT157 induces the Ser-phosphorylation and degradation of IRS1/2 in multiple cell lines. Cells were treated with 2.5 or 8 µM NT157. The Ser-phosphorylation and levels of IRS1/2 were tested 5 hrs and 24 hrs following treatment, respectively. For HepG2 cells, the levels of IRS1 were tested 4 hrs following treatment.
IRS1/2 Ser-phosphorylation increased with time (Fig. 2A), and within 20 hrs of NT treatment, IRS1/2 proteins disappeared (Fig. 2B). The autophosphorylation of IGF1R on tyrosine 1131 remained intact (Fig. 2A), and only at higher concentrations of the inhibitors was pTyr-IGF1R directly inhibited (Fig. 1B). These findings highlight the unique mode of action of the NT compounds in intact cells: The primary mechanism of the NT-induced inhibition of IGF1R signaling is a result of the inhibitory Ser-phosphorylation and the subsequent elimination of IRS1/2. In the absence of the key mediators in the pathway, signaling downstream is impeded regardless of IGF1R activity. The details of this exceptional mechanism will be further discussed below.
In fact, the targeting of IRS1/2 for Ser-phosphorylation and proteasome-dependent degradation is the major cellular mechanism for shutting off IGF1R signaling (19, 37). Indeed, when NT compounds were combined with the proteasome inhibitor, MG132, Ser-phosphorylated-IRS1/2 accumulated (Fig. 2C). Furthermore, NT157 did not decrease the mRNA levels of IRS1 or IRS2 (Fig. 2D). Treatment with NT compounds eventually led to apoptosis, as shown by the appearance of cleaved PARP (Fig. 2B). The NT-induced Ser-phosphorylation of IRS1/2 committed them to degradation. The treatment of A375 cells with NT157 for a period of 4 hrs was sufficient to induce massive Ser-phosphorylation of IRS1/2 and subsequent IRS1/2 degradation, effects that were sustained for at least 48 hrs following the removal of NT157 from the medium (Fig. 2E). Cellular proliferation was strikingly inhibited following 72 hrs of NT treatment. The IC50 values of A375 cell proliferation were unchanged when cells were treated only once with medium containing NT157 or NT75, without daily refreshment of the medium (Fig. S2A, B), or when the NT compounds were washed out 24 hrs following treatment (Fig. S2C, D). These data show that a short treatment with the NT compounds triggers an irreversible cellular cascade that culminates in cell death.
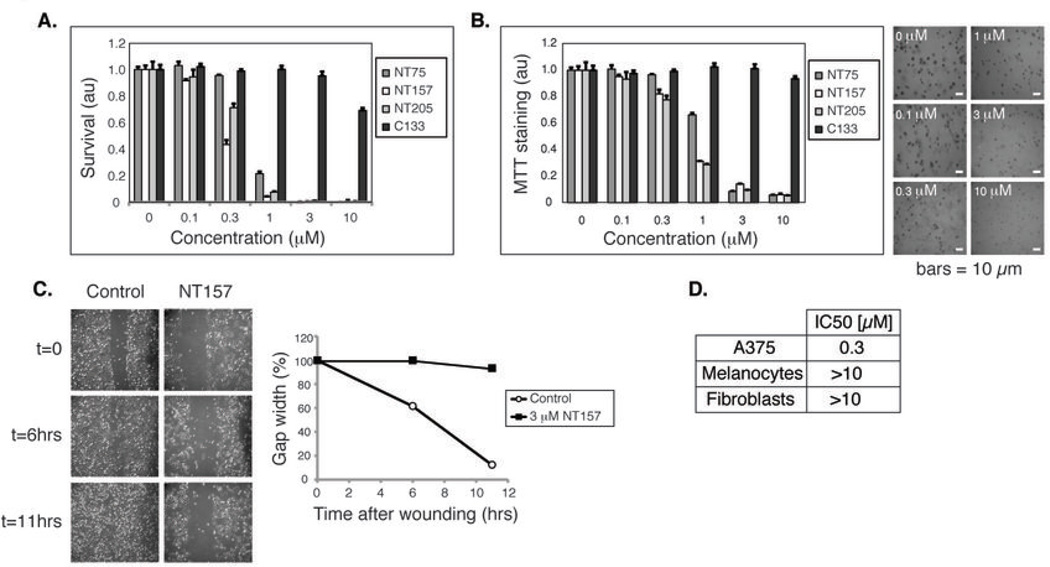
The elimination of IRS1/2 is a key feature of the NT compounds. The depletion of IRS1/2 from cancer cells should lead to the inhibition of all signals transmitted through IRS proteins. Indeed, the effects of the NT compounds on IRS1/2 were IGF1-independent (Fig. 1C, Fig. 2 and Fig. S1C). Because IRS1/2 can also mediate IR signaling, we tested whether IR activity is affected by NT157. NT157 inhibited signaling to PKB following stimulation by IGF2 and insulin, as well as by IGF1 (Fig. S3A) (27, 30). Immunoprecipitation of IR from NT157-treated A375 cells confirmed that its autophosphorylation, like that of IGF1R, was not inhibited (Fig. S3B,C). We conclude that NT compounds bring about the degradation of IRS1/2, irrespective of the upstream signal, leading to the inhibition of all signal transduction pathways that converge on IRS1/2. The Ser-phosphorylation and degradation of IRS1/2 that were induced by NT compounds were observed in various human cancer cell lines (Fig. 2F). Thus, the unique mode of inhibition of IGF1R signaling by NT compounds is widespread. The NT compounds displayed potent anti-proliferative activity on many cancer cell lines (Fig. 3A and Table S2), including lines that are resistant to anti-cancer agents. The NT compounds also demonstrated efficacy in inhibiting anchorage independent colony formation and cell migration of human melanoma A375 cells (Fig. 3B,C). Notably, NT157 displayed little to no effect on the survival of normal melanocytes and fibroblasts (Fig. 3D).
Figure 3. The NT compounds inhibit the migration and anchorage-dependent and - independent growth of A375 human melanoma cells.
(A) NT compounds, but not C133, potently block the proliferation of A375 cells. Cells were treated with inhibitors for 72 hrs and survival was quantified by methylene blue staining. (B) NT compounds inhibit A375 anchorage-independent growth. A375 cells were seeded in 0.3% agar and treated with inhibitors for 7 days. Colony formation was quantified by MTT staining. Representative images from unstained, NT157-treated cells are shown. (C) NT157 inhibits the migration of A375 cells. A375 cells were treated or not treated with NT157 for 18 hrs, and then the cell monolayer was wounded at t=0. Representative images were taken at the specified time-points. (D) NT157 displays a minor effect on the proliferation of normal melanocytes and fibroblasts. Cells were treated with NT157 as in (A). IC50 values are presented. au= arbitrary units
The NT compounds constitute a highly defined family of chemical entities (Table S1 and Fig. 1A). C133 is a structural analog of the NT family in which the 5’-OH was substituted with a Br atom (Fig. 1A). This minor modification abolished the effects on IRS1/2 (Fig. 1B). We found that the anti-IRS1/2 activity of the NT compounds highly correlated with their anti-cancer activities, C133 was only a weak inhibitor of cancer cell proliferation and of the growth of A375 cells in soft agar (Table S2 and Fig. 3A,B).
The ERKMAPK pathway in A375 melanoma cells mediates NT157-induced Ser-phosphorylation of IRS1/2
We studied the mechanism of NT action in A375 melanoma cells, with NT157 as the lead compound. We found that concomitant to the detachment of IRS1/2 from the receptor, NT157 induced an increased interaction of IGF1R with the adaptor protein Shc (Fig. 4A). Shc and IRS proteins bind the same tyrosine-phosphorylated Tyr950 of IGF1R (38), and thus the detachment of IRS1/2 may allow enhanced access of IGF1R to Shc. Following the recruitment of Shc to IGF1R, but prior to the Ser-phosphorylation of IRS1/2, NT157 treatment induced the activation of the ERKMAPK pathway in A375 cells (Fig. 4B). In melanoma cells that harbor the mutant B-RAFV600E/K, such as A375, the ERKMAPK pathway is constitutively active, and therefore plays the key role in IRS1/2 Ser-phosphorylation. The treatment of A375 cells with the MEK1/2 inhibitor, PD184352, or the B-RAFV600E inhibitor, PLX4720, resulted in nullification of the NT157-induced Ser-phosphorylation of IRS1 and in alleviation of the NT157-induced inhibition of IGF1-dependent activation of PKB (Fig. 4C), while inhibition of mTOR or PI3K exhibited no effect (Fig. S4B), confirming that the NT-induced Ser-phosphorylation of IRS1/2 is dependent on the ERKMAPK pathway in these cells. It has been previously shown that ERK1/2 is capable of phosphorylating IRS1 on certain Ser-residues, such as Ser636/639 (19). To study the mode by which NT157 induced activation of ERK1/2 beyond their basal state in A375 cells, which harbor a constitutively active B-RAFV600E, we checked the phosphorylation status of C-RAF, which can participate in the amplification of the signal elicited by IGF1R through Shc (39). C-RAF was strongly activated following NT157 treatment (Fig. 4B) in a Ras-dependent manner (Fig. 4D). The events described above occurred regardless of the kinase activity of IGF1R, namely in the absence of IGF1 stimulation (Fig. S4), or in the presence of a specific IGF1R-kinase inhibitor (Fig. S4F). Importantly, the combined treatment of A375 cells with NT157 and PLX4720 abrogated the anti-proliferative effects of NT157, confirming that these effects of NT157 are mediated by IRS1/2 phosphorylation and degradation (Fig. 4E).
Figure 4. NT157 shifts IGF1R complexation from IRS1/2 to Shc and leads to ERKMAPK-dependent Ser-phosphorylation of IRS1/2 in A375 melanoma cells.
(A) NT157 promotes recruitment of Shc to IGF1R. Serum-starved A375 cells were treated with NT157, stimulated with IGF1, and lysed. IGF1R was immunoprecipitated (IP) and Shc levels in the IP samples were detected. (B) Time-dependent activation of the ERKMAPK pathway. Serum-starved A375 cells were treated with NT157 for the indicated times, stimulated with IGF1 for 5 min, and lysed. The kinase activity of ERK was assessed by analysis of the phosphorylation of its direct substrate, RSK. (C) The ERKMAPK pathway mediates the NT157-induced Ser-phosphorylation of IRS1/2. Serum-starved A375 cells were treated with an inhibitor of mutated B-RAF (PLX4720) or of MEK1/2 (PD184352) for 30 min, and then NT157 was added for 4 hrs. Cells were then stimulated with IGF1 for 5 min and lysed. (D) The NT157-induced C-RAF activation is Ras-dependent. Serum-starved A375 cells were treated with an inhibitor of Ras (FTS) for 10 min and then NT157 was added for 4 hrs. Cells were stimulated with IGF1 for 5 min and lysed. (E) The ERKMAPK pathway mediates the anti-proliferative effect of NT157. A375 cells were treated with PLX4720 and NT157 for 72 hrs and then survival was quantified by methylene blue staining.
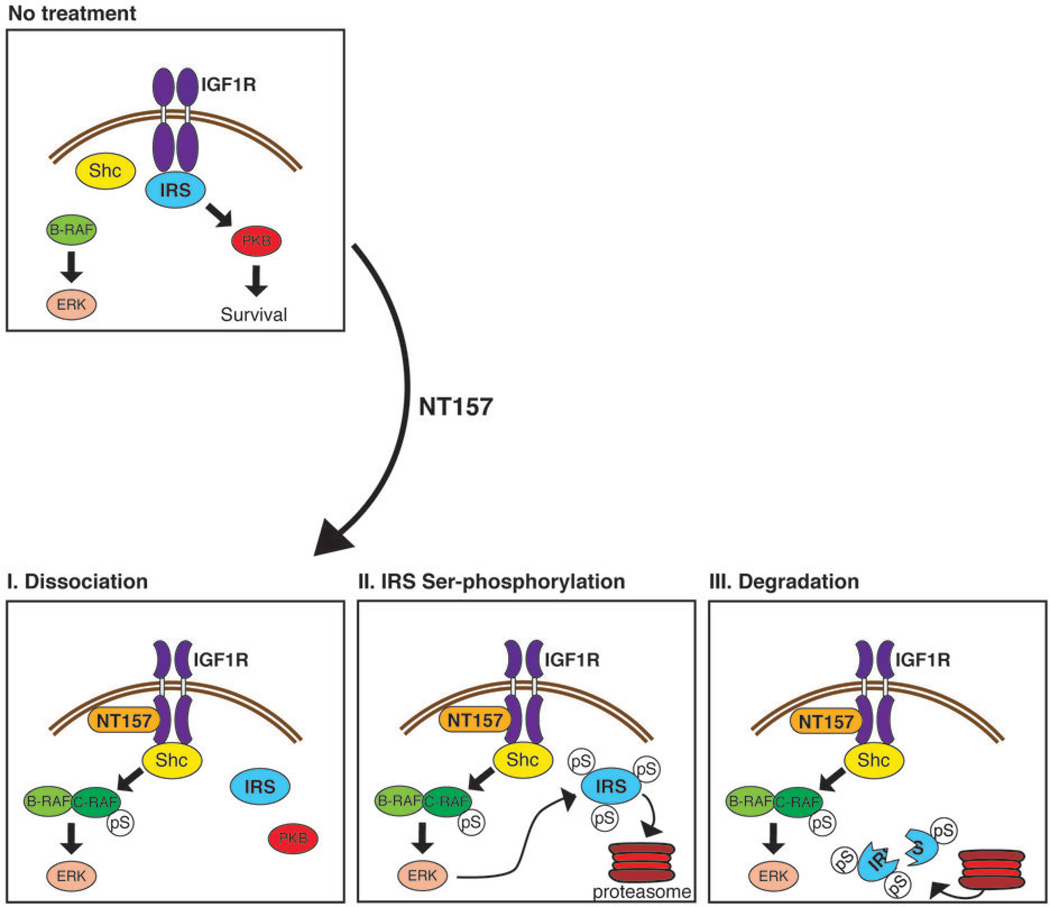
We propose a model (Fig. 5) whereby the binding of NT157 to an allosteric site on IGF1R leads to the detachment of IRS1/2 from the receptor, followed by the recruitment of Shc to the receptor. This leads to the activation of the ERKMAPK pathway, which in turn leads to Ser-phosphorylation of IRS1/2. This inhibitory Ser-phosphorylation precludes rebinding of IRS1/2 to IGF1R, blocks IGF-1 signaling and targets IRS1/2 to degradation. The downstream activation of PKB is inhibited and cell death is induced.
Figure 5. A three-step model for the inhibitory effects of NT157 on IRS1 in human melanoma A375 cells.
In human melanoma A375 cells, where B-RAF is constitutively active, IGF1R signals mainly through the IRS1/2-PKB axis, inducing cellular survival and proliferation. NT157 binds to an allosteric site on IGF1R and induces a conformational change, leading to the dissociation of IRS1/2 from the receptor (I). This allows the receptor to interact more strongly with the adaptor protein Shc, and leads to the activation of C-RAF and to enhanced signaling to ERKMAPK. In the next step, cytoplasmic IRS1/2 undergoes extensive Ser-phosphorylation, which is mediated by the ERKMAPK pathway (II). Ser-phosphorylated IRS1/2 is targeted for degradation by the proteasome (III), and IGF1R signaling becomes severely impaired. Thus, NT157 leads to long-lasting IGF1R inhibition, cancer cell apoptosis, and potent anti-tumor effects.
Melanoma tumor growth and metastasis is efficiently inhibited by NT157
We determined the effect of the NT inhibitors on tumor growth and metastasis in mice. Daily treatment with NT157 resulted in over 80% growth inhibition of established human A375 melanoma tumors (Fig. 6A). The anti-tumor effect of NT157 was dose-dependent (Fig. 6B). In models of metastasis, NT157-treated mice survived longer than vehicle-treated controls (Fig. 6C). Moreover, NT157 strongly inhibited the development of lung metastases of melanoma cells (Fig. 6D). NT157 was at least as effective as temozolomide, which is often used in the treatment of metastatic melanoma (Fig. 6D). The effect of NT157 on melanoma metastasis correlated with the inhibitory action of NT157 on melanoma cell migration and colony formation (Fig. 3B,C). Immunohistochemical analysis of the A375SM tumors confirmed our biochemical findings reported above (Fig. 1B): Treatment of mice with NT157 induced increased IRS1 Ser-phosphorylation and apoptosis of tumor cells (Fig. 6E). These observations were detected 48 hrs following the last treatment, validating the long-lasting inhibitory effect of NT157 also in vivo. At the doses applied, inhibition of IGF1R autophosphorylation was also detected. Increased expression of IRS1 has been reported in both ovary and androgen-refractory prostate cancers (26, 40). The efficacy of NT compounds on tumor growth and mouse survival was demonstrated using A2780 ovary cancer and PC3 androgen-refractory prostate cancer xenografts (Fig. S5).
Resistance to B-RAFV600E/K inhibitor drugs can be mediated by IRS1/2 up-regulation
A new drug, PLX4032/Vemurafenib (41), targeted against melanomas carrying the common B-RAFV600E/K mutation, demonstrates remarkable efficacy against metastatic melanoma, but resistance to PLX4032 emerges and tumors eventually progress in almost all patients (42). We examined the potential involvement of IRS proteins in the acquired resistance of mutated B-RAF melanomas to PLX4032. To this end we used two melanoma cell lines that have developed resistance to B-RAFV600E/K inhibition in vitro: 451-Lu-BR and Mel1617-BR. These cell lines were cloned from the parental metastatic melanoma lines 451-Lu and Mel1617, respectively, following continuous exposure to a B-RAFV600E/K inhibitor (32).
(1) IRS1/2 up-regulation is induced by short-term treatment with B-RAFV600E/K inhibitor and reversed by NT157
In melanoma cells harboring the constitutively active B-RAFV600E/K, the ERKMAPK pathway plays the most important role in the basal Ser-phosphorylation of IRS1/2. Indeed, treatment of the parental 451-Lu melanoma cells with PLX4032 led to inhibition of pERK and to inhibition of the basal Ser-phosphorylation of IRS2 (as indicated by the electrophoretic downshift of IRS2, Fig. 7A). Concomitantly, an increase in IRS2 levels was observed (Fig. 7A). Treatment of 451-Lu cells with NT157 resulted in Ser-phosphorylation and degradation of IRS1, and accordingly to inhibition of IGF1-induced PKB activation (Fig. 7B). Combined treatment with both inhibitors resulted in inhibition of both ERK and PKB pathways (Fig. 7B). Correspondingly, a synergistic cytotoxic effect was observed when the cells were treated with both inhibitors (Fig. S6A). Since the ERK pathway is crucial for the NT157-induced Ser-phosphorylation of IRS1/2, a sequential treatment in which NT157 is added to the medium a few hours ahead of PLX4032 was crucial to gain a synergic effect and avoid an antagonistic effect of the treatments.
As reported by Villanueva et al., the levels of pERK were unperturbed by PLX4032 in PLX4032-resistant 451-Lu-BR cells. Neither did PLX4032 affect IRS1/2 (Fig. 7A). In the parental Mel1617 cells, similar to 451-Lu cells, treatment with PLX4032 resulted in up-regulation of IRS1 and IRS2, and the IGF1-induced activation of PKB was concomitantly increased (Fig. S6B). The levels of pY-IGF1R were unperturbed by PLX4032, suggesting that the increase in pT-PKB levels is a results of the up-regulation of IRS1/2.
(2) Enhanced levels of IRS1 in PLX4032-resistant cells
Next we tested the relative levels of IRS1 in the PLX4032-resistant cell lines: 451-Lu-BR and Mel1617-BR. Remarkably, we found a significant increase in the levels of IRS proteins in both cell lines, compared with the levels of IRS proteins in the corresponding parental melanoma lines, which are sensitive to mutant B-RAF inhibition (Fig. 7A and Fig. S6C). The dramatic increase in IRS1 level in 451-Lu-BR cells far exceeded the up-regulation of IGF1R already reported by Villanueva et al. (Fig. 7A). Accordingly, the IGF1-induced activation of PKB was enhanced in the PLX4032-resistant 451-Lu-BR cells, as compared with the parental sensitive metastatic melanoma cells, 451-Lu (Fig. 7A). In Mel1617-BR, the levels of both IRS1 and IRS2 were highly up-regulated as compared to the parental Mel1617 cells (Fig. S6C). The fact that a similar up-regulation of IRS levels was found in both PLX4032-resistant cell lines tested suggests that this may be a common mechanism of acquired resistance to B-RAFV600E/K inhibition. To further investigate the broad relevance of this mechanism we expanded our research and analyzed a panel of six patient-derived human melanoma cell lines (Fig. 7C,D): YUMAC, YURIF, and YUSIK are human melanoma patient-derived cell lines that contain the mutated B-RAF (43). These patients were not treated with a mutated B-RAF inhibitor. M2068, M560n, and M571 are human melanoma cell lines containing the mutated B-RAF, derived from patients that had been treated with PLX4032 and developed resistance to the drug. Our biochemical analysis validated that in M2068, M560n, and M571, ERK activation was resistant to PLX4032 treatment, while in YUMAC, YURIF, and YUSIK, ERK activation was highly sensitive to PLX4032 treatment (Fig. 7C). Interestingly, we found significantly higher levels of IRS1 in the PLX4032-resistant patient-derived cells as compared with the PLX4032-sensitive cells (Fig. 7D). To summarize, we detected high levels of IRS1 in melanoma cells that acquired resistance to mutated B-RAF inhibitors both in culture and in patients.
(3) NT157 induced down-regulation of IRS1/2 in PLX4032-resistant cells and inhibited PLX4032-resistant tumor growth
In PLX4032-resistant 451-Lu-BR cells NT157 induced striking Ser-phosphorylation of IRS1/2, inhibited signaling to PKB, and led to cell death (Fig. 7B and S6A; Similar data for Mel1617-BR cells is shown in Fig. S6D,E). Correspondingly, NT157 inhibited the growth of 451-Lu-BR tumors in nude mice (Fig. 7E). Interestingly, enhanced 451-Lu-BR tumor growth was observed when mice were treated with PLX4032, possibly due to “drug addiction” (44) and up-regulation of the IGF1R/IRS1 pathway (Fig. 7E). Combined treatment with NT157 and PLX4032 suppressed this accelerated tumor growth (Fig. 7E). Notably, all the cell lines tested, which had acquired resistance to PLX4032 either in culture or in patients, demonstrated sensitivity to NT157 in a cytotoxicity assay (not shown). To summarize, we found increased levels of IRS1/2 in melanoma cells treated with PLX4032 and in melanoma cells that had acquired resistance to B-RAFV600E/K inhibition. The down-regulation of IRS1/2 by NT157 led to cell death and tumor inhibition of PLX4032-resistant cells.
Discussion
We have discovered an exceptional family of IGF1R-IRS1/2 inhibitors. Unlike existing IGF1R signaling inhibitors, the NT inhibitors represent a new concept in targeted therapy, whereby the inhibitor shuts off signaling by taking advantage of a negative feedback loop normally used by cells. This is brought about by the Ser-phosphorylation and the degradation of IRS1/2, resulting in long-lasting inhibition of signaling to PKB, and leading to cancer cell death. The anti-cancer activity of NT compounds on both IRS1 and IRS2 is highly effective, because IRS1 promotes tumor growth and IRS2 promotes metastasis. Furthermore, inhibition of both IRS proteins precludes the compensation of one for the other.
Importantly, we showed that a short exposure to NT compounds sufficed to induce Ser-phosphorylation of IRS1/2, directing IRS1/2 to degradation, even after the removal of the inhibitors. Moreover, the NT157-induced Ser-phosphorylation of IRS1/2 in A375 tumor cells in mice persisted for at least 48 hrs following the administration of the drug. The finding that a short exposure to the NT compounds suffices to gain a long-lasting anti-tumoral effect has clinical ramifications. It allows treatment at relatively low frequencies, which should lead to reduced side effects.
IR is highly homologous to IGF1R in structure and function. It has been demonstrated that selective inhibition of IGF1R, e.g. by using monoclonal antibodies, can result in compensatory activation of IR by IGF2, leading to drug resistance (45). Since IRS1 and IRS2 mediate signaling from both IGF1R and IR, the NT compounds lead to the disruption of signaling downstream of both receptors induced by IGF1, IGF2 or insulin and reduce the probability of drug resistance. Approximately 50% of melanomas are driven by B-RAFV600E/K, and resistance to B-RAFV600E/K inhibition can be mediated by different pathways (46, 47). Our results reveal that up-regulation of IRS1 and IRS2 proteins is a novel mechanism that leads to acquired resistance to B-RAFV600E/K inhibition. Similar mechanisms have been reported for acquired resistance to EGFR and mTOR inhibitors (16, 17). In all of these cases, the inhibitors reduce the levels of Ser-phosphorylation of IRS1/2, and consequently stabilize IRS1/2 and increase pro-survival signaling via PKB.
It has been previously shown that in some cases of melanoma IGF1R is up-regulated, leading to enhanced signaling through IRS proteins (32, 48). The up-regulation of IGF1R and/or IRS proteins can be effectively reversed by treatment with NT compounds, leading to tumor cell death. The present study suggests that combining NT compounds with B-RAFV600E/K inhibitors should be effective for the treatment of metastatic melanoma patients harboring the mutated B-RAF, and that NT compounds should be effective for patients showing resistance to B-RAFV600E/K inhibitors (49). Furthermore, NT compounds may re-sensitize resistant tumors to B-RAFV600E/K inhibitors. Our results highlight IRS proteins as critical mediators of tumor progression, metastasis and drug-resistance, and further establish them as important drug targets in cancer.
Supplementary Material
Acknowledgements
We thank Dr. Shoshana Klein from our laboratory for advice and editing, Prof. Yoel Kloog from Tel Aviv University for kindly providing us with FTS, Dr. Gideon Bollag from Plexxikon for providing us with PLX4032, Prof. Ruth Halaban (Yale University) and Yale SPORE in Skin Cancer as the source of the melanoma cells, and Dr. Michal Lotem (Hadassah Hospital) for providing us the patient-derived melanoma cell lines and with the normal cells.
None of the material has been published or is under consideration elsewhere, including the Internet. The animals’ care was in accordance with institutional guidelines.
Grant support:
This study was supported by grants from the Office of the Chief Scientist in the Ministry of Industry, Trade and Labor of Israel to NovoTyr (H.R., 2005–2012), by funds from Meytav Technological Incubator, Israel to NovoTyr (H.R., 2005–2008), by Teva Pharmaceutical Industries LTD. to NovoTyr (H.R., 2008–2010), by a grant from the Melanoma Research Alliance of USA to A.L. (2009–2010), by a grant from the ERC to A.L.: ERC/B3/JM/NL/MW/gk/D (2009) 600950, by a NIH grant to M.H. (CA-114046), and by a NIH Specialized Programs of Research in Skin Cancer grant to M.B.E. (P50-CA093459).
Footnotes
The authors declare no conflict of interests.
References
- 1.Chen W, Wang S, Tian T, Bai J, Hu Z, Xu Y, Dong J, Chen F, Wang X, Shen H. Phenotypes and genotypes of insulin-like growth factor 1, IGF-binding protein-3 and cancer risk: evidence from 96 studies. Eur J Hum Genet. 2009;17:1668–1675. doi: 10.1038/ejhg.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalmizrak O, Wu A, Chen J, Sun H, Utama FE, Zambelli D, Tran TH, Rui H, Baserga R. Insulin receptor substrate-1 regulates the transformed phenotype of BT-20 human mammary cancer cells. Cancer Res. 2007;67:2124–2130. doi: 10.1158/0008-5472.CAN-06-3954. [DOI] [PubMed] [Google Scholar]
- 3.Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Akiyama M, Hideshima T, Chauhan D, Joseph M, Libermann TA, Garcia-Echeverria C, Pearson MA, Hofmann F, Anderson KC, Kung AL. Inhibition of the insulin-like growth factor receptor-1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma, other hematologic malignancies, and solid tumors. Cancer Cell. 2004;5:221–230. doi: 10.1016/s1535-6108(04)00050-9. [DOI] [PubMed] [Google Scholar]
- 4.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 5.Ryan PD, Goss PE. The emerging role of the insulin-like growth factor pathway as a therapeutic target in cancer. Oncologist. 2008;13:16–24. doi: 10.1634/theoncologist.2007-0199. [DOI] [PubMed] [Google Scholar]
- 6.Li R, Pourpak A, Morris SW. Inhibition of the insulin-like growth factor-1 receptor (IGF1R) tyrosine kinase as a novel cancer therapy approach. J Med Chem. 2009;52:4981–5004. doi: 10.1021/jm9002395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouban A, Muraca P, Yeatman T, Coppola D. Expression and distribution of insulin-like growth factor-1 receptor in human carcinomas. Hum Pathol. 2003;34:803–808. doi: 10.1016/s0046-8177(03)00291-0. [DOI] [PubMed] [Google Scholar]
- 8.Xie Y, Skytting B, Nilsson G, Brodin B, Larsson O. Expression of insulin-like growth factor-1 receptor in synovial sarcoma: association with an aggressive phenotype. Cancer Res. 1999;59:3588–3591. [PubMed] [Google Scholar]
- 9.Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer. 2003;107:873–877. doi: 10.1002/ijc.11487. [DOI] [PubMed] [Google Scholar]
- 10.Heron-Milhavet L, LeRoith D. Insulin-like growth factor I induces MDM2-dependent degradation of p53 via the p38 MAPK pathway in response to DNA damage. J Biol Chem. 2002;277:15600–15606. doi: 10.1074/jbc.M111142200. [DOI] [PubMed] [Google Scholar]
- 11.Peretz S, Jensen R, Baserga R, Glazer PM. ATM-dependent expression of the insulin-like growth factor-I receptor in a pathway regulating radiation response. Proc Natl Acad Sci U S A. 2001;98:1676–1681. doi: 10.1073/pnas.041416598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albanell J, Baselga J. Unraveling resistance to trastuzumab (Herceptin): insulin-like growth factor-I receptor, a new suspect. J Natl Cancer Inst. 2001;93:1830–1832. doi: 10.1093/jnci/93.24.1830. [DOI] [PubMed] [Google Scholar]
- 13.Jones HE, Goddard L, Gee JM, Hiscox S, Rubini M, Barrow D, Knowlden JM, Williams S, Wakeling AE, Nicholson RI. Insulin-like growth factor-I receptor signalling and acquired resistance to gefitinib (ZD1839; Iressa) in human breast and prostate cancer cells. Endocr Relat Cancer. 2004;11:793–814. doi: 10.1677/erc.1.00799. [DOI] [PubMed] [Google Scholar]
- 14.Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther. 2005;4:1533–1540. doi: 10.1158/1535-7163.MCT-05-0068. [DOI] [PubMed] [Google Scholar]
- 15.Tamburini J, Chapuis N, Bardet V, Park S, Sujobert P, Willems L, Ifrah N, Dreyfus F, Mayeux P, Lacombe C, Bouscary D. Mammalian target of rapamycin (mTOR) inhibition activates phosphatidylinositol 3-kinase/Akt by up-regulating insulin-like growth factor-1 receptor signaling in acute myeloid leukemia: rationale for therapeutic inhibition of both pathways. Blood. 2008;111:379–382. doi: 10.1182/blood-2007-03-080796. [DOI] [PubMed] [Google Scholar]
- 16.Buck E, Eyzaguirre A, Rosenfeld-Franklin M, Thomson S, Mulvihill M, Barr S, Brown E, O'Connor M, Yao Y, Pachter J, Miglarese M, Epstein D, Iwata KK, Haley JD, Gibson NW, Ji QS. Feedback mechanisms promote cooperativity for small molecule inhibitors of epidermal and insulin-like growth factor receptors. Cancer Res. 2008;68:8322–8332. doi: 10.1158/0008-5472.CAN-07-6720. [DOI] [PubMed] [Google Scholar]
- 17.Crose LE, Linardic CM. Receptor tyrosine kinases as therapeutic targets in rhabdomyosarcoma. Sarcoma. 2011:756982. doi: 10.1155/2011/756982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baserga R. The insulin receptor substrate-1: a biomarker for cancer? Exp Cell Res. 2009;315:727–732. doi: 10.1016/j.yexcr.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Boura-Halfon S, Zick Y. Serine kinases of insulin receptor substrate proteins. Vitam Horm. 2009;80:313–349. doi: 10.1016/S0083-6729(08)00612-2. [DOI] [PubMed] [Google Scholar]
- 20.DeAngelis T, Chen J, Wu A, Prisco M, Baserga R. Transformation by the simian virus 40 T antigen is regulated by IGF-I receptor and IRS-1 signaling. Oncogene. 2006;25:32–42. doi: 10.1038/sj.onc.1209013. [DOI] [PubMed] [Google Scholar]
- 21.Dearth RK, Cui X, Kim HJ, Kuiatse I, Lawrence NA, Zhang X, Divisova J, Britton OL, Mohsin S, Allred DC, Hadsell DL, Lee AV. Mammary tumorigenesis and metastasis caused by overexpression of insulin receptor substrate 1 (IRS-1) or IRS-2. Mol Cell Biol. 2006;26:9302–9314. doi: 10.1128/MCB.00260-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang Q, Li Y, White MF, Fletcher JA, Xiao S. Constitutive activation of insulin receptor substrate 1 is a frequent event in human tumors: therapeutic implications. Cancer Res. 2002;62:6035–6038. [PubMed] [Google Scholar]
- 23.D'Ambrosio C, Keller SR, Morrione A, Lienhard GE, Baserga R, Surmacz E. Transforming potential of the insulin receptor substrate 1. Cell Growth Differ. 1995;6:557–562. [PubMed] [Google Scholar]
- 24.Chan BT, Lee AV. Insulin receptor substrates (IRSs) and breast tumorigenesis. J Mammary Gland Biol Neoplasia. 2008;13:415–422. doi: 10.1007/s10911-008-9101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoang CD, Zhang X, Scott PD, Guillaume TJ, Maddaus MA, Yee D, Kratzke RA. Selective activation of insulin receptor substrate-1 and-2 in pleural mesothelioma cells: association with distinct malignant phenotypes. Cancer Res. 2004;64:7479–7485. doi: 10.1158/0008-5472.CAN-04-1898. [DOI] [PubMed] [Google Scholar]
- 26.Ravikumar S, Perez-Liz G, Del Vale L, Soprano DR, Soprano KJ. Insulin receptor substrate-1 is an important mediator of ovarian cancer cell growth suppression by all-trans retinoic acid. Cancer Res. 2007;67:9266–9275. doi: 10.1158/0008-5472.CAN-07-2088. [DOI] [PubMed] [Google Scholar]
- 27.Belfiore A. The role of insulin receptor isoforms and hybrid insulin/IGF-I receptors in human cancer. Curr Pharm Des. 2007;13:671–686. doi: 10.2174/138161207780249173. [DOI] [PubMed] [Google Scholar]
- 28.Knowlden JM, Jones HE, Barrow D, Gee JM, Nicholson RI, Hutcheson IR. Insulin receptor substrate-1 involvement in epidermal growth factor receptor and insulin-like growth factor receptor signalling: implication for Gefitinib ('Iressa') response and resistance. Breast Cancer Res Treat. 2008;111:79–91. doi: 10.1007/s10549-007-9763-9. [DOI] [PubMed] [Google Scholar]
- 29.Sun H, Baserga R. The role of insulin receptor substrate-1 in transformation by v-src. J Cell Physiol. 2008;215:725–732. doi: 10.1002/jcp.21352. [DOI] [PubMed] [Google Scholar]
- 30.Tognon CE, Sorensen PH. Targeting the insulin-like growth factor 1 receptor (IGF1R) signaling pathway for cancer therapy. Expert Opin Ther Targets. 2012;16:33–48. doi: 10.1517/14728222.2011.638626. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Price JE, Fan D, Zhang RD, Bucana CD, Fidler IJ. Correlation of growth capacity of human tumor cells in hard agarose with their in vivo proliferative capacity at specific metastatic sites. J Natl Cancer Inst. 1989;81:1406–1412. doi: 10.1093/jnci/81.18.1406. [DOI] [PubMed] [Google Scholar]
- 32.Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, Wubbenhorst B, Xu X, Gimotty PA, Kee D, Santiago-Walker AE, Letrero R, D'Andrea K, Pushparajan A, Hayden JE, Brown KD, Laquerre S, McArthur GA, Sosman JA, Nathanson KL, Herlyn M. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–695. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizrachy-Schwartz S, Cohen N, Klein S, Kravchenko-Balasha N, Levitzki A. Up-regulation of AMP-activated protein kinase in cancer cell lines is mediated through c-Src activation. J Biol Chem. 2011;286:15268–15277. doi: 10.1074/jbc.M110.211813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dobroff AS, Wang H, Melnikova VO, Villares GJ, Zigler M, Huang L, Bar-Eli M. Silencing cAMP-response element-binding protein (CREB) identifies CYR61 as a tumor suppressor gene in melanoma. J Biol Chem. 2009;284:26194–26206. doi: 10.1074/jbc.M109.019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reuveni H, Levitzki A, Steiner L, Sasson R, Ben-David I, Weissberg A. Novel protein kinase modulators and therapeutic uses thereof. 2008/068751 A1 International patent application.
- 36.Posner I, Engel M, Gazit A, Levitzki A. Kinetics of inhibition by tyrphostins of the tyrosine kinase activity of the epidermal growth factor receptor and analysis by a new computer program. Mol Pharmacol. 1994;45:673–683. [PubMed] [Google Scholar]
- 37.Fritsche L, Neukamm SS, Lehmann R, Kremmer E, Hennige AM, Hunder-Gugel A, Schenk M, Haring HU, Schleicher ED, Weigert C. Insulin-induced serine phosphorylation of IRS-2 via ERK1/2 and mTOR: studies on the function of Ser675 and Ser907. Am J Physiol Endocrinol Metab. 2011;300:E824–E836. doi: 10.1152/ajpendo.00409.2010. [DOI] [PubMed] [Google Scholar]
- 38.Craparo A, O'Neill TJ, Gustafson TA. Non-SH2 domains within insulin receptor substrate-1 and SHC mediate their phosphotyrosine-dependent interaction with the NPEY motif of the insulin-like growth factor I receptor. J Biol Chem. 1995;270:15639–15643. doi: 10.1074/jbc.270.26.15639. [DOI] [PubMed] [Google Scholar]
- 39.Maurer G, Tarkowski B, Baccarini M. Raf kinases in cancer-roles and therapeutic opportunities. Oncogene. 2011 doi: 10.1038/onc.2011.160. [DOI] [PubMed] [Google Scholar]
- 40.Hellawell GO, Turner GD, Davies DR, Poulsom R, Brewster SF, Macaulay VM. Expression of the type 1 insulin-like growth factor receptor is up-regulated in primary prostate cancer and commonly persists in metastatic disease. Cancer Res. 2002;62:2942–2950. [PubMed] [Google Scholar]
- 41.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O'Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, Spevak W, Zhang C, Zhang Y, Habets G, Burton EA, Wong B, Tsang G, West BL, Powell B, Shellooe R, Marimuthu A, Nguyen H, Zhang KY, Artis DR, Schlessinger J, Su F, Higgins B, Iyer R, D'Andrea K, Koehler A, Stumm M, Lin PS, Lee RJ, Grippo J, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, Chapman PB, Flaherty KT, Xu X, Nathanson KL, Nolop K. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halaban R, Zhang W, Bacchiocchi A, Cheng E, Parisi F, Ariyan S, Krauthammer M, McCusker JP, Kluger Y, Sznol M. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res. 2010;23:190–200. doi: 10.1111/j.1755-148X.2010.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suda K, Tomizawa K, Osada H, Maehara Y, Yatabe Y, Sekido Y, Mitsudomi T. Conversion from the "oncogene addiction" to "drug addiction" by intensive inhibition of the EGFR and MET in lung cancer with activating EGFR mutation. Lung Cancer. 2012;76:292–299. doi: 10.1016/j.lungcan.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Buck E, Gokhale PC, Koujak S, Brown E, Eyzaguirre A, Tao N, Rosenfeld-Franklin M, Lerner L, Chiu MI, Wild R, Epstein D, Pachter JA, Miglarese MR. Compensatory insulin receptor (IR) activation on inhibition of insulin-like growth factor-1 receptor (IGF-1R): rationale for cotargeting IGF-1R and IR in cancer. Mol Cancer Ther. 2010;9:2652–2664. doi: 10.1158/1535-7163.MCT-10-0318. [DOI] [PubMed] [Google Scholar]
- 46.Fedorenko IV, Paraiso KH, Smalley KS. Acquired and intrinsic BRAF inhibitor resistance in BRAF V600E mutant melanoma. Biochem Pharmacol. 2011;82:201–209. doi: 10.1016/j.bcp.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi H, Kong X, Ribas A, Lo RS. Combinatorial treatments that overcome PDGFRbeta-driven resistance of melanoma cells to V600EB-RAF inhibition. Cancer Res. 2011;71:5067–5074. doi: 10.1158/0008-5472.CAN-11-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tworkoski K, Singhal G, Szpakowski S, Zito CI, Bacchiocchi A, Muthusamy V, Bosenberg M, Krauthammer M, Halaban R, Stern DF. Phosphoproteomic screen identifies potential therapeutic targets in melanoma. Mol Cancer Res. 2011;9:801–812. doi: 10.1158/1541-7786.MCR-10-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marais R, Sellers W, Livingston D, Mihich E. Twenty-fourth annual Pezcoller symposium: Molecular basis for resistance to targeted agents. Cancer Res. 2013;73:1046–1049. doi: 10.1158/0008-5472.CAN-12-3236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.