Abstract
Respiratory assist devices seek optimized performance in terms of gas transfer efficiency and thromboresistance to minimize device size and reduce complications associated with inadequate blood biocompatibility. The exchange of gas with blood occurs at the surface of the hollow fiber membranes (HFMs) used in these devices. In this study, three zwitterionic macromolecules were attached to HFM surfaces to putatively improve thromboresistance: (1) carboxyl-functionalized zwitterionic phosphorylcholine (PC) and (2) sulfobetaine (SB) macromolecules (mPC or mSB-COOH) prepared by a simple thiol-ene radical polymerization and (3) a low-molecular weight sulfobetaine (SB)-co-methacrylic acid (MA) block copolymer (SBMAb-COOH) prepared by reversible addition–fragmentation chain transfer (RAFT) polymerization. Each macromolecule type was covalently immobilized on an aminated commercial HFM (Celg-A) by a condensation reaction, and HFM surface composition changes were analyzed by X-ray photoelectron spectroscopy. Thrombotic deposition on the HFMs was investigated after contact with ovine blood in vitro. The removal of CO2 by the HFMs was also evaluated using a model respiratory assistance device. The HFMs conjugated with zwitterionic macromolecules (Celg-mPC, Celg-mSB, and Celg-SBMAb) showed expected increases in phosphorus or sulfur surface content. Celg-mPC and Celg-SBMAb experienced rates of platelet deposition significantly lower than those of unmodified (Celg-A, >95% reduction) and heparin-coated (>88% reduction) control HFMs. Smaller reductions were seen with Celg-mSB. The CO2 removal rate for Celg-SBMAb HFMs remained comparable to that of Celg-A. In contrast, the rate of removal of CO2 for heparin-coated HFMs was significantly reduced. The results demonstrate a promising approach to modifying HFMs using zwitterionic macromolecules for artificial lung devices with improved thromboresistance without degradation of gas transfer.
1. INTRODUCTION
Respiratory assist devices, including those utilized for extracorporeal membrane oxygenation (ECMO), have sought to improve overall device performance by increasing gas transfer efficiency and minimizing thrombotic deposition.1–3 To achieve the clinically required transport rates for O2 and CO2, these devices have large surface areas (~2 m2) for gas transfer in the form of hollow fiber membranes (HFMs). Such large areas of contact between patient blood and synthetic surfaces can lead to thrombotic deposition, which reduces gas transfer efficiency and depletes critical elements from the patient’s hemostatic system.4 To minimize this effect, patient blood is anticoagulated. There is tremendous interest in creating HFMs that allow high gas transfer rates but are minimally thrombogenic. Higher HFM gas transfer efficiency and resistance to thrombotic fouling may also allow the surface area of the device to be reduced, thus further minimizing the insult provided by the synthetic surfaces to the blood elements. Alternatively, anticoagulation levels might be reduced with more hemocompatible HFMs, which would reduce the risk for bleeding complications in these patients.5,6 Both of these effects would increase the likelihood that patients in end-stage respiratory failure might be supported by respiratory assist devices for longer periods of time to allow healing and recovery, or until lung transplantation might be possible.7,8
Zwitterionic phosphorylcholine (PC)-bearing polymers have been applied to a variety of blood-contacting surfaces, including HFMs for blood purification9,10 and blood oxygenators.11 Zwitterionic sulfobetaine (SB)-bearing polymers are relatively inexpensive and readily obtained compared to PC-bearing polymers, while SB-bearing polymer-modified surfaces have also exhibited resistance to protein adsorption, bacterial adhesion, and improved hemocompatibility.12–15 For application of zwitterionic moieties to HFMs, an important consideration is the maintenance of the gas transfer rates of the underlying membrane. Improved thromboresistance at the expense of reduced gas transfer efficiency would theoretically increase the total HFM surface area needed for gas transfer, thus resulting in more blood contact with membrane surfaces, offsetting the improvement in hemocompatibility per HFM.1–3,11 Approaches in which individual molecules are attached to the HFM surface, as opposed to polymerization of precursors to form films on the surface, should thus be more attractive.
In this study, we hypothesized that HFMs used in an artificial lung device could exhibit markedly improved resistance to thrombotic deposition by the covalent coupling of carboxyl-functionalized zwitterionic macromolecules onto the membrane surfaces without detrimental effects on gas transfer capacity. Specifically, two approaches were pursued. In the first, PC or SB macromolecules functionalized with a single carboxyl end group (mPC-COOH or mSB-COOH) were prepared by a simple thiol-ene radical polymerization16 and attached. In the second approach, a low-molecular weight (LMW) SB-co-methacrylic acid (MA) block copolymer (SBMAb-COOH), which contained multiple carboxyl groups in the MA block, was prepared by a reversible addition–fragmentation chain transfer (RAFT) polymerization technique.17–19 HFMs modified with these zwitterionic agents were compared to control HFMs modified either with heparin, a common anticoagulant and surface-modifying agent for improved hemocompatibility, or with aminated HFMs, which served as the base HFM onto which the conjugation reactions were performed. Thromboresistance, assessed in terms of platelet deposition, and gas transfer efficiency, assessed in terms of the transfer of CO2 from whole blood, were the parameters employed to investigate the hypothesis.
2. MATERIALS AND METHODS
2.1. Materials
N-(3-Sulfopropyl)-N-(methacryloxyethyl)-N,N-dimethylammonium betaine (SMDAB) monomer, 4-cyano-4-(phenylcarbonothioylthio)pentanoic acid as a chain transfer agent (CTA), and 4,4′-azobis(4-cyanopentanoic acid) (V-501) (ACPA, initiator), 3-mercaptopropionic acid (MPA), N-[3-(dimethylamino)-propyl]-N′-ethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), and 2,2,2-trifluoroethanol (TFE) were purchased from Sigma-Aldrich. Methacrylic acid (MA) (Sigma-Aldrich) was distilled before being used. Commercial modified microporous polypropylene hollow fibers [CelgardTM; outside diameter of 300 µm, inside diameter of 240 µm (Membrana GmbH, Wuppertal, Germany)] were obtained from ALung Technologies, Inc., with an aminated siloxane (Celg-A) or aminated siloxane/heparin (Celg-Hep) coating. Heparin anticoagulated bovine blood was purchased from Lampire Biological Laboratories (Pipersville, PA) for gas exchange studies. 2-Methacryloyloxyethyl phosphorylcholine (MPC) was obtained from NOF Corp. (Tokyo, Japan).
2.2. Synthesis of Carboxyl-Functionalized Zwitterionic Macromolecules or SB Block Copolymer with Carboxyl Groups
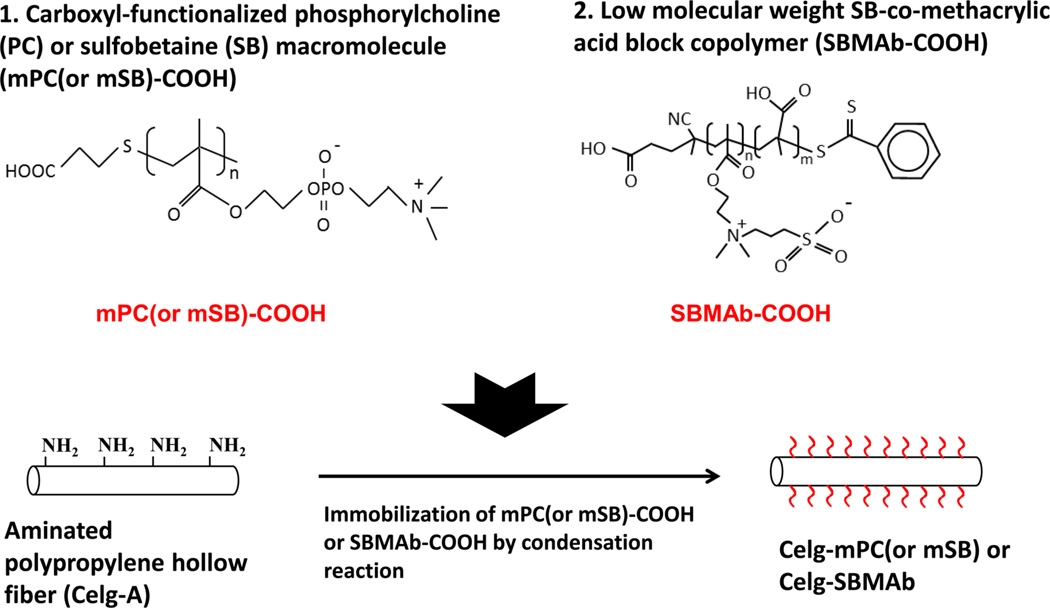
Carboxyl-functionalized PC (or SB) macromolecules (mPC or mSB-COOH) were synthesized from 3-mercaptopropionic acid and MPC or SMDAB monomer by a simple thiol-ene radical polymerization technique described in a previous study.16 A low-molecular weight SB block copolymer with carboxyl groups was also synthesized using a typical reversible addition–fragmentation chain transfer (RAFT) polymerization technique (Figure 1).17–19 In detail, SMDAB monomer, CTA, and ACPA initiator were dissolved in TFE solvent in a flask with a magnetic stirrer at a determined molar ratio (20:1:0.5 SMDAB:CTA:ACPA). Argon gas was injected and bubbled into the solution for 30 min to remove oxygen, and the solution was then sealed under argon protection. The reaction mixture was then stirred for 15 h at 70 °C, and after the reaction, synthesized product was precipitated into methanol. The obtained pink gel-like product (SB-CTA prepolymer) was further rinsed with excess methanol and dried in a vacuum oven for 24 h. Next, the obtained macro SB-CTA and MA were dissolved in TFE with a determined molar ratio (1:20 SB-CTA:MA). After ACPA initiator had been added and argon gas bubbled into the solution for 30 min, the reaction mixture was stirred for 15 h at 70 °C. The synthesized block copolymer (SBMAb-COOH) was obtained after precipitation in methanol, centrifugation, and vacuum drying after further rinsing with methanol to remove unreacted monomer and initiator. The chemical structure of SBMAb-COOH was confirmed using a proton nuclear magnetic resonance (1H NMR) instrument (BrukerBiospin Co., Billerica, MA). The carboxyl group content of the synthesized SBMAb-COOH was estimated using a typical titration assay using a phenolphthalein solution (Sigma-Aldrich).
Figure 1.
Carboxyl-functionalized zwitterionic macromolecules (mPC or mSB), a low-molecular weight SB-co-methacrylic acid block copolymer (SBMAb-COOH), and the hollow fiber surface modification scheme.
2.3. Hollow Fiber Surface Modification and Characterization
The HFMs modified with either mPC or mSB-COOH macromolecules, as well as those modified with SBMAb-COOH block copolymer fibers, were prepared by the conjugation of aminated Celg-A fibers through EDC/NHS condensation chemistry using the carboxyl groups and amino groups of Celg-A (Figure 1). Specifically, the synthesized mPC-COOH, mSB-COOH, or SBMAb-COOH was dissolved in 20 mL of deionized (DI) water (0.1 wt %) and preactivated by adding EDC (2 equiv of the COOH) at room temperature for 3 h. Then, the solution was added into a screw thread glass tube (Thermo Fisher Scientific Inc.) in which the Celg-A HFM bundle was placed, and the solution was continuously mixed with the hollow fibers for an additional 24 h at room temperature after adding NHS (1 equiv of the EDC) and sodium carbonate to adjust the solution pH to >8. After the reaction, the hollow fibers were washed with an excess of DI water for 24 h before being used. The hollow fiber surface composition was analyzed by X-ray photoelectron spectroscopy (XPS) using a Surface Science Instruments S-probe spectrometer with a takeoff angle of 55° (performed at NESAC-BIO, University of Washington). The surface composition of a given sample was averaged from three composition spots, and the mean value for three different samples was determined.
2.4. In Vitro Blood Contact Testing
Whole ovine blood was collected by jugular venipuncture using an 18 gauge 1.5 in. needle directly into a syringe after discarding the first 3 mL. National Institutes of Health guidelines for the care and use of laboratory animals were observed, and all animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pittsburgh. The blood was quickly distributed into S-Monovette tubes (3 mL of 9NC) (Sarstedt) containing sodium citrate. Thrombotic deposition on the polymer surfaces was assessed in vitro with a simple rocking test. The fiber samples were cut into 5 cm segments, and the fiber ends were sealed using a hot melt glue gun with a 3M glue stick (Uline, Pleasant Prarie, WI). For a given fiber type, five single fibers were placed into a Vacutainer tube (BD Vacutainer, no additive, catalog no. 366703). The tube was placed on a hematology mixer (Fisher Scientific, Pittsburgh, PA) after the tube had been filled with 5 mL of citrated ovine blood and rocked for 3 h at 37 °C. After blood contact, the fiber samples were rinsed with PBS at least 10 times and immersed in a 2.5% glutaraldehyde solution for 2 h at 4 °C to fix the surface-deposited platelets. The samples were then serially dehydrated with increasing ethanol solutions and sputter-coated with gold/palladium. Each sample surface was observed by scanning electron microscopy (SEM) (JSM-6330F, JEOL USA, Inc., Peabody, MA). Deposited platelets on each surface were quantified by a lactate dehydrogenase (LDH) assay20 with an LDH Cytotoxicity Detection Kit (Clontech Laboratories, Inc., Mountain View, CA). Similar blood contacting studies were performed with fiber bundle segments by using a 1 cm × 5 cm fiber bundle segment with binding fibers instead of five individual fibers. Binding fibers are nylon fibers that are often used in commercial HFM devices to hold groups of individual HFMs together.
2.5. In Vitro Removal of CO2 from Blood
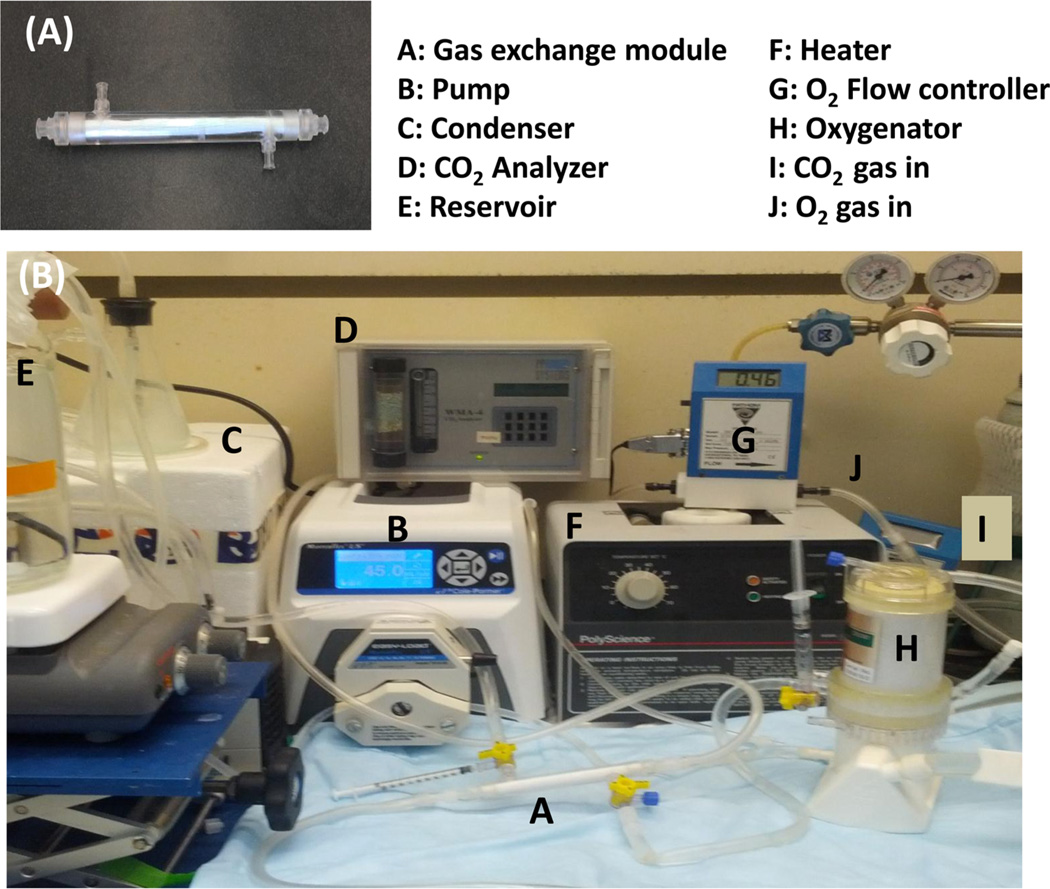
In vitro gas exchange was quantified as previously detailed.21,22 Briefly, HFMs were fabricated into a scaled-down gas exchange module (Figure 2A) by inserting HFMs (183 fibers, 12 cm length) into a ¼ in. inside diameter polycarbonate tubing (McMaster Carr, Elmhurst, IL) to which single luer locks were UV-glued 0.75 in. from each end in opposing directions. Both ends of the HFM were secured to the tubing using a 5 min epoxy adhesive (Devcon, Danvers, MA) and then trimmed to the length of the tubing to expose the HFM lumens, yielding 6.9 cm of HFM uncovered within the module for a total active surface area of 0.0119 m2.
Figure 2.
(A) Scaled-down gas exchange module and (B) in vitro gas exchange test setting loop.
An in vitro recirculating test loop was used to assess CO2 exchange rates using Celg-A, Celg-Hep, and Celg-SBMAb HFM under steady fluid and sweep gas flow (Figure 2B). CO2 exchange was monitored rather than oxygen exchange, because CO2 exchange is more sensitive to membrane diffusional resistance because of its greater effective solubility and diffusivity in blood.23,24 The zwitterionic macromolecule- modified HFMs were not evaluated because the blood compatibility of the Celg-SBMAb was found to be superior. Heparinized bovine blood (1 L) flowed from a reservoir, through a peristaltic pump, into Minimax Plus pediatric oxygenator (Medtronic, Minneapolis, MN) to balance the fluid gases, then to the model oxygenator testing module, and finally back to the reservoir. The partial pressure of CO2 (pCO2) at the model oxygenator inlet was adjusted to 50 mmHg and measured with a RAPIDLAB 248 blood gas analyzer (Siemens, Deerfield, IL). Pure oxygen sweep gas was pulled by vacuum and regulated with a GR Series mass flow controller (Fathom Technologies, Georgetown, TX) through the model oxygenator HFM lumens, moisture trap condenser, and finally an infrared CO2 analyzer (WMA-4 CO2 Analyzer, PP Systems, Amesbury, MA). The oxygen sweep gas flow rate was adjusted to maintain approximately 3000 ppm of CO2 in the sweep gas exiting the model oxygenator. This approach minimized buildup of CO2 within the HFM sweep gas exiting the device that adversely affects the trans-HFM CO2 partial pressure gradient. In this manner, we compared gas exchange modules with different CO2 removal efficiencies by maintaining a constant CO2 concentration in the sweep gas stream and varied the sweep gas flow rate to determine overall CO2 removal rates. The blood was maintained at 37 °C, ~50 mmHg pCO2, and a liquid flow rate of 30, 45, or 90 mL/min. Under these conditions, the HFM CO2 removal efficiency (milliliters per minute per cubic meter) matches those of commercially available devices. The rate of CO2 removal (VCO2) for each model oxygenator device was calculated using the sweep gas flow rate () and CO2 fraction (FCO2) exiting the scaled-down gas exchange module and then normalized to 50 mmHg to correct for small deviations in the inlet pCO2: .
For each device, the fluid inlet pCO2 and resulting FCO2 were measured in triplicate. The VCO2 for each model gas exchange device with a different fiber type is reported as an average of these measurements (n = 4 for each group).
The results are presented as means ± the standard deviation (SD). Data were analyzed by one-way analysis of variance followed by a post hoc Neuman–Keuls test. Significant differences were considered to exist at p < 0.05.
3. RESULTS
3.1. Characterization of Carboxyl-Functionalized Zwitterionic Macromolecules, SBMAb-COOH, and Modified HFMs
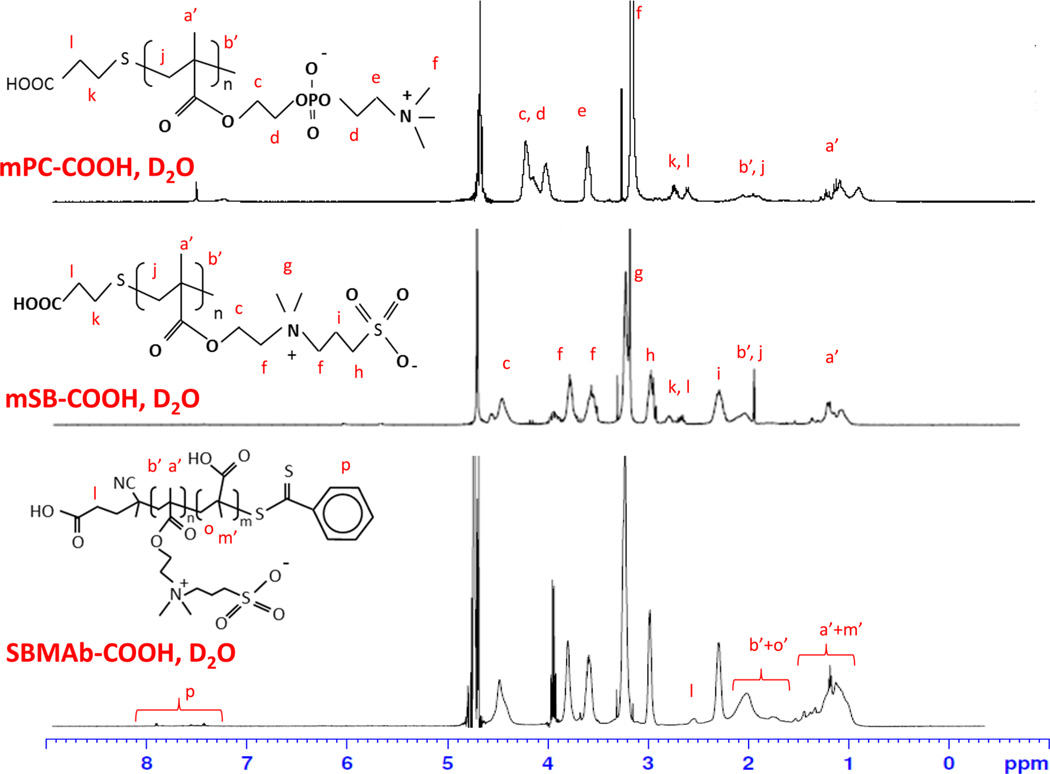
The chemical structure of the synthesized macromolecules (mPC or mSB) and SBMAb-COOH block copolymer was confirmed by 1H NMR (Figure 3 and Figures S1–S3 of the Supporting Information). For mPC in deuterium oxide, the following peaks were found: δ 0.80–1.25 (α-CH3), 1.85–2.30 (SCH2C and CH2C), 2.50–2.75 (CH2CH2S and CH2CH2S), 3.05–3.20 [CH2N(CH3)3], 3.60–3.70 [CH2N-(CH3)3], 3.90–4.40 (OCH2 and CH2PO4CH2). For mSB in D2O the following peaks were found: δ 0.90–1.30 (α-CH3), 1.75–2.20 (SCH2C and CH2C), 2.25–2.35 (CH2CH2SO3), 2.50–2.85 (CH2CH2S and CH2 CH2S), 2.90–3.00 (CH2CH2SO3), 3.20–3.40 [N(CH3)2], 3.50–3.85 [CH2N-(CH3)2CH2], 4.35–4.55 (OCH2). For SBMAb-COOH in D2O, the following peaks were found: δ 0.90–1.30 (α-CH3), 1.70–2.20 (CH2C), 2.25–2.35 (CH2CH2SO3), 2.45–2.50 (CH2COOH), 2.90–3.00 (CH2CH2SO3), 3.20–3.40 [N-(CH3)2], 3.50–3.85 [CH2N(CH3)2CH2], 4.35–4.55 (OCH2).
Figure 3.
1H NMR spectra of zwitterionic macromolecules (mPC or mSB-COOH) and the block copolymer (SBMAb-COOH).
The molecular weights of the synthesized macromolecules were 1104 ± 237 for mPC-COOH, 1488 ± 390 for mSB-COOH, and 5125 ± 494 for SBMAb-COOH as calculated from the 1H NMR spectra (n = 3) using the peak integration ratio of CH2N or CH2S (originating from the PC or SB groups) and CH2-COOH, which is implied from the degree of polymerization. The estimated number of carboxyl groups in SBMAb-COOH, synthesized from a 20:20 SB:MA molar ratio, was 18.2 ± 2.5, which was calculated by a titration assay.
The surface composition for the HFMs as determined by XPS is shown in Table 1. All of the modified surfaces consistently showed a decrease in the level of silicon (Si) and an increase in the level of carbon compared with those of the control (Celg-A) with an aminated siloxane coating. Celg-mPC and Celg-mSB showed increased levels of phosphorus (P) and sulfur (S) on the surfaces, respectively. Celg-SBMAb showed a significant increase in the level of S (1.1 ± 0.3%) consistent with successful conjugation of SBMAb-COOH onto the aminated Celg-A fiber where S was not detected.
Table 1.
Atomic Percentages on Hollow Fiber Surfaces As Determined by X-ray Photoelectron Spectroscopy
| C 1s at 285 eV | O 1s at 532 eV | N 1s at 403 eV | Si 2p at 106 eV | P 2p at 133 eV | S 2p at 168 eV | |
|---|---|---|---|---|---|---|
| Celg-A | 42.3 ± 2.8 | 33.8 ± 1.3 | 1.7 ± 0.9 | 21.9 ± 2.1 | – | – |
| Celg-mPC | 52.8 ± 2.3a | 28.1 ± 1.2 | 2.1 ± 0.4 | 16.5 ± 1.7a | 0.2 ± 0.1a | – |
| Celg-mSB | 57.9 ± 0.8a | 27.6 ± 1.0 | 1.6 ± 0.5 | 10.6 ± 3.5a | – | 0.3 ± 0.2a |
| Celg-SBMAb | 58.5 ± 3.7a | 26.6 ± 0.2 | 2.4 ± 0.2 | 11.3 ± 3.4a | – | 1.1 ± 0.3a |
p < 0.05 vs Celg-A control (n = 3; mean ± SD).
3.2. Deposition of Platelet on HFMs after Ovine Blood Contact
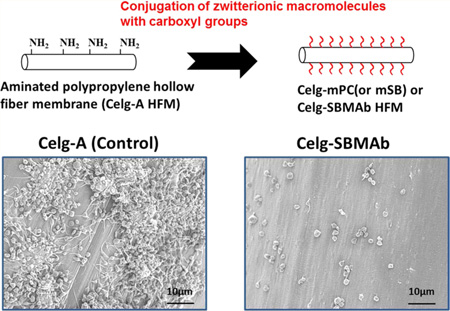
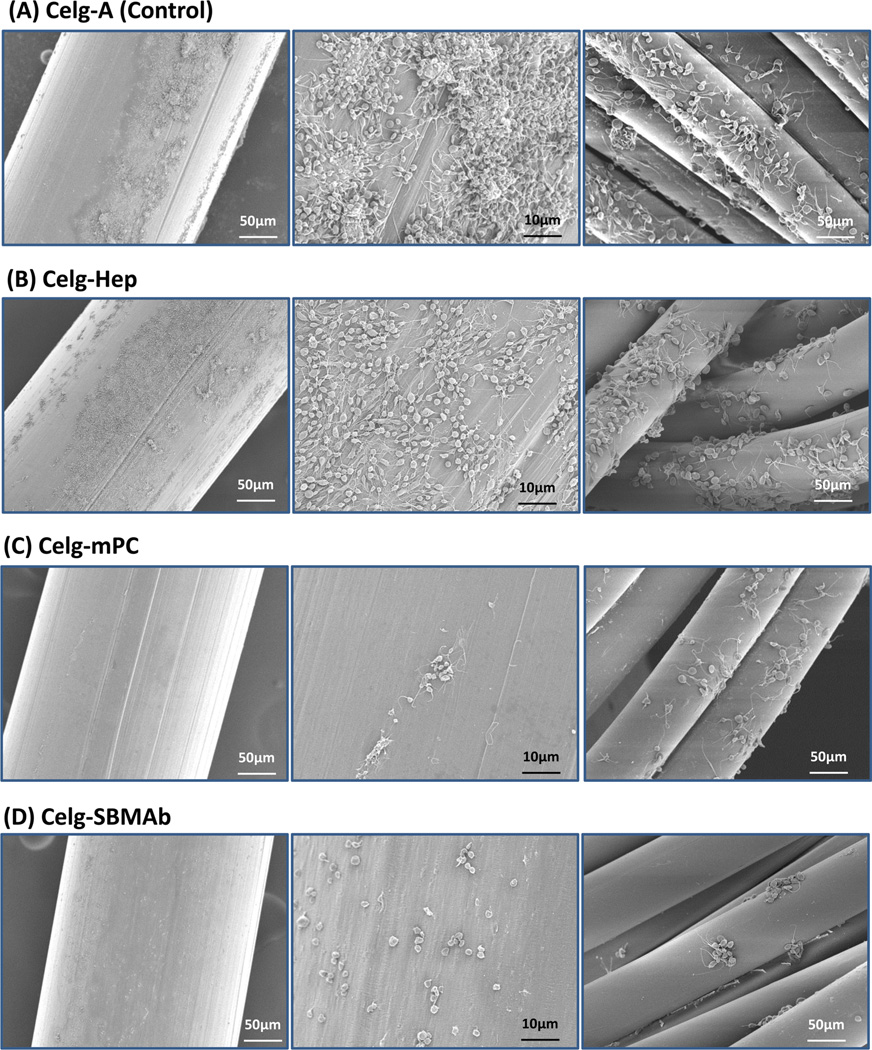
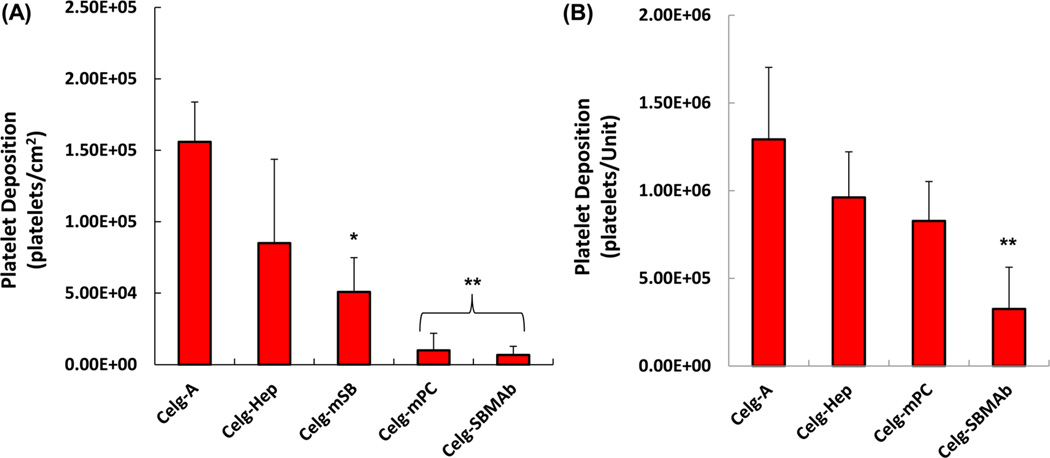
Thrombogenic deposition on each fiber surface after contact with ovine blood for 3 h is shown in Figure 4. The surfaces of Celg-A HFMs, as well as the binding fibers for the HFMs (right column of Figure 4), showed consistent platelet deposition over the whole area with large aggregates and a spread morphology for the deposited platelets (Figure 4A). The heparin coated HFM surface (Celg-Hep) still showed many deposited platelets with spread morphology (Figure 4(B), although the deposition and the aggregates were less than seen on Celg-A. In contrast, platelet deposition was significantly decreased and sparse on Celg-mPC or Celg-SBMAb surfaces (Figure 4 (C) & (D)). Platelet deposition quantified using a lactate dehydrogenase (LDH) assay (Figure 5) confirmed the visual result. Both Celg-mPC and Celg-SBMAb HFMs showed significantly lower platelet deposition than the control (Celg-A, >95% reduction) and heparin coated (Celg-Hep, >88% reduction) surfaces (Figure 5 (A)). Platelet deposition onto HFMs that included the binding fibers was also evaluated by the LDH assay (Figure 5 (B)). Celg-SBMAb HFM bundles still showed statistically lower platelet deposition compared to Celg- and Celg-Heparin fiber bundle samples (p<0.05, n=5), although the number of deposited platelets was increased by including the binding fibers which exhibit higher thrombogenicity (right column of Figure 4) and Celg-mPC was no longer significantly different from the control fibers.
Figure 4.
Scanning electron micrographs after citrated ovine blood contact for 3 h for (A) aminated polypropylene control (Celg-A) and (B) heparin-modified (Celg-Hep), (C) mPC-modified (Celg-mPC), and (D) SBMAb block copolymer-modified (Celg-SBMAb) hollow fibers (left, middle columns). Binding fibers are seen in the right column.
Figure 5.
Platelet deposition on (A) a single fiber without binding fibers present and (B) a fiber bundle unit including binding fibers after contact with ovine blood for 3 h by a lactate dehydrogenase (LDH) assay (n = 5; *p < 0.05 vs Celg-A, **p < 0.05 vs Celg-A and Celg-Hep).
3.3. Gas Exchange Performance (CO2 removal) from Whole Blood
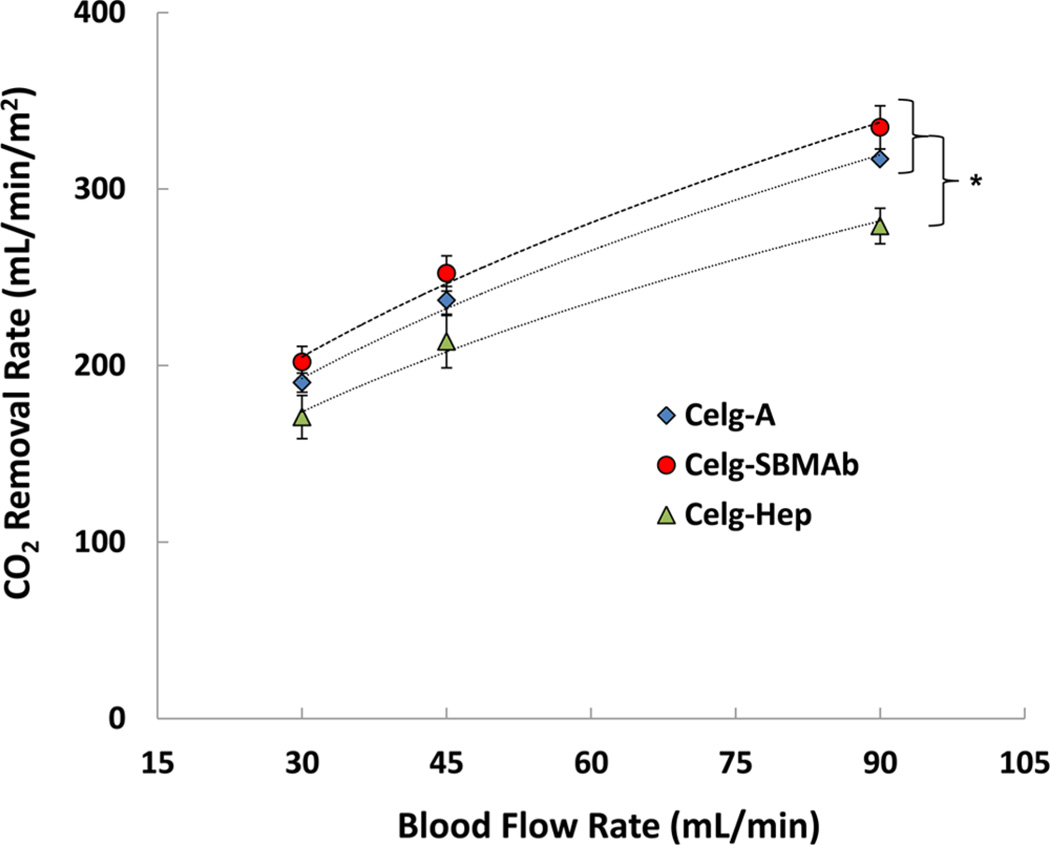
CO2 removal rates were assessed on Celg-A, Celg-Hep, and the modified HFM determined to have the greatest reduction in the extent of platelet deposition, Celg-SBMAb. The SBMAb modification showed no negative effects on CO2 removal performance compared to that of the Celg-A control and was statistically similar to the control Celg-A HFMs for all flow rates (Figure 6). At 90 mL/min, Celg-A HFM removed 317 mL min−1 m−2 while Celg-SBMAb removed 334 mL min−1 m−2. Conversely, the rate of CO2 removal of Celg-Hep was lower than those of the Celg-A and Celg-SBMAb HFMs for all flow rates (p < 0.05; n = 4), and the rate of CO2 removal was 279 mL min−1 m−2 at 90 mL/min, which is reduced by 12 and 17% relative to those of Celg-A and Celg-SBMAb, respectively. After the removal of CO2 from circulating bovine blood, the HFM surfaces were observed by SEM (Figure S4 of the Supporting Information) and the surfaces exhibited the expected trends. For Celg-A, most surfaces were covered with adherent and activated platelets, whereas the SBMAb-modified surface showed very limited adhesion without a spread morphology for the platelets. These bovine blood results confirmed the similar results found after contact with fresh ovine blood (Figure 4).
Figure 6.
CO2 removal rate (VCO2) by Celg-A control (◇), Celg-Hep (△), and Celg-SBMAb (○) hollow fibers in bovine blood in a model respiratory assist device (n = 4; *p < 0.05).
4. DISCUSSION
A broad variety of designs have been proposed to accomplish the task of respiratory gas transfer with device designs that include extracorporeal, paracorporeal, intrathoracic, and intravenous approaches, as well as microfluidic artificial lungs and tissue engineering strategies for generating pulmonary tissue or endothelialized blood-contacting membranes.1–3,25 Despite this exploration, obstacles remain to developing effective artificial lungs that can be used in an ambulatory setting, provide adequate gas exchange, and minimize blood trauma in terms of platelet consumption, coagulation cascade activation, and the triggering of inflammatory pathways.7,8 Furthermore, systemic anticoagulation leads to bleeding risks in these patients. In this study, we demonstrated that the surface modification of HFMs with carboxyl-functionalized zwitterionic PC or SB macromolecules or a low-molecular weight SB-bearing block copolymer could reduce acute thrombotic deposition significantly and, for the latter SB block copolymer, not negatively affect the gas transfer compared to that of the control HFMs (Celg-A and Celg-Hep) under bovine blood circulation. One might be concerned that enhancing the hydrophilicity of the HFM surface by zwitterionic modification might cause a side effect by increasing the wettability of HFM. However, the approach is to apply these coatings to hollow fiber membranes that have a thin, nonporous coating on the outside surface of the hollow fiber membrane. This coating prevents plasma wetting into the gas flow pathway. In future work, these coatings may be applied to asymmetric microporous hollow fiber membranes, in which the surfaces of the fibers do not have pores. Thus, the modification strategy is not anticipated to cause decreased gas transfer due to fiber wetting in respiratory assist devices, even in longer-term applications.
Previous reports indicate that a high-molecular weight PC zwitterionic polymeric coating applied to HFMs can reduce thrombogenicity;9–11,26 however, a polymeric film coating on HFMs might increase concerns regarding negative effects on gas transfer efficiency and manufacturability. Recently, Wang et al.26 showed a cross-linkable PC polymer film applied to HFMs had no negative effect on oxygen transfer efficiency, although CO2 transfer was not reported and is more likely to be impacted. CO2 exchange is more sensitive to changes in the membrane diffusional resistance than oxygen exchange because of its greater effective solubility and diffusivity in blood. The diffusional resistance of CO2 in the blood boundary layer contacting the membrane is approximately 1 order of magnitude lower than that of O2.23,24 Some SB-bearing polymeric coating approaches for HFMs have been reported, including UV-photoinitiated grafting or radical-initiated polymerization techniques.12,13,27,28 However, there are no reports of the direct conjugation of low-molecular weight zwitterionic block copolymer onto HFMs. Recently, Kuo et al.29 prepared poly(sulfobetaine methacrylate-co-acrylic acid) random copolymers and immobilized these onto polymeric substrates (tissue culture polystyrene, polyurethane, and polydimethylsiloxane) with a layer-by-layer polyelectrolyte film technique. The films were shown to reduce fibrinogen adsorption, platelet adhesion, and plasma coagulation. Lin et al.30 also prepared an SB block copolymer with a short poly(methacrylic acid) block for protein conjugation to improve separation efficiency without decreasing bioactivity.
Of primary interest in this study was achieving HFM surface modification with SB-bearing macromolecules that are relatively inexpensive and readily obtainable compared to PC-bearing analogues. In comparing the modification of aminated HFMs by SB or PC macromolecules with a single carboxyl end group, The SB macromolecule (mSB-COOH) modification significantly reduced the rate of platelet deposition versus the aminated control HFM but was less effective than the mPC-COOH. However, the S or P compositions of mSB-COOH- or mPC-COOH-modified HFMs (Table 1) are lower than those found in a previous surface modification study employing siloxane-functionalized macromolecular coatings on a magnesium alloy.16 Thus, the packing density might not achieve ideal conditions and might benefit from optimization. An important parameter for study would be the density of the reactive amino groups on the substrate Celg-A HFMs, which showed a relatively high variation of N composition (1.7 ± 0.9). Further control of the macromolecule conjugation conditions might also yield improvements in surface density. The use of RAFT polymerization to synthesize a low-molecular weight SB block copolymer with multiple carboxyl groups (SBMAb-COOH) was successful and allowed for improved HFM modification efficiency in terms of surface content (as evidenced by an increased level of sulfur on the surface) and a reduced rate of platelet deposition. However, in this report, only a single fixed monomer ratio (20:20 SB:MA) for SBMAb-COOH synthesis was evaluated. Further studies might optimize the surface modification by adjusting the SB chain length31 and the number of carboxyl groups in the SBMAb-COOH block copolymer.32 This would be dependent on the density of amino groups on the HFM and the efficiency of the condensation reaction that also could be affected by the temperature, pH, and concentration of the react solution.33,34
A notable limit in this report is the lack of extended blood contact studies with the HFMs for periods that would represent attractive clinical targets (i.e., days instead of hours). Extended testing periods (over 24 or 48 h) would need to be part of in vivo evaluations because such extended in vitro blood testing has limited value because of alterations in the contacting blood. This is important in terms of assessing the maintenance of thromboresistance and, specifically, the stability of the HFM surface modification. The amide bonding of SBMAb to the aminated siloxane coating on Celg-A was assumed, but the propensity of the surface-modified siloxane to resist surface rearrangement35 under blood flow for extended periods is also a consideration. SBMAb-modified siloxane chain mobility may lead to a reduction in SB surface availability with time, depending on the environment.36 Although our study was studied only on an aminated HFM to conjugate three carboxyl functional zwitterionic macromolecules, the significant reduction of the rate of thrombotic deposition as well as high CO2 removal rates of the HFMs in comparison with those of the heparin-coated HFMs suggests the potential for application of similar strategies to other current respiratory assist devices or those under development. Furthermore, the covalent attachment of zwitterionic macromolecules with other functional groups (e.g., siloxane, N-hydroxysuccinimide, or thiol groups) using alternative conjugation pathways might be utilized on the various substrates used for artificial lungs, including non-HFMbased devices.1–3
5. CONCLUSIONS
Carboxyl-functionalized PC (or SB) macromolecules and a low-molecular weight (LMW) SB block copolymer (SBMAb-COOH) containing carboxyl groups were synthesized by a simple thiol-ene radical polymerization and a RAFT polymerization technique, respectively. Modification of HFMs with these functional zwitterionic macromolecules or the LMW block copolymer effectively improved the surface thromboresistance and maintained a high gas exchange rate in bovine blood. The results demonstrate a promising approach to modifying the HFMs for application in future high-performance artificial lung devices.
ACKNOWLEDGMENTS
This research was supported by a National Institutes of Health Contract (HL117637-NIH R01). We thank the Center for Biological Imaging (CBI) of the University of Pittsburgh for their kind assistance in collecting the SEM images. The surface analysis experiments done at NESAC/BIO were supported by National Institute of Biomedical Imaging and Bioengineering Grant EB-002027. We also thank Joseph Hanke, Teri Horgan, and others who obtained the fresh ovine blood used in this study.
Footnotes
ASSOCIATED CONTENT
s Supporting Information
1H NMR spectra of MPC, MPA, and mPC-COOH (Figure S1), 1H NMR spectra of SMDAB, MPA, and mSB-COOH (Figure S2), 1H NMR spectra of SB-CTA and SBMAb-COOH (Figure S3), and scanning electron micrographs of hollow fiber surfaces after contact with bovine blood for the gas exchange analysis (1.5 h) (Figure S4). This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- 1.Nolan H, Wang D, Zwischenberger JB. Artificial lung basics: Fundamental challenges, alternative designs and future innovations. Organogenesis. 2011;7:23–27. doi: 10.4161/org.7.1.14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Potkay JA. The promise of microfluidic artificial lungs. Lab Chip. 2014;14:4122–4138. doi: 10.1039/c4lc00828f. [DOI] [PubMed] [Google Scholar]
- 3.Strueber M. Artificial lungs: Are we there yet? Thoracic Surgery Clinics. 2015;25:107–113. doi: 10.1016/j.thorsurg.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Esper SA, Levy JH, Waters JH, Welsby IJ. Extracorporeal membrane oxygenation in the adult: A review of anticoagulation monitoring and transfusion. Anesth. Analg. (Hagerstown, MD, U.S.) 2014;1184:731–743. doi: 10.1213/ANE.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 5.Ovrum E, Holen EA, Tangen G, Brosstad F, Abdelnoor M, Ringdal MA, Oystese R, Istad R. Completely heparinized cardiopulmonary bypass and reduced systemic heparin: Clinical and hemostatic effects. Annals of Thoracic Surgery. 1995;602:365–371. doi: 10.1016/0003-4975(95)00366-s. [DOI] [PubMed] [Google Scholar]
- 6.Wagner WR, Griffith BP. Reconstructing the lung. Science. 2010;329:520–522. doi: 10.1126/science.1194087. [DOI] [PubMed] [Google Scholar]
- 7.Gorbet MB, Sefton MV. Biomaterial-associated thrombosis: Roles of coagulation factors, complement, platelets and leukocytes. Biomaterials. 2004;25:5681–5703. doi: 10.1016/j.biomaterials.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 8.Schopka S, Schmid T, Schmid C, Lehle K. Current strategies in cardiovascular biomaterial functionalization. Materials. 2010;3:638–655. [Google Scholar]
- 9.Ye SH, Watanabe J, Iwasaki Y, Ishihara K. Antifouling blood purification membrane composed of cellulose acetate and phospholipid polymer. Biomaterials. 2003;24:4143–4152. doi: 10.1016/s0142-9612(03)00296-5. [DOI] [PubMed] [Google Scholar]
- 10.Ye SH, Watanabe J, Iwasaki Y, Takai M, Ishihara K. Design of functional hollow fiber membranes modified with phospholipid polymers for application in total hemopurification system. Biomaterials. 2005;26:5032–5041. doi: 10.1016/j.biomaterials.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 11.Pieri M, Turla OG, Calabrò MG, Ruggeri L, Agracheva N, Zangrillo A, Pappalardo F. A new phosphorylcholine-coated polymethylpentene oxygenator for extracorporeal membrane oxygenation: A preliminary experience. Perfusion. 2013;28:132–137. doi: 10.1177/0267659112469642. [DOI] [PubMed] [Google Scholar]
- 12.Razi F, Sawada I, Ohmukai Y, Maruyama T, Matsuyama H. The improvement of antifouling efficiency of polyethersulfone membrane by functionalization with zwitterionic monomers. J. Membr. Sci. 2012;401–402:292–299. [Google Scholar]
- 13.Li Q, Bi QY, Zhou B, Wang XL. Zwitterionic sulfobetaine-grafted poly(vinylidene fluoride) membrane surface with stably anti-protein-fouling performance via a two-step surface polymerization. Appl. Surf. Sci. 2012;258:4707–4717. [Google Scholar]
- 14.Wu J, Lin W, Wang Z, Chen S, Chang Y. Investigation of the hydration of nonfouling material poly(sulfobetaine methacrylate) by low-field nuclear magnetic resonance. Langmuir. 2012;28:7436–7441. doi: 10.1021/la300394c. [DOI] [PubMed] [Google Scholar]
- 15.Liu Q, Li W, Wang H, Liu L. A facile method of using sulfobetaine-containing copolymers for biofouling resistance. J. Appl. Polym. Sci. 2014;131 [Google Scholar]
- 16.Ye SH, Jang YS, Yun YH, Shankarraman V, Woolley JR, Hong Y, Gamble LJ, Ishihara K, Wagner WR. Surface modification of a biodegradable magnesium alloy with phosphorylcholine (PC) and sulfobetaine (SB) functional macromolecules for reduced thrombogenicity and acute corrosion resistance modifiers to reduce thrombogenicity. Langmuir. 2013;29:8320–8327. doi: 10.1021/la401341y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donovan MS, Sumerlin BS, Lowe AB, McCormick CL. Controlled/“living” polymerization of sulfobetaine monomers directly in aqueous media via RAFT. Macromolecules. 2002;35:8663–8666. [Google Scholar]
- 18.Moad G, Chong YK, Postma A, Rizzardo E, Thang SH. Advances in RAFT polymerization: The synthesis of polymers with defined end-group. Polymer. 2005;46:8458–8468. [Google Scholar]
- 19.Semsarilar M, Perrier S. ‘Green’ reversible addition-fragmentation chain-transfer (RAFT) polymerization. Nat. Chem. 2010;2:811–820. doi: 10.1038/nchem.853. [DOI] [PubMed] [Google Scholar]
- 20.Oh HI, Ye SH, Johnson CA, Jr, Woolley JR, Federspiel WJ, Wagner WR. Hemocompatibility assessment of carbonic anhydrase modified hollow fiber membranes for artificial lungs. Artif. Organs. 2010;34(5):439–442. doi: 10.1111/j.1525-1594.2009.00882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arazawa DT, Oh HI, Ye SH, Johnson CA, Jr, Woolley JR, Wagner WR, Federspiel WJ. Immobilized carbonic anhydrase on hollow fiber membranes accelerates CO2 removal from blood. J. Membr. Sci. 2012;403–404:25–31. doi: 10.1016/j.memsci.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimmel JD, Arazawa DT, Ye SH, Shankarraman V, Wagner WR, Federspiel WJ. Carbonic anhydrase immobilized on hollow fiber membranes using glutaraldehyde activated chitosan for artificial lung applications. J. Mater. Sci. Mater. Med. 2013;24:2611–2621. doi: 10.1007/s10856-013-5006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eash HJ, Jones HM, Hattler BG, Federspiel WJ. Evaluation of plasma resistant hollow fiber membranes for artificial lungs. ASAIO J. 2004;50:491–497. doi: 10.1097/01.mat.0000138078.04558.fe. [DOI] [PubMed] [Google Scholar]
- 24.Federspiel WJ, Henchir KA. Lung, Artificial: Basic principles and current applications. In: Wnek GE, Bowlin GL, editors. Encyclopedia of Biomaterials and Biomedical Engineering. New York: Marcel Dekker, Inc.; 2004. pp. 910–921. [Google Scholar]
- 25.Song JJ, Ott HC. Bioartificial lung engineering. Am. J. Transplant. 2012;12:283–288. doi: 10.1111/j.1600-6143.2011.03808.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang YB, Gong M, Yang S, Nakashima K, Gong YK. Hemocompatibility and film stability improvement of crosslinkable MPC copolymer coated polypropylene hollow fiber membrane. J. Membr. Sci. 2014;452:29–36. [Google Scholar]
- 27.Lie Q, Lin HH, Wang XL. Preparation of sulfobetainegrafted PVDF hollow fiber membranes with a stably anti-protein-fouling performance. Membranes. 2014;4:181–199. doi: 10.3390/membranes4020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiang T, Wang R, Zhao WF, Sun SD, Zhao CS. Covalent deposition of zwitterionic polymer and citric acid by click chemistry-enabled layer-by-layer assembly for improving the blood compatibility of polysulfone membrane. Langmuir. 2014;30:5115–5125. doi: 10.1021/la5001705. [DOI] [PubMed] [Google Scholar]
- 29.Kuo WH, Wang MJ, Chien HW, Wei TC, Lee C, Tsai WB. Surface modification with poly(sulfobetaine methacrylateco-acrylic acid) to reduce fibrinogen adsorption, platelet adhesion, and plasma coagulation. Biomacromolecules. 2011;12:4348–4356. doi: 10.1021/bm2013185. [DOI] [PubMed] [Google Scholar]
- 30.Lin W, Zhang H, Wu J, Wang Z, Sun H, Yuan J, Chen S. A novel zwitterionic copolymer with a short poly(methyl acrylic acid) block for improving both conjugation and separation efficiency of a protein without losing its bioactivity. J. Mater. Chem. B. 2013;1:2482–2488. doi: 10.1039/c3tb00474k. [DOI] [PubMed] [Google Scholar]
- 31.Sin MC, Chen SH, Chang Y. Hemocompatibility of zwitterionic interfaces and membranes. Focus Review. Polym. J. 2014;46:436–443. [Google Scholar]
- 32.Shao Q, Jiang S. Molecular understanding and design of zwitterionic materials. Adv. Mater. (Weinheim, Ger.) 2014 doi: 10.1002/adma.201404059. [DOI] [PubMed] [Google Scholar]
- 33.Bütün V, Liu S, Weaver JVM, Bories-Azeau X, Cai Y, Arme SP. A brief review of ‘schizophrenic’ block copolymers. React. Funct. Polym. 2006;66:157–165. [Google Scholar]
- 34.Willcock H, Lu A, Hansell CF, Chapman E, Collins IR, O’Reilly RK. One-pot synthesis of responsive sulfobetaine nanoparticles by RAFT polymerisation: The effect of branching on the UCST cloud point. Polym. Chem. 2014;5:1023–1030. [Google Scholar]
- 35.Zhou J, Khodakov DA, Ellis AV, Voelcker NH. Surface modification for PDMS-based microfluidic devices. Electrophoresis. 2012;33:89–104. doi: 10.1002/elps.201100482. [DOI] [PubMed] [Google Scholar]
- 36.Griesser HJ. Surface modification of polyurethanes. In: Vermette P, Griesser HJ, Laroche G, Guidoin R, editors. Biomedical Applications of Polyurethanes. Vol. 6. Georgetown, TX: Landes Bioscience; 2001. pp. 175–219. [Google Scholar]