Abstract
Intensive chemotherapy for newly diagnosed acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) is associated with significant treatment-related morbidity and mortality. Herein, we investigate how pre-treatment characteristics relate to early adverse outcomes in such patients. Among 205 consecutive patients, grade 4 neutropenia (i.e. absolute neutrophil count <500/µL) was associated with the development of fever (P=0.0378), documented infection (P<0.0001), bacteremia (P=0.002), delayed neutrophil count recovery (P<0.0001), and death within 35 days of chemotherapy (P=0.04) but not the requirement for intensive care unit-level care. Low monocyte and lymphocyte counts at baseline were similarly associated with increased risk of documented infection or bacteremia. After adjustment for age, gender, disease type, cytogenetic risk, and performance status, the risk of documented infection or bacteremia was 2.51 (95% confidence interval: 1.59–3.96)-fold and 1.82 (1.04–3.19)-fold higher in patients with initial grade 4 neutropenia. There was no significant relationship between adverse events and age, gender, disease type, disease risk, anthracycline type/dose, or initial peripheral blood blast count. Together, our studies identify severe baseline neutropenia as a risk factor for infection-associated adverse events and early death after induction chemotherapy and may provide the rationale for the risk-adapted testing of myeloid growth factor support in this high-risk AML/MDS patient subset.
Keywords: acute myeloid leukemia (AML), adverse events, chemotherapy-induced neutropenia, febrile neutropenia, induction chemotherapy, myelodysplastic syndrome (MDS)
INTRODUCTION
It has long been recognized that curative-intent chemotherapy is associated with a considerable risk of morbidity and mortality in patients with acute leukemias, in particular with regard to infections and bleeding, because of the prolonged duration of severe treatment-related cytopenias [1–4]. Surprisingly, while numerous studies on patients undergoing intensive chemotherapies for various malignancies have identified determinants of poor outcome at the time febrile neutropenia develops, including age, performance status, and low blood counts [5, 6], little information is available regarding risk factors that could be identified before initiation of chemotherapy; this is also true for patients with acute myeloid leukemia (AML) or, by extension, myelodysplastic syndromes (MDS) receiving conventional induction chemotherapy. The identification of baseline characteristics that can reliably denote a subset of patients at particularly high risk for the development of chemotherapy-induced adverse events would have major clinical implications as preemptive strategies aimed at reducing the frequency and severity of such events could be devised and tested in these patients. We reasoned that the presence of baseline cytopenias could constitute such an adverse characteristic for AML/MDS patients undergoing intensive induction chemotherapy. In this retrospective study, we therefore investigated how baseline cytopenias and early peripheral blood count dynamics are related to the development of adverse events following initiation of curative-intent chemotherapy in patients treated at our institution over the last decade.
METHODS
Study cohort
We retrospectively identified 205 adult patients with newly diagnosed MDS or AML who received conventional, curative-intent induction chemotherapy with cytarabine and an anthracycline (“7+3”) or a “7+3”-like regimen between March 2003 and August 2012 at our institution. Electronic medical records were reviewed to obtain detailed information on patient demographics, disease characteristics (molecular/cytogenetic abnormalities), peripheral blood counts at baseline and during chemotherapy until the day of neutrophil recovery (defined as an absolute neutrophil count [ANC] ≥500 cells/μL), treatment regimen, response to chemotherapy, and adverse events as defined below. Modified Medical Research Council (MRC) criteria [7] were used for disease risk assignment, whereas treatment responses were categorized as proposed by International Working Groups [8, 9]. This study was approved by the Institutional Review Board at the Fred Hutchinson Cancer Research Center.
Definition and measurement of adverse events
We evaluated the occurrence of fever, documented infection, bacteremia, requirement for intensive care unit (ICU)-level care, and death that occurred after the initiation of chemotherapy until day 35 of chemotherapy, hospital discharge without subsequent follow-up at our institution, or death, whichever came first. Fever was defined as a single temperature of 38.3°C or a temperature of 38.0°C on two temporally related but distinct occasions [10]. Documented infections included both microbiologically defined infections (i.e. the identification of a clinically significant pathogen from a normally sterile site) and clinically defined infections (i.e. the presence of fever with an appropriate clinical finding such as pulmonary infiltrates or inflammation of the skin or soft tissue) [11]. Positive cultures with common contaminants such as coagulase negative Staphylococcus were considered to reflect true infections only if there were two or more positive cultures in the same episode, if there was clinical evidence of a catheter infection, or if the positive culture was associated with fever not attributable to any other source of infection.
Modeling of peripheral blood count dynamics
In patients with ≥3 measurable neutrophil counts within the first 5 days after the start of neutrophil decline, the rate of neutrophil clearance was calculated by fitting an exponential decay curve to the data points starting on day 1 of chemotherapy [12].
Statistical analysis
To investigate the relationship between baseline peripheral blood counts and fever or infection, patients were categorized based on absolute neutrophil counts (ANCs), absolute monocyte counts (AMCs), absolute lymphocyte counts (ALCs), or blast counts into roughly equally-sized groups as follows: ANC, <500/μL (corresponding to grade 4 neutropenia based on Common Terminology Criteria for Adverse Events [CTCAE] vers. 4.03; n=75), 500 - <1,500/μL (n=51), 1,500 - <5,000/μL (n=39), and ≥5,000/μL (n=40); AMC, <50/μL (n=51), 50 - <250/μL (n=44), 250 - <2,000/μL (n=53), and ≥2,000/μL (n=52); ALC, <1,200/μL (n=47), 1,200 - <2,000/μL (n=53), 2,000 - <4,000/μL (n=49), and ≥4,000/μL (n=52); and blasts: 0/μL (n=49), >0 - <1,000/μL (n=43), 1,000 - <20,000/μL (n=59) and >=20,000/μL (n=52). Baseline blood counts that appeared to have significant associations with adverse events were then analyzed as continuous variables rather than in quartiles. Patient characteristics were tabulated. The association between baseline patient characteristics, blood counts, or neutrophil decay kinetics and adverse events was assessed using Kaplan-Meier survival curves with a log-rank test (for two groups) or log-rank test for trend (for three or more groups); patients requiring salvage therapy for persistent disease were censored on the day such therapy was initiated. Cox proportional hazards models [13] were used to estimate the hazard ratio (HR) for the associations between defined groups of patients and adverse outcomes in univariate and multivariate analyses. Differences in medians were compared with the Mann-Whitney U-test. To compare differences in proportions, an unpaired t test with Welch’s correction was used. A P-value of <0.05 was considered statistically significant.
RESULTS
Patient characteristics
A total of 205 patients with newly diagnosed AML/MDS (including 4 with acute promyelocytic leukemia and 2 with biphenotypic leukemia) who underwent initial curative-intent therapy with a “7+3” (n=175) or a “7+3”-like (n=30) regimen were identified and included in our analysis; their baseline characteristics are summarized in Table 1. Of these 205 patients, only 17 received granulocyte-colony stimulating factor (G-CSF), all between days 14 and 30. After induction, 153 patients (74.6%) achieved complete remission (CR), whereas 47 (22.9%) were refractory and 5 (2.4%) died before treatment response could be assessed; forty-one patients (20.0%) received re-induction therapy before day 35 for refractory disease. The vast majority of patients was followed for 35 days following initiation of chemotherapy either in the hospital or the outpatient clinic; only 5 patients were discharged from the hospital/clinic system prior to day 35 (range: 29–34 days) and could not be followed for a full period of 35 days.
Association between baseline blood counts and adverse events
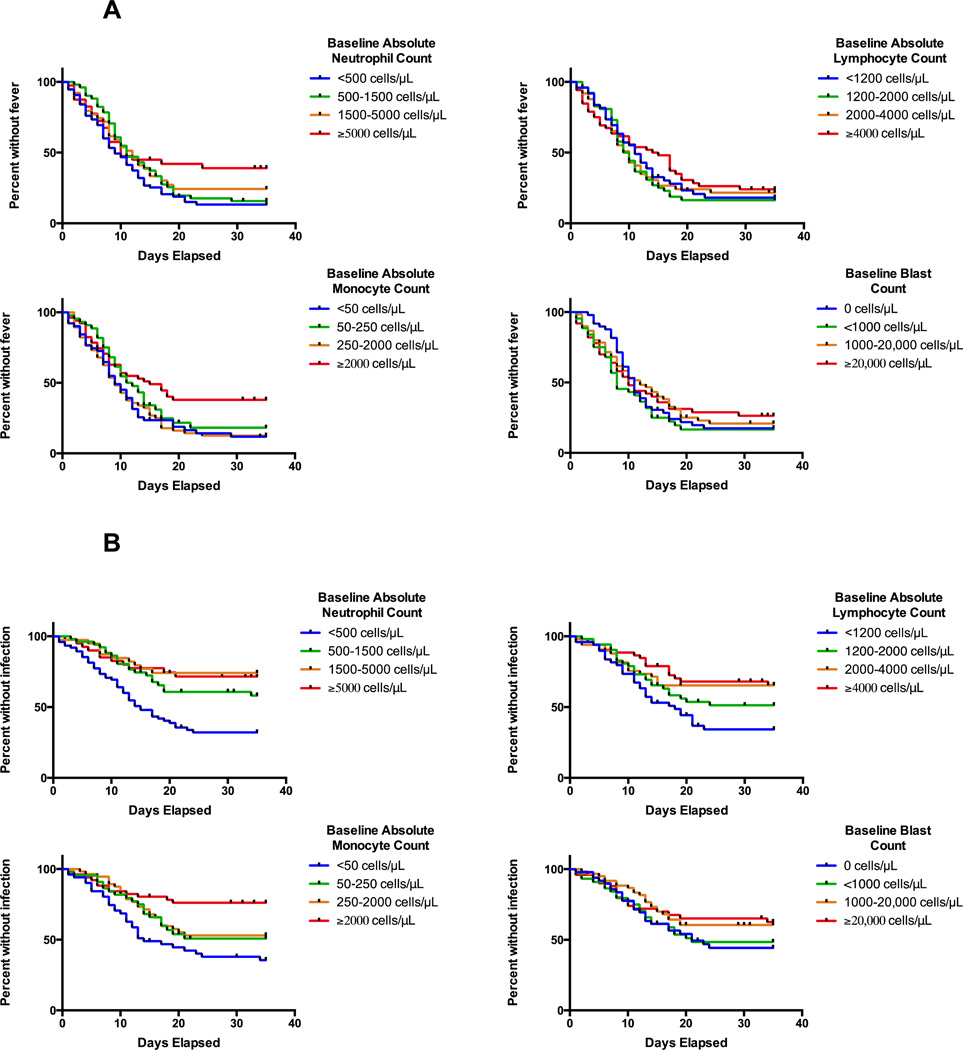
We first assessed the relationship between baseline blood counts and likelihood of developing fever or being diagnosed with a documented infection or bacteremia by dividing the study cohort in quartiles based on levels of baseline neutrophil, monocyte, lymphocyte, and peripheral blood blast count. Although fever was common in all patient subgroups, the likelihood of freedom from fever was statistically significantly lower in patients with lower baseline neutrophil (Ptrend=0.02) and monocyte (Ptrend=0.03) but not lymphocyte (Ptrend=0.45) or blast (Ptrend=0.56) counts (Figure 1A). Likewise, patients with lower baseline neutrophil (Ptrend<0.0001), monocyte (Ptrend=0.0001), and lymphocyte (Ptrend=0.0015) but not blast (Ptrend=0.08) counts were significantly more likely to develop a documented infection (Figure 1B). Similarly, patients with lower neutrophil (Ptrend=0.005), monocyte (Ptrend<0.0001), lymphocyte (Ptrend=0.004), and blast (Ptrend=0.002) counts were significantly more likely to be diagnosed with bacteremia by day 35 (Supplemental Figure 1). When analyzed as a continuous variable, the baseline neutrophil count was statistically significantly associated with fever (hazard ratio [HR]=0.97 [95% confidence interval: 0.95–0.999] for each increase of 1000 cells/µL, P=0.04) and infection (HR=0.92 [0.87–0.98], P=0.01), but not bacteremia (HR=0.95 [0.89–1.01], P=0.10) or requirement for intensive care unit (ICU) care (HR=1.00 [0.93–1.07], P=0.99). Of note, patients presenting with grade 4 neutropenia were more likely to develop fever (P=0.04), documented infection (P<0.0001), bacteremia (P=0.002), and death within 35 days of chemotherapy (P=0.04; Supplemental Figure 2). After exclusion of the 30 patients who received “7+3”-like induction rather than standard “7+3”, baseline grade 4 neutropenia was associated with documented infection (P<0.0001) and bacteremia (P=0.0005), but was no longer significantly associated with fever (P=0.16) or death (P=0.23). To investigate whether changes in supportive care over the 10-year study period resulted in differences in adverse events in later years, we determined the rates of infection in earlier versus later years and found similar results for the period of 2002–2007 vs. 2008–2012 (P=0.72). Furthermore, in the subset of patients treated in 2008–2012, lower baseline neutrophil count was associated with an increased risk of fever (P=0.03) and infection (P<0.0001) using the same 4-bin analysis used above, and baseline grade 4 neutropenia remained significantly associated with increased risk of early mortality (P=0.007), similar to the findings obtained in the entire study cohort (Supplemental Figure 3).
Figure 1. Association between baseline blood counts and development of fever or documented infection.
Kaplan-Meier estimates of the freedom from fever (A) and freedom from documented infection (B) in our cohort, stratified by quartiles of baseline neutrophil, monocyte, lymphocyte, and peripheral blood blast counts, from the first day of induction chemotherapy until day 35; patients who received salvage chemotherapy were censored on the first day of initiation of such therapy.
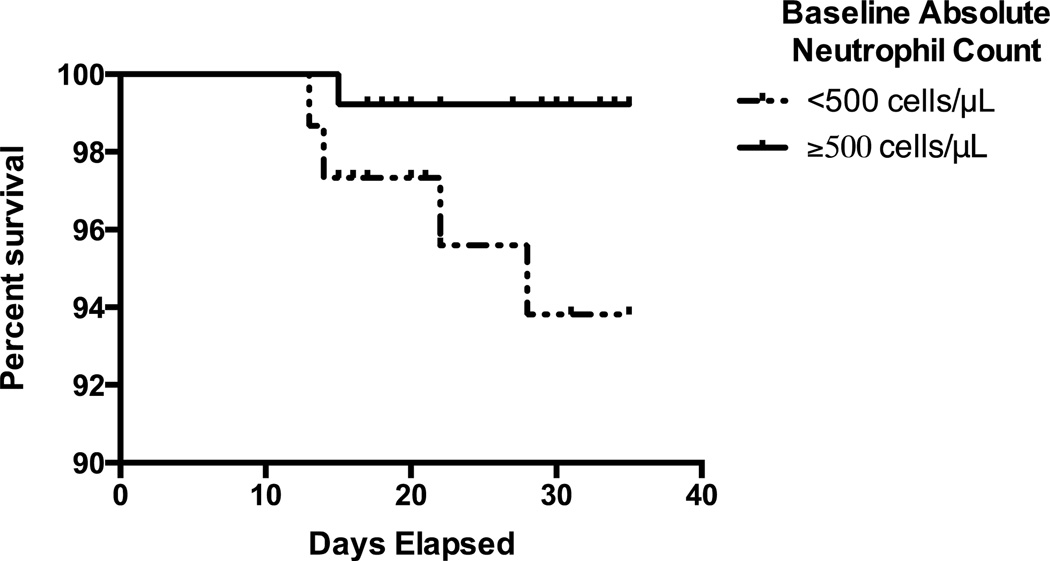
As requirement for ICU-level care or death within 35 days of therapy initiation were uncommon outcomes, we dichotomized patients based on baseline blood counts to study the relationship between pre-treatment peripheral blood counts and these outcomes. As shown in Figure 2, patients presenting with grade 4 neutropenia were more likely to experience death within 35 days of therapy initiation than those higher neutrophil counts (P=0.04). In contrast, baseline monocyte (≤800/μL vs. >800/μL), lymphocyte (≤4,800/μL vs. >4,800/μL), and peripheral blood blast (<1000/μL vs. ≥1000/μL) counts were not associated with death (all P>0.41; Supplemental Figure 4). Using identical cut-offs, we found no statistically significant difference in the requirement for ICU treatment between patients with lower and those with higher baseline blood counts (all P>0.49; Supplemental Figure 5). Interestingly, there were no statistically significant differences in the estimates for freedom from fever, documented infection, bacteremia, requirement of ICU care, or death when we assessed the following potential risk factors: age (<50 years vs. 50–65 years vs. ≥65 years; all Ptrend>0.30), gender (all P>0.21), type of disease (primary vs. secondary; all P>0.10), or type (all P>0.13) or dose (all P>0.06) of anthracycline used for induction. There was, however, a statistically significant correlation between cytogenetic risk (favorable vs. intermediate vs. adverse) and fever (P=0.02), infection (P=0.02), and bacteremia (P=0.03), with adverse risk being associated with adverse outcomes, whereas no such association was found for requirement for ICU care (P=0.67) or death (P=0.59).
Figure 2. Association between baseline neutropenia and early mortality.
Kaplan-Meier estimates of overall survival in our study cohort for patients presenting with grade 4 neutropenia at baseline (i.e. ANC <500/μL) and those with higher initial neutrophil counts from the first day of curative-intent chemotherapy until day 35; patients who received salvage chemotherapy were censored on the first day of initiation of such therapy.
Baseline grade 4 neutropenia as independent prognostic factor
These initial studies indicated that patients with baseline grade 4 neutropenia were more likely to experience bloodstream infections or death than those presenting with higher neutrophil counts in the first 35 days after initiation of chemotherapy. We therefore evaluated whether baseline grade 4 neutropenia was associated with the development of fever, bloodstream infection, or requirement for ICU level care in multivariate Cox regression models. After adjustment for age, gender, type disease, cytogenetic risk, performance status, and FLT3 as well as NPM1 mutation status, grade 4 neutropenia remained statistically significantly associated with development of fever (HR=1.87 [1.04–3.34]; P=0.04) and infection (HR=4.95 [2.2–11.16]; P<0.001), and almost reached statistical significance for the association with bacteremia (HR=3.14 [0.99–9.98]; P=0.05). In contrast, there was no statistically significant association between grade 4 neutropenia and requirement for ICU level care after multivariate adjustment (HR=1.83 [0.64–5.28]; P=0.26).
Association between neutrophil count and duration of neutropenia
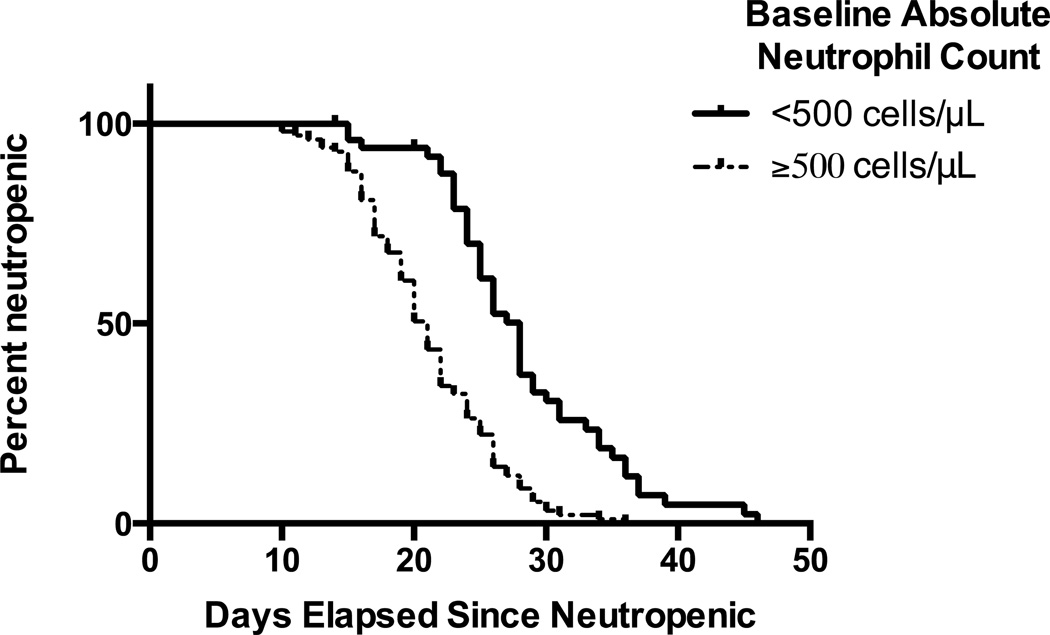
As grade 4 neutropenia was independently associated with a higher risk of infection and bacteremia in our study population, we explored whether baseline neutrophil counts might correlate with duration of neutropenia, thereby predisposing to infectious complications. In order to reduce bias from neutropenia due to persistent leukemia, we restricted this analysis to the 153 patients who achieved CR after a single course of induction therapy. Within this cohort, the median duration of grade 4 neutropenia, measured from day 1 of induction chemotherapy, was 23 (inter-quartile range [IQR]: 19–28) days. After censoring the patients who received G-CSF, the 44 patients that presented with grade 4 neutropenia had a significantly longer duration of severe neutropenia than the 92 patients that had higher neutrophil counts at baseline (P<0.0001; Figure 3). Of course, one could argue that the prolonged duration of grade 4 neutropenia was accounted for by the fact that these patients had an ANC <500/μL from the initiation of chemotherapy. To assess this possibility, we analyzed the time interval between initiation of chemotherapy and neutrophil recovery and found that this interval was longer for patients presenting with ANC <500/μL at baseline than those presenting with higher neutrophil counts (P=0.002), indicating that patients with low ANC at baseline had a slower neutrophil recovery. One might also suppose that a longer duration of neutropenia could be related to other patient factors but there was no difference in neutropenia duration between patients with primary vs. secondary disease (P=0.09), or between patients with different cytogenetic disease risk (P=0.13).
Figure 3. Association between baseline neutropenia and duration of neutropenia.
Patients with initial grade 4 neutropenia have a significantly longer duration of neutropenia after censoring for patients receiving G-CSF.
Association between neutrophil kinetics and duration of neutropenia
Reasoning that the dynamics of neutrophil counts after initiation of chemotherapy could serve as a surrogate for the sensitivity of normal myelopoiesis to the effect of chemotherapy, we also investigated the relationship between neutrophil kinetics and duration of grade 4 neutropenia. For these studies, sequential neutrophil counts, obtained for 4–5 days following the start of neutrophil decline, were used for the determination of neutrophil kinetics in the 91 patients with available data who achieved CR after a single course of induction chemotherapy and did not receive G-CSF. Modeling with an exponential decay curve [12] resulted in excellent goodness of fit (mean r2=0.954). Yet, in this subset, we did not find a significant relationship between the duration of neutropenia and rate of neutrophil decay (<1 (n=61) vs. ≥1 (n=30), median 22 [IQR: 17–26] vs. 20 [IQR: 17–25.25] days, P=0.70).
Rationale for future studies on prophylactic use of granulocyte colony stimulating factor
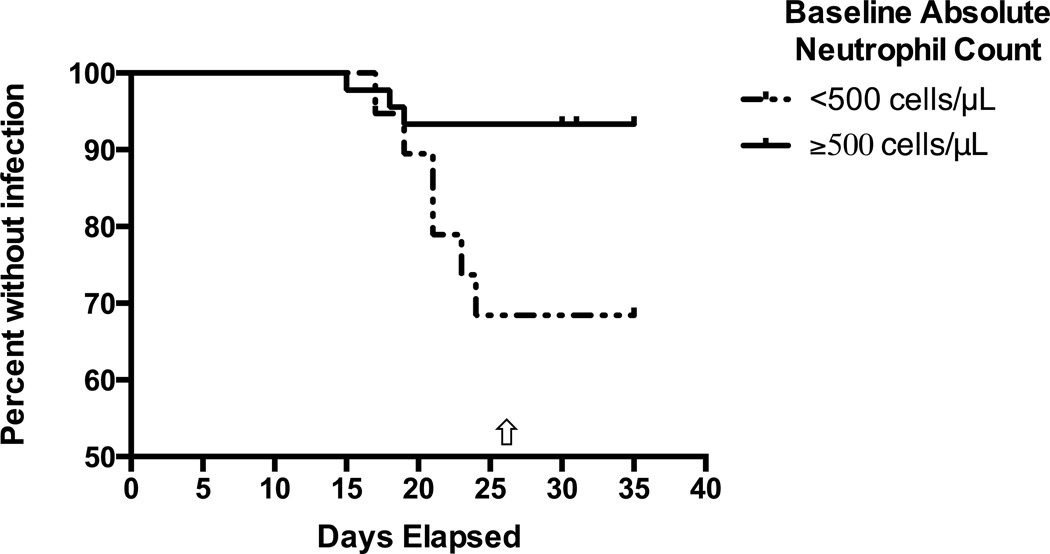
Our results presented thus far indicated that baseline grade 4 neutropenia was associated with both duration of chemotherapy-induced neutropenia as well as treatment-related adverse events. Based on these findings, we hypothesized that patients presenting with grade 4 neutropenia could encompass a patient subset in which targeted use of such growth factors could be beneficial in order to shorten the duration of neutropenia and thus decrease the risk of adverse events. As the use of myeloid growth factors in patients with active AML remains controversial and many physicians consider their use only after documentation of effective cytoreduction at the time of early disease assessment (“day 14 marrow”) we investigated whether baseline grade 4 neutropenia was also associated with increased risk of late adverse events occurring after day 14 (“late”) in patients with no residual disease in the bone marrow around day 14. Indeed, in this subgroup, initial grade 4 neutropenia was associated with a higher risk of adverse outcomes on or after day 14, including infection (P=0.01) and bacteremia (P=0.002) but not fever (P=0.11); ICU stay and death were outcomes too rare in this subgroup to study and could not be assessed conclusively. As shown in Figure 4, patients with initial grade 4 neutropenia and leukemia-free day 14 bone marrow studies tended to develop neutropenia-associated infections between days 15 and 25. It is therefore tempting to speculate that prophylactic use of myeloid growth factors on day 14 in these patients could reduce both duration of neutropenia and risk of infection. While it might also be tempting to draw conclusions from the fates of the 17 patients in our cohort who received G-CSF (all between days 14 and 30), this could not be done as in all but 2 cases, the G-CSF was administered after patients had already developed fever and/or infection, i.e. the use of G-CSF in our cohort was not “prophylactic”.
Figure 4. Estimates of late infection, stratified by baseline ANC.
In patients with a day 14 bone marrow biopsy showing no evidence of residual disease by flow cytometry, initial ANC is associated with increased risk of infection after day 14. The median day of recovery from grade 4 neutropenia was 26 in both groups (arrow).
DISCUSSION
Despite continuous improvements in supportive care over the last several years [14], it is a daily clinical observation that patients with newly diagnosed AML/MDS undergoing intensive chemotherapy remain at high risk of experiencing early life-threatening or fatal adverse events related to severe cytopenias. The data presented in this article indicate that severe cytopenias, and grade 4 neutropenia in particular, at baseline before initiation of chemotherapy can identify a subset of patients at especially high risk for adverse outcomes. This finding may not only be useful for informing patients in a risk-stratified manner about the likelihood of experiencing these adverse events but, perhaps, may provide the rationale for targeted interventions with myeloid growth factors to hasten blood count recovery in these high-risk individuals.
Our observation that low baseline blood counts are associated with adverse infection-related outcomes is not unprecedented. Previous studies have indicated that a pretreatment ANC <1,000/µL is associated with an increased risk of febrile neutropenia in patients with aggressive non-Hodgkin lymphomas [15]. Moreover, Ray-Coquard et al. reported that lymphocyte counts <700/µL before initiation of chemotherapy are correlated with an increased risk of febrile neutropenia in patients with various types of cancer [6]. Perhaps not surprisingly, our studies highlight baseline severe neutropenia as a pivotal, independent risk factor for adverse outcomes in patients undergoing curative-intent chemotherapy for newly diagnosed AML/MDS. Specifically, we found in our study cohort that grade 4 neutropenia was associated with the development of documented infection, bacteremia, delayed neutrophil count recovery, and death within 35 days of chemotherapy, but not the requirement for ICU-level care; however, the latter was a relatively uncommon occurrence in our cohort, and larger patient populations may be required to examine such an association in more detail.
Importantly, our multivariate models indicate that grade 4 neutropenia is an independent risk factor for the development of infection-related events in that, after adjustment for age, gender, performance status, disease type, and cytogenetic/molecular risk, the risk of documented infection or bacteremia was statistically significantly more than 2–2.5 fold increased for patients presenting with grade 4 neutropenia. On the other hand, we found no significant association between the occurrence of adverse events and several other baseline patient- or disease-related factors, including age, gender, disease type, and type as well as dose of the anthracycline used. As one limitation of these multivariate analyses, we did not have information on comorbidity scores available, and performance status may be a surrogate that does not capture the degree of comorbidities in their entirety.
We considered several explanations for the relationship between baseline neutropenia and increased risk of these adverse events. First, baseline neutropenia could serve as surrogate for heavy disease burden at the time of diagnosis; yet, other than an increased risk of bacteremia, we found no correlation between initial bone marrow blast percentage and any of the other assessed outcomes, arguing against this possibility. Second, we considered the possibility that baseline grade 4 neutropenia was a surrogate for a more chemotherapy-resistant disease that is associated with a higher risk of adverse events; however, multivariate Cox models indicated that the risk associated with grade 4 neutropenia was independent of classic disease-risk factors such as cytogenetic abnormalities and secondary disease, arguing against this possibility. Moreover, this association persisted after exclusion of patients with MDS (data not shown) and was also seen in the subset of patients who achieved CR after the first course of induction chemotherapy, arguing against this possibility. And third, we considered the possibility that myelosuppressive effects of infection could lead to both baseline and prolonged neutropenia, but patients who were initially neutropenic were diagnosed with an infection a median of 11 days after the start of chemotherapy, and only 6 of the 51 patients were diagnosed within the first three days, rendering undiagnosed infection an unlikely explanation. It is conceivable that immune-mediated effects from the malignant clone could impact both the degree of neutropenia and, consequently, the patient’s susceptibility to infection-related adverse events, but further studies would be necessary to test such an idea.
It is interesting to speculate about the practical implications of our finding beyond its use for risk prediction. Importantly, besides being associated with overall risk for documented infection and bacteremia, we found grade 4 neutropenia to be associated with delayed neutrophil recovery and “late” occurrence of these events in patients who achieved a CR with initial chemotherapy, with “late” being defined as occurrence on or after day 14, i.e. the time an early treatment response can be established. This observation might provide a window of opportunity for the targeted use of myeloid growth factors such as G-CSF in this high-risk patient subset after documentation of effective cytoreduction in the early disease assessment that is typically done around day 14. While it is widely appreciated that previous randomized studies provided no evidence for the prophylactic use such agents for the prevention of infectious complications in unselected AML patients [16], it is conceivable that patients at particularly high risk may derive a benefit from growth factor support. It is therefore tempting to speculate that patients presenting with grade 4 neutropenia, whom our studies identify as being a subset of patients at high risk for infectious complications, could benefit from myeloid growth factor support. Our findings may therefore provide a scientific rationale for further investigations of risk-stratified preemptive treatment strategy that, ultimately, could test this hypothesis.
Together, our studies identify severe baseline neutropenia as a risk factor for infection-associated adverse events and early death after induction chemotherapy. As our investigations were of retrospective nature and included consecutive patients treated at a single academic center, future studies in different cohorts will be required to validate our results. If validated, they may provide the rationale for the risk-adapted testing of myeloid growth factor support in this high-risk AML/MDS patient subset.
Supplementary Material
ACKNOWLEDGEMENTS
Financial support: Supported by a grant from the National Cancer Institute/National Institutes of Health (P30-CA015704-35S6 to R.B.W.). S.A.B. is the recipient of a Trainee Research Award and a “Hematology Opportunity for the Next-Generation of Research Scientists” (HONORS) Award from the American Society of Hematology.
Footnotes
AUTHORSHIP AND CONFLICT OF INTEREST
Authorship statement: S.A.B. and R.B.W. designed and performed research, analyzed and interpreted data and wrote the manuscript. M.O. performed statistical analyses, interpreted data, and revised the manuscript. V.V., J.L.A., and E.H.E. interpreted data and revised the manuscript.
Conflict of interest: V.V. is a clinical advisor for Neuromedicines Inc., Pasadena, CA. The other authors declare no competing financial interests.
REFERENCES
- 1.Bodey GP, Buckley M, Sathe YS, et al. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64:328–340. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- 2.Bodey GP, Rodriguez V, Chang HY, et al. Fever and infection in leukemic patients: a study of 494 consecutive patients. Cancer. 1978;41:1610–1622. doi: 10.1002/1097-0142(197804)41:4<1610::aid-cncr2820410452>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Gaydos LA, Freireich EJ, Mantel N. The quantitative relation between platelet count and hemorrhage in patients with acute leukemia. N Engl J Med. 1962;266:905–909. doi: 10.1056/NEJM196205032661802. [DOI] [PubMed] [Google Scholar]
- 4.Freireich EJ. Supportive care for patients with blood disorders. Br J Haematol. 2000;111:68–77. doi: 10.1046/j.1365-2141.2000.02144.x. [DOI] [PubMed] [Google Scholar]
- 5.Blay JY, Chauvin F, Le Cesne A, et al. Early lymphopenia after cytotoxic chemotherapy as a risk factor for febrile neutropenia. J Clin Oncol. 1996;14:636–643. doi: 10.1200/JCO.1996.14.2.636. [DOI] [PubMed] [Google Scholar]
- 6.Ray-Coquard I, Borg C, Bachelot T, et al. Baseline and early lymphopenia predict for the risk of febrile neutropenia after chemotherapy. Br J Cancer. 2003;88:181–186. doi: 10.1038/sj.bjc.6600724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 8.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 9.Döhner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 10.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52:e56–e93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 11.From the Immunocompromised Host Society. The design, analysis, and reporting of clinical trials on the empirical antibiotic management of the neutropenic patient. Report of a consensus panel. J Infect Dis. 1990;161:397–401. doi: 10.1093/infdis/161.3.397. [DOI] [PubMed] [Google Scholar]
- 12.Vainstein V, Buckley SA, Shukron O, et al. Rapid rate of peripheral blood blast clearance accurately predicts complete remission in acute myeloid leukemia. Leukemia. 2013 doi: 10.1038/leu.2013.341. Epub ahead of print November 18, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Cox DR. Regression models and life tables (with discussion) . J R Statist Soc. 1972;34(B):187–220. [Google Scholar]
- 14.Othus M, Kantarjian H, Petersdorf S, et al. Declining rates of treatment-related mortality in patients with newly diagnosed AML given 'intense' induction regimens: a report from SWOG and MD Anderson. Leukemia. 2013 doi: 10.1038/leu.2013.176. Epub ahead of print June 13, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyman GH, Dale DC, Friedberg J, et al. Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin's lymphoma: a nationwide study. J Clin Oncol. 2004;22:4302–4311. doi: 10.1200/JCO.2004.03.213. [DOI] [PubMed] [Google Scholar]
- 16.Gurion R, Belnik-Plitman Y, Gafter-Gvili A, et al. Colony-stimulating factors for prevention and treatment of infectious complications in patients with acute myelogenous leukemia. Cochrane Database Syst Rev. 2012;6:CD008238. doi: 10.1002/14651858.CD008238.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.