Abstract
Vitamin D is an important nutrient involved in bone mineral metabolism, and vitamin D status is reflected by serum total 25-hydroxyvitamin D (25[OH]D) concentrations. Vitamin D deficiency is highly prevalent in patients with chronic kidney disease (CKD), and nutritional vitamin D supplementation decreases elevated parathyroid hormone concentrations in subgroups of these patients. Furthermore, vitamin D is supposed to have pleiotropic effects on various diseases such as cardiovascular diseases, malignancies, infectious diseases, diabetes, and autoimmune diseases. Indeed, there is cumulative evidence showing the associations of low vitamin D with the development and progression of CKD, cardiovascular complication, and high mortality. Recently, genetic polymorphisms in vitamin D-binding protein have received great attention because they largely affect bioavailable 25(OH)D concentrations. This finding suggests that the serum total 25(OH)D concentrations would not be comparable among different gene polymorphisms and thus may be inappropriate as an index of vitamin D status. This finding may refute the conventional definition of vitamin D status based solely on serum total 25(OH)D concentrations.
1. Introduction
Vitamin D is a fat soluble secosteroid that interacts with a specific nuclear receptor similar to other steroid hormones and plays a central role in calcium and phosphate homeostasis and musculoskeletal health [1]. The past decade has brought an increasing awareness of the effects of vitamin D on several other organ systems. Ecological and observational studies have demonstrated that vitamin D deficiency, defined as a low serum total 25-hydroxyvitamin D (25[OH]D) concentration, is associated with increased risks of death and diseases such as various cardiovascular diseases, malignancies, infectious diseases, diabetes, autoimmune diseases, and kidney diseases [2]. However, more than a billion people worldwide are thought to have vitamin D deficiency or insufficiency.
Chronic kidney disease (CKD) has been identified as a risk factor for vitamin D deficiency. Indeed, the prevalence of vitamin D deficiency or insufficiency is high among patients with CKD, especially patients with end-stage renal disease and kidney transplant recipients [3, 4]. Similar to the general population, vitamin D deficiency in these patients is associated with elevated concentrations of parathyroid hormone and bone turnover markers as well as low bone mineral density [5–7]. Accumulating evidence indicates the associations of vitamin D deficiency with morbidities and mortality in patients with CKD.
In this review, we summarize the previous findings in the epidemiological and interventional studies of vitamin D in patients with CKD. The importance and implications of a genetic polymorphism of vitamin D-binding protein, which potentially overturn the current concept of vitamin D status, are also discussed.
2. Vitamin D Metabolism
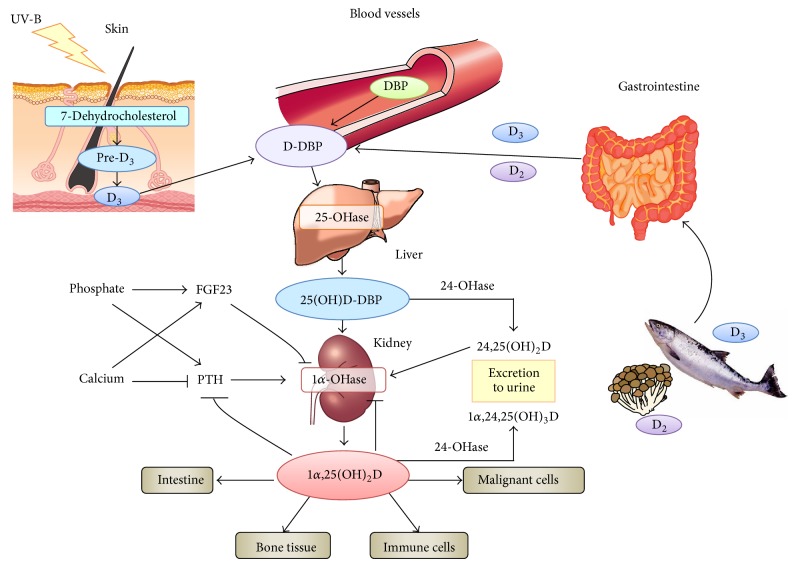
Vitamin D is synthesized via skin exposure to ultraviolet irradiation or oral intake and binds to vitamin D-binding protein (DBP) in the blood. After being converted into 25(OH)D by 25-hydroxylase CYP2R1 of the liver in a relatively unregulated manner, it forms stable 25(OH)D-DBP complex which has a long circulating half-life of 480 hours (Figure 1, Table 1). This complex is excreted into urine and reabsorbed via megalin at renal proximal tubule cells and then converted to the active form of vitamin D, 1,25-hydroxyvitamin D (1,25(OH)2D), by 1α-hydroxylase CYP27B1. Compared to 25(OH)D, 1,25(OH)2D has a shorter half-life because of its lower affinity for DBP, and 1α-hydroxylase is also regulated by various factors including parathyroid hormone (PTH) and fibroblast growth factor-23 (FGF23). Accordingly, serum total 25(OH)D concentration, rather than the 1,25(OH)2D concentration, is considered as an indicator of vitamin D sufficiency.
Figure 1.
Vitamin D metabolism. DBP: vitamin D-binding protein; PTH: parathyroid hormone; FGF23: fibroblast growth factor 23.
Table 1.
The difference in characteristics of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D.
| Affinity to vitamin D receptor | Serum total concentration | Half-life | Risk of hypercalcemia | |
|---|---|---|---|---|
| 25(OH)D | (1)∗ | 9.0–34.0 ng/mL (500)∗ |
480 hrs | Low |
|
| ||||
| 1,25(OH)2D | (100–200)∗ | 20–60 pg/mL (1)∗ |
15 hrs | High |
∗Relative value.
Conventionally, 25(OH)D has been considered merely a precursor before activation; however, serum total 25(OH)D concentration is also suggested to have clinical significance. First, 25(OH)D has a binding capacity, albeit weak, for vitamin D receptors (VDR), and has hundred times higher blood concentration than 1,25(OH)2D (Table 1). Additionally, various extrarenal cells express megalin and cubilin as well as 1α-hydroxylase [8, 9]. Thus, 25(OH)D is taken up into these cells via megalin and cubilin and exerts autocrine or paracrine effects after being locally converted into the active form 1,25(OH)2D [10, 11]. Indeed, a number of studies have reported elevated PTH concentrations, low bone mineral density, high bone turnover, and high prevalence of fracture in population with low serum 25(OH)D concentrations even if serum calcium and 1,25(OH)2D concentrations were within the normal range [12, 13].
It should be noted here that extrarenal 1α-hydroxylase is regulated in different ways from that in renal tubular cells. For example, contrary to its effect in renal proximal tubular cells, FGF23 increases 1α-hydroxylase expression in parathyroid cells [14]. Also, PTH and calcium do not affect the expression and activity of 1α-hydroxylase in osteoblasts while interleukin-1β, a NF-κB activator, increases its expression [15].
3. Vitamin D Status in CKD
FGF23 secretion from bone cells is enhanced at a relatively early stage of CKD to compensate for the phosphorus retention associated with a decrease in the number of nephrons. It inhibits the renal expression of 1α-hydroxylase resulting in the decrease in serum 1,25(OH)2D concentrations, followed by secondary hyperparathyroidism. Several studies demonstrated that FGF23 concentrations actually increase before PTH concentrations [16, 17]. Meanwhile, serum total 25(OH)D concentrations did not decrease until stage 5 CKD, while vitamin D deficiency was highly prevalent over all.
The risk factors of vitamin D deficiency include severely impaired renal function, hypoalbuminemia, urine protein/urine albumin concentrations, and diabetes [18–22]. Patient's nutritional status and inflammation influence the production of DBP in the liver, similar to that of albumin. 25(OH)D-DBP complex is excreted into urine in patients with overt proteinuria, and tubular damage in diabetic patients with CKD decreases megalin expression in the epithelial cells and thus causes decreased reabsorption of urinary 25(OH)D-DBP complex [23]. Actually, the increase in serum total 25(OH)D concentrations by nutritional vitamin D supplementation increases is diminished in patients with vitamin D deficiency and overt proteinuria [24].
4. Current Clinical Practice Guidelines for Vitamin D Status in Patients with CKD
According to the Kidney Disease: Improving Global Outcomes (K/DIGO) guidelines, although there is only limited evidence, nutritional vitamin D supplementation is suggested when predialysis patients with CKD were vitamin D deficient or insufficient, adjusting serum total 25(OH)D to the recommended concentration for the general population [25]. Nevertheless, the definition of insufficiency and deficiency varies among researchers and organizations. The Institute of Medicine proposed 20 ng/mL or more of serum total 25(OH)D as an indicator of sufficiency [26], while many other views support the conventional concentration of 30 ng/mL as stated in the 2003 Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines [27]. However, the largest problem is that neither value has been sufficiently evaluated with respect to the association with clinical outcomes in patients with CKD. For end-stage renal disease patients on dialysis, calcitriol and active vitamin D analogues are recommended to manage secondary hyperparathyroidism if necessary, but there is no clear statement regarding optimal serum total 25(OH)D concentration or nutritional vitamin D supplementation.
5. Association between the Vitamin D Status and Clinical Outcomes in CKD
As mentioned above, vitamin D is a fat soluble secosteroid that interacts with a specific nuclear receptor VDR and exerts nonclassical effects on various target organs. Nevertheless, studies have consistently shown that vitamin D deficiency is highly prevalent among patients with CKD and strongly associated with various clinical outcomes. For example, serum total 25(OH)D concentrations showed significant relationships to renal outcomes such as doubling of serum creatinine or ESRD, independent of conventional risk factors in predialysis patients with CKD [28–30]. Serum total 25(OH)D concentrations were also associated with anemia and muscle weakness independent of 1,25(OH)2D [31, 32]. Furthermore, among patients with wide range of renal dysfunctions including ESRD, vitamin D deficiency showed the associations with vascular calcification, vascular endothelial function, cardiovascular events, and cardiovascular mortality [33–37]. A meta-analysis of these observational studies revealed an inverse association between all-cause mortality and serum total 25(OH)D concentrations [38].
In a prospective observational study of renal transplant recipients, Obi et al. identified serum total 25(OH)D concentrations as an independent predictor of annual allograft function decline [39]. Vitamin D deficiency also showed a similar relationship to the incidence of allograft rejection using intravenous methylprednisolone administration as an index. These associations were more pronounced in patients with shorter intervals after transplantation but not observed at ≥10 years after transplantation. Bienaimé et al. also reported that patients with lower serum total 25(OH)D concentrations at 3 months after transplantation exhibited lower kidney allograft function at 1 year after transplantation and had higher risk of the progression of interstitial fibrosis and tubular atrophy [40]. No association with allograft rejection was found in this study, but other cohort studies showed significant associations with cellular rejection [41, 42], consistent with the findings by Obi et al.
Those nonclassical associations of vitamin D found in observational studies have been supported by a large body of basic biological science. Supposed mechanisms of the organ-protective effects of vitamin D are as follows: the inhibition of the renin-angiotensin system and NF-κB pathway [43], direct upregulation of the nitric oxide synthase transcription in vascular endothelial cells [44], and the activation of the antioxidative Keap1-Nrf2 pathway [45]. The association of vitamin D with allograft rejection in renal transplant recipients described above might be explained by the induction of regulatory T cells and the inhibition of activated T cells and B cells [36].
6. Interaction between the Vitamin D Status and FGF23 in Clinical Outcomes of CKD
There is a rapidly increasing number of evidences demonstrating that FGF23 is associated with mortality, the progression of CKD, and the development of cardiovascular events in patients with CKD [46]. This phosphaturic hormone secreted by bones regulates 1α-hydroxylase and thus confounds with calcium, phosphorus, PTH, 25(OH)D, and 1,25(OH)2D. Nevertheless, few studies have evaluated all of these factors simultaneously. Nakano et al. examined each independent association using a prospective cohort study of predialysis patients with CKD and identified serum concentrations of total 25(OH)D and intact FGF23, but not 1,25(OH)2D, as significant predictors of renal composite outcome of doubling serum creatinine and ESRD [17]. Furthermore, Hamano et al. found a nonlinear association between serum total 25(OH)D concentrations and annual kidney function decline in the same cohort; FGF23 showed a linear negative association at <23 ng/mL of total 25(OH)D while it leveled off above that concentration [47]. Meanwhile, although both low serum total 25(OH)D and high intact FGF23 concentrations were associated with cardiovascular events including heart failure in the univariate analysis, only intact FGF23 remained significant in a multivariate analysis [48]. Similarly, in an Italian study of general elderly individuals, people with higher FGF-23 concentrations and lower serum 25(OH)D concentrations had greater left ventricular hypertrophy and higher mortality while high FGF-23 concentrations but not low serum total 25(OH)D concentrations were independently associated with mortality after adjustment for other risk factors [49].
However, the extent to which FGF23 increases along with the progression of CKD could be affected by vitamin D status. The Renal Risk in Derby (RRID) study in the United Kingdom reported that intact FGF23 increased prior to PTH in patients with vitamin D sufficiency (≥20 ng/mL of serum total 25[OH]D), consistent with the previous findings [50]. On the other hand, PTH concentrations increased earlier and were relatively higher than FGF23 in patients with <20 ng/mL of serum total 25(OH)D. These results could be explained by the inhibitory effect of vitamin D against parathyroid gland and the mutual compensation of PTH and FGF23 for their phosphaturic effects. Based on these results, vitamin D status would become more important than before if FGF23 measurement becomes clinically available because FGF23 concentrations should be interpreted with caution taking both kidney function and serum total 25(OH)D concentrations into account. Further studies are still necessary to make these complicated biomarkers practically useful in clinical settings.
7. Nutritional Vitamin D Supplementation and CKD
Nutritional vitamin D (cholecalciferol and ergocalciferol) is widely available as an over-the-counter supplement in most countries. Notably, nutritional vitamin D is unlikely to induce hypercalcemia unless given continuously at a high dose because its 1α-hydroxylase-mediated activation process is regulated by many factors such as PTH, FGF23, and 24-hydroxylase (Figure 1). This is in contrast to the effects of calcitriol and active vitamin D analogues that directly increase calcium absorption and reabsorption in the intestine and kidney tubular cells, respectively. A total serum 25(OH)D concentration of <100 mg/mL is generally considered safe [1], and the United States Institute of Medicine has suggested a tolerable upper oral intake level of 4,000 IU/day [26]. Another advantage of nutritional vitamin D is that it forms a complex with DBP after being converted into 25(OH)D, which yields a long half-life (480 hours, Table 1). These characteristics allow the prescription of nutritional vitamin D at doses equivalent to weeks or months.
Patients with CKD are considered at a low risk for nutritional vitamin D-induced hypercalcemia CKD because of elevated FGF23 concentrations and tubular cell injuries. Indeed, several studies, including randomized controlled trials, a systematic review, and a metabolic balance study, have shown that although nutritional vitamin D supplementation does not significantly affect serum calcium and phosphate concentrations, it increases serum 1,25(OH)2D concentrations and decreases plasma PTH concentrations [51–54]. Furthermore, most studies have reported no effects of nutritional vitamin D supplementation on the concentrations of FGF23 [55–59]. Given the supposed pleiotropic effects, the safety profile, and the low cost of nutritional vitamin D, the prevalence of vitamin D supplementation has rapidly increased in the United States during the past decade (from 10% in 2003 to 44% in 2011) [60]. This may explain the reason why vitamin D status was not associated with long-term clinical outcomes including cardiovascular disease, ESRD, and death in the HOST (Homocysteinemia in Kidney and End Stage Renal Disease) study [61].
Interestingly, a randomized controlled trial showed that cholecalciferol prevented hospitalization for bone fractures or falls in ESRD patients on hemodialysis [59]. This result was consistent with those in the previous nutritional vitamin D trials that involved elderly subjects [62, 63]. These effects of vitamin D could be explained by the recent findings that skeletal muscle expresses VDR and that ligand-dependent 25-hydroxyvitamin D3 uptake is regulated and modulated by vitamin D in the primary myofibers [64]. In fact, nutritional vitamin D supplementation enhances muscle strength, especially in elderly individuals and those with vitamin D deficiency [65].
Besides its effects on musculoskeletal health, there is little concrete evidence regarding the pleiotropic effects of nutritional vitamin D. Observational studies have shown promising results such as improved endothelial function and reduced urinary albumin and TGF-β concentrations in predialysis patients [57, 66, 67], as well as decreased inflammatory cytokine concentrations and a reduced requirement for the erythropoiesis-stimulating agents used to manage anemia in patients on dialysis [68–70]. A small pilot study found that high-dose cholecalciferol decreased the concentration of monocyte chemoattractant protein-1 (MCP-1) but did not affect the blood concentrations of IL-6, TNF-α, and IL-10 [71]. Another pilot randomized controlled trial also failed to show significant beneficial effects of nutritional vitamin D on muscle strength, exercise tolerability, and health-related quality-of-life [72]. However, well-designed randomized controlled trials with adequate power have not yet been conducted.
8. Genetic Polymorphisms of the Vitamin D-Binding Protein and Bioavailable 25(OH)D
It has often been noted that the results of vitamin D trials vary inconsistently in terms of not only nonclassical pleiotropic effects on ill health such as cardiovascular diseases, but also the classical treatment effect against osteoporosis in general population. Even in meta-analyses, which are considered as the highest evidence, some articles reported beneficial effects of vitamin D on musculoskeletal health whereas others did not [73–75]. This discrepancy is supposed to be due to the difference in the baseline vitamin D status, baseline risk of events, doses and dosing periods of nutritional vitamin D supplementation, and adherence among the study subjects. In addition, VDR genetic polymorphism has also been suggested as an influence [76].
Powe et al. recently reported an interesting study regarding the association between bioavailable vitamin D concentrations and genetic polymorphism of DBP [77]. Although a large part (85–90%) of 25(OH)D in the blood binds to DBP as mentioned above, previous reports have reported that DBP-bound 25(OH)D could not exert its effects on target cells [78, 79]. Based on these reports, only free (<1% of total 25[OH]D) and albumin- or lipoprotein-bound fractions (10–15% of total 25[OH]D) are considered as biologically available. In fact, an observational study showed that bioavailable 25(OH)D, rather than total 25(OH)D, was associated with serum calcium and plasma PTH concentrations in patients on hemodialysis [80]. Furthermore, unique combinations of 2 common polymorphisms at rs7041 and rs4588 polymorphisms induce amino acid changes and produce 3 different phenotypes of DBP (Gc1F, Gc1S, and Gc2) which have different binding capacities to 25(OH)D [81, 82]. Accordingly, Powe et al. examined the genotypes and serum concentrations of DBP using African-Americans and Caucasians homozygous for the DBP variant and evaluated the association between bioavailable vitamin D and PTH concentrations. The following 3 important findings should be noted.
Regarding DBP phenotype, the frequency of Gc1F (high binding capacity) was dominant among African-Americans, whereas that of Gc1S (low binding capacity) was high among Caucasian subjects.
DBP blood concentrations were lowest in subjects homozygous for Gc1F (mean 93 μg/mL) and highest in subjects homozygous for Gc1S (mean 468 μg/mL).
Compared to Caucasians, African-Americans had lower serum total 25(OH)D concentrations over all but had the similar concentrations of bioavailable 25(OH)D within the quintiles of PTH concentrations.
These findings suggest that it may be inappropriate to determine vitamin D status of each individual by serum total 25(OH)D concentrations unless we examine the DBP phenotype (i.e., affinity). It might refute the conventional definition of vitamin D status using only serum total 25(OH)D concentrations. For example, the Multiethnic Study of Atherosclerosis (MESA) reported a significant association between serum total 25(OH)D concentrations and coronary heart disease in Caucasian subjects, but no association in African-American subjects. DBP polymorphisms potentially explain this racial difference [83] as well as the previously reported poor response to nutritional vitamin D supplementation in patients with low serum total 25(OH)D concentrations [84].
An in vitro study has also demonstrated that monocytes cultured with lower-affinity DBPs showed more potent induction of cathelicidin by 25(OH)D or 1,25(OH)2D [85]. Thus, DBP genetic polymorphisms may influence the association of serum total 25(OH)D concentrations with clinical outcomes and the effect of nutritional vitamin D supplementation. Conversely, another report found that DBP concentrations did not modify the effect of cholecalciferol on PTH [86]. These results will require further verification in additional intervention studies.
9. Conclusion
A large body of evidence concerns the beneficial effects of vitamin D on musculoskeletal health as well as various diseases. Observational studies have also shown an association of the vitamin D status with clinical outcomes in patients with CKD. However, vitamin D status is affected by various factors, including the seasonality of measurement, physical activity, nutritional and inflammatory status, diabetes, and urinary protein excretion. This raises significant concerns that those associations except for musculoskeletal health might be the result and not the cause of the patient's condition [87]. However, given the fact that the association with mortality is stronger in observational studies in which the prevalence of nutritional supplementation was low, nutritional vitamin D might have some pleiotropic effect on health [88]. Nonetheless, observational studies tend to overestimate true causal relationships.
Additionally, DBP gene polymorphism might be of particular importance in future human vitamin D studies. For example, Japanese studies have reported prevalence rates of 67%, 20%, and 13%, for the DBP polymorphisms Gc1F, Gc1S, and Gc2, respectively, suggesting heterogeneity of this gene among Asian populations [89–91]. Although the importance of vitamin D in CKD is well acknowledged, further observational and interventional studies of various races are still needed to evaluate the clinical associations of vitamin D and its related genes and to test the clinical efficacy of nutritional vitamin D supplementation.
Conflict of Interests
Takayuki Hamano has served as a consultant to Kyowa Medex, Co., Ltd. (Tokyo, Japan), which measured serum 25(OH)D concentrations in this study. Kyowa Medex, Co., Ltd., was not involved in writing the paper. None of the other authors had a potential conflict of interests.
References
- 1.Holick M. F. Medical progress: vitamin D deficiency. The New England Journal of Medicine. 2007;357(3):266–281. doi: 10.1056/nejmra070553. [DOI] [PubMed] [Google Scholar]
- 2.Plum L. A., Deluca H. F. Vitamin D, disease and therapeutic opportunities. Nature Reviews Drug Discovery. 2010;9(12):941–955. doi: 10.1038/nrd3318. [DOI] [PubMed] [Google Scholar]
- 3.González E. A., Sachdeva A., Oliver D. A., Martin K. J. Vitamin D insufficiency and deficiency in chronic kidney disease: a single center observational study. American Journal of Nephrology. 2004;24(5):503–510. doi: 10.1159/000081023. [DOI] [PubMed] [Google Scholar]
- 4.Stavroulopoulos A., Cassidy M. J. D., Porter C. J., Hosking D. J., Roe S. D. Vitamin D status in renal transplant recipients. American Journal of Transplantation. 2007;7(11):2546–2552. doi: 10.1111/j.1600-6143.2007.01978.x. [DOI] [PubMed] [Google Scholar]
- 5.Tomida K., Hamano T., Mikami S., et al. Serum 25-hydroxyvitamin D as an independent determinant of 1-84 PTH and bone mineral density in non-diabetic predialysis CKD patients. Bone. 2009;44(4):678–683. doi: 10.1016/j.bone.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Elder G. J., Mackun K. 25-hydroxyvitamin D deficiency and diabetes predict reduced BMD in patients with chronic kidney disease. Journal of Bone and Mineral Research. 2006;21(11):1778–1784. doi: 10.1359/jbmr.060803. [DOI] [PubMed] [Google Scholar]
- 7.Milinković N., Majkić-Singh N. T., Mirković D. D., Beletić A. D., Pejanović S. D., Vujanić S. T. Relation between 25(OH)-vitamin D deficiency and markers of bone formation and resorption in haemodialysis patients. Clinical Laboratory. 2009;55(9-10):333–339. [PubMed] [Google Scholar]
- 8.Christensen E. I., Birn H. Megalin and cubilin: multifunctional endocytic receptors. Nature Reviews Molecular Cell Biology. 2002;3(4):256–266. doi: 10.1038/nrm750. [DOI] [PubMed] [Google Scholar]
- 9.Townsend K., Evans K. N., Campbell M. J., Colston K. W., Adams J. S., Hewison M. Biological actions of extra-renal 25-hydroxyvitamin D-1α-hydroxylase and implications for chemoprevention and treatment. The Journal of Steroid Biochemistry and Molecular Biology. 2005;97(1-2):103–109. doi: 10.1016/j.jsbmb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi T., Tsugawa N., Okano T., et al. The binding properties, with blood proteins, and tissue distribution of 22-Oxa-1α,25-dihydroxyvitamin D3, a noncalcemic analogue of 1α,25-dihydroxyvitamin D3, in rats. Journal of Biochemistry. 1994;115(3):373–380. doi: 10.1093/oxfordjournals.jbchem.a124346. [DOI] [PubMed] [Google Scholar]
- 11.Dusso A. S., Tokumoto M. Defective renal maintenance of the vitamin D endocrine system impairs vitamin D renoprotection: a downward spiral in kidney disease. Kidney International. 2011;79(7):715–729. doi: 10.1038/ki.2010.543. [DOI] [PubMed] [Google Scholar]
- 12.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocrine Reviews. 2001;22(4):477–501. doi: 10.1210/er.22.4.477. [DOI] [PubMed] [Google Scholar]
- 13.Sahota O., Mundey M. K., San P., Godber I. M., Lawson N., Hosking D. J. The relationship between vitamin D and parathyroid hormone: calcium homeostasis, bone turnover, and bone mineral density in postmenopausal women with established osteoporosis. Bone. 2004;35(1):312–319. doi: 10.1016/j.bone.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Krajisnik T., Björklund P., Marsell R., et al. Fibroblast growth factor-23 regulates parathyroid hormone and 1α-hydroxylase expression in cultured bovine parathyroid cells. Journal of Endocrinology. 2007;195(1):125–131. doi: 10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- 15.van Driel M., Koedam M., Buurman C. J., et al. Evidence for auto/paracrine actions of vitamin D in bone: 1α-Hydroxylase expression and activity in human bone cells. The FASEB Journal. 2006;20(13):2417–2419. doi: 10.1096/fj.06-6374fje. [DOI] [PubMed] [Google Scholar]
- 16.Isakova T., Wahl P., Vargas G. S., et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney International. 2011;79(12):1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakano C., Hamano T., Fujii N., et al. Combined use of vitamin D status and FGF23 for risk stratification of renal outcome. Clinical Journal of the American Society of Nephrology. 2012;7(5):810–819. doi: 10.2215/CJN.08680811. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka H., Hamano T., Fujii N., et al. The impact of diabetes mellitus on vitamin D metabolism in predialysis patients. Bone. 2009;45(5):949–955. doi: 10.1016/j.bone.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Ishimura E., Nishizawa Y., Inaba M., et al. Serum levels of 1,25-dihydroxyvitamin D, 24,25-dihydroxyvitamin D, and 25-hydroxyvitamin D in nondialyzed patients with chronic renal failure. Kidney International. 1999;55(3):1019–1027. doi: 10.1046/j.1523-1755.1999.0550031019.x. [DOI] [PubMed] [Google Scholar]
- 20.Chonchol M., Scragg R. 25-Hydroxyvitamin D, insulin resistance, and kidney function in the Third National Health and Nutrition Examination Survey. Kidney International. 2007;71(2):134–139. doi: 10.1038/sj.ki.5002002. [DOI] [PubMed] [Google Scholar]
- 21.de Boer I. H., Ioannou G. N., Kestenbaum B., Brunzell J. D., Weiss N. S. 25-hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III) American Journal of Kidney Diseases. 2007;50(1):69–77. doi: 10.1053/j.ajkd.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Hamano T., Fujii N., Matsui I., et al. Guideline-practice gap in the management of predialysis chronic kidney disease mineral bone disorder in Japan. Therapeutic Apheresis and Dialysis. 2011;15(1):2–8. doi: 10.1111/j.1744-9987.2011.00918.x. [DOI] [PubMed] [Google Scholar]
- 23.Thrailkill K. M., Jo C.-H., Cockrell G. E., Moreau C. S., Fowlkes J. L. Enhanced excretion of vitamin D binding protein in type 1 diabetes: A role in vitamin D deficiency? Journal of Clinical Endocrinology and Metabolism. 2011;96(1):142–149. doi: 10.1210/jc.2010-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim S. M., Choi H. J., Lee J. P., et al. Prevalence of vitamin D deficiency and effects of supplementation with cholecalciferol in patients with chronic kidney disease. Journal of Renal Nutrition. 2014;24(1):20–25. doi: 10.1053/j.jrn.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney International Supplements. 2009;113:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 26.Ross A. C., Taylor C. L., Yaktine A. L., del Valle H. B., editors. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC, USA: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 27.National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. American Journal of Kidney Diseases. 2003;42(4, supplement 3):S1–S201. [PubMed] [Google Scholar]
- 28.Fernández-Juárez G., Luño J., Barrio V., et al. 25 (OH) vitamin D levels and renal disease progression in patients with type 2 diabetic nephropathy and blockade of the renin-angiotensin system. Clinical Journal of the American Society of Nephrology. 2013;8(11):1870–1876. doi: 10.2215/cjn.00910113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravani P., Malberti F., Tripepi G., et al. Vitamin D levels and patient outcome in chronic kidney disease. Kidney International. 2009;75(1):88–95. doi: 10.1038/ki.2008.501. [DOI] [PubMed] [Google Scholar]
- 30.Melamed M. L., Astor B., Michos E. D., Hostetter T. H., Powe N. R., Muntner P. 25-Hydroxyvitamin D levels, race, and the progression of kidney disease. Journal of the American Society of Nephrology. 2009;20(12):2631–2639. doi: 10.1681/asn.2009030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel N. M., Gutiérrez O. M., Andress D. L., Coyne D. W., Levin A., Wolf M. Vitamin D deficiency and anemia in early chronic kidney disease. Kidney International. 2010;77(8):715–720. doi: 10.1038/ki.2009.551. [DOI] [PubMed] [Google Scholar]
- 32.Boudville N., Inderjeeth C., Elder G. J., Glendenning P. Association between 25-hydroxyvitamin D, somatic muscle weakness and falls risk in end-stage renal failure. Clinical Endocrinology. 2010;73(3):299–304. doi: 10.1111/j.1365-2265.2010.03821.x. [DOI] [PubMed] [Google Scholar]
- 33.García-Canton C., Bosch E., Ramírez A., et al. Vascular calcification and 25-hydroxyvitamin D levels in non-dialysis patients with chronic kidney disease stages 4 and 5. Nephrology Dialysis Transplantation. 2011;26(7):2250–2256. doi: 10.1093/ndt/gfq650. [DOI] [PubMed] [Google Scholar]
- 34.London G. M., Guérin A. P., Verbeke F. H., et al. Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. Journal of the American Society of Nephrology. 2007;18(2):613–620. doi: 10.1681/asn.2006060573. [DOI] [PubMed] [Google Scholar]
- 35.Drechsler C., Verduijn M., Pilz S., et al. Vitamin D status and clinical outcomes in incident dialysis patients: results from the NECOSAD study. Nephrology Dialysis Transplantation. 2011;26(3):1024–1032. doi: 10.1093/ndt/gfq606. [DOI] [PubMed] [Google Scholar]
- 36.Wang A. Y.-M., Lam C. W.-K., Sanderson J. E., et al. Serum 25-hydroxyvitamin D status and cardiovascular outcomes in chronic peritoneal dialysis patients: a 3-y prospective cohort study. The American Journal of Clinical Nutrition. 2008;87(6):1631–1638. doi: 10.1093/ajcn/87.6.1631. [DOI] [PubMed] [Google Scholar]
- 37.Wolf M., Shah A., Gutierrez O., et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney International. 2007;72(8):1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 38.Pilz S., Iodice S., Zittermann A., Grant W. B., Gandini S. Vitamin D status and mortality risk in CKD: a meta-analysis of prospective studies. American Journal of Kidney Diseases. 2011;58(3):374–382. doi: 10.1053/j.ajkd.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 39.Obi Y., Hamano T., Ichimaru N., et al. Vitamin D deficiency predicts decline in kidney allograft function: a prospective cohort study. Journal of Clinical Endocrinology and Metabolism. 2014;99(2):527–535. doi: 10.1210/jc.2013-2421. [DOI] [PubMed] [Google Scholar]
- 40.Bienaimé F., Girard D., Anglicheau D., et al. Vitamin D status and outcomes after renal transplantation. Journal of the American Society of Nephrology. 2013;24(5):831–841. doi: 10.1681/asn.2012060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee J. R., Dadhania D., August P., Lee J. B., Suthanthiran M., Muthukumar T. Circulating levels of 25-hydroxyvitamin d and acute cellular rejection in kidney allograft recipients. Transplantation. 2014;98(3):292–299. doi: 10.1097/tp.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lowery E. M., Bemiss B., Cascino T., et al. Low vitamin D levels are associated with increased rejection and infections after lung transplantation. Journal of Heart and Lung Transplantation. 2012;31(7):700–707. doi: 10.1016/j.healun.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 43.Li Y. C. Renoprotective effects of vitamin D analogs. Kidney International. 2010;78(2):134–139. doi: 10.1038/ki.2009.175. [DOI] [PubMed] [Google Scholar]
- 44.Andrukhova O., Slavic S., Zeitz U., et al. Vitamin D is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Molecular Endocrinology. 2014;28(1):53–64. doi: 10.1210/me.2013-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakai K., Fujii H., Kono K., et al. Vitamin D activates the Nrf2-keap1 antioxidant pathway and ameliorates nephropathy in diabetic rats. The American Journal of Hypertension. 2014;27(4):586–595. doi: 10.1093/ajh/hpt160. [DOI] [PubMed] [Google Scholar]
- 46.Wolf M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney International. 2012;82(7):737–747. doi: 10.1038/ki.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamano T., Nakano C., Obi Y., et al. Fibroblast growth factor 23 and 25-hydroxyvitamin D levels are associated with estimated glomerular filtration rate decline. Kidney International Supplements. 2013;3(5):469–475. doi: 10.1038/kisup.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakano C., Hamano T., Fujii N., et al. Intact fibroblast growth factor 23 levels predict incident cardiovascular event before but not after the start of dialysis. Bone. 2012;50(6):1266–1274. doi: 10.1016/j.bone.2012.02.634. [DOI] [PubMed] [Google Scholar]
- 49.Masson S., Agabiti N., Vago T., et al. The fibroblast growth factor-23 and Vitamin D emerge as nontraditional risk factors and may affect cardiovascular risk. Journal of Internal Medicine. 2014;277(3):318–330. doi: 10.1111/joim.12232. [DOI] [PubMed] [Google Scholar]
- 50.Taal M. W., Thurston V., McIntyre N. J., Fluck R. J., McIntyre C. W. The impact of vitamin D status on the relative increase in fibroblast growth factor 23 and parathyroid hormone in chronic kidney disease. Kidney International. 2014;86:407–413. doi: 10.1038/ki.2013.537. [DOI] [PubMed] [Google Scholar]
- 51.Kandula P., Dobre M., Schold J. D., Schreiber M. J., Jr., Mehrotra R., Navaneethan S. D. Vitamin D supplementation in chronic kidney disease: a systematic review and meta-analysis of observational studies and randomized controlled trials. Clinical Journal of the American Society of Nephrology. 2011;6(1):50–62. doi: 10.2215/cjn.03940510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alvarez J. A., Law J., Coakley K. E., et al. High-dose cholecalciferol reduces parathyroid hormone in patients with early chronic kidney disease: a pilot, randomized, double-blind, placebo-controlled trial. The American Journal of Clinical Nutrition. 2012;96(3):672–679. doi: 10.3945/ajcn.112.040642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marckmann P., Agerskov H., Thineshkumar S., et al. Randomized controlled trial of cholecalciferol supplementation in chronic kidney disease patients with hypovitaminosis D. Nephrology Dialysis Transplantation. 2012;27(9):3523–3531. doi: 10.1093/ndt/gfs138. [DOI] [PubMed] [Google Scholar]
- 54.Armas L. A. G., Zena M., Lund R., Heaney R. P. Calcium absorption response to cholecalciferol supplementation in hemodialysis. Clinical Journal of the American Society of Nephrology. 2013;8(6):1003–1008. doi: 10.2215/cjn.08610812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scholze A., Liu Y., Pedersen L., et al. Soluble α-klotho and its relation to kidney function and fibroblast growth factor-23. The Journal of Clinical Endocrinology & Metabolism. 2014;99(5):E855–E861. doi: 10.1210/jc.2013-4171. [DOI] [PubMed] [Google Scholar]
- 56.Seibert E., Heine G. H., Ulrich C., Seiler S., Köhler H., Girndt M. Influence of cholecalciferol supplementation in hemodialysis patients on monocyte subsets: a randomized, double-blind, placebo-controlled clinical trial. Nephron Clinical Practice. 2013;123(3-4):209–219. doi: 10.1159/000354717. [DOI] [PubMed] [Google Scholar]
- 57.Chitalia N., Ismail T., Tooth L., et al. Impact of vitamin D supplementation on arterial vasomotion, stiffness and endothelial biomarkers in chronic kidney disease patients. PLoS ONE. 2014;9(3) doi: 10.1371/journal.pone.0091363.e91363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alshayeb H., Showkat A., Wall B. M., Gyamlani G. G., David V., Quarles L. D. Activation of FGF-23 mediated vitamin D degradative pathways by cholecalciferol. The Journal of Clinical Endocrinology & Metabolism. 2014;99(10):E1830–E1837. doi: 10.1210/jc.2014-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Massart A., Debelle F. D., Racapé J., et al. Biochemical parameters after cholecalciferol repletion in hemodialysis: results from the vitadial randomized trial. The American Journal of Kidney Diseases. 2014;64(5):696–705. doi: 10.1053/j.ajkd.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 60.Mariani L. H., White M. T., Shults J., et al. Increasing use of vitamin D supplementation in the chronic renal insufficiency cohort study. Journal of Renal Nutrition. 2014;24(3):186–193. doi: 10.1053/j.jrn.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kendrick J., Cheung A. K., Kaufman J. S., et al. Associations of plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D concentrations with death and progression to maintenance dialysis in patients with advanced kidney disease. The American Journal of Kidney Diseases. 2012;60(4):567–575. doi: 10.1053/j.ajkd.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bischoff-Ferrari H. A., Dawson-Hughes B., Staehelin H. B., et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. The British Medical Journal. 2009;339(7725) doi: 10.1136/bmj.b3692.b3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Avenell A., Gillespie W. J., Gillespie L. D., O'Connell D. L. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. The Cochrane Database of Systematic Reviews. 2005;(3) doi: 10.1002/14651858.CD000227.pub2.CD000227 [DOI] [PubMed] [Google Scholar]
- 64.Girgis C. M., Mokbel N., Cha K. M., et al. The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25-hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology. 2014;155(9):3227–3237. doi: 10.1210/en.2014-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beaudart C., Buckinx F., Rabenda V., et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. The Journal of Clinical Endocrinology & Metabolism. 2014;99(11):4336–4345. doi: 10.1210/jc.2014-1742. [DOI] [PubMed] [Google Scholar]
- 66.Kim M. J., Frankel A. H., Donaldson M., et al. Oral cholecalciferol decreases albuminuria and urinary TGF-β1 in patients with type 2 diabetic nephropathy on established renin-angiotensin-aldosterone system inhibition. Kidney International. 2011;80(8):851–860. doi: 10.1038/ki.2011.224. [DOI] [PubMed] [Google Scholar]
- 67.Molina P., Górriz J. L., Molina M. D., et al. The effect of cholecalciferol for lowering albuminuria in chronic kidney disease: a prospective controlled study. Nephrology Dialysis Transplantation. 2014;29(1):97–109. doi: 10.1093/ndt/gft360. [DOI] [PubMed] [Google Scholar]
- 68.Stubbs J. R., Idiculla A., Slusser J., Menard R., Quarles L. D. Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. Journal of the American Society of Nephrology. 2010;21(2):353–361. doi: 10.1681/ASN.2009040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matias P. J., Jorge C., Ferreira C., et al. Cholecalciferol supplementation in hemodialysis patients: effects on mineral metabolism, inflammation, and cardiac dimension parameters. Clinical Journal of the American Society of Nephrology. 2010;5(5):905–911. doi: 10.2215/cjn.06510909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumar V. A., Kujubu D. A., Sim J. J., Rasgon S. A., Yang P. S. Vitamin D supplementation and recombinant human erythropoietin utilization in vitamin D-deficient hemodialysis patients. Journal of Nephrology. 2011;24(1):98–105. doi: 10.5301/jn.2010.1830. [DOI] [PubMed] [Google Scholar]
- 71.Alvarez J. A., Zughaier S. M., Law J., et al. Effects of high-dose cholecalciferol on serum markers of inflammation and immunity in patients with early chronic kidney disease. European Journal of Clinical Nutrition. 2013;67(3):264–269. doi: 10.1038/ejcn.2012.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hewitt N. A., O'Connor A. A., O'Shaughnessy D. V., Elder G. J. Effects of cholecalciferol on functional, biochemical, vascular, and quality of life outcomes in hemodialysis patients. Clinical Journal of the American Society of Nephrology. 2013;8(7):1143–1149. doi: 10.2215/CJN.02840312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pittas A. G., Chung M., Trikalinos T., et al. Systematic review: vitamin D and cardiometabolic outcomes. Annals of Internal Medicine. 2010;152(5):307–314. doi: 10.7326/0003-4819-152-5-201003020-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang L., Manson J. E., Song Y., Sesso H. D. Systematic review: vitamin D and calcium supplementation in prevention of cardiovascular events. Annals of Internal Medicine. 2010;152(5):315–323. doi: 10.7326/0003-4819-152-5-201003020-00010. [DOI] [PubMed] [Google Scholar]
- 75.Cranney A., Weiler H. A., O'Donnell S., Puil L. Summary of evidence-based review on vitamin D efficacy and safety in relation to bone health. The American Journal of Clinical Nutrition. 2008;88(2):513S–519S. doi: 10.1093/ajcn/88.2.513S. [DOI] [PubMed] [Google Scholar]
- 76.Levin G. P., Robinson-Cohen C., de Boer I. H., et al. Genetic variants and associations of 25-hydroxyvitamin D concentrations with major clinical outcomes. The Journal of the American Medical Association. 2012;308(18):1898–1905. doi: 10.1001/jama.2012.17304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Powe C. E., Evans M. K., Wenger J., et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. The New England Journal of Medicine. 2013;369(21):1991–2000. doi: 10.1056/nejmoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Safadi F. F., Thornton P., Magiera H., et al. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. The Journal of Clinical Investigation. 1999;103(2):239–251. doi: 10.1172/jci5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bikle D. D., Gee E. Free, and not total, 1,25-dihydroxyvitamin D regulates 25-hydroxyvitamin D metabolism by keratinocytes. Endocrinology. 1989;124(2):649–654. doi: 10.1210/endo-124-2-649. [DOI] [PubMed] [Google Scholar]
- 80.Bhan I., Powe C. E., Berg A. H., et al. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney International. 2012;82(1):84–89. doi: 10.1038/ki.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arnaud J., Constans J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP) Human Genetics. 1993;92(2):183–188. doi: 10.1007/BF00219689. [DOI] [PubMed] [Google Scholar]
- 82.Braun A., Bichlmaier R., Cleve H. Molecular analysis of the gene for the human vitamin-D-binding protein (group-specific component): allelic differences of the common genetic GC types. Human Genetics. 1992;89(4):401–406. doi: 10.1007/BF00194311. [DOI] [PubMed] [Google Scholar]
- 83.Robinson-Cohen C., Hoofnagle A. N., Ix J. H., et al. Racial differences in the association of serum 25-hydroxyvitamin D concentration with coronary heart disease events. The Journal of the American Medical Association. 2013;310(2):179–188. doi: 10.1001/jama.2013.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parikh A., Chase H. S., Vernocchi L., Stern L. Vitamin D resistance in chronic kidney disease (CKD) BMC Nephrology. 2014;15(1, article 47) doi: 10.1186/1471-2369-15-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chun R. F., Lauridsen A. L., Suon L., et al. Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. Journal of Clinical Endocrinology and Metabolism. 2010;95(7):3368–3376. doi: 10.1210/jc.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ponda M. P., McGee D., Breslow J. L. Vitamin D-binding protein levels do not influence the effect of vitamin D repletion on serum PTH and calcium: data from a randomized, controlled trial. The Journal of Clinical Endocrinology & Metabolism. 2014;99(7) doi: 10.1210/jc.2014-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Autier P., Boniol M., Pizot C., Mullie P. Vitamin D status and ill health: a systematic review. The Lancet Diabetes & Endocrinology. 2014;2(1):76–89. doi: 10.1016/s2213-8587(13)70165-7. [DOI] [PubMed] [Google Scholar]
- 88.Chowdhury R., Kunutsor S., Vitezova A., et al. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. The British Medical Journal. 2014;348 doi: 10.1136/bmj.g1903.g1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Niino M., Kikuchi S., Fukazawa T., Yabe I., Tashiro K. No association of vitamin D-binding protein gene polymorphisms in Japanese patients with MS. Journal of Neuroimmunology. 2002;127(1-2):177–179. doi: 10.1016/S0165-5728(02)00099-1. [DOI] [PubMed] [Google Scholar]
- 90.Hirai M., Suzuki S., Hinokio Y., et al. Variations in vitamin D-binding protein (group-specific component protein) are associated with fasting plasma insulin levels in Japanese with normal glucose tolerance. The Journal of Clinical Endocrinology & Metabolism. 2000;85(5):1951–1953. doi: 10.1210/jcem.85.5.6569. [DOI] [PubMed] [Google Scholar]
- 91.Ohkura K., Nagasawa H., Uto Y., Okamura N., Murakami A., Hori H. The role of Gc protein oligosaccharide structure as a risk factor for COPD. Anticancer Research. 2006;26(6):4073–4078. [PubMed] [Google Scholar]