Abstract
Purpose of review
Discuss the recent progress on the clinical use of Mesenchymal stromal (stem) cells (MSC) in solid organ transplantation (SOT).
Recent findings
Tissue repair and immunomodulatory properties have been recognized for MSC obtained from different human tissues. MSC-based therapy has been proposed to reduce ischemia-reperfusion injury and to promote immune tolerance. The results of recent clinical trial support the safety and promising effects of autologous and allogeneic MSC in SOT. Collectively, the use of MSC in recipients of living donor kidney transplantation (LDKT) was associated with improved graft function, reduced rejection, ability to omit induction and/or lower maintenance immunosuppression regimen, as well as to treat rejection episodes.
Summary
We are living very exciting times with the implementation of novel clinical trials aimed at establishing safety, feasibility and efficacy of cellular therapies including MSC to improve SOT outcomes. The results of the initial clinical trials support the safety of MSC-based therapy and justifying cautious optimism for the immediate future.
Keywords: Mesenchymal Stem Cells (MSC), Mesenchymal Stromal Cells (MSC), Bone Marrow, Adipose Tissue, Solid Organ Transplantation (SOT), Cellular Therapies, Regenerative Medicine, Living-Donor Kidney Transplantation (LDKT), Clinical Trial, Ischemia/Reperfusion Injury, Acute Cellular Rejection (ACR), Immunomodulation, Immune Tolerance, Immunosuppression, Opportunistic Infections
Introduction
Transplanting cells, tissues and organs aims at the long-lasting restoration of function lost to genetic defect, inflammation, toxicity, or trauma. Cellular therapies are emerging as therapeutic options to ameliorate, reduce, modify, correct and cure medical conditions. Mesenchymal stromal (stem) cells (MSC) are particularly appealing because of their tissue repair and immunomodulatory potential (1). Herein, we review and discuss the recent progress on the clinical use of MSC in solid organ transplantation (SOT)(2-10).
Rationale for the use of MSC in solid organ transplantation
MSC comprise a heterogeneous cell population of putative perycytic origin (11-13). The 2006 guidelines of the International Society for Cellular Therapy (ISCT) identify MSC based on: (i) adherence to plastic; (ii), ≥95% of the MSC population must express CD105, CD73 and CD90; must lack expression ≤2% positive of CD45, CD34, CD14 or CD11b, CD79a or CD19 and HLA class II); and (iii) multipotent differentiation (osteoblast, adipocyte and chondroblast under standard in vitro differentiating conditions)(12). Bona fide MSC are obtained from bone marrow (BM), adipose tissue (AT), umbilical cord (UC), and other human tissues (14-17), likely due to their perivascular (pericyte) origin (18-20).
Autologous MSC are obtained from the patient's own tissues or from HLA-identical siblings. Allogeneic MSC are obtained from ‘donor-specific’ (if also donate the solid organ) or ‘third-party’ HLA-matched/mismatched individuals (Figure 1). MSC are obtained in adequate numbers prior to transplantation from either a prospective living donor or from the recipient and used fresh or cryopreserved. Third-party (off-the-shelf) allogeneic MSC may represent a practical choice if other options are unavailable.
Figure 1. Sources of MSC.
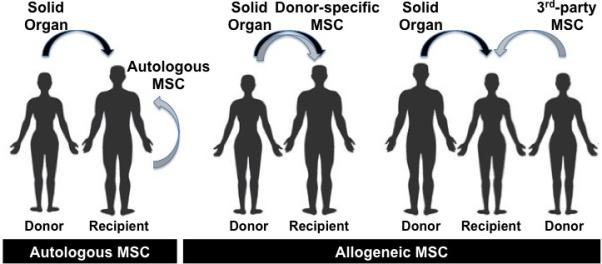
Possible donor:recipient combinations for MSC therapy in SOT.
Ischemia-reperfusion injury (IRI) of SOT is the result of hypoxia-mediated cellular death and activation of stress-induced signal transduction pathways in vascular endothelium and organ parenchyma, which are triggered by cerebral or cardiac death and organ preservation, and after reperfusion. Consequences of IRI include delayed function and primary non-function, heightened organ immunogenicity due to increased expression of major histocompatibility complex (MHC) molecules, pro-inflammatory mediators, and activation of adaptive immunity. In fact, deceased donor organs have higher rates of rejection than living-donor organs (21, 22).
After inoculum, MSCs preferentially home at the site of vascular damage or inflammation where they likely function as the native resident pericytes/MSCs do in small, minor injuries (23, 24). This property may help mitigating IRI (25, 26), rescuing marginal donor organs, reducing activation of innate immunity leading to progressive tissue fibrosis, and blunting ‘danger signals’ that could synergize with immune tolerance-inducing strategies (Table 1).
Table 1.
Therapeutic Potential of MSC for Solid Organ Transplantation
| Therapeutic Application |
|---|
| Immunomodulation |
| Immunotherapy induction agent |
| Promotion of Immune Tolerance |
| Treatment of Acute Rejection |
| Tissue Repair |
| Reduction/Prevention of Ischemia Reperfusion Injury |
| Mitigate/Counteract Drug Toxicity (?) |
| Contribute to Tissue Remodeling |
Immunosuppressive protocols generally combine agents, including lymphodepletion [i.e., rabbit anti-lymphocyte globulin (RATG), anti-CD25 antibody (targeting the interleukin-2 receptor), anti-CD52 antibody (campath-1H, alemtuzumab), anti-CD3 antibody, amongst others], calcineurin inhibitors (CNI: cyclosporine A, CsA; and tacrolimus), molecular target of Rapamycin (mTOR) inhibitors (sirolimus and everolimus), purine/pyrimidine synthesis inhibitors (mycophenolate mofetil–MMF; mycophenolic acid–MPA; azathioprine–AZA), cyclophosphamide (CyP), and/or steroids, amongst others. Immunosuppression heightens the risk of opportunistic infections (OI) and organ toxicity (which may progress to end-stage failure)(27, 28), and may affect quality of life of transplanted patients, as well as graft survival. Achieving permanent acceptance of transplanted tissues reproducibly without the need for life-long anti-rejection therapy represents the ‘Holy Grail’ of transplant immunobiology, and has been reported only sporadically or in limited patient cohorts (29-34).
Immunomodulatory effects of MSC have been recognized on T, B, Natural Killer (NK), dendritic (DC), and monocyte cell functions, as well as on the induction of ‘regulatory’ immune circuits (35-38). Bartholomew et al. described the immunomodulatory properties of MSC in allogeneic nonhuman primate skin grafts (35). LeBlanc et al. demonstrated that BM-MSC administration, irrespective of the HLA matching of the MSC donors, effectively treats severe graft-versus host disease (GVHD) refractory to steroids in hematopoietic stem cell (HSC) transplant recipients (39, 40). In SOT, MSC treatment may help reducing the burden of immunosuppressive regimen, treat rejection episodes, and promote induction of immune tolerance (Table 1)(36-38).
Clinical trials of MSC and SOT
Multiple MSC clinical trials in SOT are registered with ClinicalTrials.gov (Table 2)(3, 5, 7, 8), but the overall worldwide number is likely higher (4, 9). The results from recent MSC trials in SOT are encouraging (Table 3).
Table 2.
Registered clinical trials of MSC in solid organ transplantation (ClinicalTrials.gov)
| NCT* | Title | Site | Settings | Type of MSC | MSC Inoculum | Type of Study | Start Date |
|---|---|---|---|---|---|---|---|
00646724 
|
Cotransplantation of islet and mesenchymal stem cell in Type 1 diabetic patients | Fuzhou, China | Islet Transplant | UC-MSC | 1-2 × 106/kg bw simultaneous islet and MSC transplantation via hepatic portal vein | Interventional Safety/Efficacy Single Group Assignment Open Label | Jan 2008 |
00658073 
|
Induction therapy with autologous mesenchymal stem cells for kidney allografts | Fuzhou, China | Living Donor Kidney Transplant | Autologous BM-MSC | 1-2 × 106/kg bw, IV at reperfusion and day14 | Interventional Randomized Safety/Efficacy Parallel Assignment Open Label | Mar 2008 |
00659620 
|
Mesenchymal stem cell transplantation in the treatment of chronic allograft nephropathy | Fuzhou, China | Chronic Kidney Rejection | Autologous BM-MSC | 1-2 × 106/kg bw, IV once a week for 4 weeks (total 4 injections) | Interventional Safety/Efficacy Single Group Assignment Open Label | May 2008 |
00734396 
|
Mesenchymal stem cells and subclinical rejection | Leiden, Netherland | Kidney Rejection | Autologous BM-MSC | 106/kg bw, IV 7 days apart (total 2 injections) | Interventional Non-Randomized Safety/Efficacy Single Group Assignment Open Label | Feb 2009 |
00752479 
|
Mesenchymal stem cells under Basiliximab/low dose RATG to induce renal transplant tolerance | Bergamo, Italy | Living Donor Kidney Transplant | Autologous BM-MSC | 2×106/kg bw IV | Interventional Randomized Safety/Efficacy Parallel Assignment Open Label | May 2008 |
01175655 
|
A study to evaluate the potential of mesenchymal stromal cells to treat obliterative bronchiolitis after lung transplantation | Chermside, Australia | Lung Transplant | HLA identical and allogeneic (Third-party) BM-MSC ? | 2×106/kg bw IV, twice weekly, 2 wks | Interventional Single Group Assignment Safety/Efficacy Open Label | Feb 2010 |
01429038 
|
Mesenchymal stem cells after renal or liver transplantation | Liege, Belgium | Liver or Kidney Transplant | Allogeneic (Third-party) BM-MSC | 1.3-3.0×106/kg bw day 3±2 | Interventional Non-Randomized Safety/Efficacy Parallel Assignment Open Label | Feb 2012 |
01668576 
|
Properties of mesenchymal stem cells in lung transplant candidates | Atlanta, USA | Lung Transplant | Autologous BM-MSC | In vitro assessment only | Observational Cohort Cross-sectional | Aug 2012 |
01690247 
|
Human mesenchymal stem cells induce liver transplant tolerance | Beijing, China | Liver Transplant | UC-MSC | 106/kg bw IV once every 4 weeks day 0 to 12 weeks | Interventional Randomized Safety/Efficacy Parallel Assignment Open Label | Feb 2012 |
Status:  Recruiting
Recruiting  Completed
Completed  Unknown
Unknown
Abbreviations: BM: Bone Marrow; bw: body weight; HLA: Human Leukocyte Antigen; IV: intravenous; MSC: Mesenchymal Stromal (stem) Cell; NCT: CLinicaTrial.gov Identifier; UC: Umbilical Cord
Table 3.
Effects associated with MSC therapy in recent clinical SOT trials
| Observed Benefit | AE | No. Subjects (vs. control) |
Follow- up (months) |
Random | Setting | Type of MSC | MSC inoculum Time & Route |
Immunosuppression | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Minimization of Immunosuppression Reduced rejection episodes Increased hematopoietic chimerism Donor-hyporesponsiveness in vitro | None | 100 vs. 100 | 18 | No | LDKT | Donor-specific, Allogeneic AT-MSC | Day -9, IP or IV | DST/HST/TBI aCD20/RATG/CyP CNI/AZA/Steroids | Vanikar 2011 |
| Induction of Treg Inhibition of memory T cells Donor-hyporesponsiveness in vitro | Elevated sCr | 2 | 12 | No | LDKT | Autologous BM-MSC | Day 7, IV | RATG/aCD25 CsA/MMF/Steroids | Perico 2011 |
| Alternative to anti-CD25 blockade Reduced maintenance CNI dose Early graft function Reduced acute rejection Reduced opportunistic infections | None | 102 vs. 51 | 12 | Yes | LDKT | Autologous BM-MSC | Day 0 and 14, IV | CNI/MMF/Steroids | Tan 2012 |
| Reduced maintenance CNI dose Transient increase of memory B cells | None | 6 vs. 6 | 12 | Yes | LDKT | Donor-specific, Allogeneic BM-MSC | Day 0 (IA) Day 30 (IV) | CyP CNI/Steroids | Pong 2012 |
| Minimization of Immunosuppression | None | 606 vs. 310 | 48 | No | LDKT | Donor-specific, Allogeneic AT-MSC | Day 3, IV | DST/HST/TBI RATG/CyP/IVIg CNI/MMF(AZA)/Steroids | Vanikar 2012 |
| Reduced tubulitis Reduced interstitial fibrosis/tubular atrophy Donor-hyporesponsiveness in vitro | Viral Infections | 6 | 5 | No | LDKT | Autologous BM-MSC | 6 months, IV | aCD25 CNI/MMF/Steroids | Reinders 2012 |
| Inhibition of memory T cells Donor-hyporesponsiveness in vitro | Acute Rejection | 2 | 12 | No | LDKT | Autologous BM-MSC | Day -1, IV | RATG CNI/MMF/Steroids | Perico 2013 |
| Increased Tregs Donor-hyporesponsiveness in vitro Increased IL10 levels | Acute Rejection | 7 | 12 | No | LDKT | Donor-specific, Allogeneic BM-MSC | Day 0, IO | RATG CNI/MMF/Steroids | Lee 2013 |
*Abbreviations:
aCD20: anti-CD20 antibody (Rituximab); aCD25: anti-CD25 andibody (Basiliximab); AT: Adipose Tissue; AZA: Azathioprine; BM: Bone Marrow; CNI: Calcineurin Inhibitor(s); CyP: Cyclophosphamide; DST: Donor specific leukocyte Transfusion; HSC: Hematopoietic Stem Cells; IA: Intra-Arterial; IO: Intra-Osseous; IP: Intraportal; IV: Intra-Venous; LDKT: Living Donor Kidney Transplant; MMF: Mycophenolate Mofetil; MSC: Mesenchymal Stromal (stem) Cell ; RATG: rabbit Anti-Thymoglobulin
Vanikar et al. (2) first reported the use of donor-specific AT-MSC as part of a non-randomized protocol aimed at the induction of donor hyporesponsiveness in 100 recipients of Living Donor Kidney Transplantation (LDKT) for end-stage renal disease (ESRD). Treatment included: donor-specific leukocyte transfusion (DST; days -27,-25); anti-CD20 antibody (Rituximab; 6mg/kg, Day -18; RATG (1.5mg/kg, day -17); unmodified donor-marrow HSC (200ml, day -16); nonmyeloablative conditioning with total body irradiation (TBI; targeted to subdiaphragmatic lymph nodes, spleen, pelvic bones and lumbar vertebrae; 200CGy x5 days); intra-portal (IP) infusion of donor-specific AT-MSC, 10-day cultured BM cells, and peripheral blood stem cells (PBSC; G-CSF mobilization in the prospective donor; day −9); methylprednisone (500mg IV, days −1,0,+1); CNI (CsA 3mg/kg/day) plus prednisone (20mg/day) for first trimester, then AZA plus prednisone (5-10mg/day). Control subjects (n=100) received the same treatment without AT-MSC. The AT-MSC group displayed improved graft survival, sustained chimerism levels using low-dose immunosuppression than controls over an 18-month follow-up period. Similar outcomes over a 4-year period were observed in a subsequent large-scale, nonrandomized LDKT trial (patients declining HSC/MSC protocol enrolled as controls)(4) testing the induction of hyporesponsiveness protocol with donor-specific AT-MSC in 606 patients vs. 310 controls receiving conventional triple immunosuppression. The results of the study are promising, though lack of randomization in both studies, and lack of a group of patients receiving the conditioning without AT-MSC in the latter limits the generalization of the results.
Perico et al. tested autologous BM-MSC in two patients with end-stage renal disease (ESRD) receiving LDKT (3). One week after LDKT, intravenous BMMSC (1.7-2.0×106/kg BW) was given under conventional immunosuppression with fractioned RATG (0.5mg/kg, days 0 through 6), anti-CD25 antibody (basiliximab, 20mg intravenously pre-transplant and day 4), steroids (tapered and weaned by 1-week post-transplant), and CNI (CsA) plus MMF maintenance. Transient increase in serum creatinine was observed in both patients, who displayed good graft function at one-year. Increased frequency of CD4+CD25highFoxP3+CD127– T-regulatory (Treg) cells and reduction of CD8+CD45RO+RA– T-memory cells were observed in these two patients, when compared to historical controls receiving the same immunosuppression without MSC. A subsequent pilot trial on two additional patients evaluated the impact of (i) timing of autologous BM-MSC inoculum and (ii) omission of CD25 blockade from standard immunosuppression (10). Intravenous BM-MSC (2.0×106/kg BW) was given on the day before LKDT. One patient with higher HLA haplotype mismatch developed transient elevation of serum creatinine 2-wks after transplant with pathology compatible with acute cellular rejection (ACR) that resolved after steroid pulses. In vitro CD8+ T cell cytolytic function appeared more suppressed to donor than to third-party antigens in both MSC recipients; response to donor antigens progressively returned to baseline, while response to third-party antigens was unaffected by immunosuppression in historical controls by 12 months. T-memory/effector cell proportions in historical controls with standard immunosuppression (plus anti-CD25 antibody) increased over time, but markedly decreased by day 7 and remained lower than pre-transplant throughout the one-year follow-up in both MSC recipients. Unlike their previous trial (3), Treg proportions appeared unaffected by MSC inoculum, except for a transient decreased soon after transplantation (10). These two pilot trials preliminary confirmed safety and provided encouraging mechanistic observations after inoculum of autologous MSC in immunosuppressed SOT recipients (Table 3), though small sample size, lack of concomitant controls and of randomization limit generalizations.
Our group (Tan et al.)(5, 6) completed the one-year follow-up of a prospective, open-label, randomized clinical trial on 159 patients with ESRD receiving LDKT and tested the risk/benefit profile of autologous BM-MSC infusion compared to anti-CD25 antibody (basiliximab) induction therapy (5). All patients received MMF and corticosteroids. Controls (n=51) received anti-CD25 antibody plus standard dose CNI (either CsA or tacrolimus). In the two experimental arms, anti-CD25 treatment was replaced by BM-MSC inoculum (1−2×106/kg intravenously at reperfusion and day 14 post-transplant) with either standard (n=52) or reduced-dose CNI (80% of standard dose; n=52), the latter to prevent organ toxicity (41, 42). The primary outcome was one-year incidence of biopsy-confirmed ACR and estimated glomerular filtration rate (eGFR). The secondary outcome was one-year patient and graft survival and incidence of adverse events. Replacement of CD25 blockade with autologous MSC in LDKT transplant recipients did not compromise graft and patient safety, while yielding, when compared to controls: (i) faster recovery of renal graft function during the first month post-transplant (suggesting a possible effect on IRI, a recognized risk factor for graft failure and ACR)(43, 44); (ii) lower frequency of and less severe biopsy-confirmed ACR in the first semester post-transplant (none steroid-resistant requiring RATG, vs. 7.8% in the controls); and (iii) fewer adverse events, particularly OI. One-year graft function was comparable in all groups. Similar graft survival rates with reduction of maintenance CNI therapy in LDKT recipients was previously reported only in recipients of whole or fractionated donor-specific BM cell transplantation (45) or using anti-CD52 antibody lymphodepletion (in absence of cellular therapy), though the latter increased the rates of severe OI (46). Notably, OI occurring mostly in the first two trimesters heighten mortality rates in kidney transplant recipients in China (47). Secretion by MSC of anti-microbial/immunomodulatory molecules (i.e., cathelicidin hCAP-18/LL-37)(48) might have contributed, at least in part, to the significantly lower rates of OI in recipients of MSC plus low and standard CNI doses in our trial.
Peng et al. (7) assessed safety and efficacy of donor-BM-MSC in LDKT recipients (nonrandomized trial). All patients received induction with cyclophosphamide and steroids followed by maintenance immunosuppression with MMF and prednisone; CNI (tacrolimus) was started on day 4; the control group received standard-dose (0.07–0.08mg/kg/day; n=6), whereas the experimental group (n=6) received low dose (0.04–0.05mg/kg) along with donor 5×106 BM-MSC into the renal allograft artery at reperfusion, plus 2×106/kg intravenously a month later. Direct MSC injection into the renal artery was uneventful. Recipients of MSC plus low-CNI dose maintained stable graft function during the one-year follow-up and displayed higher numbers of peripheral B-memory (CD27+) cells at 3 months. No other differences amongst study groups were observed (i.e., lymphocyte phenotypes, intracellular cytokines, one-way mixed lymphocyte responses in vitro, chimerism, etc.).
Lee et al. (9) tested donor-BM-MSC in seven HLA mismatched LDKT recipients under conventional immunosuppression based on fractionated RATG (8–10 days at 1.5 mg/kg/day) with maintenance CNI, MMF and steroids. Donor-BM-MCS (1×106/kg BW) intra-osseous (IO; into the recipient's right iliac bone) on the day of kidney transplantation was uneventfully. Reduction of donor-specific lymphocyte and mitogen-induced T-cell proliferation were observed in two patients, though chimerism was never detected. Donor-specific lymphocyte or T-cell proliferation and Treg priming responses were observed in few patients. Three patients displayed biopsy-proven ACR controlled well with steroid pulse therapy; one patient received Intravenous immunoglobulin (IVIG) and plasmapheresis for acute antibody-mediated rejection on day 9 post-transplantation; ACR responsive to steroids was observed at 43 days and 613 days post-transplantation; another ACR at 12 months was detected by protocol biopsy; two patients displayed borderline change without clinical signs of rejection not requiring treatment. While the study supports the feasibility of intra-BM administration of MSC, further studies should ascertain the impact of allogeneic BM-MSC on graft outcome on a larger number of study subjects and concomitant controls.
Reinders et al. (8) used autologous BM-MSC to treat ACR episodes and renal interstitial fibrosis and tubular atrophy (IF/TA) in six recipients of fully HLA mismatched LDKT immunosuppressed with anti-CD25 antibody (basiliximab), CNI (tacrolimus or CsA), MMF and prednisone, under 3-month antiviral prophylaxis. Patients displaying ACR or increased IF/TA (compared to the 4-week biopsy) at the 6-month protocol biopsy were given intravenous BM-MSC (106/kg) a week apart without modifying immunosuppression. Two subjects with biopsy-confirmed allograft rejection (Banff 1A with mild IF/TA and Banff 1B, respectively) had resolution of tubulitis without IF/TA at post-MSC treatment biopsy. A BK virus-associated nephropathy occurred 21 weeks after MSC infusion and resolved without reduction of immunosuppression. A de novo CMV infection occurred two weeks post-MSC infusion (6 months after discontinuing prophylaxis) resolved without reduction of immunosuppression. A patient showed persistent (months) low-grade CMV viral load post-MSC infusion despite reduction of immunosuppression. Reduced in vitro leukocyte proliferative responses were demonstrated 12-weeks post-MSC inoculum. The potential direct effect of MSC therapy in promoting resolution of the features of rejection in clinical allogeneic renal grafts emerges from this pilot trial. Large size clinical trials with concomitant controls will be of assistance in determining the reproducibility of the positive effects of MSC treatment on graft pathology, as well as defining the actual relationship with opportunistic viral infections.
Considerations regarding the clinical use of MSC in SOT
The methods utilized for MSC isolation (i.e., enzymatic vs. non-enzymatic), selection (i.e., adherence to plastic, cell sorting based on surface cell markers, etc.), expansion (i.e., culture media and supplements, oxygen tension, etc.), and assessment are not yet fully standardized amongst facilities and based on the anatomical source (16). Since the 2006 ISCT guidelines (12), improved criteria for MSC isolation and characterization have been proposed (49-51).
It remains to be elucidated whether comorbidities (i.e., chronic medical conditions: diabetes, ESRD, etc.) of MSC donor and/or recipient may negatively affect efficacy and potency of the cellular products (52), and whether these effects can be reverted under the appropriate conditions (i.e., in vitro culture and/or in vivo treatments)(53-57).
Multipotency and immunomodualtory properties of MSC may represent, at least hypothetically, a safety threat for transplant recipients who are immunosuppressed. Development of MSC-derived neoplasm is possible, though never reported in relation to MSC inoculum in humans. A meta-analysis on a sample of 1,012 MSC recipients confirmed clinical safety (58), even though heterogeneity of both medical conditions and protocols analyzed should suggest caution. Potentiation of immunosuppression by MSC may heighten risk fo (de novo and/or reactivation) viral infections, lymhoproliferative diseases and progressive multifocal leukoencephalopathy. Common practice prophylaxis, close monitoring and careful assessment of immune and viral status of the recipients could allow for timely interventions aimed at minimizing risks.
The effects (synergy or competition) of concomitant therapy on MSC viability, potency and efficacy are being investigated. Preliminary studies suggest that CNI's and of mTOR inhibitors, but not purine/pyrimidine synthesis inhibitors (MMF and MPA), may interfere with the immunomodulatory properties of MSC (3, 59-62). RATG binds to human MSC in a dose-dependent fashion in vitro (3, 63) and this phenomenon is associated with MSC death, impaired in vitro immunosuppressive effects, and susceptibility to lysis by cytokine-activated CD8+ cytotoxic cells and NKT cells (63). Human MSC exposed to serum collected from renal transplant recipients who had received RATG treatment displayed only minimal RATG binding with no impairment of in vitro MSC immunomodulatory effects in mixed lymphocyte reactions (3); addition of CsA, MMF or steroids to the cultures did interfere with MSC suppression of T cell responses to mitogenic stimulation with anti-CD3/CD28 antibodies in vitro, while synergy was rather observed with MMF (3).
The immunogenicity of transplanted MSC (donor-specific or third-party) may negatively impact SOT survival. Griffin et al. (64) collected literature evidence in support of specific cellular (T-cell) and humoral (B-cell/antibody) immune responses against donor antigens following administration of non-manipulated, interferon (IFN)-γ-activated and differentiated allo-MSCs. These important aspects deserve further studies in the clinical setting.
While overall safety of MSC therapies emerges from recent clinical SOT trials, the heterogeneity in the design (MSC source, route and schedule of administration, concomitant immunotherapy, and study endpoints) amongst the published clinical trials limits the possibility of meaningful comparisons at the present time.
For a widespread application of clinical MSC products, ‘regional’ (centralized) Cell Processing facilities or the use of off-the-shelf, ‘standardized’ MSC products (i.e., centralized or industry manufacturing) may allow containing the costs. The remarkable financial burden imposed by the regulatory framework requiring proof safety and efficacy of cellular therapies under the Investigational New Drug (IND) classification before a Biological License is obtained from regulatory agencies represents an important hurdle to the transition of cellular therapies from academic initiatives into widespread clinical applications. This issue has steamed an intense debate, as elevated costs may hinder the development of potentially promising cellular therapies that could benefit humankind even beyond SOT (65).
Patients’ safety is paramount. Clinical studies should be performed under ethically approved protocols and appropriate Data Safety Monitoring Board oversight. Establishment of a ‘Cell Therapy in SOT’ Registry to gather critical parameters and outcomes will be of assistance in assessing the safety and efficacy of MSC therapy in SOT and guide the design of future trials. The selection of ‘standardized’ tests (i.e., cell product identity, potency and clinical outcomes) and endpoints to be adopted across centers would further promote the progress of the field.
Conclusion
We are living very exciting times with the implementation of novel clinical trials aimed at establishing safety, feasibility and efficacy of cellular therapies and MSC to improve SOT outcomes. The results of the initial clinical trials are quite promising supporting the safety of the procedure and beneficial effects on SOT justifying cautious optimism for the immediate future.
Key points.
Tissue repair and immunomodulatory properties have been recognized for MSC obtained from different human tissues.
MSC-based therapy has been proposed to reduce IRI, reduce immunosuppression, treat rejection episodes and possibly induce immune tolerance.
Initial clinical reports support the safety of MSC in SOT and reveal encouraging positive effects on engraftment, reduction of immunosuppression burden, reduction of rejection and possibly inducing immune modulation.
MSC may represent a viable adjuvant therapy to improve clinical outcomes in SOT.
Acknowledgements
This work is part of The Cure Focus Research Alliance (TheCureAlliance.org), an international not-for-profit, collegial association of scientists, physicians, surgeons, and other professional and/or committed individuals who share the vision and primary objective to develop effective strategies for the cure and eventual eradication of disease conditions now afflicting humankind, and to do so in the fastest, most efficient and safest ways possible. The work at the University of Miami was supported in part by grants from the National Institutes of Health (5U19AI050864-10, U01DK089538, 5U42RR016603-08S1, 1DP2DK083096-01, 1R01EB008009-02, 5R01DK059993-06, 1 R21 DK076098-01, 1 U01 DK70460-02, 5R01DK25802-24, 5R01DK56953-05), the Juvenile Diabetes Research Foundation International (17-2012-361, 17-2010-5, 4-2008-811, 6-39017G1, 4-2004-361, 4-2000-947), The Leona M. and Harry B. Helmsley Charitable Trust, the American Diabetes Association (7-13-IN-32), the University of Miami Interdisciplinary Research Development Initiative, the Diabetes Research Institute Foundation (www.DiabetesResearch.org), and Converge Biotech. The work at Affiliated Fuzhou General Hospital of Xiamen University was supported in part by grants from the Fujian Province Key Science Research Project (2009Y4001) and from the Fujian Province Key Laboratory (2008J1006). Notably, the funding agencies at US and China institutions had no role in the design and conduct of the study, collection, management, analysis and interpretation of the data, content, presentation, decision to publish, or preparation of the manuscript.
Abbreviations
- ACR
Acute Cellular Rejection
- AT
Adipose Tissue
- AZA
Azathioprine
- BM
Bone Marrow
- BW
Body Weight
- CGy
Centigray
- cGMP
current Good Manufacturing Practice
- CNI
Calcineurin Inhibitor
- CMV
Cytomegalovirus
- CsA
Cyclosporin A
- CyP
Cyclophosphamide
- DC
Dendritic Cells
- DSMB
Data Safety Monitoring Board
- DST
Donor-Specific Leukocyte Transfusion
- EBV
Epstein Barr Virus
- eGFR
estimated Glomerular Filtration Rate
- ESRD
End-Stage Renal Disease
- G-CSF
Granulocyte–Colony Stimulating Factor
- GVHD
Graft Versus Host Disease
- HSC
Hematopoietic Stem Cell
- HLA
Human Leukocyte Antigens
- IF
Interstitial Fibrosis
- IFN
Interferon
- IND
Investigational New Drug
- IL
Interleukin
- IO
Intra-Osseous
- IP
Intra-Portal
- IRB
Institutional Review Board
- IRI
Ischemia-Reperfusion Injury
- ISCT
International Society for Cellular Therapy
- IV
Intra-Venous
- IVIG
Intravenous Immunoglobulin
- LDKT
Living Donor Kidney Transplant
- MMF
Mycophenolate Mofetil
- MPA
Mycophenolic acid
- MSC
Mesenchymal Stromal (Stem) Cells
- mTOR
molecular Target of Rapamycin
- NHBD
Non Heart-Beating Donors
- NK
Natural Killer
- NKT
Natural Killer T cells
- OI
Opportunistic Infections
- PBSC
Peripheral Blood Stem Cells
- RATG
rabbit Anti-Lymphocyte Globulin
- SOP
Standard Operating Procedure
- SOT
Solid Organ Transplantation
- TA
Tubular Atrophy
- TBI
Total Body Irradiation
- Treg
T regulatory cell
- UC
Umbilical Cord
Footnotes
The Authors have no conflict of interests to disclose regarding the content of this manuscript.
References
- 1.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nature reviews Immunology. 2008;8(9):726–36. doi: 10.1038/nri2395. Epub 2009/01/28. [DOI] [PubMed] [Google Scholar]
- 2.Vanikar AV, Trivedi HL, Feroze A, Kanodia KV, Dave SD, Shah PR. Effect of co-transplantation of mesenchymal stem cells and hematopoietic stem cells as compared to hematopoietic stem cell transplantation alone in renal transplantation to achieve donor hypo-responsiveness. International urology and nephrology. 2011;43(1):225–32. doi: 10.1007/s11255-009-9659-1. Epub 2010/01/20. [DOI] [PubMed] [Google Scholar]
- 3.Perico N, Casiraghi F, Introna M, Gotti E, Todeschini M, Cavinato RA, et al. Autologous mesenchymal stromal cells and kidney transplantation: a pilot study of safety and clinical feasibility. Clinical journal of the American Society of Nephrology : CJASN. 2011;6(2):412–22. doi: 10.2215/CJN.04950610. Epub 2010/10/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanikar AV, Trivedi HL. Stem cell transplantation in living donor renal transplantation for minimization of immunosuppression. Transplantation. 2012;94(8):845–50. doi: 10.1097/TP.0b013e3182664000. Epub 2012/09/21. [This study describes the use of donor-specific allogeneic AT-MSC in the context of a hyposensitivity inducing protocol.] [DOI] [PubMed] [Google Scholar]
- **5.Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2012;307(11):1169–77. doi: 10.1001/jama.2012.316. Epub 2012/03/23. [This study is the first large-scale randomized clinical trial reporting on the use of autologous BM-MSC in LDKT recipients. It demonstrates that MSC inoculum may replace induction therapy with anti-CD25 antibody and even reduce maintenance CNI dose without compromising graft survival, while associating with faster recovery of function, reduced ACR and lower OI.] [DOI] [PubMed] [Google Scholar]
- 6.Tan J, Pileggi A, Ricordi C. Stem Cell Therapy in Kidney Transplantation—Reply. JAMA : the journal of the American Medical Association. 2012;308(2):130–1. doi: 10.1001/jama.2012.6370. [DOI] [PubMed] [Google Scholar]
- *7.Peng Y, Ke M, Xu L, Liu L, Chen X, Xia W, et al. Donor-derived mesenchymal stem cells combined with low-dose tacrolimus prevent acute rejection after renal transplantation: a clinical pilot study. Transplantation. 2013;95(1):161–8. doi: 10.1097/TP.0b013e3182754c53. Epub 2012/12/25. [This pilot study describes that donor-specific allogeneic MSC treatment may allow for the reduction of CNI maintenance dose in LDKT recipients.] [DOI] [PubMed] [Google Scholar]
- 8.Reinders ME, de Fijter JW, Roelofs H, Bajema IM, de Vries DK, Schaapherder AF, et al. Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: results of a phase I study. Stem cells translational medicine. 2013;2(2):107–11. doi: 10.5966/sctm.2012-0114. Epub 2013/01/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9*.Lee H, Park JB, Lee S, Baek S, Kim H, Kim SJ. Intra-osseous injection of donor mesenchymal stem cell (MSC) into the bone marrow in living donor kidney transplantation; a pilot study. Journal of translational medicine. 2013;11:96. doi: 10.1186/1479-5876-11-96. Epub 2013/04/13. [This pilot study demonstrates the safety of intra-osseous inoculum of donor-specific allogeneic BM-MSC in LDKT recipients under conventional immunosuppression.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.Perico N, Casiraghi F, Gotti E, Introna M, Todeschini M, Cavinato RA, et al. Mesenchymal stromal cells and kidney transplantation: pretransplant infusion protects from graft dysfunction while fostering immunoregulation. Transpl Int. 2013;26(9):867–78. doi: 10.1111/tri.12132. Epub 2013/06/07. [This case report study evaluates the effect of timing of autologous BM-MSC in two recipients of LDKT and compares outcomes to historical controls.] [DOI] [PubMed] [Google Scholar]
- 11.Caplan AI, Correa D. The MSC: an injury drugstore. Cell stem cell. 2011;9(1):11–5. doi: 10.1016/j.stem.2011.06.008. Epub 2011/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. Epub 2006/08/23. [DOI] [PubMed] [Google Scholar]
- 13.Ricordi C. Back to the Future: Mesenchimal Stem Cells. CellR4. 2013;1(2):152–4. [PMC free article] [PubMed] [Google Scholar]
- 14.Hoogduijn MJ, Crop MJ, Peeters AM, Van Osch GJ, Balk AH, Ijzermans JN, et al. Human heart, spleen, and perirenal fat-derived mesenchymal stem cells have immunomodulatory capacities. Stem cells and development. 2007;16(4):597–604. doi: 10.1089/scd.2006.0110. Epub 2007/09/06. [DOI] [PubMed] [Google Scholar]
- 15.Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem cells and development. 2012;21(14):2724–52. doi: 10.1089/scd.2011.0722. Epub 2012/04/04. [DOI] [PubMed] [Google Scholar]
- **16.Menard C, Pacelli L, Bassi G, Dulong J, Bifari F, Bezier I, et al. Clinical-grade mesenchymal stromal cells produced under various good manufacturing practice processes differ in their immunomodulatory properties: standardization of immune quality controls. Stem cells and development. 2013;22(12):1789–801. doi: 10.1089/scd.2012.0594. Epub 2013/01/24. [This study compared the immunomodulatory properties of clinica-grade MSC and demonstrates the different potency based on different techniques of cell isolation and expansion.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Coppi P. Regenerative Medicine for Congenital Malformation: New Opportunities for Therapy. CellR4. 2013;1(2):123–7. [Google Scholar]
- 18.Crisan M, Deasy B, Gavina M, Zheng B, Huard J, Lazzari L, et al. Purification and long-term culture of multipotent progenitor cells affiliated with the walls of human blood vessels: myoendothelial cells and pericytes. Methods in cell biology. 2008;86:295–309. doi: 10.1016/S0091-679X(08)00013-7. Epub 2008/04/30. [DOI] [PubMed] [Google Scholar]
- 19.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell stem cell. 2008;3(3):301–13. doi: 10.1016/j.stem.2008.07.003. Epub 2008/09/13. [DOI] [PubMed] [Google Scholar]
- 20.Corselli M, Crisan M, Murray IR, West CC, Scholes J, Codrea F, et al. Identification of perivascular mesenchymal stromal/stem cells by flow cytometry. Cytometry Part A : the journal of the International Society for Analytical Cytology. 2013;83(8):714–20. doi: 10.1002/cyto.a.22313. Epub 2013/07/03. [DOI] [PubMed] [Google Scholar]
- 21.Tilney NL, Guttmann RD. Effects of initial ischemia/reperfusion injury on the transplanted kidney. Transplantation. 1997;64(7):945–7. doi: 10.1097/00007890-199710150-00001. Epub 1997/11/05. [DOI] [PubMed] [Google Scholar]
- 22.Gasser M, Waaga AM, Laskowski IA, Tilney NL. The influence of donor brain death on short and long-term outcome of solid organ allografts. Annals of transplantation : quarterly of the Polish Transplantation Society. 2000;5(4):61–7. Epub 2001/08/14. [PubMed] [Google Scholar]
- 23.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells, tissues, organs. 2001;169(1):12–20. doi: 10.1159/000047856. Epub 2001/05/08. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Xiang LX, Shao JZ, Pan RL, Wang YX, Dong XJ, et al. Recruitment of endogenous bone marrow mesenchymal stem cells towards injured liver. Journal of cellular and molecular medicine. 2010;14(6B):1494–508. doi: 10.1111/j.1582-4934.2009.00912.x. Epub 2009/09/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Vries DK, Schaapherder AF, Reinders ME. Mesenchymal stromal cells in renal ischemia/reperfusion injury. Frontiers in immunology. 2012;3:162. doi: 10.3389/fimmu.2012.00162. Epub 2012/07/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Souidi N, Stolk M, Seifert M. Ischemia-reperfusion injury: beneficial effects of mesenchymal stromal cells. Current opinion in organ transplantation. 2013;18(1):34–43. doi: 10.1097/MOT.0b013e32835c2a05. Epub 2012/12/21. [DOI] [PubMed] [Google Scholar]
- 27.Therasse A, Wallia A, Molitch ME. Management of post-transplant diabetes. Current diabetes reports. 2013;13(1):121–9. doi: 10.1007/s11892-012-0346-8. Epub 2012/11/29. [DOI] [PubMed] [Google Scholar]
- 28.Guerra G, Ilahe A, Ciancio G. Diabetes and kidney transplantation: past, present, and future. Current diabetes reports. 2012;12(5):597–603. doi: 10.1007/s11892-012-0306-3. Epub 2012/08/09. [DOI] [PubMed] [Google Scholar]
- 29.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. The New England journal of medicine. 2008;358(4):353–61. doi: 10.1056/NEJMoa071074. Epub 2008/01/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LoCascio SA, Morokata T, Chittenden M, Preffer FI, Dombkowski DM, Andreola G, et al. Mixed chimerism, lymphocyte recovery, and evidence for early donor-specific unresponsiveness in patients receiving combined kidney and bone marrow transplantation to induce tolerance. Transplantation. 2010;90(12):1607–15. doi: 10.1097/TP.0b013e3181ffbaff. Epub 2010/11/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strober S, Benike C, Krishnaswamy S, Engleman EG, Grumet FC. Clinical transplantation tolerance twelve years after prospective withdrawal of immunosuppressive drugs: studies of chimerism and anti-donor reactivity. Transplantation. 2000;69(8):1549–54. doi: 10.1097/00007890-200004270-00005. Epub 2000/06/03. [DOI] [PubMed] [Google Scholar]
- 32.Starzl TE. Immunosuppressive therapy and tolerance of organ allografts. The New England journal of medicine. 2008;358(4):407–11. doi: 10.1056/NEJMe0707578. Epub 2008/01/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leventhal J, Abecassis M, Miller J, Gallon L, Tollerud D, Elliott MJ, et al. Tolerance induction in HLA disparate living donor kidney transplantation by donor stem cell infusion: durable chimerism predicts outcome. Transplantation. 2013;95(1):169–76. doi: 10.1097/TP.0b013e3182782fc1. Epub 2012/12/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leventhal J, Abecassis M, Miller J, Gallon L, Ravindra K, Tollerud DJ, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Science translational medicine. 2012;4(124):124ra28. doi: 10.1126/scitranslmed.3003509. Epub 2012/03/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Experimental hematology. 2002;30(1):42–8. doi: 10.1016/s0301-472x(01)00769-x. Epub 2002/02/02. [DOI] [PubMed] [Google Scholar]
- 36.Hoogduijn MJ, Popp FC, Grohnert A, Crop MJ, van Rhijn M, Rowshani AT, et al. Advancement of mesenchymal stem cell therapy in solid organ transplantation (MISOT). Transplantation. 2010;90(2):124–6. doi: 10.1097/TP.0b013e3181ea4240. Epub 2010/07/08. [DOI] [PubMed] [Google Scholar]
- 37*.Franquesa M, Hoogduijn MJ, Reinders ME, Eggenhofer E, Engela AU, Mensah FK, et al. Mesenchymal Stem Cells in Solid Organ Transplantation (MiSOT) Fourth Meeting: Lessons Learned from First Clinical Trials. Transplantation. 2013;96(3):234–8. doi: 10.1097/TP.0b013e318298f9fa. Epub 2013/06/14. [This is a white paper issued by the Mesenchymal Stem Cells and Solid Organ Transplantation (MiSOT) Study Group regarding the translation of MSC therapies in SOT.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dahlke MH, Hoogduijn M, Eggenhofer E, Popp FC, Renner P, Slowik P, et al. Toward MSC in solid organ transplantation: 2008 position paper of the MISOT study group. Transplantation. 2009;88(5):614–9. doi: 10.1097/TP.0b013e3181b4425a. Epub 2009/09/11. [DOI] [PubMed] [Google Scholar]
- 39.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–41. doi: 10.1016/S0140-6736(04)16104-7. Epub 2004/05/04. [DOI] [PubMed] [Google Scholar]
- 40.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–86. doi: 10.1016/S0140-6736(08)60690-X. Epub 2008/05/13. [DOI] [PubMed] [Google Scholar]
- 41.Ekberg H, Tedesco-Silva H, Demirbas A, Vitko S, Nashan B, Gurkan A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. The New England journal of medicine. 2007;357(25):2562–75. doi: 10.1056/NEJMoa067411. Epub 2007/12/21. [DOI] [PubMed] [Google Scholar]
- 42.Zhao WY, Zhang L, Han S, Zhu YH, Wang LM, Zhou MS, et al. Evaluation of living related kidney donors in China: policies and practices in a transplant center. Clinical transplantation. 2010;24(5):E158–62. doi: 10.1111/j.1399-0012.2010.01229.x. Epub 2010/03/20. [DOI] [PubMed] [Google Scholar]
- 43.Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. International journal of molecular medicine. 2004;14(6):1035–41. Epub 2004/11/18. [PubMed] [Google Scholar]
- 44.Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. Journal of the American Society of Nephrology : JASN. 2004;15(7):1794–804. doi: 10.1097/01.asn.0000128974.07460.34. Epub 2004/06/24. [DOI] [PubMed] [Google Scholar]
- 45.Ciancio G, Burke GW, Garcia-Morales R, Suzart K, Rosen A, Ricordi C, et al. Effect of living-related donor bone marrow infusion on chimerism and in vitro immunoregulatory activity in kidney transplant recipients. Transplantation. 2002;74(4):488–96. doi: 10.1097/00007890-200208270-00010. Epub 2002/09/28. [DOI] [PubMed] [Google Scholar]
- 46.Hanaway MJ, Woodle ES, Mulgaonkar S, Peddi VR, Kaufman DB, First MR, et al. Alemtuzumab induction in renal transplantation. The New England journal of medicine. 2011;364(20):1909–19. doi: 10.1056/NEJMoa1009546. Epub 2011/05/20. [DOI] [PubMed] [Google Scholar]
- 47.Tan J, Qiu J, Lu T, Liu Z, Guo Y, Zhang S, et al. Thirty years of kidney transplantation in two Chinese centers. Clinical transplants. 2005:203–7. Epub 2007/04/12. [PubMed] [Google Scholar]
- 48.Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28(12):2229–38. doi: 10.1002/stem.544. Epub 2010/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49*.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15(6):641–8. doi: 10.1016/j.jcyt.2013.02.006. Epub 2013/04/11. [This study provides guidelines for the definition and characterization of AT-MSC.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grisendi G, Anneren C, Cafarelli L, Sternieri R, Veronesi E, Cervo GL, et al. GMP-manufactured density gradient media for optimized mesenchymal stromal/stem cell isolation and expansion. Cytotherapy. 2010;12(4):466–77. doi: 10.3109/14653241003649510. Epub 2010/04/01. [DOI] [PubMed] [Google Scholar]
- 51*.Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L. Immunological characterization of multipotent mesenchymal stromal cells-The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy. 2013;15(9):1054–61. doi: 10.1016/j.jcyt.2013.02.010. Epub 2013/04/23. [This paper provides the guidelines of the ISCT on the immunological characterization of MSCs.] [DOI] [PubMed] [Google Scholar]
- 52.Crop MJ, Baan CC, Korevaar SS, Ijzermans JN, Pescatori M, Stubbs AP, et al. Inflammatory conditions affect gene expression and function of human adipose tissue-derived mesenchymal stem cells. Clinical and experimental immunology. 2010;162(3):474–86. doi: 10.1111/j.1365-2249.2010.04256.x. Epub 2010/09/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan J, Tie G, Wang S, Messina KE, DiDato S, Guo S, et al. Type 2 diabetes restricts multipotency of mesenchymal stem cells and impairs their capacity to augment postischemic neovascularization in db/db mice. Journal of the American Heart Association. 2012;1(6):e002238. doi: 10.1161/JAHA.112.002238. Epub 2013/01/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y, Li Z, Liu T, Xue X, Jiang H, Huang J, et al. Impaired cardioprotective function of transplantation of mesenchymal stem cells from patients with diabetes mellitus to rats with experimentally induced myocardial infarction. Cardiovascular diabetology. 2013;12:40. doi: 10.1186/1475-2840-12-40. Epub 2013/03/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fiorina P, Jurewicz M, Augello A, Vergani A, Dada S, La Rosa S, et al. Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol. 2009;183(2):993–1004. doi: 10.4049/jimmunol.0900803. Epub 2009/06/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56*.Roemeling-van Rhijn M, Reinders ME, de Klein A, Douben H, Korevaar SS, Mensah FK, et al. Mesenchymal stem cells derived from adipose tissue are not affected by renal disease. Kidney international. 2012;82(7):748–58. doi: 10.1038/ki.2012.187. Epub 2012/06/15. [This study suggests that renal disease is not affecting AT-MSCs qualities and that they may be suitable for autologous use.] [DOI] [PubMed] [Google Scholar]
- 57.Reinders ME, Roemeling-van Rhijn M, Khairoun M, Lievers E, de Vries DK, Schaapherder AF, et al. Bone marrow-derived mesenchymal stromal cells from patients with end-stage renal disease are suitable for autologous therapy. Cytotherapy. 2013;15(6):663–72. doi: 10.1016/j.jcyt.2013.01.010. Epub 2013/02/20. [DOI] [PubMed] [Google Scholar]
- **58.Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PloS one. 2012;7(10):e47559. doi: 10.1371/journal.pone.0047559. Epub 2012/11/08. [This meta-analysis study on a large sample size of patients who received MSC therapies supports the safety of the procedure.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoogduijn MJ, Crop MJ, Korevaar SS, Peeters AM, Eijken M, Maat LP, et al. Susceptibility of human mesenchymal stem cells to tacrolimus, mycophenolic acid, and rapamycin. Transplantation. 2008;86(9):1283–91. doi: 10.1097/TP.0b013e31818aa536. Epub 2008/11/14. [DOI] [PubMed] [Google Scholar]
- 60.Buron F, Perrin H, Malcus C, Hequet O, Thaunat O, Kholopp-Sarda MN, et al. Human mesenchymal stem cells and immunosuppressive drug interactions in allogeneic responses: an in vitro study using human cells. Transplantation proceedings. 2009;41(8):3347–52. doi: 10.1016/j.transproceed.2009.08.030. Epub 2009/10/28. [DOI] [PubMed] [Google Scholar]
- 61.Eggenhofer E, Renner P, Soeder Y, Popp FC, Hoogduijn MJ, Geissler EK, et al. Features of synergism between mesenchymal stem cells and immunosuppressive drugs in a murine heart transplantation model. Transplant immunology. 2011;25(2-3):141–7. doi: 10.1016/j.trim.2011.06.002. Epub 2011/06/28. [DOI] [PubMed] [Google Scholar]
- 62.Popp FC, Eggenhofer E, Renner P, Slowik P, Lang SA, Kaspar H, et al. Mesenchymal stem cells can induce long-term acceptance of solid organ allografts in synergy with low-dose mycophenolate. Transplant immunology. 2008;20(1-2):55–60. doi: 10.1016/j.trim.2008.08.004. Epub 2008/09/03. [DOI] [PubMed] [Google Scholar]
- 63*.Franquesa M, Baan CC, Korevaar SS, Engela AU, Roemeling-van Rhijn M, Weimar W, et al. The effect of rabbit antithymocyte globulin on human mesenchymal stem cells. Transpl Int. 2013;26(6):651–8. doi: 10.1111/tri.12109. Epub 2013/05/21. [This study demonstrates that RATG may bind to MSC impairing viability and immunomodulatory properties in vitro.] [DOI] [PubMed] [Google Scholar]
- **64.Griffin MD, Ryan AE, Alagesan S, Lohan P, Treacy O, Ritter T. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: what have we learned so far? Immunology and cell biology. 2013;91(1):40–51. doi: 10.1038/icb.2012.67. Epub 2012/12/05. [This study summarizes the literature evidence of the potential alloresponse elicited by allogeneic MSCs.] [DOI] [PubMed] [Google Scholar]
- 65*.Ricordi C. Towards a constructive debate and collaborative efforts to resolve current challenges in the delivery of novel cell based therapeutic strategies. CellR4. 2013;1(1):2–7. [This manuscript contributes to the debate on stem cell therapy and regulatory challenges of its clinical translation.] [PMC free article] [PubMed] [Google Scholar]


