Abstract
Importance
Guidelines recommend individualizing screening mammography decisions for women 75 and older. However, little pragmatic guidance is available to inform this approach.
Objective
To provide an evidence-based approach to individualizing decision-making about screening mammography that considers older women's risk of breast cancer and the potential benefits and harms of screening in the context of varying life expectancies and preferences.
Evidence Acquisition
We searched PubMed for English-language studies in peer-reviewed journals published from January 1, 1990 to February 1, 2014 to identify risk factors for late-life breast cancer in women 65 and older and to quantify the benefits and harms of screening mammography for women 75 and older.
Findings
Age is the major risk factor for late-life breast cancer. In general, traditional breast cancer risk factors (e.g., age at first birth, age at menarche) that represent hormonal exposures in the distant past are less predictive of late-life breast cancer than factors indicating recent exposure to endogenous hormones (e.g., bone mass, obesity). None of the randomized trials of screening mammography included women over age 74, such that it is uncertain whether screening mammography is beneficial in these women. Observational data favor extending screening mammography to older women who have a life expectancy > 5-10 years. Modeling studies suggest approximately 2 fewer women per 1,000 die from breast cancer if women in their 70's continue biennial screening for 10 years, versus stopping screening at age 69. Potential benefits must be weighed with potential harms of continued screening over ten years, which include false-positive mammograms (~200 per 1,000 women screened) and overdiagnosis (~13 per 1,000 women screened). Providing these frequencies both verbally and graphically may help inform older women's decision-making.
Conclusions and Relevance
For women with less than a 5-10 year life expectancy, recommendations to stop screening mammography should be framed around increased harms and the need to refocus health promotion on interventions likely to be beneficial over a shorter timeframe. For women with a life expectancy > 5-10 years, the decision about whether potential benefits of screening outweigh harms is a value judgment that requires a realistic understanding of screening outcomes.
Keywords: Mammography screening, older women, individualized decisions
THE PATIENT's STORY
Mrs. M is a 91-year-old woman who has had annual screening mammograms since age 50. She lives alone in her apartment and independently performs all activities of daily living. Her chronic medical conditions include hypertension and osteopenia, and she was diagnosed with intermittent claudication in 2010. Her medications include valsartan, furosemide and isosorbide dinitrate. Mrs. M had her only child at age 16, had menopause at age 50 and never used hormone therapy. She had a negative breast biopsy in 1984. Her daughter died of breast cancer at age 37.
In 2008, at age 87, she had an abnormal screening mammogram with microcalcifications in the left medial inferior breast, interpreted as BI-RADS (Breast Imaging-Reporting Data System) category 3 (probably benign). She subsequently underwent three 6-month follow-up diagnostic mammograms. The third mammogram, in 2010, showed interval increase in the number of heterogeneous microcalcifications and was classified as BI-RADS category 5 (highly suggestive of malignancy). The lesion was not amenable to biopsy under stereotactic guidance. Therefore, she underwent excisional biopsy of the left breast lesion using needle localization. The biopsy identified ductal carcinoma in situ (DCIS), intermediate grade without comedo-type necrosis, on 2 of 9 slides. Estrogen receptor staining was strongly positive. One area of DCIS was < 1mm from the anterior margin so she underwent re-excision and no residual DCIS was identified. She met with a radiation oncologist who did not recommend radiation therapy, and Ms. M declined hormone therapy due to concerns about side effects. She continues to have annual mammograms, which have been negative, and she is seen by breast oncology every 6 months.
PERSPECTIVES
Mrs. M: I get mammograms every year. I know you don't get them all your life. Dr. P: I think people might say: “What are you doing getting mammograms in a 91-year-old?” but you have to meet this lady. She is a lot more likely to live to be 100 than I am.
There is considerable uncertainty about the benefit of screening mammography in women age 75 years and older. While meta-analyses of randomized controlled trials for women ages 50 to 74 years indicate screening mammography is associated with a reduction in breast cancer mortality of 15% to 25% after 10 to 15 years, none of the trials included women over age 74.1-3 Given this lack of trial data, most guideline panels and organizations recommend decisions about screening mammography in older women be individualized, weighing potential benefits and harms of screening in the context of a woman's overall health, life expectancy and preferences (Table 1).4-10 However, little pragmatic guidance is available to inform this approach to individualizing screening mammography decisions in older women.
Table 1.
Guidelines for Screening Mammography among Women 75 years or Older*
| Organization | Year Guideline Issued | Screening Mammography Recommendations |
|---|---|---|
| American Cancer Society4 | 2010 | There is no specific upper age at which mammography screening should be discontinued. Rather, the decision to stop regular mammography screening should be individualized based on the potential benefits and risks of screening in the context of overall health and estimated longevity. As long as a woman is in good health and would be a candidate for breast cancer treatment, she should continue to be screened with mammography. If performed, recommend screening every 1 year. |
| American College of Obstetricians and Gynecologists5 | 2011 | Women aged 75 or older should, in consultation with their physicians, decide whether or not to continue mammographic screening. Medical comorbidity and life expectancy should be considered. If performed, recommend screening every 1 year. |
| American College of Radiology6 | 2008 | It is unclear at what age, if any, women cease to benefit from screening mammography. Because this age is likely to vary depending on the individual's overall health, the decision as to when to stop routine mammography screening should be made on an individual basis by each woman and her physician. If performed, recommend screening every 1 year. |
| Canadian Task Force on Preventive Health Care7 | 2011 | A tailored approach to screening mammography is warranted in women aged 70 years or older. If a woman desires to continue screening mammography, it is justified if her life expectancy exceeds 5-10 years (weak recommendation; low quality evidence). If performed, recommend screening every 2-3 years. |
| National Comprehensive Cancer Network8 | 2013 | In older women, mammography screening should be individualized, weighing its potential benefits/risks in the context of the patient's overall health and estimated longevity. If a patient has severe comorbid conditions limiting her life expectancy and no intervention would occur based on the screening findings, then the patient should not undergo screening. If performed, recommend screening every 1 year. |
| National Health Service, United Kingdom9 | 2010 | Women aged 74 or older can request continued mammography screening, but they do not receive routine invitations. If performed, recommend screening every 3 years. |
| US Preventive Services Task Force10 | 2009 | Evidence is insufficient to assess the additional benefits and harms of screening mammography in women 75 years or older. No recommendation (I statement—If the service is offered, patients should understand the uncertainty about the balance of benefits and harms). If performed, recommend screening every 2 years. |
Recommendations are based on each organization's literature review, consensus process and expert opinion.
METHODS
We searched PubMed for English-language studies in peer-reviewed journals published from January 1, 1990 to February 1, 2014, focused on women 65 years and older, screening mammography and breast cancer. Systematic searches were completed to: (1) identify risk factors for breast cancer in women 65 years and older; and (2) estimate potential benefits and harms of screening mammography in women 75 years and older. Explanations of search strategies and publications resulting from each search are presented in the eAppendix. Studies were excluded if they lacked outcomes specific to the subgroups of women in the above age ranges.
RISK STRATIFICATION IN OLDER WOMEN
Because the probability that a woman will benefit from screening mammography depends on her risk for developing clinically significant breast cancer in her lifetime, most screening algorithms start by stratifying women into average- and increased-risk categories. However, the process for identifying women at increased risk for developing breast cancer differs for older women, as the relative importance of risk factors changes with advancing age and consideration of life expectancy becomes more salient.
Estimating Late-life Breast Cancer Risk
The Gail model, which integrates multiple breast cancer risk factors into a risk score, is commonly used to identify women at increased risk for developing breast cancer.11 However, its performance was evaluated in a cohort of Vermont women 70 and older and was found to predict breast cancer only slightly better than flipping a coin (c-statistic 0.54).12 The Gail model includes family history and reproductive factors which become less predictive of breast cancer in older women.13 Table 2 presents results from a systematic literature review to identify risk factors for late-life breast cancer. We included studies that focused on women 65 and older because focusing on women 75 and older would have included only four studies. In general, factors that represent hormonal exposures in the distant past (e.g., age at first birth, age at menarche) are less predictive of late-life breast cancer than factors indicating recent exposure to endogenous hormones (e.g., life-long obesity, high bone mass, high breast density). Moreover, use of estrogen plus progesterone increases the incidence of breast cancer even among women 75 and older, but the risk declines rapidly within 2 years after discontinuation and few older women currently use this medication.14 Whether race is a risk factor for late-life breast cancer is uncertain. White women ages 75-84 have a higher incidence of breast cancer than African American women in this age range, but the difference may be a result of differential use of mammography.15 In addition, while family history of breast cancer highly influences older women's decisions to continue screening, as was the case for Mrs. M, advancing age is actually the major risk factor for breast cancer.16 The incidence of breast cancer increases substantially with age, peaking between ages 75-79.17
Table 2.
Risk Factors for Breast Cancer among Women Aged 65 Years and Oldera
| Risk Factor | |||
|---|---|---|---|
| AGE, years | (Incidence rates per 100,000 women per year; SEER data 2006-2010)1 | ||
| Overall | Whites | Blacks | |
| 50-54 | 223 | 227 | 221 |
| 55-59 | 268 | 274 | 275 |
| 60-64 | 346 | 359 | 329 |
| 65-69 | 413 | 431 | 382 |
| 70-74 | 425 | 445 | 397 |
| 75-79 | 440 | 462 | 405 |
| 80-84 | 420 | 436 | 396 |
| 85+ | 357 | 365 | 372 |
| OTHER RISK FACTORS | Range of 95% Confidence Intervals of Adjusted Valuesb |
|---|---|
| Family history of breast cancer | RRs and HRs |
| At least 1 first degree relative | 0.90-1.99 (significant2-4, not significant5,6) |
| Increasing Body Mass Index | RRs, ORs, and HRs |
| 0.32-2.98 (significant2,3,5,7-11, not significant12,13) | |
| Reproductive Factors | RRs, ORs, and HRs |
| Age >14 years at menarche (reference <11-13 y) | 0.55-1.92 (significant14, not significant2,3,5-7,15,16) |
| Age >30 years at first live birth (reference <19-22 y)c | 0.69-2.31 (significant2,14, not significant3,5-7,16) |
| Age ≥ 50 years at menopause (reference <45 y)d,e | 0.73-2.6 (significant14,15, not significant2,3,6,7,16) |
| 4 or more live births (reference =1) | 0.37-1.53 (significant2,3,6,7,14, not significant16) |
| Nulliparity (reference =parity) | 0.59-1.65 (significant14, not significant2,3,7,16) |
| >12 months breastfeeding (reference =never) | 0.21-1.27 (significant16, not significant6,7) |
| Bone Density | RRs |
| Increasing Bone Density: Hip | 1.01-4.8 (significant17,18) |
| Increasing Bone Density: Distal radius | 1.1-1.95 (significant6,17,19) |
| RRs (not significant5)f | |
| Breast density: Extremely dense | 0.56-2.92 |
| Previous breast biopsy | RR 1.06-1.60 (significant5) |
| Smoking (reference=never) | ORs and HRs |
| Former | 1.0-1.5 (significant20, not significant2) |
| Current | 0.7-1.9 (significant20, not significant2,6) |
| Alcohol Consumption | ORs and HRs |
| 0.86-4.20 (significant21,22, not significant2,6) | |
| Physical Activity | RR |
| Highly active | 0.05-1.2 (significant23, not significant6,21) |
| Hormone Replacement Therapy (reference=placebo) | HR |
| Estrogen plus progesterone | 0.88-2.04 (not significant24)g |
| Estrogen alone | 0.53-1.23 (not significant25,26) |
We included studies that presented risk ratios specific for women at least 65 years and older. References 1-28 for Table 2 are listed in the eAppendix, Search #1 Strategy.
We present the range of 95% confidence intervals for each risk factor. Significant refers to references where the 95% confidence interval does not cross 1.0. Abbreviations: ORs, odds ratios; RRs, risk ratios; HRs, hazards ratios.
In 1 study the maximum age was ≥28 years.16
In 1 study the cut-off was ≥52 years.16
One study used <48 years as the reference range.16
Two additional studies showed incidence of breast cancer increases among women aged 70 years and older who have increased breast density but did not present a measure of association.27,28
Although the HR for estrogen plus progesterone was not significant when stratified by age, overall use of estrogen plus progesterone increased invasive breast cancer by a HR of 1.24, (weighted p<0.001) in the Women's Health Initiative Randomized Trial and there was no interaction by age.
Estimating Life Expectancy
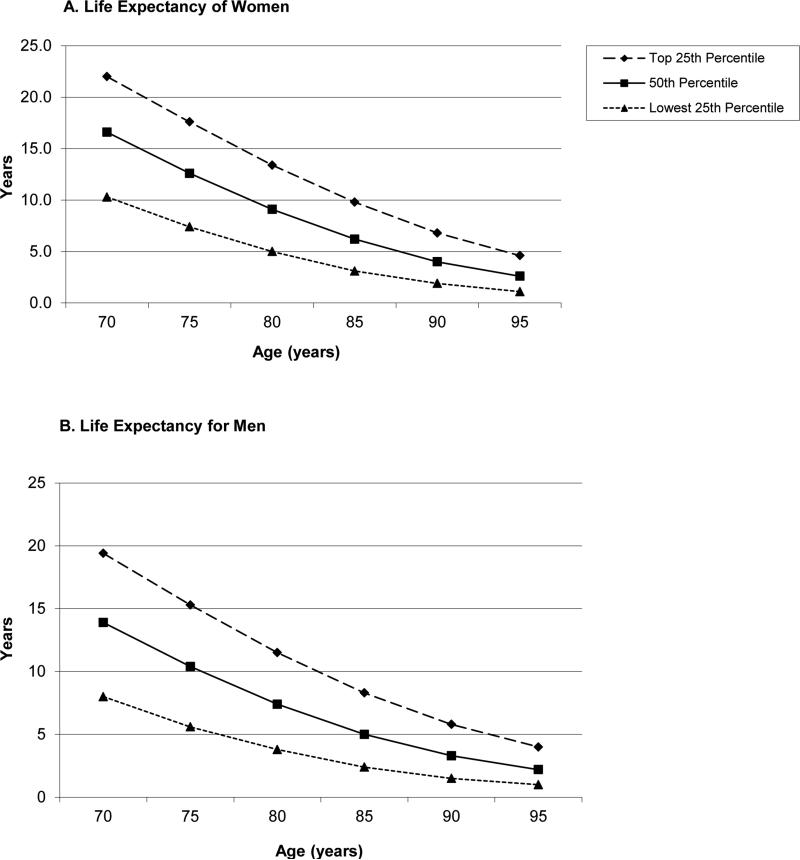
While the risk of developing and dying from breast cancer increases with advancing age, which favors screening, decreases in overall life expectancy reduce the chance of dying of an asymptomatic screen-detectable cancer. Risk stratification in older women must weigh these opposing factors to identify older women with substantial life expectancy, who are most likely to benefit from screening.18 Age alone is a crude predictor of life expectancy as illustrated by Figure 1, which shows the substantial variability in life expectancy that exists at each age for women and men in the U.S. (updated from Walter 2001,18 based on 2008 U.S. Life Tables).19 For example, Mrs. M had no significant comorbid conditions or functional impairments when she underwent screening mammography at age 87 in 2008, suggesting that she was in the upper quartile of life expectancy for her age-sex subgroup. This clinical judgment is corroborated by prognostic indices for predicting 4-10 year mortality in community dwelling elders described in a recent systematic review and available on the ePrognosis website.20 These indices incorporate age, comorbidities, and functional status and were developed and validated using data from national surveys of older adults. Based on these indices, in 2008 Mrs. M had greater than a 50% probability of living 10 years or more, meaning her life expectancy exceeded 10 years. While the effectiveness of these indices across diverse clinical settings requires further study, some clinicians find these indices useful in corroborating their judgments about prognosis.20
Figure 1.
Upper, middle, and lower quartiles of life expectancy for women and men at selected ages.*
*Data are from the 2008 Life Tables of the United States. This figure shows, for example, that 25% of 90-year-old women in the United States will live more than 6.8 years, 50% will live at least 4.0 years and 25% will live less than 1.9 years. See eAppendix Calculations for Figure 1.
ESTIMATING BENEFITS AND HARMS OF SCREENING MAMMOGRAPHY IN OLDER WOMEN
Benefits of Screening Mammography in Older Women
Dr. P: Breast cancer can be awful at any age and it does increase as women get older. I would hate to have her come in with a big mass in her breast because that is a much harder situation than dealing with something small on a mammogram.
Mrs. M: Whatever it was in my breast was found through a mammogram. I didn't even know it was there.
The benefit of screening mammography is finding breast cancer at an early, asymptomatic stage when treatment is expected to be more effective in reducing breast cancer mortality than if treatment was begun later when the cancer presents symptomatically. The appropriate measure of screening benefit, therefore, is reduction in mortality from breast cancer in women offered screening mammography compared to women not offered screening.2,21 However, none of the randomized controlled trials evaluating screening mammography included women over age 74, such that there is no direct evidence that screening is beneficial in older women.
In the absence of randomized trial data, observational data are often used to provide evidence about the effectiveness of interventions in older adults. In general, retrospective cohort studies and case-control studies have found a reduction in breast cancer mortality associated with mammographic detection of breast cancer among women 75 years and older, although there was no reduction in breast cancer mortality for older women in poor health defined by Charlson comorbidity scores ≥ 2 (Table 3).22-24 However, the results of these studies may represent lead-time, length-time and selection biases rather than screening benefit.25 The significant methodological limitations of these studies are listed in Table 3. Data from prospective cohort studies suggest the accuracy of mammography for detecting cancers increases with age (Table 3). Sensitivity and specificity of mammography are highest in women older than 80 years, like Mrs. M, in whom sensitivity=86% and specificity=94% (versus sensitivity=73% and specificity=92% in 50-year-old women).26
Table 3.
Summary of Observational Data on Mortality Benefit and Downstream Harms of Screening Mammography in Women Aged 75 and Older.
| Study | Design | Limitations | Data Source | Results if Continued Screening | Results if Stopped Screening | Summary |
|---|---|---|---|---|---|---|
| Modeling Studies | ||||||
| Barratt et al. 20051 | -Markov model comparing biennial screening from age 50 to 79 vs. stopping at age 69 -Calculated outcomes over 10 years |
Applied same relative risk reduction in breast cancer mortality from screening for women > 70 years as for women 50-69 years; Non-U.S. data | BreastScreen Australia, Australian Institute of Health and Welfare and Australian Bureau of Statistics | -6 in 1,000 women die from breast cancer -35 in 1,000 women diagnosed with invasive breast cancer -6 in 1,000 women diagnosed with DCIS -140 in 1,000 women with false-positive mammogram |
-8 in 1,000 women die of breast cancer -25 in 1,000 women diagnosed with invasive breast cancer -0.5 in 1,000 women diagnosed with DCIS -0 false-positive mammograms |
Benefits: Over 10 years, 2 fewer women per 1,000 die from breast cancer vs. women who stop biennial screening at age 69 Harms: 140 more women with false-positive mammograms and 5.2 more women diagnosed with DCIS per 1,000 women screened to age 79 vs. age 69 |
| Mandelblatt et al. 20092 | -Multiple decision models comparing: a) biennial screening from age 50 to 79 vs. stopping at age 69 b)annual screening from age 50 to 79 vs. stopping at age 69 -Calculated outcomes over lifetime of cohort |
Estimates of age-specific tumor natural history are limited by paucity of data; Model uses stage distributions among screened and unscreened women to calculate screening benefit. | Breast Cancer Surveillance Consortium (BCSC) | a)7% median improvement in percentage of mortality reduction from breast cancer b)8% median improvement in percentage of mortality reduction from breast cancer |
Not reported |
Benefits: Over a lifetime, 1-4 fewer women per 1,000 die from breast cancer vs. women who stop annual or biennial screening at age 69 Harms: a)240 more false-positive mammograms and 16 more unnecessary biopsies per 1,000 women biennially screened to age 79 vs. age 69. b)390 more false-positive mammograms and 27 more unnecessary biopsies per 1,000 annually screened to age 79 vs. age 69. |
| Schousboe et al. 20113 | Markov model comparing screening every 3-4 years vs. no screening in women 70-79 years, stratified by breast density. -Calculated outcomes over 10 years |
Results sensitive to assumptions about rates of DCIS detection and overdiagnosis with mammography; Model uses stage distributions among screened and unscreened women to calculate screening benefit. | BCSC | -Number needed to screen to prevent 1 death from breast cancer: 704 for women with breast density BIRADS 1; 491 for BIRADS 2; 339 for BIRADS 3; 337 for BIRADS 4 -False-positive results: 12% for women with BIRADS 1; 23% for women with BIRADS 2; 25% for women with BIRADS 3; 23% for women with BIRADS 4. |
Not reported |
Benefits: Over 10 years, 1-3 fewer women per 1,000 die from breast cancer vs. women who are not screened between ages 70-79. Harms: 12-25% risk of a false-positive mammogram depending on breast density. |
| Prospective Cohort Studies | ||||||
| Braithwaite et al 20134 | -Cohort study of 137,949 women aged 66 to 89 years who underwent screening mammography 1999-2006 and were not diagnosed with breast cancer -Outcomes calculated over 10 years |
Screening interval was based on time since previous mammogram and may not represent long-term patterns for individuals. | BCSC |
Ages 66-74 -Annual screening: 50% false-positive rate (48-52%, 95% CI) -Biennial screening: 30% false-positive rate (29-31%, 95% CI) Ages 75-89 -Annual screening: 47% false-positive rate (45-50%, 95% CI) -Biennial screening: 27% false-positive (26-28%, 95% CI) |
Not reported |
Benefits: Mortality benefit not determined Harms: Over 10 years the likelihood of a false-positive result is 1.7 times higher with annual versus biennial screening mammograms |
| Nelson et al. 20095 | -Cohort study of regularly screened women 70 and older (Number of women analyzed was not mentioned) -Outcomes calculated from a single screening round. |
Results based on single screening round; Incomplete capture of additional imaging and biopsies following screening mammography. | BCSC |
Ages 70-79: -69 in 1,000 women with a false-positive mammogram -1.4 in 1,000 women with screen-detected DCIS Ages 80-89: -59 in 1,000 women with a false-positive mammogram -1.5 in 1,000 women with screen-detected DCIS |
Not reported |
Benefits: Not determined Harms: 59-69 more women with false-positive mammograms and 1.5 more women diagnosed with DCIS per 1,000 women after one mammogram vs. women who are not screened. |
| Schonberg et al. 20096 | -Cohort study of 2,011 women ≥ 80 years comparing women screened with mammography since age 80 vs. those not screened after age 80 -Calculated outcomes over a median of 5 years of follow-up. |
Not powered to detect differences in breast cancer mortality between screened and unscreened women; Selection bias. | Screen-eligible women receiving care at one academic and two community health centers in Boston | -1 in 1,034 women died of breast cancer -20 in 1,034 women diagnosed with invasive breast cancer -8 in 1,034 women diagnosed with DCIS -110 false-positive mammograms and 19 more unnecessary biopsies among 1,034 screened |
-2 in 977 women died of breast cancer -20 in 997 diagnosed with invasive breast cancer -0 diagnosed with DCIS -0 false-positive mammograms |
Benefits: Over 5 years, 1 less woman per ~1,000 die from breast cancer vs. women who stop screening at age 80 Harms: 110 more women with false-positive mammograms and 8 more women diagnosed with DCIS per ~1,000 women screened after age 80 vs. stopping screening at age 80. |
| Carney et al. 20037 | -Cohort study of 329,495 regularly screened women aged 40-89 years who underwent screening mammograms between 1996 and 1998. -Described mammography outcomes over 1 year. |
Varying amounts of missing data across facilities; Only 3.9% of women were 80 and older. | BCSC |
Ages 70-79: -5.7 true-positive results per 1,000 mammograms -1.4 false-negative results per 1,000 mammograms -Sensitivity=81%; Specificity=94% Ages 80-89: -5.9 true-positive results per 1,000 mammograms -1.5 false-negative results per 1,000 mammograms -Sensitivity=86%; Specificity=94% |
Not reported |
Benefits: Mammography is most effective in detecting breast cancer in women ≥ 80 years. Harms: 1.5 in 1,000 mammograms are false-negatives in women ≥ 80 years. |
| Walter et al. 20018 | -Cohort study of 216 frail women (mean age = 81; 49% had cognitive impairment) who underwent a screening mammogram on enrollment in a health program. -Described outcomes over a mean of 3 years of follow-up. |
Small sample size; Includes only a single site in San Francisco | Women who enrolled in On Lok (a health program for nursing-home-eligible adults in San Francisco) | -0 women died of breast cancer -2 in 216 women diagnosed with invasive breast cancer -2 in 216 women diagnosed with clinically insignificant breast cancer or DCIS -28 false-positive mammograms and 6 women refused work-up of abnormal mammogram among 216 screened |
Not reported |
Benefits: Not determined Harms: 17% of frail screened women experienced a false-positive mammogram or had clinically insignificant disease identified after one screening mammogram. |
| Welch et al. 19989 | -Cohort study of 23,172 women ≥ 65 years who underwent screening mammography 1/95–4/95. -Described outcomes over 8 months. |
Some diagnostic mammograms may have been misclassified as screening; Medicare claims likely undercount cases of cancer | Medicare's National Claims History System |
Ages > 70: -81 in 1,000 women had additional imaging or biopsy -11 in 1,000 women were diagnosed with breast cancer |
Not reported |
Benefits: Not determined Harms: 70 in 1,000 women had additional imaging or biopsy in the 8 months after a screening mammogram and were not diagnosed with cancer. |
| Retrospective Cohort Studies and Case-Control Studies | ||||||
| Badgwell et al. 200810 | -Retrospective cohort study of 12,358 women ≥ 80 years diagnosed with breast cancer comparing women who received 0, 1-2, or 3+ screening mammograms during the 5 years before their breast cancer diagnosis | Lead-time; length-time and selection biases. | Surveillance, Epidemiology, and End Results-Medicare database | 5-year breast cancer-specific survival was 94% for women who received 3+ mammograms and 88% for women who received 1-2 mammograms | 5-year breast cancer-specific survival was 82% for women without any screening |
Benefits: Breast cancer survival was greater in screened women; However, similar improvements were seen for non-breast cancer-related survival suggesting the bias that healthier older women obtain mammography. Harms: Not determined |
| McPherson et al. 200211 | -Retrospective cohort study of 5,186 women aged 65 to 101 diagnosed with breast cancer, comparing women with tumors detected by mammography vs. women with tumors not detected by mammography, stratified by comorbidity. | Lead-time; length-time and selection biases; Disease-specific cause of death was not available. | Upper Midwest Tumor Registry System (regional database in Minnesota, North Dakota, and South Dakota) | All-cause mortality in screened vs. non-screened: No comorbidity: Ages 75-79: RR=0.36 (0.26-0.49); ages 80-84: RR=0.66 (0.52-0.83) 2+ Charlson comorbidities: Ages 75-79: RR=0.53 (0.2-1.36); ages 80-84: RR=0.64 (0.03-1.87) |
Not reported |
Benefits: All-cause mortality was lower in women with mammographically detected breast cancer, except when women had severe or multiple comorbidities. Harms: Not determined |
| McCarthy et al. 200012 | -Retrospective cohort study of 9,767 women aged 65+ diagnosed with breast cancer, comparing women who received mammography within 2 years of their cancer diagnosis vs. women who did not. | Lead-time, length-time and selection biases; No distinction was made between screening and diagnostic mammography | Three Surveillance, Epidemiology, and End Results Program regions | Not reported | 5-year breast cancer-specific mortality in nonusers vs. mammography users: Ages 75-84: adjusted HR=2.47 (1.70-3.58); Ages 85+: adjusted HR=1.45 (0.63-3.32) |
Benefits: 5-year breast cancer mortality was higher among women aged 75-84 who did not receive mammography within 2 years of their breast cancer diagnosis. Harms: Not determined |
| Van Dijck et al. 199613 | -Case-control study of women aged 65+, including 82 women with breast cancer (cases) and 410 age-matched controls. Compared women who attended screening vs. those who did not. | Lead-time, length-time and selection biases; Only included a small number of women 75 years and older. | Women invited to screening program in Nijmegan, the Netherlands | Breast cancer mortality in those who recently attended screening vs. those who did not: Ages 65-74: RR=0.45 (0.2-1.02); Ages 75+: RR=1.05 (0.27-4.14) |
Not reported |
Benefits: No significant difference in breast cancer mortality in screened vs. non-screened women aged 75+. Harms: Not determined |
The benefit of screening mammography is also dependent on there being effective treatment for early-stage breast cancer in older women. Unfortunately, few clinical trials of breast cancer treatments have included women 75 years and older, especially those with multiple comorbidities or frailty.27 Therefore, the benefits of some treatments are uncertain in this population. In practice, older women with DCIS or early-stage breast cancer are generally initially treated with lumpectomy with or without radiotherapy.28 Mastectomy is associated with equivalent survival outcomes as lumpectomy and is generally reserved for older women with large primary tumors or multicentric disease.29 Although radiotherapy is associated with a reduction in 10-year risk of local or regional recurrence (from 10% to 2%) among women 70 years and older with early-stage breast cancer also treated with hormone therapy, radiotherapy has not been shown to improve survival.30 Hormone therapy is recommended to older women with hormone-receptor-positive breast cancers because it has been associated with a reduction in cancer recurrence of 30-50% after 10 years and improved survival.31 Chemotherapy is generally reserved for healthy older women with lymph node-positive or hormone receptor-negative invasive cancers because chemotherapy is associated with improved breast cancer survival among these women.32 Among women with biopsy-detected DCIS there is a desire to identify women based on age, comorbidities and tumor characteristics who could forgo surgery and be followed with observation, but no study has identified such a group.33 However, as with screening, guidelines agree that DCIS and invasive breast cancer treatment decisions should be individualized based on treatment benefits and harms and patient preferences.34
Modeling studies combine the numerous factors that may influence screening mammography outcomes, such as breast cancer incidence and mortality, competing causes of death, mammography test characteristics and breast cancer treatment effects in order to estimate plausible benefits of extending screening to older women. There are three modeling studies that estimate benefits over various time horizons if screening mammography is continued in women aged 70 to 79 versus stopping screening at age 69 (Table 3).35-37 These modeling studies must make assumptions about the natural history of breast cancer in older women or the efficacy of screening mammography because of limited data. Also, many models assume that women invited for screening gain little or no mortality benefit in the first 5-10 years after starting screening.2,37 This lag-time to benefit timeframe is supported by a recent survival meta-analysis of the major trials of screening mammography.38 Also, despite differing methodologies and assumptions, modeling studies generally suggest some benefit for women who continue screening past age 69.35-37 The modeling study by Baratt et al estimates that after 10 years, compared with women who stop screening at age 69 years, women who continue biennial screening mammography into their 70s have 2 fewer women per 1000 die from breast cancer (6 vs 8 deaths from breast cancer per 1000 women). Findings from this modeling study were consistent with those from a prospective cohort study evaluating breast cancer screening for women screened after 80 years.37, 39 Cost-effectiveness analyses similarly suggest that it is cost-effective to conduct biennial screening mammography up until a life expectancy of 9.5 years, which can be expected for about 50% of 80-year-old women and 25% of 85-year-old women (Figure 1).40-42
Harms of Screening Mammography in Older Women
Dr. P: I thought she was very low risk for having any problems from an excisional biopsy and I knew that at any step along the way we could decide if we didn't need to do anything further.
Mrs. M: I had to have 2 surgeries because the first time they weren't sure if they got it all [the ductal carcinoma in situ]. I had no problems from the surgeries and it was a relief to know it was gone and that God would take care of it.
While the potential benefit of screening (e.g., reducing breast cancer mortality) occurs on average 5-10 years after mammography screening, the potential harms of screening occur immediately. Harms of mammography screening include pain, anxiety and complications from follow-up procedures after a false-positive mammogram (i.e., an abnormal mammogram requiring further assessment in a woman ultimately found not to have cancer) or after overdiagnosis (i.e., cancer detected by screening that would not otherwise have come to attention in the woman's lifetime).
Pain and anxiety are experienced in varying degrees by nearly every woman who has a false-positive mammogram and systematic reviews have found that cancer-specific psychological distress may persist for up to 3 years after a false-positive mammogram.43,44 However, few studies included women 75 and older. Among women 75 and older who undergo biennial screening the cumulative probability of a false-positive mammogram over 10 years ranges from 14-27%, and this risk nearly doubles if women are screened annually (Table 3). 26, 36, 37,45 Diagnostic mammography, breast ultrasound and/or breast biopsy are used to determine if an abnormal screening mammogram is a false alarm.39,46 These follow-up tests are considered low-risk procedures although a breast biopsy can cause distress, scarring and infections.47 Moreover, some older women may have cognitive impairment and other comorbidities that make follow-up procedures more painful (e.g., arthritis or hemiparesis causing discomfort with positioning for procedures), difficult (e.g., transportation challenges), or frightening (e.g., agitation in women with dementia who do not understand what is being done to them).48-50
Overdiagnosis is the major harm of cancer screening and increases with age due to decreasing life expectancy and an increasing proportion of slower growing cancers.51 Detection of invasive or in situ breast cancers that would not otherwise have clinically surfaced in the absence of screening leads to treatments that only cause harm because, by definition, treatments cannot improve outcomes of overdiagnosed cancers.21 However, establishing the risk of overdiagnosis has been challenging because different study designs and perspectives produce different estimates of overdiagnosis, which range from 0-54% for mammography.21,52 In addition, 20-30% of screen-detected breast cancers are DCIS.53 From the perspective of a woman considering screening mammography, studies with reasonable assumptions suggest approximately 30% of breast cancers (invasive and in situ) detected during the screening period are overdiagnosed cancers;54-56 however, this estimate has not been calculated specifically for women ≥ 70 years. Data from Barratt et al. suggest approximately 41 per 1,000 women ≥ 70 years who continue biennial mammography will be diagnosed with cancer (invasive or in situ) over 10 years.37 We therefore estimate that 13 of these women (13/41=32%) will experience the harm of overdiagnosis. The risk for overdiagnosis will be higher among screened women with less than a 5-10 year life expectancy because of their increased risk of dying from other causes before a screen-detected cancer can progress to symptoms.51
Currently, it is not possible to definitively determine which individual cases of breast cancer represent overdiagnosed cancers. Mrs. M's screening mammogram led to her being diagnosed with non-comedo intermediate-grade DCIS, which is a type of DCIS that is unlikely to recur or develop into invasive cancer during her lifetime and most likely represents overdiagnosis.57 However, given the uncertain natural history of untreated DCIS, she underwent lumpectomy and additional excision for close margins. In fact, 97% of U.S. women diagnosed with DCIS undergo surgery.57 Yet, harms of breast cancer treatment increase with age. Approximately 20% of women 65 and older experience complications from breast cancer surgery and the risk increases with age.58 Short-term decreases in cognition may occur among older women following general anesthesia and chemotherapy.34,59 Toxicity and mortality from chemotherapy increase with age.60 Breast radiotherapy can cause fatigue, breast pain and edema and increases the risk of ischemic heart disease.61,62 Tamoxifen can cause endometrial cancer and increase the risk of thromboembolism, particularly for older women, and aromatase inhibitors can cause joint pain, myalgias, heart disease, and fractures.21,34
Mrs. M chose not to pursue radiotherapy or hormone therapy and does not feel she was harmed by screening. Rather, she is thankful that her DCIS was detected and removed. Like most women, she has little awareness of DCIS or overdiagnosis as a possibility and continues to be screened and seen by oncology.63 There are no guidelines about when to stop screening women with a history of DCIS to inform care. To reduce the frequency of overdiagnosis and overtreatment requires finding appropriate ways to talk with women about these possibilities. Most women want information about screening harms and report that this knowledge would influence their decision-making.64
DISCUSSING SCREENING MAMMOGRAPHY WITH OLDER WOMEN
Mrs. M: I talked with my doctor [about mammography] but I never considered not having it. My daughter had breast cancer and she died.
Dr. P: I think I know when making a recommendation to stop screening is going to be received well and when it might not be received well. I have no problem having that discussion and having it differently with each patient.
Many women ≥ 75 years continue screening mammography, but few are informed of potential benefits and harms before being screened.16,65 This is likely because such discussions can be challenging and time consuming.64,66-67 Clinicians often report feeling ill-prepared for these discussions due to the complexity of the issues and uncertainties about screening outcomes in older women.16 In addition, clinician discussions and patient brochures about mammography screening tend to be uniformly positive, since the dominant public health approach has been to promote uptake of screening.65,68 The Affordable Care Act includes coverage of screening mammography regardless of life expectancy. No medical test has been as aggressively promoted as the mammogram. As a result, many older adults overestimate the benefits of screening and underestimate the harms.65, 67 Continuation of screening is generally viewed not as a decision but as something that is done automatically or as morally obligatory, whereas stopping screening is considered a major decision.65,66 Therefore, a clinician's recommendation to stop screening mammography may be jarring to some older patients, who expect clinicians to uniformly endorse screening.
Framing cancer screening conversations in terms of increasing harms in relation to decreasing benefits has been found to be most acceptable to older patients and may maintain or promote trust more than citing national guideline recommendations to stop screening based on age cutoffs.16,66 For women with less than a 5-10 year life expectancy, recommendations to stop screening mammography should be framed around how a woman's health problems increase the harms of screening (e.g., overdiagnosis) and shift the focus to interventions likely to be beneficial over a shorter time frame (e.g., falls prevention, depression screening). For women with > 5-10 year life expectancy, screening discussions should start by informing women that there is a choice to be made about whether or not to continue mammography. Clinicians should also inquire about a woman's preferred role in decision-making about mammography. Some women will prefer their clinician to make the final decision while others will prefer to share the decision with their clinician, or to make the final decision on their own.69 Regardless, most women want information about a decision and for their clinician to have a clear understanding or their values.69
The best method to elicit older women's values and preferences is not clear. Describing the harms and benefits of a decision and having patients weigh-in is the most common method used.70 Visual displays or graphics have been shown to improve risk communication and may enhance decision-making even among adults with low numeracy.71 The graphical format most recommended for conveying risk information is the pictograph, which visually represents frequencies rather than probabilities and simultaneously conveys both the numerator and denominator.71,72 While numerical information can be difficult for many older adults, words like “a low chance” are imprecise and can have very different numerical meaning to different patients. When possible, it is best to present the absolute risk or natural frequency of an outcome (e.g., 200 out of 1,000 women ≥ 75 years who are screened over 10 years will experience a false-alarm). To maximize comprehension, use the same denominator (e.g., 1,000 women) and time frame (e.g., 10 years) for communicating every outcome.71,72 Because older adults tend to focus more on positive aspects of a decision, presenting the harms of mammography before the benefits may aid in comprehension.73 Furthermore, full disclosure of all the potential harms and benefits of screening may result in information overload and poor quality decision-making.73 Therefore, it is important to focus on the major benefits and harms critical to a patient's decision-making.
Pictographs are particularly good at conveying the gist of risk information, which may be especially important for older adults who increasingly rely on their intuition to make decisions.73 Presenting a summary table of the pros and cons of screening has also been shown to improve patient understanding and can be used to help older women clarify their preferences around screening (eAppendix Table 1).74 Currently available decision aids aimed to inform older women's screening mammography decisions are listed in eAppendix Table 2.
CONCLUSIONS
For healthy older women with a life expectancy > 5-10 years there is no single correct answer for how to balance the harms and benefits of screening at a particular age. Instead, clinicians may start by explaining that for women ≥ 75 years it is not known whether getting a mammogram decreases the risk of dying of breast cancer, therefore, a choice needs to be made whether to continue screening. Clinicians may discuss breast cancer risk and then refer to or use the decision-aids in eAppendix Table 2 to help patients understand the trade-offs of screening. It is important to ask women how they feel about the potential benefits and harms of screening and factor in their goals and values to make an individualized screening decision. Different individuals with the same trade-offs might reasonably make different choices.
Mrs. M strongly values the potential to avert death from breast cancer and is less concerned about screening harms. However, as newly diagnosed peripheral vascular disease advances, reducing her life expectancy to < 5-10 years, potential benefits of future mammography disappear, leaving only potential harms. Future discussions with Mrs. M will likely describe the importance of changing the focus of preventive care away from cancer screening and instead focus on her vascular disease, mobility, and maintaining her independence in order to meet her goals of living longer and better. Of course, the time available in clinical practice to discuss and provide the numerous preventive care recommendations is inadequate.75 This makes prioritization and personalization of preventive care all the more essential, allowing more time to be spent on medical care that is most likely to help an older individual achieve his/her goals and is least likely to cause harm.
Supplementary Material
ACKNOWLEDGEMENT
This work was supported by the National Cancer Institute at the National Institutes of Health (grant number R01 CA134425) to [LW]; the National Institute on Aging at the National Institutes of Health (grant number K24AG041180) to [LW] and the Paul B. Beeson Career Development Award in Aging (grant number K23AG028584) to [MS]; as well as support from the John A. Hartford Foundation, the Atlantic Philanthropies, the Starr Foundation and the American Federation for Aging Research to [MS].
The funding sources had no role in the design, conduct, or analysis of this study or in the decision to submit the manuscript for publication. The authors report no conflicts of interest related to the work described in this manuscript.
The corresponding author, Louise Walter, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(10):727–737. doi: 10.1059/0003-4819-151-10-200911170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Independent UK panel on breast cancer screening The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380(9855):1778–1786. doi: 10.1016/S0140-6736(12)61611-0. [DOI] [PubMed] [Google Scholar]
- 3.Gotzsche PC, Jorgensen KJ. Screening for breast cancer with mammography (review). Cochrane Database of Systematic Reviews. 2013;(6) doi: 10.1002/14651858.CD001877.pub5. Art. No.: CD001877. DOI: 10.1002/14651858.CD001877.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2010;60(2):99–119. doi: 10.3322/caac.20063. [DOI] [PubMed] [Google Scholar]
- 5.American College of Obstetricians and Gynecologists Practice Bulletin Clinical management guidelines for obstetrician-gynecologists. Number 122, August 2011. Breast cancer screening. Obstet Gynecol. 2011;118(2):372–382. [Google Scholar]
- 6.American College of Radiology [November 11, 2013];ACR practice guideline for the performance of screening and diagnostic mammography. Available at: http://www.acr.org/~/media/ACR/Documents/PGTS/guidelines/Screening_Mammography.pdf.
- 7.Warner E, Heisey R, Carroll JC. Applying the 2011 Canadian guidelines for breast cancer screening in practice. CMAJ. 2012;184(16):1803–1807. doi: 10.1503/cmaj.120392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network [November 11, 2013];Breast cancer screening and diagnosis. Version 2.2013. Available at: http://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf.
- 9.National Health System [November 11, 2013];NHS breast screening programme: extending the screening age range. Available at: http://www.cancerscreening.nhs.uk/breastscreen/over-70.html.
- 10.U.S. Preventive Services Task Force Screening for breast cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2009;151(10):716–726. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- 11.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 12.Vacek PM, Skelly JM, Geller BM. Breast cancer risk assessment in women aged 70 and older. Breast Cancer Res Treat. 2011;130(1):291–299. doi: 10.1007/s10549-011-1576-1. [DOI] [PubMed] [Google Scholar]
- 13.Sweeney C, Blair CK, Anderson KE, Lazovich D, Folsom AR. Risk factors for breast cancer in elderly women. Am J Epidemiol. 2004;160:868–875. doi: 10.1093/aje/kwh276. [DOI] [PubMed] [Google Scholar]
- 14.Chlebowski RT, Kuller LH, Prentice RL, et al. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med. 2009;360(6):573–87. doi: 10.1056/NEJMoa0807684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grabler P, Dupuy D, Rai J, Bernstein S, Ansell D. Regular screening mammography before the diagnosis of breast cancer reduces black:white breast cancer differences and modifies negative biological prognostic factors. Breast Cancer Res Treat. 2012;135(2):549–553. doi: 10.1007/s10549-012-2193-3. [DOI] [PubMed] [Google Scholar]
- 16.Schonberg MA, Ramanan RA, McCarthy EP, Marcantonio ER. Decision making and counseling around mammography screening for women aged 80 or older. J Gen Intern Med. 2006;21(9):979–85. doi: 10.1111/j.1525-1497.2006.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. [February 14, 2014];Data on SEER (Surveillance, Epidemiology and End Results) cancer statistics. available online at http://seer.cancer.gov/csr/1975_2010/browse_csr.php?sectionSEL=4&pageSEL=sect_04_table.12.html.
- 18.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 19.National Center for Health Statistics [November 11, 2013];Life Tables of the United States. 2008 Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_03.pdf.
- 20.Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. [February 14, 2014];JAMA. 2012 307(2):182–192. doi: 10.1001/jama.2011.1966. Prognostic indices available at http://www.eprognosis.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M. The Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Br J Cancer. [published online ahead of print June 6, 2013] doi: 10.1038/bjc.2013.177. [Google Scholar]
- 22.Badgwell BD, Giordano SH, Duan ZZ, et al. Mammography before diagnosis among women age 80 years and older with breast cancer. J Clin Oncol. 2008;26(15):2482–2488. doi: 10.1200/JCO.2007.12.8058. [DOI] [PubMed] [Google Scholar]
- 23.McPherson CP, Swenson KK, Lee MW. The effect of mammographic detection and comorbidity on the survival of older women with breast cancer. J Am Geriatr Soc. 2002;50(6):1061–1068. doi: 10.1046/j.1532-5415.2002.50261.x. [DOI] [PubMed] [Google Scholar]
- 24.McCarthy EP, Burns RB, Freund KM, et al. Mammography use, breast cancer stage at diagnosis, and survival among older women. J Am Geriatr Soc. 2000;48(10):1226–1233. doi: 10.1111/j.1532-5415.2000.tb02595.x. [DOI] [PubMed] [Google Scholar]
- 25.Galit W, Green MS, Lital K. Routine screening mammography in women older than 74 years: a review of the available data. Maturitas. 2007;57(2):109–119. doi: 10.1016/j.maturitas.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Carney PA, Miglioretti DL, Yankaskas BC, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138(3):168–175. doi: 10.7326/0003-4819-138-3-200302040-00008. [DOI] [PubMed] [Google Scholar]
- 27.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr., Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341(27):2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 28.Schonberg MA, Marcantonio ER, Li D, Silliman RA, Ngo L, McCarthy EP. Breast cancer among the oldest old: tumor characteristics, treatment choices, and survival. J Clin Oncol. 2010;28(12):2038–2045. doi: 10.1200/JCO.2009.25.9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wildiers H, Kunkler I, Biganzoli L, et al. Management of breast cancer in elderly individuals: recommendations of the International Society of Geriatric Oncology. Lancet Oncol. 2007;8:1101–1115. doi: 10.1016/S1470-2045(07)70378-9. [DOI] [PubMed] [Google Scholar]
- 30.Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31:2382–2387. doi: 10.1200/JCO.2012.45.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Early Breast Cancer Trialists’ Collaborative Group Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 32.Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360:2055–2065. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allegra CJ, Aberle DR, Ganschow P, et al. National Institutes of Health State-of-the-Science Conference statement: Diagnosis and Management of Ductal Carcinoma in Situ September 22-24, 2009. J Natl Cancer Inst. 2010;102:161–169. doi: 10.1093/jnci/djp485. [DOI] [PubMed] [Google Scholar]
- 34.Hurria A, Browner IS, Cohen HJ, et al. National Comprehensive Cancer Network clinical practice guidelines in oncology for senior adult oncology. JNCCN. 2012;10(2):162–209. doi: 10.6004/jnccn.2012.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151(10):738–747. doi: 10.1059/0003-4819-151-10-200911170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schousboe JT, Kerlikowske K, Loh A, Cummings SR. Personalizing mammography by breast density and other risk factors for breast cancer: analysis of health benefits and cost-effectiveness. Ann Intern Med. 2011;155(1):10–20. doi: 10.7326/0003-4819-155-1-201107050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barratt A, Howard K, Irwig I, Salkeld G, Houssami N. Model of outcomes of screening mammography: information to support informed choices. BMJ. 2005;330(7497):936. doi: 10.1136/bmj.38398.469479.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O'Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: a meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. doi: 10.1136/bmj.e8441. [published online ahead of print January 8, 2013] doi: http://dx.doi.org/10.1136/bmj.e8441. [DOI] [PMC free article] [PubMed]
- 39.Schonberg MA, Silliman RA, Marcantonio ER. Weighing the benefits and burdens of mammography screening among women age 80 years or older. J Clin Oncol. 2009;27(11):1774–1780. doi: 10.1200/JCO.2008.19.9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandelblatt JS, Schechter CB, Yabroff R, et al. Toward optimal screening strategies for older women: costs, benefits, and harms of breast cancer screening by age, biology, and health status. J Gen Intern Med. 2005;20(6):487–496. doi: 10.1111/j.1525-1497.2005.0116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandelblatt JS, Wheat ME, Monane M, Moshief RD, Hollenberg JP, Tang J. Breast cancer screening for elderly women with and without comorbid conditions: a decision-analysis model. Ann Intern Med. 1992;116(9):722–730. doi: 10.7326/0003-4819-116-9-722. [DOI] [PubMed] [Google Scholar]
- 42.Kerlikowske K, Salzmann P, Phillips KA, Cauley JA, Cummings SR. Continuing screening mammography in women aged 70 to 79 years: impact on life expectancy and cost-effectiveness. JAMA. 1999;282(22):2156–2163. doi: 10.1001/jama.282.22.2156. [DOI] [PubMed] [Google Scholar]
- 43.Bond M, Pavey T, Welch K, et al. Systematic review of the psychological consequences of false-positive screening mammograms. Health Technol Assess. 2013;17(13):1–170. doi: 10.3310/hta17130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montgomery M, McCrone SH. Psychological distress associated with the diagnostic phase for suspected breast cancer: a systematic review. Journal of Advanced Nursing. 2010;66(11):2372–2390. doi: 10.1111/j.1365-2648.2010.05439.x. [DOI] [PubMed] [Google Scholar]
- 45.Braithwaite D, Zhu W, Hubbard RA, et al. Screening outcomes in older US women undergoing multiple mammograms in community practice: does interval, age, or comorbidity score affect tumor characteristics or false-positive rates? J Natl Cancer Inst. 2013;105(5):334–341. doi: 10.1093/jnci/djs645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welch HG, Fisher ES. Diagnostic testing following screening mammography in the elderly. J Natl Cancer Inst. 1998;90(18):1389–1392. doi: 10.1093/jnci/90.18.1389. [DOI] [PubMed] [Google Scholar]
- 47.Bruening W, Fontanarosa J, Tipton K, Treadwell JR, Launders J, Schoelles K. Systematic review: comparative effectiveness of core-needle and open surgical biopsy to diagnose breast lesions. Ann Intern Med. 2010;152(4):238–246. doi: 10.7326/0003-4819-152-1-201001050-00190. [DOI] [PubMed] [Google Scholar]
- 48.Walter LC, Eng C, Covinsky KE. Screening mammography for frail older women: what are the burdens? J Gen Intern Med. 2001;16(11):779–784. doi: 10.1111/j.1525-1497.2001.10113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sox HC. Screening for disease in older people. J Gen Intern Med. 1998;13(6):424–425. doi: 10.1046/j.1525-1497.1998.00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raik BL, Miller FG, Fins JJ. Screening and cognitive impairment: ethics of forgoing mammography in older women. J Am Geriatr Soc. 2004;52(3):440–444. doi: 10.1111/j.1532-5415.2004.52119.x. [DOI] [PubMed] [Google Scholar]
- 51.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605–613. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 52.Puliti D, Duffy SW, Miccinesi G, et al. Overdiagnosis in mammographic screening for breast cancer in Europe: a literature review. J Med Screen. 2012;19(3)(Suppl 1):42–56. doi: 10.1258/jms.2012.012082. [DOI] [PubMed] [Google Scholar]
- 53.Ernster VL, Ballard-Barbash R, Barlow WE, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst. 2002;94:1546–1554. doi: 10.1093/jnci/94.20.1546. [DOI] [PubMed] [Google Scholar]
- 54.Etzioni R, Gulati R, Mallinger L, Mandelblatt J. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Intern Med. 2013;158(11):831–838. doi: 10.7326/0003-4819-158-11-201306040-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367(21):1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 56.Barratt AL, Glasziou PP. Do the benefits of screening mammography outweigh the harms of overdiagnosis and unnecessary treatment? Med J Aust. 2012;196(11):681. doi: 10.5694/mja12.10236. [DOI] [PubMed] [Google Scholar]
- 57.Baxter NN, Virnig BA, Durham SB, Tuttle TM. Trends in the treatment of ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2004;96(6):443–448. doi: 10.1093/jnci/djh069. [DOI] [PubMed] [Google Scholar]
- 58.De Glas NA, Kiderlen M, Bastiaannet E, et al. Postoperative complications and survival of elderly breast cancer patients: a FOCUS study analysis. Breast Cancer Res Treat. 2013;138:561–569. doi: 10.1007/s10549-013-2462-9. [DOI] [PubMed] [Google Scholar]
- 59.Dijkstra JB, Houx PJ, Jolles J. Cognition after major surgery in the elderly: test performance and complaints. Br J Anaesth. 1999;82(6):867–874. doi: 10.1093/bja/82.6.867. [DOI] [PubMed] [Google Scholar]
- 60.Muss HB, Woolf S, Berry D, et al. Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA. 2005;293:1073–1081. doi: 10.1001/jama.293.9.1073. [DOI] [PubMed] [Google Scholar]
- 61.Buchholz TA. Radiation therapy for early-stage breast cancer after breast-conserving surgery. N Engl J Med. 2009;360(1):63–70. doi: 10.1056/NEJMct0803525. [DOI] [PubMed] [Google Scholar]
- 62.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 63.Hersch J, Jansen J, Barratt A, et al. Women's views on overdiagnosis in breast cancer screening: a qualitative study. BMJ. doi: 10.1136/bmj.f158. [published online ahead of print January 23, 2013] doi: 10.1136/bmj.f158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wegwarth O, Gigerenzer G. Overdiagnosis and overtreatment: evaluation of what physicians tell their patients about screening harms. JAMA Int Med. doi: 10.1001/jamainternmed.2013.10363. [published online ahead of print October 21, 2013] doi: 10.1001/jamainternmed.2013.10363. [DOI] [PubMed] [Google Scholar]
- 65.Schwartz LM, Woloshin S, Fowler FJ, Jr., Welch HG. Enthusiasm for cancer screening in the United States. JAMA. 2004;291(1):71–78. doi: 10.1001/jama.291.1.71. [DOI] [PubMed] [Google Scholar]
- 66.Torke AM, Schwartz P, Holtz LR, Montz K, Sachs GA. Older adults and forgoing cancer screening: “I think it would be strange.”. Arch of Intern Med. 2013;173(7):526–531. doi: 10.1001/jamainternmed.2013.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoffman RM, Lewis CL, Pignone MP, et al. Decision-making processes for breast, colorectal, and prostate cancer screening: the DECISIONS survey. Med Decis Making. 2010;30(5 Suppl):53S–64S. doi: 10.1177/0272989X10378701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stefanek ME. Uninformed compliance or informed choice? A needed shift in our approach to cancer screening. J Natl Cancer Inst. 2011;103(24):1821–1826. doi: 10.1093/jnci/djr474. [DOI] [PubMed] [Google Scholar]
- 69.Degner LF, Kristjanson LJ, Bowman D, et al. Information needs and decisional preferences in women with breast cancer. JAMA. 1997;277(18):1485–1492. [PubMed] [Google Scholar]
- 70.Fagerlin A, Pignone M, Abhyankar P, et al. Clarifying values: an updated review. BMC Med Inform Decis Mak. doi: 10.1186/1472-6947-13-S2-S8. [published online ahead of print November 29, 2013] doi: 10.1186/1472-6947-13-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garcia-Tetamero R, Okan Y, Cokely ET. Using visual aids to improve communication of risks about health: a review. Scientific World Journal. doi: 10.1100/2012/562637. [published online ahead of print May 2, 2012] doi: 0.1100/2012/562637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fagerlin A, Zikmund-Fisher BJ, Ubel PA. Helping patients decide: ten steps to better risk communication. J Natl Cancer Inst. 2011;103(19):1436–1443. doi: 10.1093/jnci/djr318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peters E, Diefenbach MA, Hess TM, Vastfjall D. Age differences in dual information-processing modes: implications for cancer decision making. Cancer. 2008;113(12 Suppl):3556–3567. doi: 10.1002/cncr.23944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morrow DG, Leirer VO, Andrassy JM, Hier CM, Menard WE. The influence of list format and category headers on age differences in understanding medication instructions. Exp Aging Res. 1998;24(3):231–256. doi: 10.1080/036107398244238. [DOI] [PubMed] [Google Scholar]
- 75.Taksler GB, Keshner M, Fagerlin A, Hajizadeh N, Braithwaite RS. Personalized estimates of benefit from preventive care guidelines: a proof of concept. Ann Intern Med. 2013;159(3):161–168. doi: 10.7326/0003-4819-159-3-201308060-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.