Abstract
Background
Adhesion of the Trypanosoma cruzi trypomastigotes, the causative agent of Chagas' disease in humans, to components of the extracellular matrix (ECM) is an important step in host cell invasion. The signaling events triggered in the parasite upon binding to ECM are less explored and, to our knowledge, there is no data available regarding •NO signaling.
Methodology/Principal Findings
Trypomastigotes were incubated with ECM for different periods of time. Nitrated and S-nitrosylated proteins were analyzed by Western blotting using anti-nitrotyrosine and S-nitrosyl cysteine antibodies. At 2 h incubation time, a decrease in NO synthase activity, •NO, citrulline, arginine and cGMP concentrations, as well as the protein modifications levels have been observed in the parasite. The modified proteins were enriched by immunoprecipitation with anti-nitrotyrosine antibodies (nitrated proteins) or by the biotin switch method (S-nitrosylated proteins) and identified by MS/MS. The presence of both modifications was confirmed in proteins of interest by immunoblotting or immunoprecipitation.
Conclusions/Significance
For the first time it was shown that T. cruzi proteins are amenable to modifications by S-nitrosylation and nitration. When T. cruzi trypomastigotes are incubated with the extracellular matrix there is a general down regulation of these reactions, including a decrease in both NOS activity and cGMP concentration. Notwithstanding, some specific proteins, such as enolase or histones had, at least, their nitration levels increased. This suggests that post-translational modifications of T. cruzi proteins are not only a reflex of NOS activity, implying other mechanisms that circumvent a relatively low synthesis of •NO. In conclusion, the extracellular matrix, a cell surrounding layer of macromolecules that have to be trespassed by the parasite in order to be internalized into host cells, contributes to the modification of •NO signaling in the parasite, probably an essential move for the ensuing invasion step.
Author Summary
Interaction of Trypanosoma cruzi with the extracellular matrix (ECM) is an essential step in the invasion of mammalian cells. However, the nature of the signaling triggered in the parasite is poorly understood. Herein the key role of nitric oxide in T. cruzi signaling is described, using an ECM preparation, in the absence of host cells. Inhibition of NOS activity, with the expected decrease in •NO production, as well as decrease in cGMP concentration were observed by the incubation of T. cruzi trypomastigotes with ECM. Additionally, lower levels of protein S-nitrosylation and nitration were detected. These post-translational modifications have been analyzed by biotin-switch and protein immunoprecipitation approaches coupled to mass spectrometry. The presence of both modifications was confirmed for specific proteins, as mucin II (S-nitrosylation), histones, enolase and tubulins. To our knowledge, decrease in the •NO signaling pathway upon T. cruzi trypomastigotes adhesion to ECM, affecting both the canonical pathway (•NO-soluble guanylyl cyclase-cGMP) and protein S-nitrosylation and nitration is described for the first time in this parasite.
Introduction
Trypanosoma cruzi is the etiological agent of Chagas disease, an infectious disease affecting areas of poor socioeconomic development. The parasite infects a wide range of mammalian hosts, including humans, from which 7–8 million are infected and other 25 million are at risk of contamination [1]. T. cruzi trypomastigotes, the classical parasite infective form, invade almost all mammalian cells, including macrophages [2,3,4], being exposed to nitrosative and oxidative stress during the life cycle [5,6,7]. The cytotoxic effect of •NO and its derivatives on pathogens such as T. cruzi is well known.
In mammals and other organisms, the free radical •NO is endogenously synthesized by nitric oxide synthase catalyzing the conversion of L-arginine to L-citrulline [8], a reaction that depends on heme, FAD, FMN and tetrahydro-L-biopterin (BH4) as co-factors. •NO is highly reactive towards O2, but reactions with biological molecules preferentially occur with •NO- derived species (N2O3, NO2 • or ONOO-) [9]. Biologically, •NO plays essential role in cell signaling, acting by two main mechanisms: (i) activation of guanylyl cyclase, yielding cGMP—the classical pathway; or (ii) acting in post-translational modifications such as S-nitrosylation and tyrosine nitration- the non-classical pathway [10,11]. Protein S-nitrosylation and tyrosine nitration affect the activity of many relevant targets of several biological processes [12,13].
Proteins are S-nitrosylated (SNO) by the addition of a nitroso group into a cysteine residue in a non-enzymatic process, dependent on the local nitric oxide concentration or by transnitrosylation, a key mechanism in •NO signaling (acquisition of a •NO from another S-nitrosothiol) [14,15,16]. Denitrosylation may occurs by nonenzymatic mechanisms or by the action of denitrosylases [17,18,19]. New targets of S-nitrosylation are being extensively described in different organisms due to the development of tools such as the traditional biotin-switch technique associated with proteomic analysis [20,21]. As an example, 319 putative S-nitrosylation targets, as well as enzymatic denitrosylating and transnitrosylating activities in Plasmodium falciparum were recently described [22]. Of note, P. falciparum lacks a NOS ortholog and probably produces •NO from a nitrate/nitrite chemical reduction pathway [23].
In contrast to S-nitrosylation, tyrosine nitration of proteins was classically regarded as an undesired byproduct of radical species with greater reactivity capable of oxidizing tyrosine to 3-nitro-tyrosine. However, tyrosine nitration of proteins occurs under physiological conditions, with an increment of 3-nitro-tyrosine in many physiopathological and aging processes. Protein nitration is mediated by free radical reactions, with the intermediate •Tyr reacting with •NO or •NO2 [24]. Not all proteins are nitrated, pointing out to the specificity of the modification. Only one or two specific protein residues are preferentially modified and a close relationship between protein tyrosine nitration and the presence of a transition metal has been made [rev. 25]. Although not well established as S-nitrosylation, evidence gathered in the past few years suggests that tyrosine-nitrated proteins regulate several biological processes, such as stress response in plants [26], cytochrome c regulation [27], protein degradation [28], control of the redox environment [29] and PKC signaling [30].
There is a limited knowledge of •NO signaling in T. cruzi, as happens with other parasites [31]. S-nitrosylation or tyrosine nitration of T. cruzi proteins remains largely unexplored, despite the relevance of •NO and •NO-derived species produced by mammals in response to T. cruzi infection. In vitro treatment of cruzipain, the major T. cruzi papain-like cysteine proteinase [32], with •NO donors led to inhibition of the enzyme activity [33], but this modification has not been reported in vivo. Additionally, the putative signaling in response to the endogenous •NO formation is mostly unknown in T. cruzi.
Biochemical evidence of NOS activity, with •NO donors leading to an increase in the cGMP concentration was described in T. cruzi extracts [34]. Probing with an anti-neuronal NOS antibody the enzyme was localized in the inner surface of cell membranes, cytosol, flagellum and apical extremity [35]. However, NOS and guanylyl cyclase orthologs seem to be lacking in the parasite genome. An adenylyl cyclase containing a putative guanylyl cyclase domain was suggested to be responsible for cGMP production [36,37] and a soluble dual-specificity phosphodiesterase (TcrPDEC), capable of cleaving both cAMP and cGMP with similar Km (20–31.6 μM and 78.2 μM, respectively) would be responsible for the degradation of cGMP [38,39,40,41]. The downstream effector of cGMP is assumed to be the cGMP-dependent protein kinase (PKG). While the involvement of cAMP is relatively well known in biological processes, such as T. cruzi metacyclogenesis [40,42,43] and downstream proteins that interact with PKA were characterized [44] the knowledge of cGMP signaling is far from being understood. Although the role of cGMP signaling pathway is currently unresolved in T. cruzi and other kinetoplastids, the presence of cGMP-specific kinase in T. brucei [45] and Leishmania [46] points out to the presence of the pathway in these parasites.
In addition to a structural role in tissues, the extracellular matrix (ECM), a complex tridimensional structure composed of more than 300 proteins and glycoproteins [47], is relevant for many cellular signaling pathways, including •NO signaling in mammalians. T. cruzi trypomastigotes bind to components of the extracellular matrix (ECM), such as laminin, fibronectin, collagen, heparan sulfate, thrombospondin or galectin-3, as an early event of the infection process of mammalian cells [2,3,48,49]. Despite this, the signaling pathways triggered in T. cruzi after adhesion to ECM or its components are less characterized. Adhesion to laminin or fibronectin leads to changes in the phosphorylation level of T. cruzi proteins, including paraflagellar rod proteins and tubulins, probably involving the ERK1/2 pathway [50].
Herein, the role of nitric oxide in post-translational modification of proteins as a consequence of trypomastigotes adhesion to ECM is focused. A decrease of the •NO signaling pathway, including S-nitrosylation and tyrosine nitration of proteins was observed in T. cruzi trypomastigotes upon adhesion to host cell-derived ECM, an essential event for mammalian host cell invasion. To our knowledge this phenomenon is described for the first time.
Materials and Methods
Chemicals
S-methyl-methanethiosulfonate (MMTS), imidazole, L-arginine and L-citrulline, 3-isobutyl-1-methylxanthine (IBMX), GTP, sulfanilamide, N-(1-naphthylethylenediamine dichloride, HgCl2, S-nitrosoglutathione, neocupreine and also the antibodies: anti-alfa tubulin, anti-nitrosocysteine, anti-mouse FITC conjugated, anti-rabbit HRP conjugated were purchased from Sigma-Aldrich (St. Louis, USA). Phosphoric acid and sodium hydroxide were acquired from Synth (São Paulo, Brazil). Sodium dihydrogenphosphate was purchased from Merck (Darmstadt, Germany). The antibody anti-nitro-tyrosine was obtained from Millipore (Billerica, USA). Sepharose beads, anti-rabbit Alexafluor 555 conjugated and DAPI were acquired from Invitrogen (Carlsbad, USA). EZ-link HPDP Biotin was from Thermo Scientific (Waltham, USA). The L-[3H]-Arginine was from PerkinElmer (Waltham, USA).
Parasite cultures
T. cruzi epimastigotes, Y strain, were cultivated at 28°C in Liver infusion Tryptose (LIT) medium supplemented with 10% fetal bovine serum (FBS), up to 107 epimastigotes per mL. [51]. T. cruzi trypomastigotes, Y strain, were maintained by infection in LLC-MK2 cells in DMEM supplemented with 2% FBS at 37°C and 5% CO2. Five days after infection, trypomastigotes released into the medium were collected, washed in DMEM 2% FBS (10,000 x G for 12 minutes) and resuspended to adequate cell density accordingly to the experiment [52].
Parasite incubation with extracellular matrix
Trypomastigotes (1x109/mL) were incubated with 10 mg/mL ECM (Gibco) for 2 h, unless otherwise stated, at 37°C and 5% CO2. After incubation, parasites were washed twice in PBS containing 5 mM NaF, 2 mM Na3VO4, 50 μM Na β-glicerophosphate, 1 mM PMSF and protease inhibitor cocktail (Sigma-Aldrich), and kept at -80°C until used.
Nitric Oxide quantification
After incubation with ECM, parasites were centrifuged (10,000 x G, 5 minutes) and the supernatant separated for nitric oxide quantification, as described by the manufacturer (Measure-iT High-Sensitivity Nitrite Assay Kit, Invitrogen)
NOS activity assay
Nitric oxide synthase activity was determined by following the conversion of L[3H]-arginine to L[3H]-citrulline in T. cruzi cell lysates (5x108), accordingly to the manufacturer (Cayman), and as previously described [34]. The presence of [3H]-citrulline was confirmed by thin layer chromatography and radioactivity measurement of the spots, as described [34].
Determination of cGMP levels
cGMP was measured in T. cruzi extracts (5x108 cells) accordingly to the commercial EIA test Biotrak instructions (GE Healthcare). Due to the intrinsic presence of extracellular matrix proteins in some of the experimental assays specific activity could not be calculated and, thus, results are expressed in total femtomoles produced.
Total S-NO quantification
Total S-NO was quantified by the Saville-Griess method, as described elsewhere [53]. Briefly, the parasite pellet (109) was lysed in 20 mM Tris-HCl buffer, pH 7.4, containing 0.1% Triton X-100, 5 mM NaF, 2 mM Na3VO4, 50 μM Na β-Glycerophosphate, 1 mM PMSF and protease inhibitor cocktail (Sigma-Aldrich) and centrifuged (10 minutes at 14,000 x G, 4°C). Twenty μL of supernatant were then added to 180 μL reaction buffer (57 mM Sulfanilamide, 1.2 mM N-(1-Naphthyl) ethylenediamine dihydrochloride in PBS, pH 7.4) and the reaction started by the addition of 100 μM HgCl2. After 30 minutes at room temperature and in the dark, the absorbance at 496 nm was measured. Controls without HgCl2 were included to account for NO already present in the sample. The amount of total S-NO was estimated against a standard curve with S-nitrosoglutathione.
Determination of L-Arginine and L-Citrulline contents
The parasite pellet was lysed in H2O: methanol (1:1, v:v), centrifuged for 10 minutes at 14,000 x G, 4°C and the supernatant collected was dried in SpeedVac. The resulting dried pellet was resuspended with 200 μL MilliQ water and centrifuged (10 minutes at 14,000 x G, 4°C) to remove impurities. The supernatant was analyzed by a capillary electrophoresis system (model PA 800, Beckman Coulter Instruments, Fullerton, USA), equipped with DAD detector and a temperature control device. Data acquisition and treatment were carried out by the vendor software (32 Karat Software version 8.0, Beckman Coulter). A fused silica capillary (Polymicro Technologies, Phoenix, USA) of 50.2 cm total length, 40.0 cm effective length and 50 μm i.d. was used. The capillary was preconditioned as follows: 1 mol.L-1 NaOH (5 minutes/20 psi), MilliQ water (5 min/20 psi) and background electrolyte (BGE) (5 minutes/20 psi). The BGE was comprised of 50 mmol.L-1 of sodium dihydrogenophosphate at pH 2.5, adjusted with phosphoric acid. Samples were injected hydrodynamically by applying a 0.5 psi pressure during 2 s. The conditions applied during separation were voltage of 25 kV and detection at 200 nm. To quantify arginine and citrulline in the samples, analytical curves were constructed with background electrolyte the linear range of 50–200 mg.mL-1 and 1–50 mg.mL-1, respectively. Imidazole was used as internal standard (50 mg.mL-1).
Immunofluorescence
After incubation with ECM and subsequent washes, parasites were fixed in 2% paraformaldehyde for 15 minutes at room temperature, pelleted by centrifugation (5,000 x G for 5 minutes), resuspended in PBS and added to a cover glass and left to dry for 16 hours at room temperature. After permeabilization of the parasites (PBS containing 1% BSA and 0.1% Triton X-100 for one hour at 37°C), anti-nitrosocysteine (rabbit, 1:200), anti-nitro-tyrosine (rabbit, 1:500) or anti-alpha-tubulin (mouse, 1:500) were added and incubated for 2 h at 37°C. After exhaustive washes with PBS containing 1% BSA, the correspondent secondary antibodies were added (anti-rabbit Alexa 555 conjugated, 1:500; anti-mouse FITC conjugated, 1:100), followed by one hour incubation at 37°C. After successive washes in PBS-1% BSA, the slides were mounted in a solution containing 50% glycerol, 50% milliQ H2O and 10 μg DAPI. The images were taken on an ExiBlue camera (Qimaging) coupled to a Nikon Eclipse E 600 optical microscope and deconvoluted using the software Huygens Essential (Scientific Volume Imaging).
Immunoblotting
Proteins from the parasite were extracted with Laemmli Buffer [54] without reducing agents. SDS polyacrylamide gel electrophoresis was performed in a 6–16% gradient polyacrylamide gel and transferred to a 0.45 μm nitrocellulose membrane for 16 hours at 15 V. The membrane was blocked in 5% BSA and incubated with the primary antibody (anti-nitrosocysteine or anti-nitro-tyrosine, produced in rabbit, 1:2000), washed thrice in PBS-0.1% Tween 20, incubated with secondary antibody (anti-rabbit conjugated with HRP, 1:8000 dilution), washed five times in PBS-0.1% Tween 20 and developed by electrochemiluminescence.
Biotin switch
Conversion of protein SNO to biotinylated groups was performed as described [20]. Briefly, parasites were lysed by sonication in 25 mM HEPES, 50 mM NaCl, 0.1 mM EDTA, 1% NP-40, 5 mM NaF, 2 mM Na3VO4, 50 μM Na β-glicerophosphate, 1 mM PMSF and protease inhibitor cocktail (Sigma), then clarified by centrifugation at 14,000 x G for 10 min at 4°C. The supernatant was blocked in 250 mM Hepes-NaOH buffer, pH 7.7, containing 1 mM EDTA, 0.1 mM Neocuproine, 2.5% SDS and 20 mM MMTS for 20 minutes at 50°C, protected from light. Proteins were then precipitated by the addition of six volumes of ice cold acetone for one hour at -20°C. After successive washes in 70% acetone, the precipitate was resuspended in 250 mM Hepes-NaOH buffer, pH 7.7, containing 1 mM EDTA, 0.1 mM neocuproine, 1% SDS, 4 mM Biotin-HPDP and 1 mM sodium ascorbate. The mix was incubated for one hour at 25°C at dark. Proteins were then precipitated in ice cold acetone for one hour at -20°C and washed extensively in 70% acetone.
Biotin-containing proteins were prepared for sequencing as described [21] Proteins were digested by trypsin (sequencing grade 1:100, mass/mass) for 16 hours at 37°C and the reaction stopped by the addition of 0.5 mM PMSF. Then, biotin-containing proteins were pulled-down using streptavidin beads. After 2 hours incubation at room temperature under gentle shaking, the beads were washed thrice in 20mM Tris-HCl buffer pH 7.7, containing 1 mM EDTA and 0.4% Triton X-100. Proteins were eluted with 5 mM ammonium bicarbonate containing 150 μL of 50 mM 2-mercaptoethanol for 5 minutes. Sequencing was determined (Veritas Life Sciences, Brasil) using a LTQ-Orbitrap coupled to nLC-MS/MS. Acquired data were automatically processed by CPAS (Computational Proteomics Analysis System) [55] and only peptides with high quality were considered (expected score <0.2). The TryTripDB was used for protein search combining Esmeraldo-like, non-Esmeraldo-like and unassigned.
For Mucin II validation, proteins were biotinylated as described above, ressuspended in Tris-HCl 20 mM and incubated at 12°C for 16 hours with streptavidin beads. After three successive washes, the beads were ressuspended in Laemmli buffer without reducing agents, incubated at 100°C for 5 minutes, followed by SDS-PAGE using a 6–16% gradient gel. The proteins were transferred to a nitrocellulose membrane and the immunoblotting was performed using anti-rabbit Mucin II antibodies (1: 500 in PBS- 5% BSA).
Immunoprecipitation and identification of tyrosine-nitrated proteins
Parasites were lysed by sonication using 100 μL RIPA buffer, containing 5 mM NaF, 2 mM Na3VO4, 50 μM Na β-Glicerophosphate, 1 mM PMSF and protease inhibitor cocktail (Sigma). Samples were diluted in 1.4 mL of 20 mM Tris-HCl buffer, pH 7.4, containing the same concentration of inhibitors. After centrifugation at 14,000 x G for 10 minutes at 4°C, the supernatant was added to protein A- Agarose with the desired antibody or normal serum (control) or to covalent-linked antibody-Agarose (anti-nitro-tyrosine-resin). After 16 h at 12°C, the beads were washed with 20 mM Tris-HCl buffer, pH 7.4 containing 0.1% Triton X-100 and the bound material eluted with Laemmli buffer without reducing agent. In the particular case of immunoprecipitation using covalent-linked anti-nitro-tyrosine antibodies, the elution was performed by incubation in 5 M acetic acid for 5 minutes. The eluted proteins were identified by a commercial facility (Veritas Life Sciences, Brasil), as described above.
Results
It was previously shown that T. cruzi is able to produce •NO via a putative tcNOS [34] although no NOS ortholog can be found on the parasite genome. The synthesis of •NO was then first confirmed in T. cruzi extracts of the non infective (epimastigote) and infective (trypomastigote) stages by measuring the conversion of 3H-L-arginine to 3H-L-citrulline by thin layer chromatography, as described [34] and the product of the reaction confirmed by capillary electrophoresis. Importantly, approximately 260 fold-enrichment in NOS activity was obtained after partial purification of the enzyme from epimastigotes, as described [34].
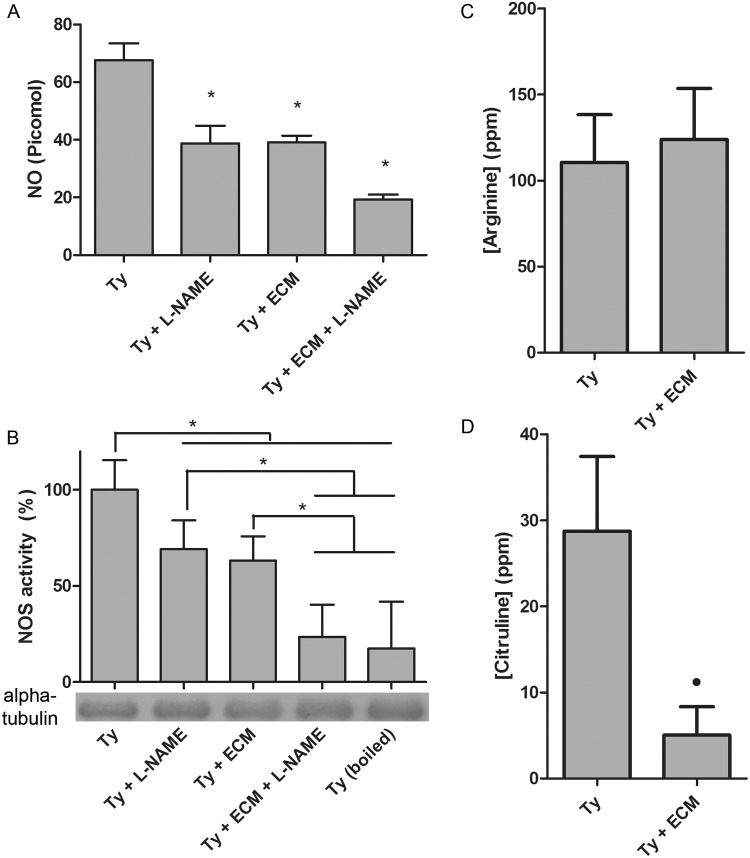
Even though the identity of the enzyme remains elusive, the possible involvement of •NO signaling during T. cruzi binding to ECM was pursued. Trypomastigotes were incubated with purified ECM for to 2 h and the amount of extracellular •NO was quantified (Fig. 1A). The extracellular •NO concentration dropped 43% under this condition, in the same order of magnitude observed when trypomastigote extracts were incubated with 10 mM L-NAME, a NOS inhibitor (Fig. 1A). The simultaneous incubation with ECM and L-NAME leads to an even higher inhibition of •NO production (approximately 72%). The data strongly suggest that interaction of the parasites with ECM hinders •NO responses.
Fig 1. Nitric oxide synthase activity during Trypanosoma cruzi trypomastigotes adhesion to extracellular matrix.
Trypomastigotes (1x109) were incubated with ECM (1.5 mg) in phenol red free-MEM, supplemented with 2% FBS, for 2 h at 37°C and 5% CO2. The inhibitor L-NAME (10 mM) was added after the extract preparation. (A) •NO concentration in the medium was measured employing a modified fluorescent reaction of DAF-2 (Measure-iT High-Sensitivity Nitrite Assay Kit). (B) NOS activity was measured in T. cruzi extracts by the conversion of [3H]arginine to [3H]citrulline in T. cruzi extracts. The immunoblots were probed with anti-tubulin antibody to guarantee that the protein loading in each track was similar. The levels of intracellular L-Arginine (C) and L-citrulline (D) were obtained by the capillary electrophoresis method. Points marked with an asterisk represent a p<0.001 (one-way ANOVA) and with a dot represent p<0.001 in unpaired t-Student test. Results in (A) and (B) are the mean of three independent experiments and (C) and (D) are the mean of five independent experiments.
Accordingly, 37% decrease in NOS activity was observed in parasite extracts previously incubated with ECM, as compared to parasites incubated in the absence of ECM under the same experimental conditions (Fig. 1B). Partial or total inhibition of •NO production by 10 mM L-NAME or by boiling the cellular extracts for 10 minutes at 100°C, respectively, confirmed that the •NO measured is a product of an enzymatic activity (Fig. 1B). The enzymatic activity was reduced to 9% by the addition of 50 mM L-NAME. Furthermore, changes in L-arginine/L-citrulline ratio upon incubation with ECM strengthen the evidence of declining NOS activity upon parasite adhesion to ECM (Fig. 1C, D). Whereas Intracellular concentration of L-citrulline decreased 83% upon adhesion of trypomastigotes to ECM, no significant change was observed in the L-arginine levels. This could be attributed to the contribution of other metabolic routes, but it is important to note that T. cruzi lacks a pathway to convert citrulline to arginine (i.e. arginase, an enzyme of the urea cycle, is absent) [56], which strongly suggests that the decline in the L-citrulline levels might be, at least in part, a consequence of NOS activity inhibition.
Since biological signaling by •NO is primarily mediated by activation of guanylyl cyclase, the production of cGMP in trypomastigotes incubated or not with ECM was quantified (Fig. 2). The levels of cGMP production fell from 3.5 to 0.6 fmoles after adhesion of the parasite to ECM (Fig. 2). Taken together, the findings strongly suggest that parasite adhesion to ECM leads to inhibition in •NO production, consequently deactivating a classical •NO signaling pathway.
Fig 2. cGMP responses during Trypanosoma cruzi trypomastigotes adhesion to extracellular matrix.
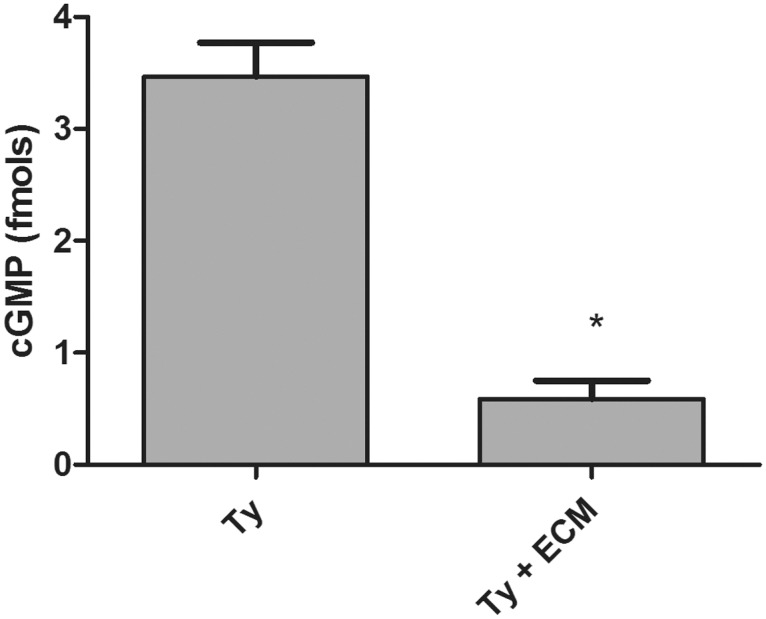
Trypomastigotes (1x109) were incubated with ECM (1.5 mg) in phenol red free-MEM, supplemented with 2% FBS, for 2 h at 37°C and 5% CO2. cGMP levels were measured in T. cruzi extracts by competitive ELISA. The asterisk represents a p<0.001 according to t-Student test. Values are the mean of four independent experiments.
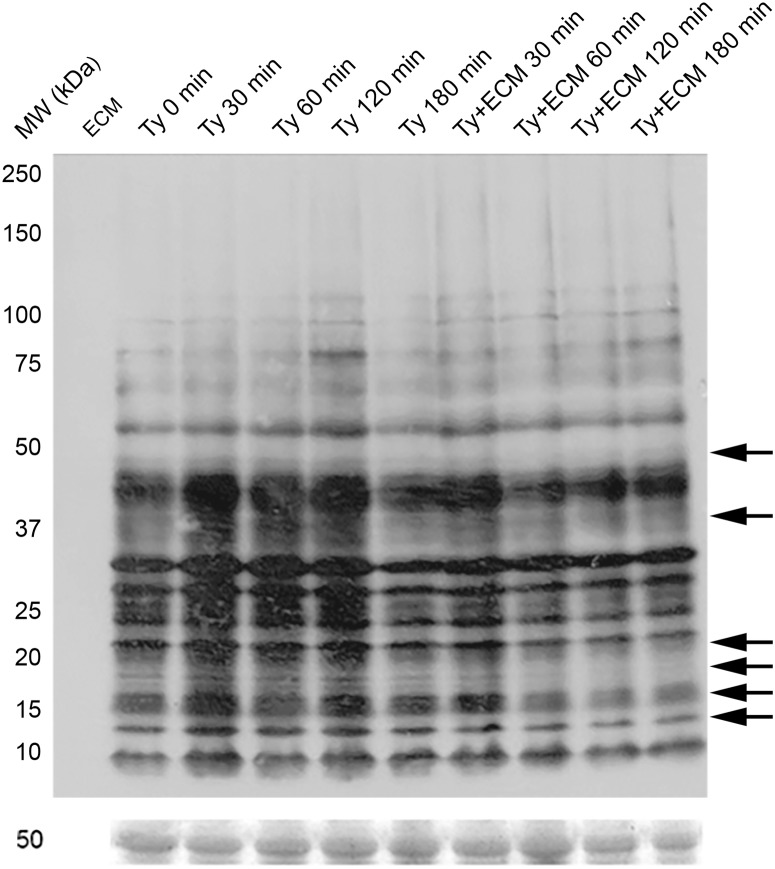
To check whether parasite adhesion to ECM would modulate protein S-nitrosylation (SNO) and tyrosine nitration, immunological assays were performed employing anti-S-nitroso-cysteine and anti-3-nitro-tyrosine antibodies. Immunoblotting experiments reveal a time-dependent decrease of SNO in specific bands, mainly in the 37 kDa region, but also noticeable in protein bands at the 47, 20, 18, 15 and 13 kDa regions (Fig. 3).
Fig 3. S-nitrosylation pattern during Trypanosoma cruzi trypomastigotes adhesion to extracellular matrix.
Trypomastigotes (1x109) were incubated with ECM (1.5 mg) in phenol red free-MEM, supplemented with 2% FBS, at 37°C and 5% CO2, for different incubation times. Proteins (50 μg) were resolved in 6–16% gradient SDS-PAGE. The immunoblotting was performed using the antibodies: rabbit anti-SNO 1:2,000, and the secondary HRP conjugated anti-rabbit 1:8,000. The Ponceau’s staining was used as a load control. Arrows indicate protein bands in which the signal intensity is decreased on the group incubated with the extracellular matrix in relation to the equivalent group incubated with growth media alone. The figure is representative of three experiments.
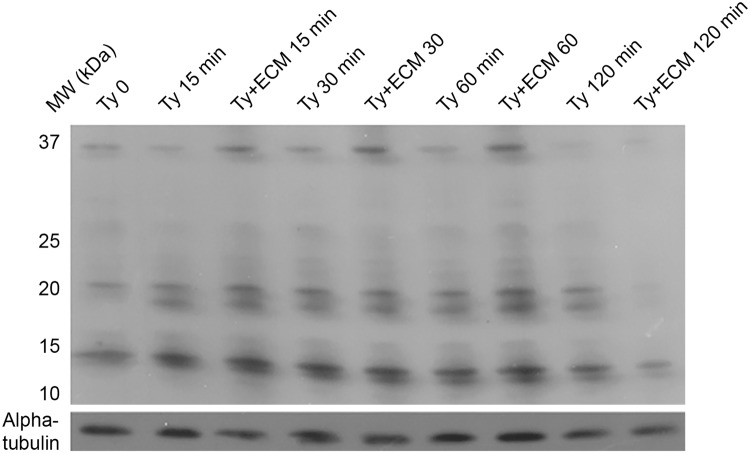
Differently from SNO, the number of nitrated-proteins detected by anti-3-nitro-tyrosine was considerably less and differences in tyrosine-nitrated proteins were not significant at the first hour of the experiment (Fig. 4). However, the levels of tyrosine-nitrated proteins were extensively reduced at 2 h incubation, affecting proteins in the range of 10 to 37 kDa (Fig. 4).
Fig 4. Tyrosine nitration pattern during Trypanosoma cruzi trypomastigotes adhesion to extracellular matrix.
Trypomastigotes (1x109) were incubated with ECM (1.5 mg) in phenol red free-MEM, supplemented with 2% FBS, at 37°C and 5% CO2, for different incubation times. Proteins (50 μg) were resolved in 6–16% gradient SDS-PAGE. The immunoblotting was performed using the antibodies: rabbit anti-TyrNO2 1:2,000, mouse anti-α-tubulin 1:5,000 as a load control and the secondary HRP conjugated anti-rabbit or anti-mouse 1:8,000 antibodies. The figure is representative of three independent experiments.
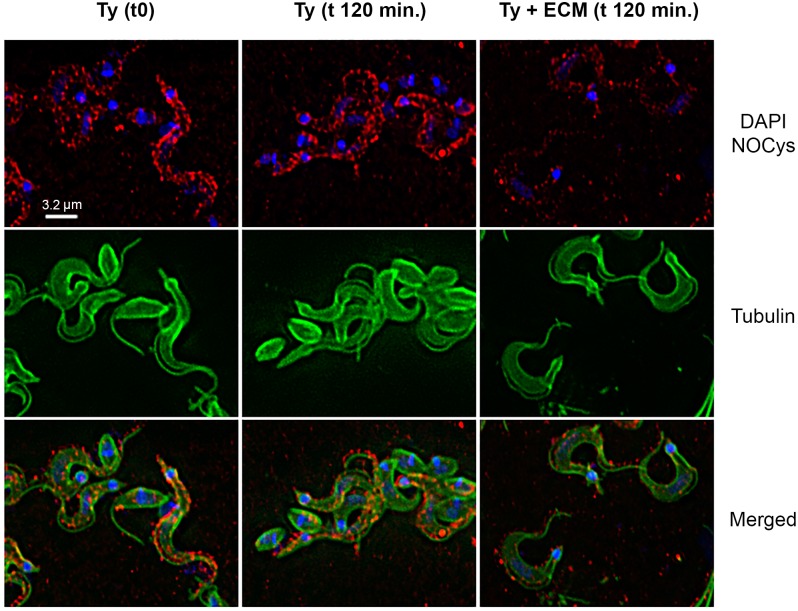
Likewise, the general decrease in SNO and nitrated-proteins can be observed by immunofluorescence microscopy. Paraformaldehyde-fixed T. cruzi trypomastigotes previously incubated with ECM for 2 h showed a significant decrease in the immune reaction for both S-nitrosylation (Fig. 5) and tyrosine nitration of proteins (Fig. 6). Indeed, image pixels/spots quantification showed a reduction higher than 50% and around 30% in the immunoreaction for S-nitrosylated and for tyrosine nitration proteins, respectively, when parasites were incubated with ECM.
Fig 5. Presence of S-nitrosylated proteins in Trypanosoma cruzi trypomastigotes during adhesion to the extracellular matrix.
Trypomastigotes (1x109) were incubated with ECM (1.5 mg) in phenol red free-MEM, supplemented with 2% FBS, for 2 h at 37°C and 5% CO2. Parasites were submitted to the immunofluorescence protocol. S-nitrosylated proteins are stained in red, alpha-tubulin in green, and nucleus and kinetoplast in blue (DAPI). The white bar represents 3.2 μm.
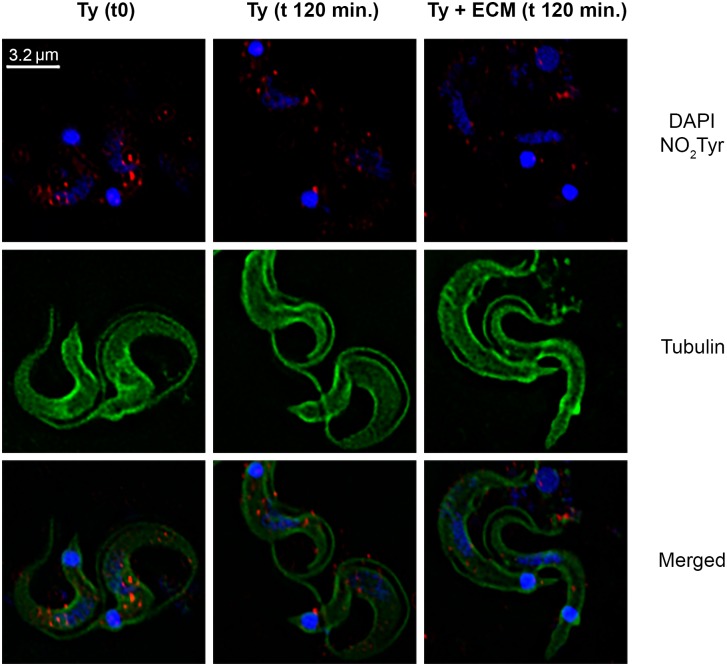
Fig 6. Presence of tyrosine nitrated proteins in Trypanosoma cruzi trypomastigotes during adhesion to the extracellular matrix.
Trypomastigotes (1x109) were incubated with ECM (1.5 mg) in phenol red free-MEM, supplemented with 2% FBS, for 2 h at 37°C and 5% CO2. Parasites were submitted to the immunofluorescence protocol. Tyrosine nitrated proteins are stained in red, alpha-tubulin is stained in green, nucleus and kinetoplast are stained in blue (DAPI). The white bar represents 3.2 μm. The image is representative of two experiments.
In order to confirm the adhesion effect on protein S-nitrosylation, total S-nitrosylated proteins in T. cruzi extracts were quantified by the Saville-Griess method [53]. A pronounced decrease of 87% was observed in the total SNO trypomastigote proteins when parasites were incubated with ECM, as compared to the control (Fig. 7A). As additional controls, parasites were also incubated in the presence or absence of 100 μM CysNO (•NO donor) or 100 μM cPTio (•NO scavenger). As expected, increasing •NO availability led to an improvement in SNO, as well removing •NO from the system resulted in SNO decrease (Fig. 7A). Also, addition of CYsNO to parasites incubated with ECM did not restore the levels observed for trypomastigotes incubated with CYsNO only, showing the predominance of the ECM effect. On the other hand, cPTio added to ECM-treated parasites reduced even more the amount of S-nytrosylated proteins. The different treatments did not affect parasite viability (Fig. 7B).
Fig 7. The effect of nitric oxide availability on Trypanosoma cruzi protein S-nitrosylation.
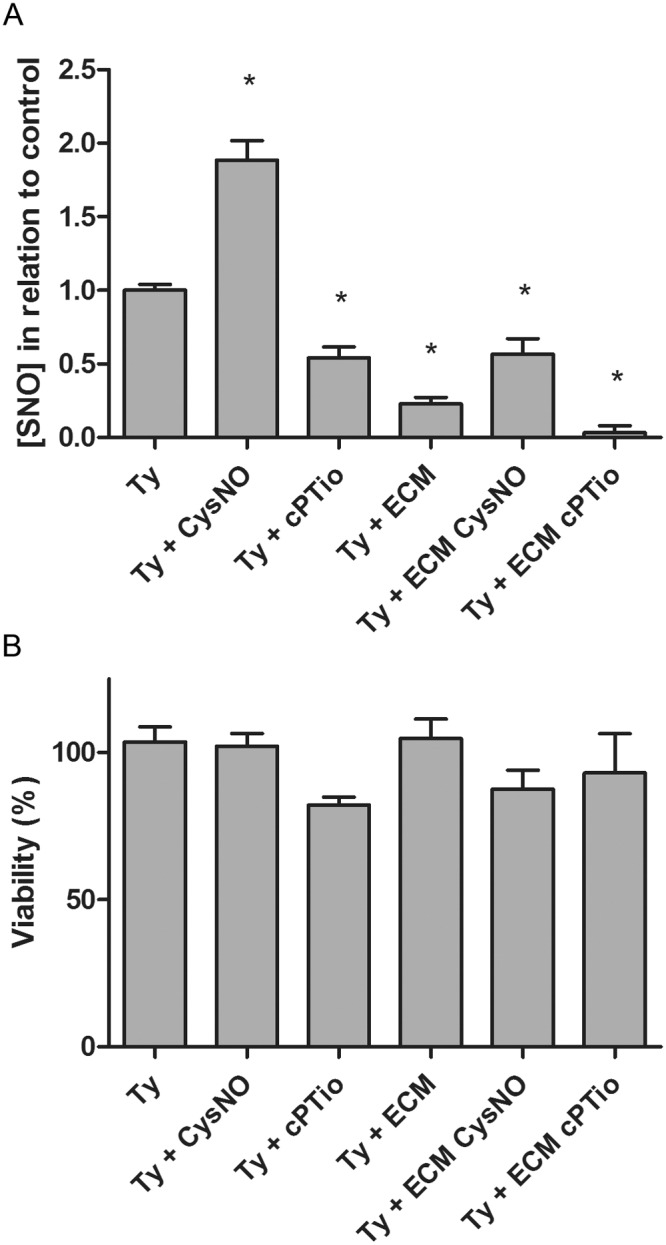
Trypomastigotes (1x109) were incubated with ECM (1.5 mg) in phenol red free-MEM, supplemented with 2% FBS, for 2 h at 37°C and 5% CO2, in presence or absence of 100 μM cPTIO or CysNO. (A) Total protein S-NO was quantified in the parasite lysate using the Saville-Griess method. Asterisks represent a p<0.01 according to one-way ANOVA. (B) Parasite cell viability (1x107/well in a 96 well plate) was carried out by following the reduction of WST-1 reagent (Roche) as described by the manufacturer. Differences are not statistically significant. Values are the mean of three independent experiments.
Preliminary data using the biotin-switch technique and mass spectrometry further confirmed the presence of S-nitrosylated proteins in T. cruzi. Although a low number of SNO proteins were detected, an even lower amount was present in ECM-incubated trypomastigotes, as predicted by the experiments herein described.
Examples of putative modified proteins that have been identified under different conditions were: (1) calpain-like cysteine peptidase, retrotransposon hot spot protein, surface protease GP63, trans-sialidase and mucin TcMUCII, in both untreated and ECM-incubated parasites; (2) fucose kinase, glycerophosphate mutase and kinesin K39 only in ECM-incubated parasites; (3) DGF-1, fatty acid elongase and helicase only in untreated parasites. Additionally, 27 hypothetical S-nitrosylated proteins were detected in ECM-treated (7) or untreated (20) trypomastigotes. However, it must be emphasized that the presence or absence of a modification in a particular protein due to the incubation of the parasite with ECM needs validation in each case, due to the possibility of a nonspecific binding during the enrichment of the SNO proteins.
The existence of S-nitrosylation in T. cruzi proteins was validated for mucin TcMUCII. SNO proteins were converted to biotin-containing proteins, pulled-down by streptavidin beads as described in Materials and Methods, and the biotinylated-proteins were subjected to immunoblotting using anti-rabbit Mucin II antibodies (Fig. 8A). As a negative control, proteins prepared by the biotin switch method in the absence of ascorbate gave no reactivity with anti-mucin II antibodies (Fig. 8A). A significant increase in the level of S-nitrosylation was detected in ECM-treated trypomastigotes in relation to the untreated parasites (Fig. 8B) and taking into account the protein loaded in each case.
Fig 8. Validation of T. cruzi cysteine nitrosylated proteins.
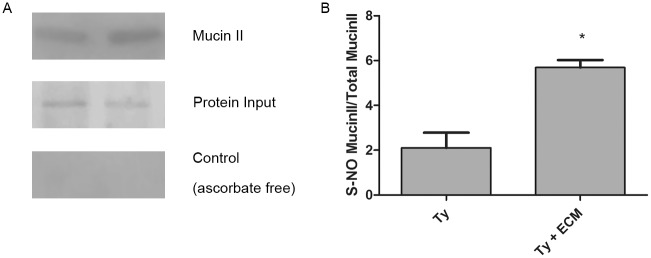
(A). S-Nitrosylated proteins from ECM-incubated and ECM-non-incubated parasites were isolated by biotin switch and streptavidin pull down methodology and submitted to immunoblotting using anti-mucin II antibodies. Control of protein input and negative control (the biotin switch methodology in the absence of ascorbate) are shown. T. cruzi control (Ty) and treated with ECM for 2 hours (Ty + ECM). (B) Quantification of the results presented in (A). The asterisk represents a p<0.001 according to t-Student test.
In relation to the tyrosine-nitrated modifications, immunoprecipitated proteins with anti-nitro-tyrosine antibodies were identified by nLC-MS/MS. A number of putative nitrated targets identified decreased in ECM-incubated trypomastigotes (Table 1). Hypothetical and ribosomal proteins comprise the majority of the sequences obtained in untreated trypomastigotes. Also, the majority of the identified proteins were detected in ECM- treated or untreated parasites.
Table 1. Putative Tyrosine nitrated T. cruzi proteins identified by nLC/MS-MS after sample enrichment by immunoprecipitation.
| Group | Protein | Gene ID | Function | Theoretical | |
|---|---|---|---|---|---|
| MM (Da) | pI | ||||
| Ty | 10 kDa heat shock protein, putative | TcCLB.508209.90 | Protein folding | 10701 | 9.07 |
| Ty | 40S ribosomal protein S10, putative | TcCLB.506679.140 | Translation | 18250 | 10.8 |
| Ty | 40S ribosomal protein S14, putative | TcCLB.508823.50 | Translation | 15568 | 10.6 |
| Ty | 40S ribosomal protein S24E, putative | TcCLB.507681.150 | Translation | 15668 | 11.7 |
| Ty | 40S ribosomal protein S3a-1 OS | TcCLB.510999.39 | Translation | 28288 | 11 |
| Ty | 40S ribosomal protein S4, putative | TcCLB.509683.117 | Translation | 30876 | 11.1 |
| Ty | 40S ribosomal protein S6, putative | TcCLB.507709.50 | Translation | 21400 | 11.4 |
| Ty | 60S ribosomal protein L10a, putative | TcCLB.506963.10 | Translation | 25146 | 10.2 |
| Ty | 60S ribosomal protein L13, putative | TcCLB.508153.280 | Translation | 25294 | 11.9 |
| Ty | 60S ribosomal protein L18, putative | TcCLB.506181.50 | Translation | 34028 | 11.1 |
| Ty | 60S ribosomal protein L23a, putative | TcCLB.508175.146 | Translation | 21268 | 11.5 |
| Ty | 60S ribosomal protein L28, putative | TcCLB.510101.30 | Translation | 16368 | 12.2 |
| Ty | 60S ribosomal protein L6, putative | TcCLB.507709.50 | Translation | 21400 | 11.4 |
| Ty | 60S ribosomal protein L7a, putative | TcCLB.510835.40 | Translation | 34550 | 11.8 |
| Ty, Ty+ECM | ADP, ATP Carrier protein 1, mitocondrial precursos, putative | TcCLB.506211.160 | Transport | 34955 | 10.3 |
| Ty, Ty+ECM | alpha tubulin, putative | TcCLB.411235.9 | Cytoskeleton | 49800 | 4.7 |
| Ty | ATP synthase, epsilon chain, putative | TcCLB.506945.240 | Metabolism | 20246 | 6.08 |
| Ty, Ty+ECM | beta tubulin, putative | TcCLB.506563.40 | Cytoskeleton | 49701 | 4.43 |
| Ty | beta-fructofuranosidase-like protein | TcCLB.506705.70 | Metabolism | 53584 | 7.79 |
| Ty | chaperonin HSP60, mitochondrial precursor | TcCLB.510187.551 | Protein folding | 30553 | 9.15 |
| Ty, Ty+ECM | cytochrome c, putative | TcCLB.506949.50 | Metabolism | 12234 | 9.88 |
| Ty, Ty+ECM | elongation factor 1-alpha, putative | TcCLB.511369.30 | Translation | 30736 | 9.33 |
| Ty | enoyl-CoA hydratase, mitochondrial precursor, putative | TcCLB.508185.10 | Metabolism | 28818 | 8.91 |
| Ty, Ty+ECM | glyceraldehyde 3-phosphate dehydrogenase, putative | TcCLB.506943.50 | Metabolism | 39033 | 9.2 |
| Ty, Ty+ECM | glycosomal malate dehydrogenase, putative | TcCLB.506503.69 | Metabolism | 34068 | 8.88 |
| Ty | guanosine monophosphate reductase, putative | TcCLB.506519.130 | Metabolism | 52311 | 9.05 |
| Ty, Ty+ECM | heat shock 70 kDa protein, putative | TcCLB.511211.170 | Protein folding | 73299 | 5.22 |
| Ty, Ty+ECM | histone H2A, putative | TcCLB.510525.80 | DNA packaging | 14354 | 11.9 |
| Ty, Ty+ECM | histone H2B, putative | TcCLB.511635.10 | DNA packaging | 12361 | 12.2 |
| Ty, Ty+ECM | histone H3, putative | TcCLB.505931.50 | DNA packaging | 14762 | 11.9 |
| Ty, Ty+ECM | histone H4, putative | TcCLB.510351.20 | DNA packaging | 11170 | 11.7 |
| Ty+ECM | hypothetical protein, conserved (pseudogene) | TcCLB.511821.179 | N/A | 143829 | 4.19 |
| Ty | hypothetical protein, conserved | TcCLB.504089.70 | N/A | 12959 | 8.48 |
| Ty | hypothetical protein, conserved | TcCLB.504001.10 | N/A | 13465 | 9.78 |
| Ty+ECM | hypothetical protein, conserved | TcCLB.510877.40 | N/A | 24454 | 10.2 |
| Ty | hypothetical protein, conserved | TcCLB.510143.5 | N/A | 7311 | 6.52 |
| Ty | hypothetical protein, conserved | TcCLB.508719.70 | N/A | 44107 | 10.6 |
| Ty, Ty+ECM | kinetoplast DNA-associated protein, putative | TcCLB.508719.60 | DNA packaging | 14361 | 12.1 |
| Ty | kinetoplast-associated protein 3 | TcCLB.509791.120 | DNA packaging | 21245 | 11.9 |
| Ty | mitochondrial oligo_U binding protein TBRGG1, putative | TcCLB.507927.20 | RNA processing | 97266 | 8.8 |
| Ty | poly(A)-binding protein, putative | TcCLB.508461.140 | RNA processing | 61411 | 9.69 |
| Ty | ribosomal protein l35a, putative | TcCLB.506559.470 | Translation | 16892 | 12.1 |
| Ty | ribosomal protein L36, putative | TcCLB.509671.64 | Translation | 12948 | 12.1 |
| Ty, Ty+ECM | ribosomal protein S25, putative | TcCLB.504105.94 | Translation | 12351 | 11.2 |
| Ty | ribosomal protein S7, putative | TcCLB.506593.19 | Translation | 23915 | 11.9 |
| Ty | RNA-binding protein, putative | TcCLB.508413.50 | RNA Processing | 31097 | 9.78 |
Tyrosine nitrated T. cruzi proteins after 2 h incubation of the parasite in the presence of bovine serum albumin (Ty) or extracellular matrix (Ty+ECM).
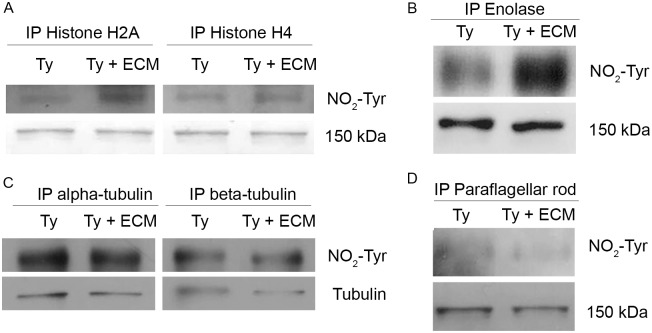
To confirm this post-translational modification, histone 2A, histone 4B, enolase, alpha-tubulin, beta-tubulin and paraflagellar rod proteins (PAR) were selected. Modified histones and tubulins were detected herein (Table 1), enolase was included since nitrated-enolase was already described in the literature [57] and PAR was chosen as a negative control of the method. The mentioned proteins were immunoprecipitated with commercial specific antibodies (except for anti-PAR monoclonal antibody prepared in the laboratory [50]) followed by Western blot developed with anti-nitro-tyrosine antibodies. An increase in the nitrosylation levels of enolase and histones 2A and 4 were observed after the incubation with ECM (Fig. 9A, B). No changes in the nitration levels were observed when a similar experiment was performed using anti-alpha and beta-tubulin antibodies, while no reactivity was detected with paraflagellar proteins immunoprecipitated with anti-PAR monoclonal antibody (Fig. 9C, D). The results have shown that in spite of the general down regulation of protein S-nitrosylation and nitration upon incubation of the parasites with ECM, there is a specific response for each protein, including the stimulation of the nitration levels of enolase and histones 2A and 4B.
Fig 9. Validation of T. cruzi tyrosine nitrated proteins.
Proteins of interest were immunoprecipitated (IP) with specific antibodies (anti histone H2A, anti-histone H4, anti-enolase, anti-alpha tubulin, anti-beta tubulin or anti-paraflagellar rod proteins antibodies) and submitted to immunoblotting employing anti-nitro-tyrosine antibodies. A 150 kDa band of the blotting (A, B and D) or anti-tubulin antibodies (C) are shown as controls of the protein loading. T. cruzi control (Ty) and treated with ECM for 2 hours (Ty + ECM).
Taken together, the results herein presented strongly suggest that T. cruzi responds to the interaction with ECM by the involvement of •NO regulated pathways.
Discussion
Nitric oxide is a key signaling molecule affecting many biological activities. Human parasites such as T. cruzi are exposed to the anti-parasitic •NO produced by the host but also to its own •NO. Since the interaction of T. cruzi trypomastigotes with ECM is an essential step during the infective process [2,48,49], the in vitro model in the absence of host cells has been used to study the role of •NO in the parasite response to the interaction. ECM is a very dynamic structure and its relevance in •NO signaling was shown, for example, by thrombospondin-1 inhibition [58] of the •NO pathway in vascular cells. Additionally, the relevance of ECM to T. cruzi signaling was previously shown by changes in parasite protein phosphorylation levels [50].
Endogenous nitric oxide is predominantly produced in T.cruzi by enzyme catalysis, probably by NOS, as described [34] since the addition of the inhibitor L-NAME drastically reduces •NO production (Fig. 1A, B). In addition to •NO, the reaction produces L-citrulline from the substrate L-arginine. Of note, a strong reduction of citrulline, but not of arginine, was measurable in ECM-incubated trypomastigotes, suggestive of an inhibited NOS activity, although other possibilities such as its utilization in another activated metabolic route could not be ruled out. Arginine, on the other hand, is a substrate for protein synthesis and a precursor of •NO and other important metabolites as phosphoarginine, an energy buffer synthesized in T. cruzi by arginine kinase. Due to its relevance, it was previously suggested that arginine concentration under different external conditions may be buffered by TcAAP3, a specific permease [59].
Adhesion of trypomastigotes to ECM resulted in a remarkable inhibition not only on NOS activity but also in •NO and cGMP concentrations (Fig. 1, 2). However, a direct correlation between •NO levels and cGMP concentrations is difficult to make since the amount of •NO that may activate cGMP synthesis in trypomastigotes is unknown, due to the fact that no typical guanylyl cyclase is present in the T. cruzi genome. Moreover, the putative cGMP synthetic activity of an ubiquitous adenylyl cyclase remains non characterized [37]. Additionally, cGMP concentrations would depend on its degradation by a soluble dual-specificity phosphodiesterase (TcrPDEC) [38,39,40,41]. Presumably, the downstream signal transmission may be dependent on a protein kinase A activated by cGMP, as described for T. brucei [45] and Leishmania [46].
The decrease in total nitration and S-nitrosylation levels of proteins as described here for ECM-incubated trypomastigotes probably reflects the lower level of nitrosative stress. Changes in S-nitrosylated proteins were easily noticed by Western blot experiments after 30 minutes incubation of the parasites with ECM, with a marked decrease after 2 h period time (Fig. 5) and confirmed by immunofluorescence and decrease in the total SNO measured (Figs. 5 and 7). S-nitrosylation of proteins is a key player in diverse biological functions of •NO and is associated with processes such as apoptosis [60] and regulation of numerous signaling pathways, for example, PKC [61] and MAPK [62]. Of interest, S-nitrosylation has been associated with activation and desensitization of the human soluble guanylyl cyclase that possesses 37 cysteine residues (review in [58]). To our knowledge, the only study describing S-nitrosylation in T. cruzi proteins used •NO-donors to investigate a possible role of the host derived •NO in the inhibition of cruzipain, a cysteine protease important for the parasite infection [33]. However, the relevance of S-nitrosylation in T. cruzi signaling was not further explored.
Here proteins putatively S-nitrosylated in normal conditions and after interaction of the T. cruzi with the extracellular matrix were analyzed for the first time, using mucin II to validate the modification (Fig. 8). A number of other interesting targets were identified including some proteins already described as S-nitrosylated with relevant modification in function, such as Dual Specificity Phosphatase (DUSP) [62], Serine-Threonine Protein Kinase [61] and HSP 90 [63]. Interestingly, phosphorylation levels in DUSP and Serine/Threonine Protein Kinase were also modified in ECM-incubated parasites [50], but the possibility that both modifications are somehow related, as happens in other cases, has not been addressed. The results described point out to a possible role of the S-nitrosylation in T.cruzi signaling pathways and will be further explored.
Tyrosine nitration is not as well understood as S-nitrosylation, although relevant processes seem to be modulated by this covalent modification, such as PKC signaling [30] and protein degradation [28]. The present study was able to identify some of the proteins previously described as potential nitration targets (Table 1), such as enoyl-CoA hydratase [64], glyceraldehyde-3-phosphate dehydrogenase [65], heat shock protein 70 [66] and histone 2A [67]. Furthermore, validation of the data for histone 2A, histone 4, enolase and tubulins was achieved (Fig. 8). In the literature, for example, nitration of histones was associated with the induction of autoimmunity in systemic lupus erythematosus and rheumatoid arthritis [67]. Remarkably, a large number of 40 and 60 S-ribosomal proteins were modified by nitration, but whether this modification affects protein expression in T. cruzi remains to be elucidated. Nitration of ribosomal proteins was also described in native and differentiated PC12 cells, but tryptophan was identified as the modified amino acid [11].
In summary, the present work was able to confirm previous claims on the existence of enzymatic NOS activity in T. cruzi and demonstrated that the •NO classic signaling pathway is greatly inhibited in the presence of ECM regarding the synthesis of •NO and cGMP. Furthermore, numerous possible S-nitrosylation and tyrosine nitration targets have been identified, with a total decrease in the level of modified proteins upon interaction of the parasite with ECM. However, in spite of general down regulation of protein S-nitrosylation and nitration, the increase in the S-nitrosylation level of mucin II or in the nitration of enolase or histones 2A and 4, in contrast to the constant nitration levels of alpha and beta-tubulins under any condition, point out to the specificity of the modification for each particular target. The biological relevance of each of these target modifications remains to be explored and may give clues to the function of each target in parasite internalization into host cells. In this regard it must be stressed that •NO availability appears to be essential for parasite motility [68]. Thus, it is tempting to speculate that adhesion of T. cruzi trypomastigotes to ECM, an obligatory path to reach the host cell, triggering decrease in •NO levels, may also decrease parasite motility, somehow facilitating its binding to the host cell plasma membrane prior to invasion.
Data Availability
All relevant data are within the paper.
Funding Statement
The work was supported by Fundação de Amparo à Pesquisa do Estado de Sao Paulo (http://www.fapesp.br/), grant numbers 2012/50188-3, 2012/03887-3 and 2012/05621-0) and by Conselho Nacional de Desenvolvimento Científico e Tecnológico (http://www.cnpq.br/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization [http://www.who.int/mediacentre/factsheets/fs340/en/]. Accessed 22 november 2014.
- 2. Alves MJM, Colli W (2007) Trypanosoma cruzi: adhesion to the host cell and intracellular survival. IUBMB Life 59:274–279. [DOI] [PubMed] [Google Scholar]
- 3. Barrias ES, de Carvalho TM, de Souza W (2013) Trypanosoma cruzi: entry into mammalian host cells and parasitophorus vacuole formation. Frontiers Immunol. 4: a186 10.3389/fimmu.2013.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fernandes MC, Andrews NW (2012) Host cell invasion by Trypanosoma cruzi: a unique strategy that promotes persistence. FEMS Microbiol Rev 36:734–747. 10.1111/j.1574-6976.2012.00333.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodrigues MM, Ribeirão M, Boscardin SB (2000) CD4 Th1 but not Th2 clones efficiently activate macrophages to eliminate Trypanosoma cruzi through a nitric oxide dependent mechanism. Immunol Lett 73:43–50. [DOI] [PubMed] [Google Scholar]
- 6. Piacenza L, Alvarez MN, Peluffo G, Radi R (2009) Fighting the oxidative assault: the Trypanosoma cruzi journey to infection. Curr Opin Microbiol. 12:415–21. 10.1016/j.mib.2009.06.011 [DOI] [PubMed] [Google Scholar]
- 7. Carvalho CM, Silverio JC, da Silva AA, Pereira IR, Coelho JM, et al. (2012) Inducible nitric oxide synthase in heart tissue and nitric oxide in serum of Trypanosoma cruzi-infected rhesus monkeys: association with heart injury. PLoS Negl Trop Dis 6:e1644 10.1371/journal.pntd.0001644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alderton WK, Cooper CE, Knowles RG (2001) Nitric oxide synthases: structure, function and inhibition. Biochem J 357: 593–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koppenol WH (1998) The basic chemistry of nitrogen monoxide and peroxynitrite. Free Radic Biol Med 25: 385–391. [DOI] [PubMed] [Google Scholar]
- 10. Martínez-Ruiz A, Cadenas S, Lamas S (2011) Nitric oxide signaling: classical, less classical, and nonclassical mechanisms. Free Radic Biol Med 51:17–29. 10.1016/j.freeradbiomed.2011.04.010 [DOI] [PubMed] [Google Scholar]
- 11. Kawasaki H, Shigenaga A, Uda M, Baba T, Ogawa H, Takamori K, Yamakura F (2011) Nitration of tryptophan in ribosomal proteins is a novel post-translational modification of differentiated and naïve PC12 cells. Nitric Oxide 25: 176–182. 10.1016/j.niox.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 12. Abello N, Kerstjens HA, Postuma DS, Bischoff R (2009) Protein tyrosine nitration: selectivity, physicochemical and biological consequences, denitration, and proteomics methods for the identification of tyrosine-nitrated proteins. J Proteome Res 8: 3222–3238. 10.1021/pr900039c [DOI] [PubMed] [Google Scholar]
- 13. Hess DT, Stamler JS (2012) Regulation by S-nitrosylation of protein post-translational modification. J Biol Chem 287: 4411–4418. 10.1074/jbc.R111.285742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kornberg MD, Sen N, Hara MR, Juluri KR, Nguyen JV, et al. (2010) GAPDH mediates nitrosylation of nuclear proteins. Nat Cell Biol 12: 1094–1100. 10.1038/ncb2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakamura T, Lipton SA (2013) Emerging role of protein-protein transnitrosylation in cell signaling pathways. Antioxid Redox Signal 18: 239–49. 10.1089/ars.2012.4703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu C, Liu T, Chen W, Oka S, Fu C, et al. (2010) Redox regulatory mechanism of transnitrosylation by thioredoxin. Mol Cellular Proteomics 9: 2262–2275. 10.1074/mcp.M110.000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benhar M, Forrester MT, Hess DT, Stamler JS (2008) Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science 320: 1050–1054. 10.1126/science.1158265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benhar M, Forrester MT, Stamler JS (2009) Protein denitrosylation: enzymatic mechanisms and cellular functions. Nature Rev Molec Cell Biol 10: 721–732. [DOI] [PubMed] [Google Scholar]
- 19. Sengupta R, Ryter SW, Zuckerbraun BS, Tzeng E, Billiar TR, et al. (2007) Thioredoxin catalyzes the denitrosation of low-molecular mass and protein S-nitrosothiols. Biochemistry 46: 8472–8483. [DOI] [PubMed] [Google Scholar]
- 20. Jaffrey SR, Snyder SH (2001) The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE 86: pl1 [DOI] [PubMed] [Google Scholar]
- 21. Derakhshan B, Wille P, Gross S (2007) Unbiased identification of cysteine S-nitrosylation sites on proteins. Nat Protoc 2: 1685–1691. [DOI] [PubMed] [Google Scholar]
- 22. Wang L, Delahunty C, Prieto JH, Rahlfs S, Jortzik E, et al. (2014) Protein S-nitrosylation in Plasmodium falciparum . Antioxid Redox Signal 20: 2923–2935. 10.1089/ars.2013.5553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ostera G, Tokumasu F, Oliveira F, Sa J, Furuya T, Teixeira C, Dvorak J. (2008) Plasmodium falciparum: food vacuole localization of nitric oxide—derived species in intraerythrocytic stages of the malaria parasite. Exp. Parasitol. 120: 29–38. 10.1016/j.exppara.2008.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Radi R (2013) Protein tyrosine nitration: biochemical mechanisms and structural basis of functional effects. Acc Chem Res 46: 550–559. 10.1021/ar300234c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Campolo N, Bartesaghi S, Radi R (2014) Metal- catalyzed protein tyrosine nitration in biological systems. Redox Rep 19: 221–231. 10.1179/1351000214Y.0000000099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cecconi D, Orzetti S, Vandelle E, Rinnalducci S, Zolla L, et al. (2009) Protein nitration during defense response in Arabidopsis thaliana . Electrophoresis 30: 2460–2468. 10.1002/elps.200800826 [DOI] [PubMed] [Google Scholar]
- 27. Nakagawa H, Komai N, Takusagawa M, Miura Y, Toda T, et al. (2007) Nitration of specific tyrosine residues of cytochrome C is associated with caspase-cascade inactivation. Biol Pharm Bull 30: 15–20. [DOI] [PubMed] [Google Scholar]
- 28. Souza JM, Choi I, Chen Q, Weisse M, Daikhin E, et al. (2000). Proteolytic degradation of tyrosine nitrated proteins. Arch Biochem Biophys 380: 360–366. [DOI] [PubMed] [Google Scholar]
- 29. Ji Y, Neverova I, van Eyk JE, Bennett BM (2006) Nitration of tyrosine 92 mediates the activation of rat microsomal glutathione s-transferase by peroxynitrite. J Biol Chem 281:1986–1991. [DOI] [PubMed] [Google Scholar]
- 30. Balafanova Z, Bolli R, Zhang J, Zhen Y, Pass JM, et al. (2002). Nitric oxide (NO) induces nitration of protein kinase Cepsilon (PKCepsilon), facilitating PKCepsilon translocation via enhanced PKCepsilon-RACK2 interactions: a novel mechanism of NO-triggered activation of PKCepsilon. J Biol Chem 277: 15021–15027. [DOI] [PubMed] [Google Scholar]
- 31. Jortzik E, Wang L, Becker K (2012) Thiol-based posttranslational modifications in parasites. Antioxid Redox Signal 17: 657–673. 10.1089/ars.2011.4266 [DOI] [PubMed] [Google Scholar]
- 32. Scharfstein J, Lima AP (2008) Roles of naturally occurring protease inhibitors in the modulation of host cell signaling and cellular invasion by Trypanosoma cruzi . Subcell Biochem 47: 140–154. [DOI] [PubMed] [Google Scholar]
- 33. Venturini G, Salvati L, Muolo M, Colasanti M, Gradoni L, et al. (2000) Nitric oxide inhibits cruzipain, the major papain-like cysteine proteinase from Trypanosoma cruzi . Biochem Biophys Res Commun 270: 437–441. [DOI] [PubMed] [Google Scholar]
- 34. Paveto C, Pereira C, Espinosa J (1995) The nitric oxide transduction pathway in Trypanosoma cruzi . J Biol Chem 270: 16576–16579. [DOI] [PubMed] [Google Scholar]
- 35. Goldstein J, Paveto C, López-Costa JJ, Pereira C, Alonso G et al. (2000) Immuno and Cytochemical Localization of Trypanosoma cruzi Nitric Oxide Synthase. Biocell 24: 217–222. [PubMed] [Google Scholar]
- 36. Alexandre S, Paindavoine P, Tebabi P, Pays A, Halleux S et al. (1990) Differential expression of a family of putative adenylate/guanylate cyclase genes in Trypanosoma brucei . Mol Biochem Parasitol 43: 279–288. [DOI] [PubMed] [Google Scholar]
- 37. Laxman S, Beavo J (2007) Cyclic nucleotide signaling mechanisms in trypanosomes: possible targets for therapeutic agents. Mol Interven 7: 203–215. [DOI] [PubMed] [Google Scholar]
- 38. Kunz S, Oberholzer M, Seebeck T (2005) A FYVE-containing unusual cyclic nycleotide phosphodiesterase from Trypanosoma cruzi. FEBS J 272: 6412–6422. [DOI] [PubMed] [Google Scholar]
- 39. Alonso GD, Schoijet AC, Torres HN, Flawia MM (2006) TCPDE4, a novel membrane- associated cAMP-specific phosphodiesterase from Trypanosoma cruzi . Mol Biochem Parasitol 145: 40–49. [DOI] [PubMed] [Google Scholar]
- 40. Gould MK, Koning HP (2011) Cyclic nucleotide signaling in protozoa. FEMS Microbiol Rev 35: 515–541. 10.1111/j.1574-6976.2010.00262.x [DOI] [PubMed] [Google Scholar]
- 41. Wang H, Kunz S, Chen G, Seebeck T, Wan Y et al. (2012). Biological and structural characterization of Trypanosoma cruzi phosphodiesterase C and implications for design of parasite selective inhibitors. J Biol Chem 287, 11788–11797. 10.1074/jbc.M111.326777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gonzales-Perdomo M, Romero P, Goldenberg S (1988) Cyclic AMP and adenylate cyclase activators stimulate Trypanosoma cruzi differentiation. Exp. Parasitol 66: 205–212. [DOI] [PubMed] [Google Scholar]
- 43. Rangel-Aldao R, Triana F, Fernandez V, Comach G, Abate T et al. (1988) Cyclic AMP as inducer of cell differentiation of Trypanosoma cruzi . Biochem Int 17: 337–344. [PubMed] [Google Scholar]
- 44. Bao Y, Weiss LM, Ma FY, Kahn S, Huang H (2010) Protein kinase A catalytic subunit interacts and phosphorylates members of trans-sialidase super family in Trypanosoma cruzi . Microbes Infect 12: 716–726. 10.1016/j.micinf.2010.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shalaby T, Liniger M, Seebeck T (2001) The regulatory subunit of a cGMP-regulated protein kinase A of Trypanosoma brucei . Eur J Biochem 268: 6197–6206. [DOI] [PubMed] [Google Scholar]
- 46. Malki-Feldman L, Jaffe CL (2009) Leishmania major: effect of protein kinase A and phosphodesterase activity on infectivity and proliferation of promastigotes. Exp parasitol 123: 39–44. 10.1016/j.exppara.2009.05.010 [DOI] [PubMed] [Google Scholar]
- 47. Hynes RO, Naba A (2012) Overview of the matrisome- an inventory of the extracellular matrix constituints and functions. Cold Spring Harb Perspect Biol 4:a004903 10.1101/cshperspect.a004903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Giordano R, Fouts DL, Tewari D, Colli W, Manning JE, et al. (1999) Cloning of a Surface Membrane Glycoprotein Specific for the Infective Form of Trypanosoma cruzi Having Adhesive Properties to Laminin. J Biol Chem 274: 3461–3468. [DOI] [PubMed] [Google Scholar]
- 49. Nde PN, Lima MF, Johnson CA, Prata S, Villalta F (2012) Regulation and use of extracellular matrix by Trypanosoma cruzi during early infection. Frontiers Immunol 3: e337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mattos EC, Schumacher RI, Colli W, Alves MJM (2012) Adhesion of Trypanosoma cruzi trypomastigotes to fibronectin or laminin modifies tubulin and paraflagellar rod protein phosphorylation. PloS One 7: e46767 10.1371/journal.pone.0046767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Camargo EP (1964) Growth and differentiation in Trypanosoma cruzi. Origin of metacyclic trypanosomes in liquid media. Rev Inst Med Trop São Paulo 12: 93–100. [PubMed] [Google Scholar]
- 52. Andrews NW, Colli W (1982) Adhesion and interiorization of Trypanosoma cruzi in mammalian cells. J Protozool 29: 264–269. [DOI] [PubMed] [Google Scholar]
- 53. Cook JA, Kim SY, Teague D, Krishna M C, Pacelli R, et al. (1996) Convenient colorimetric and fluorometric assays for S-nitrosothiols. Analytical Biochem 238:150–158. [DOI] [PubMed] [Google Scholar]
- 54. Laemmli U (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- 55. Rauch A, Bellew M, Eng J (2006) Computational Proteomics Analysis System (CPAS): an extensible, open-source analytic system for evaluating and publishing proteomic data and high throughput. J Proteome Res 5: 112–121. [DOI] [PubMed] [Google Scholar]
- 56. da Silva MF, Floeter-Winter LM (2014) Arginase in Leishmania. Subcell Biochem. 74:103–117. 10.1007/978-94-007-7305-9_4 [DOI] [PubMed] [Google Scholar]
- 57. Casoni F, Basso M, Massigan T, Gianazza E, Cheroni C, et al. (2005) Protein nitration in a mouse model of familial amyotrophic lateral sclerosis: possible multifunctional role in the pathogenesis. J Biol. Chem. 280: 16295–304. [DOI] [PubMed] [Google Scholar]
- 58. Rogers NM, Seeger F, Garcin ED, Roberts D, Isenberg JS (2014) Regulation of soluble guanylate cyclase by matricellular thrombospondins: implications for blood flow. Frontiers Physiol 5: e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Miranda MR, Sayé M, Bouvier LA, Cámara Mde L, Montserrat J, Pereira CA (2012) Cationic amino acid uptake constitutes a metabolic regulation mechanism and occurs in the flagellar pocket of Trypanosoma cruzi. PLoS One 7:e32760 10.1371/journal.pone.0032760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sen N, Hara MR, Kornberg MD, Cascio MB, Bae B-I, et al. (2008) Nitric oxide-induced nuclear GAPDH activates p300/CBP and mediates apoptosis. Nat Cell Biol 10: 866–73. 10.1038/ncb1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Choi H, Tostes R, Webb R (2011) S-Nitrosylation inhibits protein kinase C-mediated contraction in mouse aorta. J Cardiovasc Pharmacol 57: 65–71. 10.1097/FJC.0b013e3181fef9cb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Guan W, Sha J, Chen X, Xing Y, Yan J, Wang Z (2012) S-Nitrosylation of mitogen activated protein kinase phosphatase-1 suppresses radiation-induced apoptosis. Cancer Lett 314: 137–146. 10.1016/j.canlet.2011.09.022 [DOI] [PubMed] [Google Scholar]
- 63. Martínez-Ruiz A, Villanueva L, González de Orduña C, López-Ferrer D, Higueras MA, et al. (2005) S-nitrosylation of Hsp90 promotes the inhibition of its ATPase and endothelial nitric oxide synthase regulatory activities. Proc Natl Acad Sci USA 102: 8525–8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Koeck T, Fu X, Hazen SL, Crabb JW, Stuehr DJ, Aulak KS (2004) Rapid and selective oxygen-regulated protein tyrosine denitration and nitration in mitochondria. J Biol Chem 279: 27257–27262. [DOI] [PubMed] [Google Scholar]
- 65. Palamalai V, Miyagi M (2010) Mechanism of glyceraldehyde-3-phosphate dehydrogenase inactivation by tyrosine nitration. Protein Sci 19: 255–262. 10.1002/pro.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rodríguez-Ariza A, López-Sánchez LM, González R, Corrales FJ, López P, Bernardos A, Muntané J (2005) Altered protein expression and protein nitration pattern during d-galactosamine-induced cell death in human hepatocytes: a proteomic analysis. Liver Internat 25: 1259–1269. [DOI] [PubMed] [Google Scholar]
- 67. Khan MA, Dixit K, Uddin M, Malik A, Alam K (2012) Role of peroxynitrite-modified H2A histone in the induction and progression of reumathoid arthritis. Scand J Rheumatol 41: 426–423. 10.3109/03009742.2012.698300 [DOI] [PubMed] [Google Scholar]
- 68. Pereira C, Paveto C, Espinosa J, Alonso G, Flawiá MM, Torres HN (1997) Control of Trypanosoma cruzi epimastigote motility through the nitric oxide pathway. J Eukaryot Microbiol 44:155–156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.