Abstract
Microbicides may prevent HIV and sexually transmitted infections (STIs) in women; however, determining the optimal means of delivery of active pharmaceutical ingredients remains a major challenge. We previously demonstrated that a vaginal gel containing the non-nucleoside reverse transcriptase inhibitor MIV-150 partially protected macaques from SHIV-RT (simian/HIV reverse transcriptase) infection, and the addition of zinc acetate rendered the gel significantly protective. We test the activity of MIV-150 without the addition of zinc acetate when delivered from either ethylene vinyl acetate (EVA) or silicone intravaginal rings (IVRs). MIV-150 was successfully delivered, because it was detected in vaginal fluids and tissues by radioimmunoassay in pharmacokinetic studies. Moreover, EVA IVRs significantly protected macaques from SHIV-RT infection. Our results demonstrate that MIV-150–containing IVRs have the potential to prevent HIV infection and highlight the possible use of IVRs for delivering drugs that block HIV and other STIs.
INTRODUCTION
Products that block HIV sexual transmission are urgently needed. A 1% tenofovir gel applied before and after intercourse significantly reduced HIV and HSV-2 (herpes simplex virus 2) acquisition in women (1), indicating the potential for topical microbicides to curb the AIDS pandemic. In contrast, the 1% tenofovir gel arm of the VOICE (Vaginal and Oral Interventions to Control the Epidemic) trial, which used a daily dosing regimen, was dropped after interim data analysis showed that the gel was ineffective. The reasons for these different outcomes are unclear, but the discrepancies underscore the need for continued research into HIV prevention products.
Adherence remains a critical bottleneck to the success of microbicides (1–4). Intravaginal rings (IVRs) represent a sustained-release approach to microbicide delivery. They are well tolerated by women, are efficacious for contraception (5) and hormone replacement therapy (6), and have been associated with improved adherence over other drug delivery systems (7, 8). IVR technology is well suited to the delivery of small molecules like the hydrophilic nucleoside reverse transcriptase inhibitors and the hydrophobic non-nucleoside reverse transcriptase inhibitors (NNRTIs) (9–11). Recent advances in microbicide IVRs have led to their ability to release multiple active pharmaceutical ingredients (APIs) targeting HIV and other sexually transmitted infections (STIs) (6, 10–13).
We previously reported that repeated application of a carrageenan gel containing the potent NNRTI MIV-150 (Fig. 1A, not used in first-line HIV therapies) provided partial protection against vaginal SHIV-RT (simian/HIV reverse transcriptase) transmission in macaques when the last dose was given 8 hours before challenge (14). Protection was lost when the last dose was applied 24 hours before challenge; however, the addition of zinc acetate boosted the gel's protective potential to 89% (P < 0.0002) (14). We aim to develop IVRs as an alternative delivery platform for this promising API combination. Needing to first demonstrate the activity of MIV-150 delivered from IVRs, we hypothesized that IVRs would provide sustained release of MIV-150 in vivo and improve protection compared to MIV-150 gels. To that end, in this study, we developed silicone and ethylene vinyl acetate (EVA) MIV-150 IVRs. Both IVRs released MIV-150 in vitro and in vivo, with EVA IVRs significantly protecting macaques from SHIV-RT infection. Our findings demonstrate that MIV-150–releasing IVRs can protect against immunodeficiency virus challenge and support the development of IVRs that release MIV-150 and zinc acetate to prevent HIV and other STIs.
Fig. 1.
MIV-150 IVRs. (A) Chemical structure and properties of MIV-150. MF, molecular formula; MW, molecular weight; Tm, melting point; Sw, SEtOH, and SDMSO, solubility in water, ethanol, and dimethyl sulfoxide, respectively; Log P, octanol/water partition coefficient; CP, number of crystal polymorphs. (B) MIV-150 silicone IVR (50 mg) (left) and MIV-150 EVA-40 IVR (100 mg) (right).
RESULTS
Silicone IVRs release active MIV-150 and provide partial protection against vaginal SHIV-RT infection
Silicone matrix IVRs were formulated with 50 mg of MIV-150 (Fig. 1B), and in vitro MIV-150 release was evaluated with the nonionic surfactant Solutol (0.05 weight percent) in 100 ml of 25 mM sodium acetate buffer (pH 4.2) (SNaC buffer) as the release medium (15). Daily release ranged from 111 to 33 μg/day, averaging 54 μg/day with a day 15 median of 45 μg/day (Fig. 2A). The cumulative amount of MIV-150 released at 29 days was 1410 μg (mean of the area under the curve of the MIV-150 release curves) or 2.8% of the loading dose (Fig. 2B). The cumulative percentage of MIV-150 released (Q) over time exhibited partition-controlled kinetics of Q α time0.90 (Fig. 2B). The IC50 (median inhibitory concentration) value of MIV-150 released into SNaC buffer on day 1 was 2.7 nM (Table 1), whereas MIV-150 released into aqueous ethanol on day 1 (fig. S1) had an IC50 value of 0.60 nM (Table 1). Thus, ring formulation conditions did not affect MIV-150's activity.
Fig. 2.
MIV-150 silicone IVRs release MIV-150 in vitro. (A) Release of MIV-150 over 29 days from 50-mg of MIV-150 IVRs in SNaC buffer. (B) Cumulative percentage of MIV-150 released (Q) from the IVRs over 29 days. The means ± SD from four identical experiments are shown.
Table 1.
Antiviral activity of in vitro-released MIV-150. The IC50 of unformulated MIV-150 is <1 nM.
| Ring | IC50, nM (95% confidence interval) |
|
|---|---|---|
| 1:1 ethanol/water* | SNaC buffer† | |
| Silicone | 0.6 (0.52-0.76) | 2.7 (1.9-3.9) |
| EVA | 0.5 (0.42-0.82) | 2.8 (1.9-4.3) |
200 ml at 37°C for 1 day.
100 ml at 37°C for 1 day.
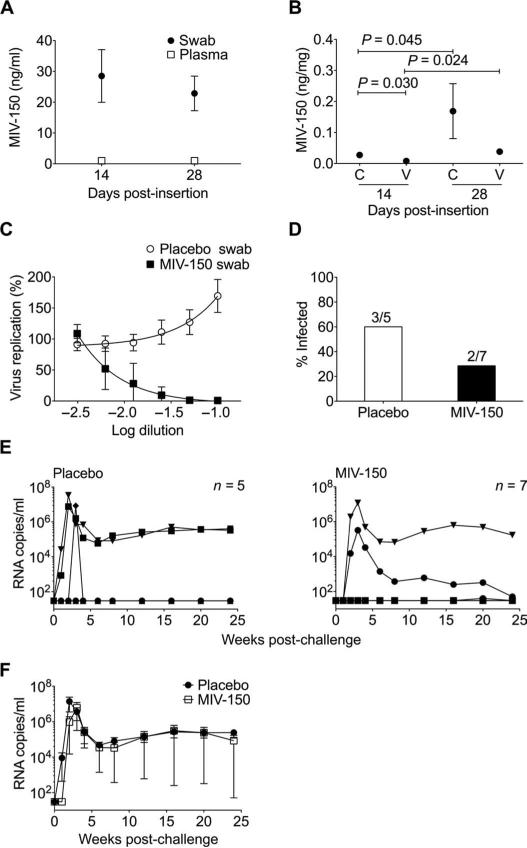
To characterize in vivo release, we quantified MIV-150 in plasma, vaginal swabs, and vaginal and cervical tissues of Depo-Provera (depo)–treated macaques that received silicone IVRs (table S1) for 4 weeks. MIV-150 was below the limit of detection in the plasma 14 and 28 days after IVR insertion [radioimmunoassay (RIA) lower limit of quantification (LLQ) was 1 ng/ml (2.7 nM)] (16) (Fig. 3A). Similar MIV-150 levels (mean ± SEM) were detected in vaginal swabs 14 days (28.5 ± 8.5 ng/ml, 77.0 nM) and 28 days after insertion (22.9 ± 5.6 ng/ml, 61.8 nM) (Fig. 3A), which was >60 times the IC50. MIV-150 was detected in cervical (0.027 ± 0.006 ng/mg wet tissue) and vaginal (0.008 ± 0.004 ng/mg) biopsies 14 days after insertion, increasing significantly by 28 days (cervix, 0.169 ± 0.089 ng/mg; vagina, 0.038 ± 0.009 ng/mg) (Fig. 3B). More MIV-150 was detected in the cervix than in the vagina (significant only 14 days after insertion). MIV-150 released in vivo was active in vitro. Vaginal swabs collected 2 to 4 weeks after insertion had a mean ± SD IC50 value of 0.40 ± 0.26 nM (Fig. 3C).
Fig. 3.
MIV-150 silicone IVRs offer partial protection against SHIV-RT infection. (A and B) MIV-150 in plasma and vaginal swabs 14 days (n = 13) and 28 days (n = 12) after insertion (A) and in cervical (C) and vaginal (V) pinch biopsies at 14 days (n = 6) and 28 days (n = 6) after insertion (B). Mean ± SEM is shown in both (A) and (B). Statistical analyses in (A) and (B) used the Mann-Whitney U test. (C) Percent of in vitro HIV replication in the presence of vaginal swab fluids from animals that received MIV-150 (n = 6) and placebo IVRs (n = 10; mean ± SEM) (relative to no swab controls, set as 100%). (D) Infection frequency in the placebo and MIV-150 IVR groups. The numbers above each bar denote the number of infected animals over the number of challenged animals in each group. (E) Viremia (SIV RNA copies per milliliter of plasma) shown over time for each animal challenged with 103 TCID50 SHIV-RT 2 weeks after receiving placebo or MIV-150 IVRs. (F) Mean ± SEM plasma viral load of infected animals from each group.
Because silicone IVRs released MIV-150 in vivo, we evaluated their ability to protect macaques from SHIV-RT. IVRs were present in the vagina for 2 weeks before vaginal challenge (inserted 3 weeks after depo) and 2 weeks after (table S2). Silicone IVRs reduced infection frequency to 29% (two of seven; 58% reduction in infection compared to three of five infected in the placebo IVR group) (Fig. 3D). This was not statistically significant (P = 0.55, Fisher's exact test) because of the small number of animals tested. Virus in both infected macaques peaked 3 weeks after infection before declining to a variable set point (Fig. 3E). Two placebo animals experienced normal viremia that peaked 2 weeks after infection and declined to a set point; the third had high viremia at week 3 but no detectable virus at every other time point (Fig. 3E and table S2). There was no difference in average viremia between infected animals that received placebo or MIV-150 IVRs (Fig. 3F). Plasma viral RNA from infected animals at peak viremia showed none of the 10 most frequent NNRTI-associated resistance mutations (table S3). Only infected animals seroconverted except for the placebo macaque with a single viremic time point (table S2).
MIV-150 release in vitro is improved from EVA-40 IVRs
As an alternative delivery vehicle, we explored EVA IVRs loaded with MIV-150 (Fig. 1B). EVA is commonly used for clinical devices and the only thermoplastic used clinically for an IVR (6, 17). We prepared EVA-40 IVRs (40% vinyl acetate content) (Fig. 1B) loaded with 50 mg (for comparison with silicone) or 100 mg (to maximize drug release) of MIV-150. Daily release (in the SNaC buffer) for the 50-mg IVRs ranged from 238 to 130 μg/day, averaging 163 μg/day with a day 14 median of 151 μg/day, and for the 100-mg IVRs ranged from 251 to 162 μg/day, averaging 195 μg/day with a day 14 median of 176 μg/day (Fig. 4A). The cumulative amounts of MIV-150 released at 28 days from the 50- and 100-mg EVA-40 IVR were 4370 μg, or 8.7% of the loading dose, and 5285 μg, or 5.3% of the loading dose, respectively (Fig. 4B). The 50- and 100-mg IVRs exhibited partition-controlled release kinetics of Q α time1.04 and Q α time1.08, respectively (Fig. 4B). The in vitro antiviral activity of MIV-150 released from 100-mg EVA-40 IVRs into SNaC buffer and aqueous ethanol (Table 1) confirmed that IVR processing conditions did not affect MIV-150's activity. The IC50 value for MIV-150 released into SNaC buffer was 2.8 and 0.5 nM for release into aqueous ethanol (Table 1).
Fig. 4.
MIV-150 release from EVA-40 IVRs is greater than that from silicone IVRs in vitro. (A) Release of MIV-150 over 28 days from 50- and 100-mg MIV-150 EVA-40 IVRs in SNaC buffer. (B) Cumulative percentage of MIV-150 released (Q) from 50- and 100-mg MIV-150 EVA-40 IVRs over 28 days. The mean ± SD from four identical experiments is shown.
Active MIV-150 is released from EVA-40 IVRs in vivo
We initially hypothesized that having IVRs in place for 2 weeks before challenge would increase MIV-150 levels in target tissues and enhance protection. However, if drug levels at the time of virus exposure were important, then IVRs might be even more effective if macaques were challenged 24 hours after the ring was inserted (see Fig. 4A). Therefore, we investigated the temporal relationship between in vitro–release kinetics and MIV-150 levels in tissues and fluids in vivo. Mimicking a scenario in which macaques would be challenged either 2 weeks or 24 hours after insertion and to maintain 5 weeks between depo treatment and challenge [for optimal transmission in the control group (14, 18)], we varied the time of IVR insertion relative to depo (table S1). MIV-150 IVRs (100 mg) were inserted either 3 weeks after depo (for challenge 2 weeks after insertion) or 5 weeks after depo (for challenge 24 hours after insertion). Plasma MIV-150 levels were below the RIA LLQ at all time points (Fig. 5A). Vaginal swabs and vaginal and cervical biopsies contained MIV-150 (Fig. 5, A and B). In the swabs, MIV-150 concentrations fluctuated over time, ranging from 17.1 to 78.5 times the IC50 in the 3-week post-depo group and from 54.1 to 97.1 times the IC50 in the 5-week post-depo group. Although there were no significant differences over time or between the groups, more MIV-150 was detected in the swabs of the 5-week post-depo group than in those of the 3-week post-depo group 14 days after insertion (P = 0.0524) (Fig. 5A). MIV-150 levels in swabs from macaques that received 100-mg MIV-150 EVA-40 IVRs 3 weeks after depo were comparable to those from macaques that received 50-mg MIV-150 silicone IVRs 3 weeks after depo (14 days, P = 0.0798; 28 days, P = 0.0512).
Fig. 5.
Active MIV-150 is released from EVA-40 IVRs (100 mg) in vivo. (A) In vivo–released MIV-150 measured in plasma and vaginal swabs over time indicated in hours or days. Closed symbols represent IVR insertion 3 weeks after depo, and open symbols indicate IVR insertion 5 weeks after depo. In 3-week post-depo animals, swabs were collected 0.5 hour (n = 11), 1, 2, 3, and 14 days (n = 11), and 28 days (n = 5) after insertion. In 5-week post-depo animals, swabs were collected 1 hour (n = 11), 1 day (n = 11), and 14 days (n = 5) after insertion. (B) MIV-150 detected in cervical (C) and vaginal (V) pinch biopsies at various time points after insertion. Closed and open symbols are used as in (A). Biopsies were taken from 3-week post-depo animals 14 days (n = 6) or 28 days (n = 5) after insertion and from 5-week post-depo animals 1 day (n = 6) or 14 days (n = 5) after insertion. Data points in (A) and (B) represent means ± SEM, and statistical comparisons were made with the Mann-Whitney U test. (b) Percent of HIV infection in vitro in the presence of vaginal swabs from animals that received MIV-150 (n = 6) and placebo IVRs (n = 10; mean ± SEM).
MIV-150 levels in cervical and vaginal tissues increased significantly over time in macaques that received IVRs 5 weeks after depo but not in animals that received IVRs 3 weeks after depo (cervix, P = 0.4286; vagina, P = 0.3290) (Fig. 5B). There was no difference in the amount of drug detected in the cervix and vagina in either group at any time point (5 weeks after depo: 1 day, P = 0.1320; 14 days, P = 0.3095; 3 weeks after depo: 14 days, P = 1.000; 28 days, P = 0.9166). However, animals that received IVRs 5 weeks after depo had significantly more MIV-150 in vaginal (but not cervical, P = 0.0823) tissues 14 days after insertion than those that received IVRs 3 weeks after depo. The concentration of MIV-150 found in the mucosal tissues was higher on the day corresponding to the day of challenge in animals receiving 100-mg MIV-150 EVA-40 IVRs (0.10 ng/mg in vagina, 0.09 ng/mg in cervix) than in those receiving 50-mg MIV-150 silicone IVRs (0.008 ng/mg in vagina, 0.03 ng/mg in cervix) [P = 0.0047 (vagina) and P = 0.045 (cervix) 14 days after insertion of EVA versus silicone IVRs 3 weeks after depo; P = 0.0047 (vagina) and P = 0.0245 (cervix) 1 day after insertion of EVA IVR versus 14 days after insertion of silicone IVR]. MIV-150 in the swabs from animals carrying EVA-40 IVRs collected 1 to 24 hours after insertion had potent antiviral activity (Fig. 5C), with a mean ± SD IC50 value of 0.37 ± 0.12 nM.
MIV-150 EVA-40 IVRs provide significant protection against SHIV-RT
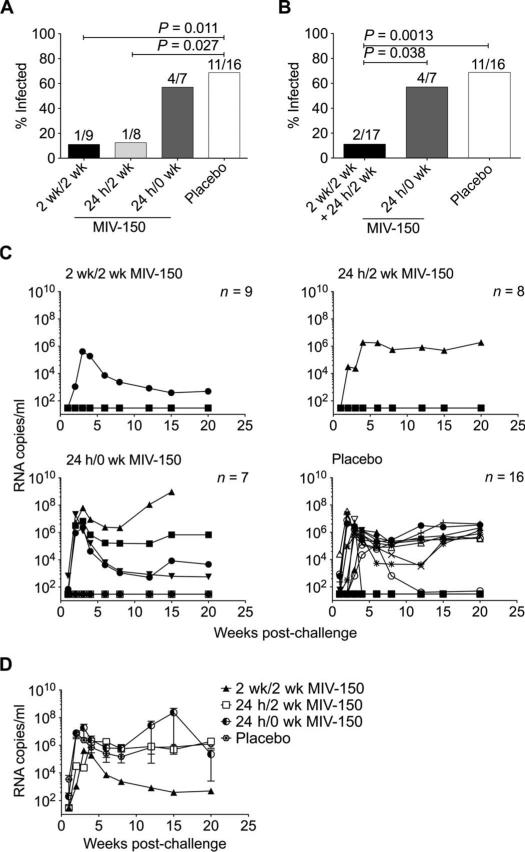
We next tested the ability of 100-mg MIV-150 EVA-40 IVRs to protect macaques from SHIV-RT infection. IVRs were inserted either 2 weeks or 24 hours before challenge and either left in place for 2 weeks after challenge or removed immediately before challenge (table S4). In the placebo EVA-40 IVR group, 8 of 11 macaques became infected (72.7%), equivalent to the placebo silicone IVR group (P = 1.0000, Fisher's exact test). Controls were pooled to increase our power to detect protection, yielding 11 of 16 animals infected (68.8%) (Fig. 6). In the EVA-40 IVR groups that were challenged 2 weeks or 24 hours after insertion and had their IVRs in place for 2 weeks after challenge (2 weeks/2 weeks or 24 hours/2 weeks), only one of nine (11.1%) and one of eight (12.5%) animals became infected, respectively (Fig. 6, A and C). Protection provided by MIV-150 EVA-40 IVRs against SHIV-RT was significant compared to placebo IVRs in both the 2 weeks/2 weeks (84%) and the 24 hours/2 weeks (82%) groups (Fig. 6A). When MIV-150 EVA-40 IVRs were inserted 24 hours before and removed immediately before challenge (24 hours/0 week), the infection frequency was similar to the placebo group (four of seven, 57.1%; P = 0.6570) (Fig. 6A). Focusing on the impact of post-challenge IVR exposure (pooling the 2 weeks/2 weeks and 24 hours/2 weeks groups, 2 of 17 infected), we found that protection was significant compared to the pooled placebo (83%) and the 24 hours/0 week groups (79%) (Fig. 6B). Potential differences in viremia among animals that became infected in the presence of EVA-40 IVRs could not be addressed due to the small number of animals (Fig. 6, C and D); however, viremia in these animals was within the range previously observed for vaginal challenge with this virus (14, 19). Viral RNA isolated at peak viremia carried wild-type RT gene sequences (table S5). All but one (DA69) infected animals and no uninfected animals seroconverted (table S4).
Fig. 6.
MIV-150 EVA-40 IVRs offer significant protection against SHIV-RT infection. (A) Infection frequency across the placebo and different 100-mg MIV-150 IVR groups. (B) Infection frequency evaluating only the role of post-challenge IVR exposure. In (A) and (B), the numbers above each bar indicate the number of infected animals over the number of challenged animals. Fisher's exact test was used for statistical analysis. (C) Viremia (SIV RNA copies per milliliter of plasma) over time for each animal challenged with 103 TCID50 SHIV-RT after receiving placebo or MIV-150 IVRs with different timing of insertion and removal. (D) Mean ± SEM plasma viral load of infected animals in each group.
DISCUSSION
This study presents evidence that MIV-150 IVRs can significantly protect macaques against SHIV-RT infection. MIV-150 silicone (50 mg) and MIV-150 EVA-40 IVRs (100 mg) both reduced infection frequency. The data suggest that EVA-40 IVRs released more MIV-150 in vitro than did silicone IVRs and protected better in vivo (although the latter may have been hampered by the small number of animals studied).
Use of nonsink conditions for in vitro release of hydrophobic HIV inhibitors from polyurethane IVRs has been shown to correlate better with in vivo–release profiles in macaques and rabbits (20, 21), whereas sink conditions correlated better with in vivo results in a silicone IVR study in HIV-negative women (22). At all time points, in vitro–released MIV-150 levels were >859 times the IC50 against HIVADA, HIVMN, and SHIV-RT (19, 23). The cumulative release profiles for all three MIV-150 IVRs in the SNaC buffer exhibited partition-controlled kinetics [typical of nonsink conditions (24)] of Q α time, where Q is the cumulative percentage of MIV-150 released. Q for the 50-mg silicone, 50-mg EVA, and 100-mg EVA IVRs was 2.8, 8.7, and 5.3%, respectively. The larger Q for the 50-mg EVA IVR versus the 100-mg EVA IVR can be due to attenuated MIV-150 release from both rings under nonsink conditions, which results in similar amounts of MIV-150 being released from both rings despite the difference in initial loading (15, 20). Cumulative MIV-150 release in the SNaC buffer was significantly greater from EVA-40 IVRs (P = 0.0001, paired t test) compared to silicone. Overall, this paralleled the larger amounts of MIV-150 detected in the fluids and tissues of animals receiving the EVA-40 IVRs than the silicone IVRs. These differences could be due to differential solubilityofMIV-150 intheIVRs.FromDSC(differentialscanning calorimetry) of formulated ring segments and in vitro–release studies with micronized MIV-150, we know that MIV-150 is insoluble in silicone. MIV-150 was partially soluble in EVA-40 as indicated by DSC. However, because we used a solvent casting method to formulate the EVA IVRs, it is possible that the residual solvent remaining in the IVRs afforded soluble MIV-150intheIVR.SiliconeIVRsweighed, on average, 3.2% more after the 29-day in vitro–release study than before, whereas the EVA IVRs weighed 0.4% less. These data could indicate that the residual solventremainingintheEVAIVRswaseluted during the release study, and support the notion that MIV-150 was at least partially soluble in the EVA IVR.
The virus inoculum used in this study [103 TCID50 (median tissue culture infectious dose)] infected ~69% of animals receiving placebo IVRs, paralleling the 64% transmission rate in controls from previous vaginal gel studies (14, 19). Challenging depo-treated animals in this single high-dose challenge model provided a rigorous test of MIV-150 IVR efficacy and also allowed us to demonstrate that insertion of the IVRs for 4 weeks resulted in no adverse effects that could promote infection (25). Many other microbicide studies use the repeated low-dose challenge model (~10 TCID50 per challenge) in cycling macaques because it more closely resembles human exposure; infection frequency per challenge in controls for these studies is only 8 to 33% (26–30). We believe that the setting of heightened susceptibility to infection in this proof-of-concept study asked more of the product because it had to prevent infection with an inoculum that results in a ~69% infection frequency per challenge. Moreover, depo normalizes the discrepancy in infection susceptibility between the follicular and the luteal phases of the menstrual cycle, which can affect efficacy studies (31).
Similar amounts of MIV-150 were found in fluids of macaques that received silicone and EVA-40 IVRs, even though EVA-40 IVRs were loaded with and released more MIV-150 in vitro and provided greater protection. However, significantly more MIV-150 was detected in the sampled tissues of animals that received EVA-40 than silicone IVRs on the day of challenge. These results suggest that mucosal tissue levels of MIV-150 (~0.1 ng/mg) on the day of challenge predicted efficacy. Alternatively, MIV-150 concentrations may have been somewhat different between the silicone and the EVA-40 studies in the depth of tissue penetration or the location within the tissue (such as penetration boundary at the lumen compared with the lamina propria), but this could not be addressed herein. Although a previous study of the partially protective MIV-150 gel demonstrated comparable levels of MIV-150 in the tissues to those observed here with EVA-40 IVRs (14), it is impossible to directly compare the two regimens given the different drug delivery methods. Future studies are needed to dissect the relationship between drug release, absorption, and protection by IVRs and gels.
MIV-150 EVA-40 IVRs protected only when they were present after challenge. Had we been able to include a group receiving IVRs 5 weeks after depo and then challenged under a 2-week/0-hour regimen, it is possible that these animals would have been protected as the result of sufficient MIV-150 accumulation in tissues over the course of 2 weeks. Because release and tissue absorption of drugs from IVRs are affected by the thickness of the vaginal epithelium and properties of the cervicovaginal fluid (CVF) (32, 33), differences in absorption are attributable to the timing of IVR insertion relative to depo treatment. CVF volume is decreased and viscosity increased under depo (34), which may affect longitudinal drug transport in the vagina (33). Tissue drug levels on the day of challenge were similar for both EVA-40 groups, but the levels at the time corresponding to 2 weeks after challenge were markedly increased in 5-week post-depo animals in accordance with reports of thinner vaginal epithelia at this time point (18). However, we do not know what the MIV-150 levels in the mucosal tissues would be in the absence of depo. In a macaque pharmacokinetic (PK) study on silicone IVRs releasing one of the CCR5 inhibitors maraviroc or CMPD167, depo affected in vivo distribution of the drugs, reducing CVF and tissue drug levels while increasing systemic levels (12). We also cannot rule out that the increased tissue MIV-150 levels we observed 14 days after insertion in animals that received IVRs 5 weeks after depo were due to depo-induced drug accumulation. Had we biopsied animals in the 3-week post-depo group at 1 day after insertion, the levels may have been correspondingly lower.
These data underscore the importance of tissue MIV-150 concentration on the day of challenge as well as post-challenge drug exposure. They also highlight that MIV-150 levels in vaginal swabs did not correlate with protection, perhaps because they were not normalized for the amount of CVF in each swab. These data differ from that seen in the CAPRISA-004 trial, where post hoc analysis of tenofovir levels in undiluted CVF indicated a statistically significant correlation between tenofovir concentrations in the CVF and likelihood of HIV infection (35). It is unclear whether the tenofovir represented released drug or residual gel (a problem with many gel studies); nevertheless, the data indicate that high adherers were better protected. Furthermore, if IVR insertion at the time of challenge or in the minutes to hours after virus exposure protects, then MIV-150–containing IVRs may be valuable for both prevention and emergency post-exposure prophylaxis.
Phase 1 studies of 25- and 200-mg dapivirine silicone reservoir IVRs in healthy women (36) as well as the macaque preclinical 400-mg maraviroc/CMPD167 IVR study (11) relied on drug release and absorption to predict efficacy. In both studies, drug levels above the IC50 were detected in CVF and tissues within hours of IVR insertion and were maintained for up to 7 days (dapivirine) or 28 days after insertion (CCR5 inhibitors) but were lower than levels measured with in vitro– release studies (12, 37), similar to our findings. Vaginal fluid and tissue-associated dapivirine levels were slightly higher with the lower-dose IVR. Minor differences in dapivirine levels are not unexpected because reservoir IVRs deliver APIs with zero-order kinetics in a concentration-independent manner. These dapivirine IVRs have entered phase 3 trials despite no efficacy testing in an animal model. Although our data suggest that IVRs delivering therapeutic drug levels to the mucosal tissues should be effective, it is impossible to know what drug level is required without performing the efficacy study. Moreover, therapeutic levels in macaques may not accurately predict therapeutic levels in women, which underscores the need to examine in vivo efficacy in combination with PK.
This study had several limitations. The efficacy of the MIV-150 IVR was assessed in only one of the two standard macaque challenge models. We challenge with a single high dose of SHIV-RT, whereas other groups administer multiple low doses (which we plan to do in future studies). Each model has its strengths and weaknesses. Neither has been correlated with clinical efficacy, due in part to poor participant adherence and to the limitations of any animal model. In addition, this was a proof-of-concept study using a formulation (a matrix IVR loaded with MIV-150) that will not be tested clinically. However, our results not only represent an important and necessary first step in the development of our final formulation (a combination IVR loaded with MIV-150 and another API to address efficacy and resistance issues and broaden the spectrum of activity) but also strongly support the notion that drug-loaded IVRs can block in vivo HIV infection, which has not been shown with any other IVRs to date. PK and efficacy were not evaluated in the same animals to avoid skewing infection rates and MIV-150 absorption. We were unable to detect MIV-150 in plasma using an RIA-based assay (LLQ, 1 ng/ml) and had to rely on vaginal swabs and tissue samples for PK studies. However, we are developing a liquid chromatography–mass spectrometry method to detect MIV-150 in plasma (estimated LLQ of 20 pg/ml), with which we may be able to detect plasma MIV-150 and identify a systemic correlate of protection. This will allow us to run future efficacy and PK studies in the same set of animals. Moreover, sampling plasma rather than mucosal tissue is preferred in clinical trials because it is less invasive and less expensive. Furthermore, the need to have MIV-150 exposure after challenge for efficacy in macaques precluded the use of pharmacodynamics to evaluate the protective potential of in vivo–released MIV-150. In vivo efficacy studies are required to determine whether this will be true for other IVRs in development. We did not perform true in vitro–in vivo correlation studies because we were unable to develop a reliable method to quantify the remaining MIV-150 levels in the EVA IVRs after insertion. However, it is clear that less MIV-150 was released from the rings in vivo compared to the SNaC buffer conditions in vitro, yet the levels (~0.1 mg/ng) in the tissues at the time of challenge were sufficient to significantly reduce infection (as long as the rings remained in place after challenge). Finally, we did not determine whether the presence of MIV-150 in mucosal tissues led to the emergence of drug-resistant virus in tissues. However, we did show that the presence of MIV-150– releasing IVRs did not drive the emergence of RT resistance in virus that was replicating at normal levels in the blood. This is not surprising because plasma MIV-150 was below detectable levels at all times, and earlier studies in rats showed that any absorbed MIV-150 is cleared within 12 hours (16), thereby reducing the likelihood of resistance development and drug toxicity based on systemic exposure. Although this study was limited to looking for resistance developing systemically, extensive studies are ongoing in chronically SHIV-RT–infected animals to determine whether MIV-150–containing IVRs drive the emergence of drug resistance locally.
Our results demonstrate the protective potential of MIV-150 EVA-40 IVRs, particularly when they are present after virus exposure. This study proves that sustained delivery of MIV-150 can afford significant protection against vaginal immunodeficiency virus infection.
In previous gel studies, the addition of 14 mM zinc acetate to a 50 μM MIV-150/carrageenan gel improved the level of protection above that of the MIV-150 gel alone (14). We are developing combination IVRs that release both MIV-150 and zinc acetate to provide enhanced protection against immunodeficiency viruses and to protect against HSV-2 (38). Moreover, a combination IVR may be loaded with less MIV-150, reducing the emergence of drug-resistant viruses, minimizing toxicity, and containing the cost of the IVR. Our data support the development of microbicide IVRs that prevent HIV and other STIs.
MATERIALS AND METHODS
Matrix IVRs
IVRs had a 2-cm outer diameter and a 4-mm cross-section (Fig. 1B). Silicone IVRs containing 50 mg of MIV-150 were prepared from MED-4211 silicone elastomer (NuSil Technology) with two-part platinum catalysis. MIV-150 was mixed with cold part A silicone. Part B silicone containing the catalyst was added, mixed until homogeneous, and de-aerated. The mixture was injected into brass molds and polymerized for 90 min at 90°C. Cooled IVRs were removed and stored dark at room temperature.
EVA-40 IVRs contained 50 or 100 mg of MIV-150. EVA-40 beads (Scientific Polymer Products) and MIV-150 were dissolved in dichloro-methane (DCM) (Sigma-Aldrich) with stirring. The solution was poured into a pan, and DCM was evaporated to afford a homogeneous thin film that was frozen, ground into 4 × 4–mm fragments, melted at 93°C, and then injected into ring molds at 75 psi. Cooled IVRs were removed from molds and stored dark at room temperature.
MIV-150 in vitro release
Saturation curves over 24 hours were established for 50-mg silicone and 50- and 100-mg EVA-40 IVRs in SNaC buffer at 37°C with shaking. For extended-release studies, MIV-150 IVRs were suspended in the same buffer at 37°C with shaking. The release medium was replaced daily (except for weekends). MIV-150 content on selected time points was measured in triplicate by high-performance liquid chromatography with a 150 × 4.6–mm, 3-mm, C18 column: injection volume 100 μl, mobile phase 50% acetonitrile and 50% 200 mM ammonium acetate buffer, pH 5.0; flow rate 1 ml/min; wavelength 260 nm; column temperature 35°C.
Antiviral activity of MIV-150 released from IVRs
Antiviral activity in samples of in vitro–released MIV-150 and vaginal swabs was evaluated against HIV-1ADA-M [lot P4189; multiplicity of infection, 0.001; AIDS Vaccine Program (SAIC-Frederick, National Cancer Institute at Frederick)] with the TZM-bl–based multinuclear-activated galactosidase indicator (MAGI) assay (39). Samples diluted 1:10 were titrated threefold for a total of six dilutions and assayed in triplicate. Stock MIV-150 diluted to 3.7 to 0.0148 ng/ml (10 to 0.04 nM) was used in each experiment to control for IC50 variations. IC50 values were calculated with a dose-response-inhibition analysis and MIV-150 concentrations previously measured in the samples (GraphPad Prism v5.0).
Macaque treatments
Adult Chinese (silicone IVRs) and Indian (EVA-40 IVRs) rhesus macaques (Macaca mulatta) were housed at the Tulane National Primate Research Center (TNPRC). Protocols were approved by the TNPRC Animal Care and Use Committee (OLAW Assurance A4499-01), which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC 000594). Procedures complied with the Animal Welfare Act (40), the Guide for the Care and Use of Laboratory Animals (41), and TNPRC standards for minimizing animal distress (42). Macaques tested negative for simian type D retroviruses and simian T cell leukemia virus-1, and those challenged with SHIV-RT also tested negative for simian immunodeficiency virus (SIV). For PK, healthy SHIV-RT–infected and uninfected macaques were recycled from previous studies. Macaques were randomized to the treatment groups. Blood, swabs, and tissues were shipped from TNPRC to our laboratories in New York overnight (42). Peripheral blood mononuclear cells (PBMCs) were isolated from EDTA blood (43). Plasma was processed as described (44). Vaginal swabs were collected with Weck-Cel spear sponges (Beaver Visitec). Sponges were pre-moistened in 1 ml of saline and inserted into the vaginal vault for 5 min. Upon removal, sponges were immersed in remaining saline in 15-ml conical tubes, sealed with Parafilm, and shipped cold.
Biopsies were collected from anesthetized macaques (Telazol, 8 mg/kg) in sternal recumbency with the pelvis elevated, using a speculum to visualize the vaginal vault and external cervical os. Vaginal biopsies were collected from the lateral or dorsal wall using forceps with a 3-mm × 5-mm cup. Biopsies (3 mm × 1.5 mm) of the external cervical os were collected with Burke biopsy punch forceps. Because of increased variability in the MIV-150 RIA at low weights, 20 mg was the minimum tissue weight per animal per site (16). The average total vaginal tissue weight obtained was 94.67 mg per macaque (silicone IVRs) and 71.33 mg per macaque (EVA-40 IVRs). The average total cervical tissue weight obtained was 61.83 mg per macaque (silicone IVRs) and 71.67 mg per macaque (EVA-40 IVRs). Analgesics were administered for 2 days after biopsy.
We quantified MIV-150 in plasma, swabs, and tissues by RIA [LLQ, 1 ng/ml (2.7 nM) for fluids, 0.01 ng/mg for tissues] (14, 42, 45). To mimic efficacy studies, we similarly depo-treated PK animals (18). PK study sampling is described in table S1. EDTA blood was collected 14 and 28 days after insertion in the silicone study and 30 min and 1 to 72 hours after insertion in the EVA study.
Details on the 50-mg MIV-150 silicone IVR efficacy study are in table S2. Details on the 100-mg MIV-150 EVA-40 IVR efficacy study are in table S4. Animals were challenged with 103 TCID50 SHIV-RT (1.6 × 106 to 1.6 × 107 viral RNA copies) (14) and followed for 24 weeks. Before initiating efficacy studies, IVR safety and tolerability were evaluated (n = 8 silicone, n = 4 EVA-40) for 28 days. IVRs caused no distress or vaginal irritation. Analysis of all macaques that received IVRs for 28 days showed that 100% of silicone IVRs (30 of 30) and 86% of EVA-40 IVRs (19 of 22) remained in place for the entire 28 days.
Determining SHIV-RT infection
Plasma viral RNA was measured by quantitative reverse transcription– polymerase chain reaction (RT-PCR) for SIV gag (42). PBMC viral DNA was detected by nested PCR for SIV gag (42). Viral DNA in co-cultures of PBMCs with CEMx174 cells was measured by quantitative PCR for SIV gag (19). SIV-specific antibodies were detected in plasma by enzyme-linked immunosorbent assay (46).
Statistical analyses
Statistical analyses were performed with GraphPad Prism v5.0 (47). In vitro–released MIV-150 was evaluated with Student's t test. Macaque infection frequency was analyzed with Fisher's exact test. All other analyses were performed with a two-tailed Mann-Whitney U test. Significance was defined by P < 0.05.
Supplementary Material
Acknowledgments
We thank the veterinary staff at the TNPRC for continued support, D. Phillips and R. Maguire for scientific discussions on the concept and design of MIV-150 rings, and E. Read for assistance with graphics. Funding: Supported by the United States Agency for International Development (USAID) Cooperative Agreement GPO-A-00-04-00019-00, the Swedish Ministry of Foreign Affairs, the Swedish International Development Cooperation Agency, the NIH base grant RR00164, and with federal funds from the National Cancer Institute, NIH, under contract HHSN261200800001E. The contents of this manuscript are the sole responsibility of the Population Council and do not necessarily reflect the views or policies of USAID or the U.S. government. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. None of the material in this article has been published or is under consideration elsewhere, including the Internet. M.R. is a 2002 Elizabeth Glaser Scientist.
Footnotes
Author contributions: T.M.Z. designed and oversaw the ring manufacturing, oversaw the in vitro IVR testing, assisted in writing the manuscript, and performed data analysis. M.R. designed and oversaw the execution of the macaque studies and assisted in writing the manuscript. R.S. performed the macaque research and assisted in the data analysis. P.M. produced and tested the IVRs in vitro and assisted in the data analysis. N.D. performed the data analysis and wrote the manuscript. A.R., L.K., and J.A.F.-R. performed all in vitro testing of MIV-150 released from rings in vitro and in vivo (RIA and MAGI). R.M. and D.G. performed the in vitro–release studies using the SNaC buffer. J.K. and M.A. provided critical assistance in the processing of macaque samples. S.S. manufactured IVRs for in vitro–release studies. A.G. and J.B. coordinated and executed the macaque treatments. M.P.Jr. performed the PCR assays to determine plasma viral loads. J.D.L. assisted in the writing of the manuscript.
Citation: R. Singer, P. Mawson, N. Derby, A. Rodriguez, L. Kizima, R. Menon, D. Goldman, J. Kenney, M. Aravantinou, S. Seidor, A. Gettie, J. Blanchard, M. Piatak Jr., J. D. Lifson, J. A. Fernández-Romero, M. Robbiani, T. M. Zydowsky, An intravaginal ring that releases the NNRTI MIV-150 reduces SHIV transmission in macaques. Sci. Transl. Med. 4, 150ra123 (2012).
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: Patents pertaining to the compound MIV-150 and related methods for making the same are exclusively licensed to The Population Council Inc. by Medivir AB and cannot be given to any outside group without a Material Transfer Agreement (MTA). The Population Council will consider reasonable requests for access to MIV-150 for research purposes, which, if granted, will require establishment of an MTA.
REFERENCES AND NOTES
- 1.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D, CAPRISA 004 Trial Group Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skoler-Karpoff S, Ramjee G, Ahmed K, Altini L, Plagianos MG, Friedland B, Govender S, De Kock A, Cassim N, Palanee T, Dozier G, Maguire R, Lahteenmaki P. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: A randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1977–1987. doi: 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]
- 3.The ART of preventing HIV. Nat. Med. 2011;17:46–47. doi: 10.1038/nm0111-46. [DOI] [PubMed] [Google Scholar]
- 4.Cohen J. AIDS research. Complexity surrounds HIV prevention advances. Science. 2011;333:393. doi: 10.1126/science.333.6041.393. [DOI] [PubMed] [Google Scholar]
- 5.Kerns J, Darney P. Vaginal ring contraception. Contraception. 2011;83:107–115. doi: 10.1016/j.contraception.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Malcolm RK, Edwards KL, Kiser P, Romano J, Smith TJ. Advances in microbicide vaginal rings. Antiviral Res. 2010;88(Suppl. 1):S30–S39. doi: 10.1016/j.antiviral.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Barentsen R, van de Weijer PH, Schram JH. Continuous low dose estradiol released from a vaginal ring versus estriol vaginal cream for urogenital atrophy. Eur. J. Obstet. Gynecol. Reprod. Biol. 1997;71:73–80. doi: 10.1016/s0301-2115(96)02612-7. [DOI] [PubMed] [Google Scholar]
- 8.Penman-Aguilar A, Swezey T, Turner AN, Bell AJ, Ramiandrisoa FN, Legardy-Williams J, Randrianasolo B, Van Damme K, Dulyx J, Behets F, Jamieson DJ. Promoting continuous use as a strategy for achieving adherence in a trial of the diaphragm with candidate microbicide. AIDS Educ. Prev. 2009;21:512–525. doi: 10.1521/aeap.2009.21.6.512. [DOI] [PubMed] [Google Scholar]
- 9.Malcolm K, Woolfson D, Russell J, Tallon P, McAuley L, Craig D. Influence of silicone elastomer solubility and diffusivity on the in vitro release of drugs from intravaginal rings. J. Control. Release. 2003;90:217–225. doi: 10.1016/s0168-3659(03)00178-0. [DOI] [PubMed] [Google Scholar]
- 10.Johnson TJ, Gupta KM, Fabian J, Albright TH, Kiser PF. Segmented polyurethane intravaginal rings for the sustained combined delivery of antiretroviral agents dapivirine and tenofovir. Eur. J. Pharm. Sci. 2010;39:203–212. doi: 10.1016/j.ejps.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Moss JA, Malone AM, Smith TJ, Kennedy S, Kopin E, Nguyen C, Gilman J, Butkyavichene I, Vincent KL, Motamedi M, Friend DR, Clark MR, Baum MM. Simultaneous delivery of tenofovir and acyclovir via an intravaginal ring. Antimicrob. Agents Chemother. 2012;56:875–882. doi: 10.1128/AAC.05662-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malcolm RK, Veazey RS, Geer L, Lowry D, Fetherston SM, Murphy DJ, Boyd P, Major I, Shattock RJ, Klasse PJ, Doyle LA, Rasmussen KK, Goldman L, Ketas TJ, Moore JP. Sustained release of the CCR5 inhibitors CMPD167 and maraviroc from vaginal rings in rhesus macaques. Antimicrob. Agents Chemother. 2012;56:2251–2258. doi: 10.1128/AAC.05810-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiser PF, Johnson TJ, Clark JT. State of the art in intravaginal ring technology for topical prophylaxis of HIV infection. AIDS Rev. 2012;14:62–77. [PubMed] [Google Scholar]
- 14.Kenney J, Aravantinou M, Singer R, Hsu M, Rodriguez A, Kizima L, Abraham CJ, Menon R, Seidor S, Chudolij A, Gettie A, Blanchard J, Lifson JD, Piatak M, Jr., Fernández-Romero JA, Zydowsky TM, Robbiani M. An antiretroviral/zinc combination gel provides 24 hours of complete protection against vaginal SHIV infection in macaques. PLoS One. 2011;6:e15835. doi: 10.1371/journal.pone.0015835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang L, Khan SU, Muhammad NA. Evaluation and selection of bio-relevant dissolution media for a poorly water-soluble new chemical entity. Pharm. Dev. Technol. 2001;l6:531–540. doi: 10.1081/pdt-120000291. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez A, Fernandez-Romero J. personal communication.
- 17.van Laarhoven JA, Kruft MA, Vromans H. In vitro release properties of etonogestrel and ethinyl estradiol from a contraceptive vaginal ring. Int. J. Pharm. 2002;232:163–173. doi: 10.1016/s0378-5173(01)00900-0. [DOI] [PubMed] [Google Scholar]
- 18.Hild-Petito S, Veazey RS, Larner JM, Reel JR, Blye RP. Effects of two progestin-only contraceptives, Depo-Provera and Norplant-II, on the vaginal epithelium of rhesus monkeys. AIDS Res. Hum. Retroviruses. 1998;14(Suppl. 1):S125–S130. [PubMed] [Google Scholar]
- 19.Turville SG, Aravantinou M, Miller T, Kenney J, Teitelbaum A, Hu L, Chudolij A, Zydowsky TM, Piatak M, Jr., Bess JW, Jr., Lifson JD, Blanchard J, Gettie A, Robbiani M. Efficacy of Carraguard-based microbicides in vivo despite variable in vitro activity. PLoS One. 2008;3:e3162. doi: 10.1371/journal.pone.0003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson TJ, Srinivasan P, Albright TH, Watson-Buckheit K, Rabe L, Martin A, Pau CP, Hendry RM, Otten R, McNicholl J, Buckheit R, Jr., Smith J, Kiser PF. Safe and sustained vaginal delivery of pyrimidinedione HIV-1 inhibitors from polyurethane intravaginal rings. Antimicrob. Agents Chemother. 2012;56:1291–1299. doi: 10.1128/AAC.05721-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark MR, Johnson TJ, McCabe RT, Clark JT, Tuitupou A, Elgendy H, Friend DR, Kiser PF. A hot-melt extruded intravaginal ring for the sustained delivery of the anti-retroviral microbicide UC781. J. Pharm. Sci. 2012;101:576–587. doi: 10.1002/jps.22781. [DOI] [PubMed] [Google Scholar]
- 22.Nel A, Smythe S, Young K, Malcolm K, McCoy C, Rosenberg Z, Romano J. Safety and pharmacokinetics of dapivirine delivery from matrix and reservoir intravaginal rings to HIV-negative women. J. Acquir. Immune Defic. Syndr. 2009;51:416–423. doi: 10.1097/qai.0b013e3181acb536. [DOI] [PubMed] [Google Scholar]
- 23.Fernández-Romero JA, Thorn M, Turville SG, Titchen K, Sudol K, Li J, Miller T, Robbiani M, Maguire RA, Buckheit RW, Jr., Hartman TL, Phillips DM. Carrageenan/MIV-150 (PC-815), a combination microbicide. Sex. Transm. Dis. 2007;34:9–14. doi: 10.1097/01.olq.0000223287.46097.4b. [DOI] [PubMed] [Google Scholar]
- 24.Chien YW, Lambert HJ. Controlled drug release from polymeric delivery devices. II. Differentiation between partition-controlled and matrix-controlled drug release mechanisms. J. Pharm. Sci. 1974;63:515–519. doi: 10.1002/jps.2600630405. [DOI] [PubMed] [Google Scholar]
- 25.Patton DL, Kidder GG, Sweeney YC, Rabe LK, Hillier SL. Effects of multiple applications of benzalkonium chloride and nonoxynol 9 on the vaginal epithelium in the pigtailed macaque (Macaca nemestrina). Am. J. Obstet. Gynecol. 1999;180:1080–1087. doi: 10.1016/s0002-9378(99)70598-3. [DOI] [PubMed] [Google Scholar]
- 26.Promadej-Lanier N, Srinivasan P, Curtis K, Adams DR, Kim C, Luo W, Jia H, Subbarao S, Otten RA, Butera S. Systemic and mucosal immunological responses during repeated mucosal SHIV162P3 challenges prior to and following infection in pigtailed macaques. Virology. 2008;375:492–503. doi: 10.1016/j.virol.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 27.Otten RA, Adams DR, Kim CN, Jackson E, Pullium JK, Lee K, Grohskopf LA, Monsour M, Butera S, Folks TM. Multiple vaginal exposures to low doses of R5 simian-human immunodeficiency virus: Strategy to study HIV preclinical interventions in nonhuman primates. J. Infect. Dis. 2005;191:164–173. doi: 10.1086/426452. [DOI] [PubMed] [Google Scholar]
- 28.Dobard C, Sharma S, Martin A, Pau CP, Holder A, Kuklenyik Z, Lipscomb J, Hanson DL, Smith J, Novembre FJ, García-Lerma JG, Heneine W. Durable protection from vaginal simian-human immunodeficiency virus infection in macaques by tenofovir gel and its relationship to drug levels in tissue. J. Virol. 2012;86:718–725. doi: 10.1128/JVI.05842-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parikh UM, Dobard C, Sharma S, Cong ME, Jia H, Martin A, Pau CP, Hanson DL, Guenthner P, Smith J, Kersh E, Garcia-Lerma JG, Novembre FJ, Otten R, Folks T, Heneine W. Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J. Virol. 2009;83:10358–10365. doi: 10.1128/JVI.01073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng-Mayer C, Huang Y, Gettie A, Tsai L, Ren W, Shakirzyanova M, Sina ST, Trunova N, Blanchard J, Jenkins LM, Lo Y, Schito ML, Appella E. Delay of simian human immunodeficiency virus infection and control of viral replication in vaccinated macaques challenged in the presence of a topical microbicide. AIDS. 2011;25:1833–1841. doi: 10.1097/QAD.0b013e32834a1d94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vishwanathan SA, Guenthner PC, Lin CY, Dobard C, Sharma S, Adams DR, Otten RA, Heneine W, Hendry RM, McNicholl JM, Kersh EN. High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J. Acquir. Immune Defic. Syndr. 2011;57:261–264. doi: 10.1097/QAI.0b013e318220ebd3. [DOI] [PubMed] [Google Scholar]
- 32.Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999;59:91–95. doi: 10.1016/s0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 33.Geonnotti AR, Katz DF. Compartmental transport model of microbicide delivery by an intravaginal ring. J. Pharm. Sci. 2010;99:3514–3521. doi: 10.1002/jps.22120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blanchard J. personal communication.
- 35.Karim SS, Kashuba AD, Werner L, Karim QA. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: Implications for HIV prevention in women. Lancet. 2011;378:279–281. doi: 10.1016/S0140-6736(11)60878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romano J, Variano B, Coplan P, Van Roey J, Douville K, Rosenberg Z, Temmerman M, Verstraelen H, Van Bortel L, Weyers S, Mitchnick M. Safety and availability of dapivirine (TMC120) delivered from an intravaginal ring. AIDS Res. Hum. Retroviruses. 2009;25:483–488. doi: 10.1089/aid.2008.0184. [DOI] [PubMed] [Google Scholar]
- 37.Malcolm RK, Woolfson AD, Toner CF, Morrow RJ, McCullagh SD. Long-term, controlled release of the HIV microbicide TMC120 from silicone elastomer vaginal rings. J. Antimicrob. Chemother. 2005;56:954–956. doi: 10.1093/jac/dki326. [DOI] [PubMed] [Google Scholar]
- 38.Fernández-Romero JA, Abraham CJ, Rodriguez A, Kizima L, Jean-Pierre N, Menon R, Begay O, Seidor S, Ford BE, Gil PI, Peters J, Katz D, Robbiani M, Zydowsky TM. Zinc acetate/carrageenan gels exhibit potent activity in vivo against high-dose herpes simplex virus 2 vaginal and rectal challenge. Antimicrob. Agents Chemother. 2012;56:358–368. doi: 10.1128/AAC.05461-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Begay O, Jean-Pierre N, Abraham CJ, Chudolij A, Seidor S, Rodriguez A, Ford BE, Henderson M, Katz D, Zydowsky T, Robbiani M, Fernández-Romero JA. Identification of personal lubricants that can cause rectal epithelial cell damage and enhance HIV type 1 replication in vitro. AIDS Res. Hum. Retroviruses. 2011;27:1019–1024. doi: 10.1089/aid.2010.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Animal Welfare Act and Regulation of 2001, Code of Federal Regulations, Chapter 1, Sub-chapter A: Animals and Animal Products. U.S. Department of Agriculture; Beltsville, MD: 2001. [Google Scholar]
- 41.Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources . Guide for the Care and Use of Laboratory Animals. U.S. Department of Health and Human Services, National Institutes of Health; Bethesda, MD: 1985. pp. 1–83. [Google Scholar]
- 42.Singer R, Derby N, Rodriguez A, Kizima L, Kenney J, Aravantinou M, Chudolij A, Gettie A, Blanchard J, Lifson JD, Piatak M, Jr., Fernández-Romero JA, Zydowsky TM, Robbiani M. The nonnucleoside reverse transcriptase inhibitor MIV-150 in carrageenan gel prevents rectal transmission of simian/human immunodeficiency virus infection in macaques. J. Virol. 2011;85:5504–5512. doi: 10.1128/JVI.02422-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frank I, Piatak M, Jr., Stoessel H, Romani N, Bonnyay D, Lifson JD, Pope M. Infectious and whole inactivated simian immunodeficiency viruses interact similarly with primate dendritic cells (DCs): Differential intracellular fate of virions in mature and immature DCs. J. Virol. 2002;76:2936–2951. doi: 10.1128/JVI.76.6.2936-2951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crostarosa F, Aravantinou M, Akpogheneta OJ, Jasny E, Shaw A, Kenney J, Piatak M, Lifson JD, Teitelbaum A, Hu L, Chudolij A, Zydowsky TM, Blanchard J, Gettie A, Robbiani M. A macaque model to study vaginal HSV-2/immunodeficiency virus co-infection and the impact of HSV-2 on microbicide efficacy. PLoS One. 2009;4:e8060. doi: 10.1371/journal.pone.0008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar N, Didolkar AK, Ladd A, Thau R, Monder C, Bardin CW, Sundaram K. Radioimmunoassay of 7 alpha-methyl-19-nortestosterone and investigation of its pharmacokinetics in animals. J. Steroid Biochem. Mol. Biol. 1990;37:587–591. doi: 10.1016/0960-0760(90)90405-a. [DOI] [PubMed] [Google Scholar]
- 46.Smith SM, Holland B, Russo C, Dailey PJ, Marx PA, Connor RI. Retrospective analysis of viral load and SIV antibody responses in rhesus macaques infected with pathogenic SIV: Predictive value for disease progression. AIDS Res. Hum. Retroviruses. 1999;15:1691–1701. doi: 10.1089/088922299309739. [DOI] [PubMed] [Google Scholar]
- 47.Agresti A. A survey of exact inference for contingency tables. Statist. Sci. 1992;7:131–153. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.