Aprepitant (and its prodrug fosaprepitant) is a neurokinin-1 receptor antagonist approved more than a decade ago for the prevention of chemotherapy-induced nausea and vomiting (CINV). This review examined safety and efficacy data for aprepitant and fosaprepitant and explored recommendations in current guidelines for their use. Future perspectives on potential uses of aprepitant and fosaprepitant for indications other than CINV are discussed.
Keywords: Aprepitant, Chemotherapy-induced nausea and vomiting, Fosaprepitant, Highly emetogenic chemotherapy, Moderately emetogenic chemotherapy, Neurokinin-1 receptor antagonist
Abstract
Chemotherapy-induced nausea and vomiting (CINV) is a common adverse event associated with anticancer treatment that can have a significant adverse impact on patient health-related quality of life and that can potentially undermine the effectiveness of chemotherapy. Traditional regimens to prevent CINV generally involved a combination of a corticosteroid plus a 5-hydroxytryptamine (5HT3) receptor antagonist (RA). In the past 10 years, antiemetic treatment has greatly advanced with the availability of the neurokinin-1 receptor antagonist (NK1 RA) aprepitant and its prodrug fosaprepitant. NK1 RAs have a different mechanism of action in CINV than corticosteroids and 5HT3 RAs, thus their use can complement traditional antiemetic drugs and can enhance control of CINV. This review examined accumulated data regarding the safety and efficacy of aprepitant and fosaprepitant over the decade since the first regulatory approval. Data from key studies of aprepitant and fosaprepitant in the prevention of CINV in patients receiving moderately and highly emetogenic chemotherapy were explored, as were recommendations in currently available guidelines for their use. In addition, their use as antiemetic therapy in special patient populations was highlighted. Future perspectives on potential uses of aprepitant and fosaprepitant for indications other than CINV are presented.
Implications for Practice:
Aprepitant (and its prodrug fosaprepitant) is a neurokinin-1 receptor antagonist approved more than a decade ago for the prevention of chemotherapy-induced nausea and vomiting (CINV). Its alternative mechanism of action complements traditional antiemetic drugs, enhancing control of CINV. This review examined safety and efficacy data for aprepitant and fosaprepitant accumulated since the first regulatory approval and explores recommendations in current guidelines for their use. The review serves as a useful reminder for the practitioner that aprepitant and fosaprepitant are valuable additions to the therapeutic armamentarium for the prevention of CINV. Future perspectives on potential uses of aprepitant and fosaprepitant for indications other than CINV are discussed.
Introduction
Chemotherapy-induced nausea and vomiting (CINV) has a significant adverse effect on health-related quality of life [1–4]. CINV often influences patient compliance with chemotherapeutic regimens and even patient decisions about whether to undergo chemotherapeutic treatment [3, 5]. The potential of chemotherapy to induce emesis has been categorized into a four-level classification scheme, according to emetogenic risk (minimal <10%, low 10%–30%, moderate 31%–90%, and high >90%) [6]. Beyond the emetogenic potential of individual chemotherapeutic agents, other factors related to treatment (e.g., route of administration, dose, number of treatment cycles, whether given singly or in combination with other chemotherapeutic agents) and individual patients (e.g., age, sex, alcohol intake, anxiety) can enhance the risk of emesis [6–9].
Great advances have been made in controlling CINV over the past two decades, but nausea and vomiting remain clinically significant problems for patients receiving highly emetogenic chemotherapy (HEC) and moderately emetogenic chemotherapy (MEC) [6, 10–14]. Treatment for CINV since the early 1990s has included corticosteroids, most commonly dexamethasone [15], but improvements quickly followed with the addition of 5-hydroxytryptamine (5HT3) receptor antagonists (RAs) [11, 15]. Furthermore, recent clinical practice guidelines from the Multinational Association for Supportive Care in Cancer (MASCC) and European Society of Medical Oncology (ESMO) [16], the American Society of Clinical Oncology (ASCO) [17], and the National Comprehensive Cancer Network (NCCN) [18] recommend the addition of a neurokinin-1 (NK1 RA) to the 5HT3 RA-corticosteroid combination, as the most effective regimen for controlling both acute and delayed CINV associated with HEC, or with anthracycline and cyclophosphamide (AC)-based regimens.
NK1 RAs are thought to act centrally, inhibiting emesis by blocking binding of substance P at the NK1 receptor in the brain stem emetic center [19]. They have demonstrated efficacy in clinical studies in controlling postoperative nausea and vomiting [20–22]. In vitro and preclinical studies have suggested that NK1 RAs could also be effective in several other therapeutic indications, including nausea, analgesia, migraine, asthma, urinary incontinence, and gastrointestinal disorders [23, 24]; however, most clinical studies have failed to demonstrate activity for conditions other than nausea and vomiting [23–25]. The exceptions are a pilot study that demonstrated NK1 RAs may be effective for the treatment of overactive bladder [26] and a study of aprepitant that suggested it may be useful for the treatment of severe pruritus associated with the use of biological agents in patients with cancer [27].
Aprepitant, an oral NK1 RA, and intravenous fosaprepitant, a prodrug of aprepitant, are currently the only NK1 RAs approved for prevention of HEC- or MEC-induced CINV, including acute- and delayed-phase CINV with aprepitant [28–30]; however, several other NK1 RAs have recently undergone or are currently undergoing clinical evaluation for the treatment of CINV, including casopitant, rolapitant, and netupitant [31, 32]. Casopitant, in particular, completed a number of phase III clinical trials studying its efficacy and safety in the control of CINV [33, 34] but was not granted approval by the U.S. Food and Drug Administration (FDA) because of insufficient safety data [32]. Consequently, further plans for casopitant as a therapeutic agent for CINV were dropped. Both rolapitant and netupitant have shown promise for preventing CINV. Rolapitant is currently undergoing phase III trials [32]. Netupitant in combination with palonosetron has recently demonstrated efficacy in preventing CINV in patients receiving HEC or MEC in phase III trials [35–37].
We reviewed key studies supporting the applicability of aprepitant and fosaprepitant for prevention of CINV and results relevant to their safety and efficacy. The potential utility of aprepitant and fosaprepitant for alternative indications was discussed with the goal of providing perspective on some anticipated applications of these NK1 RAs.
Materials and Methods
A search of PubMed was conducted for published preclinical data and clinical trials from 2003 through May 19, 2014, using the following search terms: aprepitant, CINV, fosaprepitant, HEC, MEC, and NK1 RA. Relevant reports published during this time frame were extracted; earlier reports were included if they were cited in the extracted reports and were deemed to contain information pertinent to the review topic. No combined analysis was planned because formulations of the drug (aprepitant or fosaprepitant) and populations included in each clinical trial are different. Data are presented along historical and medical drug-development lines.
Early Phase Trials
Early clinical data demonstrated that aprepitant represented a new class of antiemetic therapy, with an efficacy profile distinct from those of 5HT3 RAs or dexamethasone, providing enhanced control of both acute and delayed CINV when coadministered with a 5HT3 RA and dexamethasone compared with dual therapy alone [38–40]. An initial study showed that the triple combination of aprepitant, granisetron, and dexamethasone was statistically better than granisetron and dexamethasone for preventing cisplatin-related acute and delayed CINV, supporting further study of triple therapy [39]. Furthermore, in a small, randomized, proof-of-concept trial, fosaprepitant was superior to ondansetron in preventing delayed CINV when each was administered as a single agent, supporting the combination of fosaprepitant with a 5HT3 RA and dexamethasone [38]. Early studies also determined the most effective dose of aprepitant when combined with standard antiemetic regimens for prevention of CINV, adjusting for the pharmacokinetic interaction of aprepitant and dexamethasone [41–43].
Milestone and Efficacy and Safety Studies of Aprepitant
Multiple-Day Dosing Studies
The safety and efficacy of aprepitant were evaluated in several milestone clinical studies examining treatment of CINV during HEC, the design and endpoints of which are summarized in Table 1.
Table 1.
Study designs and endpoints of randomized, double-blind, milestone trials of aprepitant for prevention of chemotherapy-induced nausea and vomiting in highly emetogenic chemotherapy
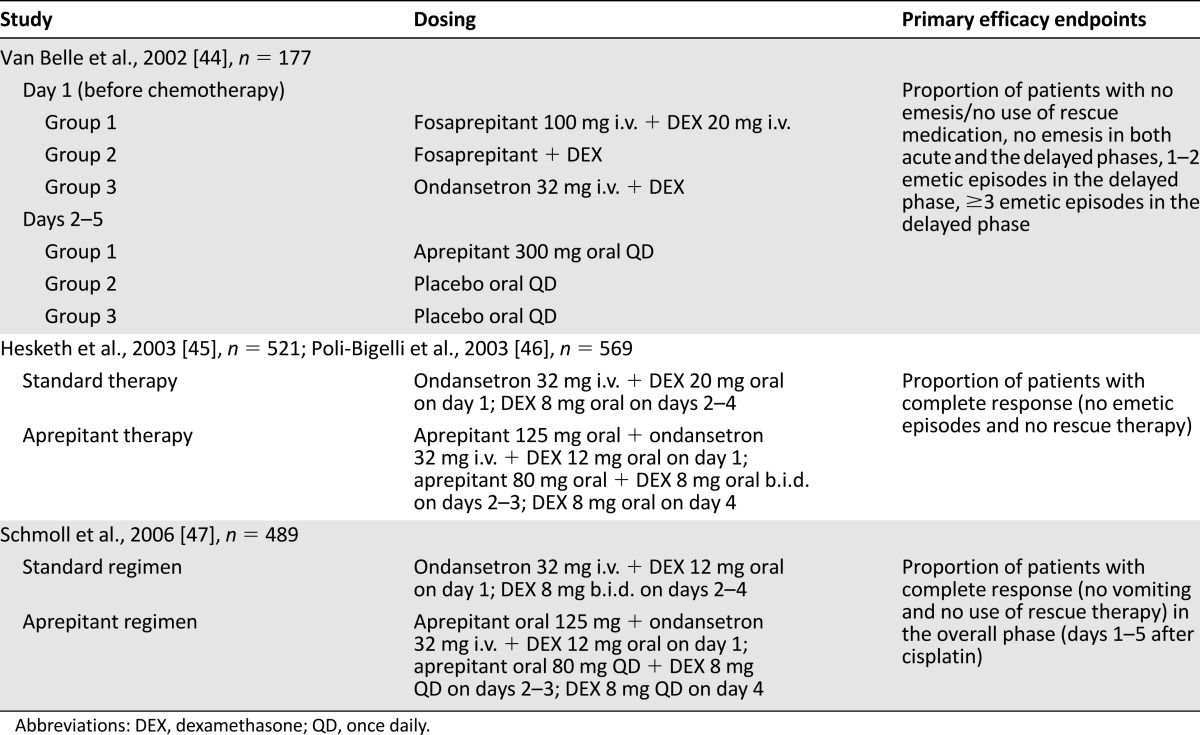
After the first phase IIa proof-of-concept study, discussed previously [38], Van Belle et al. reported results from a multicenter, randomized, double-blind, placebo-controlled study involving 177 cancer patients undergoing initial treatment with cisplatin [44]. Patients were assigned to one of three treatment groups, consisting of (a) intravenous fosaprepitant with dexamethasone before chemotherapy, followed by oral aprepitant 2–5 days after chemotherapy; (b) fosaprepitant with dexamethasone before chemotherapy, followed by placebo 2–5 days after chemotherapy; and (c) the 5HT3 RA ondansetron with dexamethasone before chemotherapy, which was the standard antiemetic combination for CINV, followed by placebo 2–5 days after chemotherapy [44]. It is important to note that the formulation of aprepitant used in this study was different from the nanoparticle formulation currently available.
Dual therapy with ondansetron plus dexamethasone was superior in controlling acute emesis compared with fosaprepitant plus dexamethasone, with 83% of patients reporting no emesis versus 44% and 36% for respective fosaprepitant groups [44]. By days 2–5, however, more patients receiving fosaprepitant plus dexamethasone, with or without oral aprepitant in the delayed phase, reported no emesis than those treated with ondansetron plus dexamethasone. This result suggests that aprepitant and fosaprepitant were more effective than ondansetron for controlling delayed-phase emesis and the need for rescue medication [44]. Both fosaprepitant and aprepitant were well tolerated with no treatment-related adverse events (AEs). The authors concluded that aprepitant plus dexamethasone was effective for control of delayed emesis in patients receiving HEC but that addition of a 5HT3 RA would likely be beneficial in the acute phase [44].
Subsequently, two multinational, identically designed, phase III, randomized, placebo-controlled studies of aprepitant were published in 2003, by Hesketh et al. [45] and Poli-Bigelli et al. [46] (Tables 1, 2). Both studies found that the rate of complete response, defined as no emesis and no rescue therapy, was significantly higher on days 1–5 for patients treated with aprepitant (Table 2) [45, 46]; these findings led to the approval of aprepitant by the FDA. Aprepitant-treated patients had patterns and incidences of AEs similar to those associated with standard antiemetic treatment, with an increased incidence of asthenia/fatigue (17% vs. 10%) after aprepitant therapy in one study [45, 46].
Table 2.
Clinical efficacy of oral aprepitant for prevention of chemotherapy-induced nausea and vomiting in highly emetogenic chemotherapy
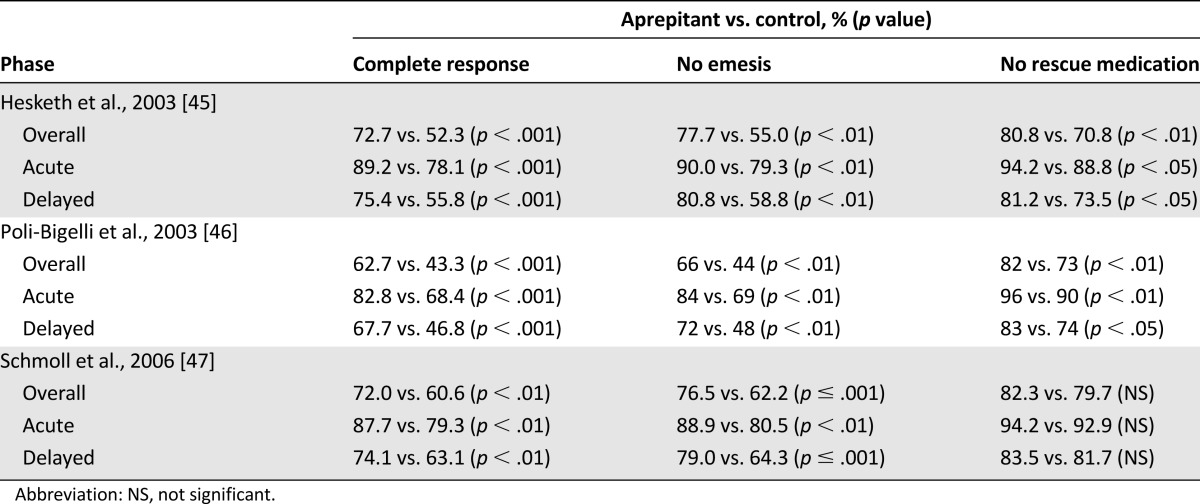
A further randomized, double-blind, parallel-group study in patients receiving cisplatin examined the effect on complete response rates of adding aprepitant to a standard regimen of ondansetron and dexamethasone (Tables 1, 2) [47]. Complete response rate was significantly higher in the group receiving aprepitant than in those receiving placebo over the total treatment period and during acute and delayed phases (Table 2) [47]. Statistically significant differences in incidences of stomatitis (4.9% vs. 1.2%), peripheral edema (0.4% vs. 3.7%), and urinary tract infection (3.7% vs. 0.8%) between aprepitant and standard therapy groups were reported [47].
Prevention of CINV in a broad population of cancer patients treated with a wide variety of MEC was examined in a multinational study [48]. Patients were randomly assigned in a double-blind manner to receive dexamethasone 12 mg plus oral ondansetron 8 mg alone or in combination with oral aprepitant 125 mg on day 1 before chemotherapy, followed by ondansetron 8 mg at 8 hours after chemotherapy and either aprepitant 80 mg once daily or ondansetron twice daily on days 2 and 3 [48]. Significantly more aprepitant recipients than controls achieved no vomiting (76.2% vs. 62.1%; p < .001) and complete response (68.7% vs. 56.3%; p < .001) in the 5 days after chemotherapy, regardless of whether they received AC-based regimens [48]. Post hoc analyses of these patients showed that the efficacy of aprepitant varied by tumor type [49–51] and that aprepitant was more efficacious than a standard regimen across sex, age, or region (North America, Central and South America, or international) [52].
A double-blind, double-dummy, parallel-group study examined the efficacy of an aprepitant-containing antiemetic regimen with dexamethasone plus ondansetron in breast cancer patients receiving MEC with AC-based chemotherapy [53]. The aprepitant-containing regimen consisted of aprepitant 125 mg plus ondansetron 8 mg and dexamethasone 12 mg before chemotherapy, ondansetron 8 mg at 8 hours after chemotherapy, and aprepitant 80 mg once daily on days 2 and 3 after chemotherapy. The control regimen consisted of ondansetron 8 mg plus dexamethasone 20 mg before chemotherapy, ondansetron 8 mg at 8 hours after chemotherapy, and ondansetron 8 mg twice daily on days 2 and 3 [53]. The rate of complete response, with no vomiting and no requirement for rescue therapy, was significantly higher for the aprepitant-containing regimen than for the control regimen (50.8% vs. 42.5%; p = .015). Aprepitant was well tolerated in this patient group [53].
An additional multicenter, double-blind, parallel study demonstrated similar delayed CINV prophylactic efficacy of aprepitant 80 mg once daily compared with dexamethasone 4 mg twice daily administered on days 2 and 3 in patients with breast cancer who were receiving AC-based chemotherapy (complete response rate 79.5% vs. 79.5%; no vomiting 89.2% vs. 91.6%; no nausea 43.9% vs. 49.1%) [54]. All patients received the same combination of oral aprepitant 125 mg and intravenous palonosetron 0.25 mg and dexamethasone 8 mg administered on day 1 for prophylaxis of acute CINV.
Single Oral Dosing
Although the originally recommended dosing of aprepitant for controlling CINV was 3 days, several studies found that single doses of oral aprepitant are effective in preventing acute and delayed CINV [40, 55, 56]. A study in 41 chemotherapy-naïve patients with solid tumors receiving cyclophosphamide with or without doxorubicin found that a single dose of oral aprepitant (285 mg) given in combination with palonosetron and dexamethasone prior to chemotherapy was effective for protection against CINV in both the acute and delayed phases [56]. A pilot study involving 75 patients with a broad variety of tumors treated with HEC compared the effectiveness of a single dose of aprepitant 125 mg administered on day 1 of chemotherapy (n = 30) versus aprepitant given over 3 days (n = 29), both of which were given in combination with palonosetron and dexamethasone [55]. Single-dose aprepitant produced a level of antiemetic activity similar to that of the 3-day regimen [55]. Although these results suggest that a single dose of aprepitant (in combination with a 5HT3 RA and dexamethasone) may be effective at preventing CINV, it is important to note that the optimal single-day dose has yet to be determined.
Studies in healthy adult volunteers demonstrated bioequivalence of a single oral dose of aprepitant 125 mg and intravenous fosaprepitant 115 mg [57] and bioequivalence of a single oral dose of 165 mg of aprepitant and intravenous fosaprepitant 150 mg [58]. This latter observation, in conjunction with the single intravenous-dosing data indicated below, reinforces the impression that aprepitant does not need to be used over several days.
Single Intravenous Dosing
Single doses of intravenous fosaprepitant have also been shown to be effective for preventing acute and delayed CINV [59]. A randomized, double-blind study showed that a single dose of fosaprepitant 150 mg (on day 1) after ondansetron and dexamethasone was noninferior to a standard aprepitant regimen (125 mg on day 1, and 80 mg on days 2 and 3) in preventing CINV in 2,247 patients receiving cisplatin [60]. Complete response rates overall and during the delayed phase, respectively, were 71.9% and 74.3% in patients treated with fosaprepitant and 72.3% and 74.2% in those who received aprepitant.
In patients receiving HEC, a single higher dose of fosaprepitant 150 mg in combination with granisetron, a 5HT3 RA, and dexamethasone on day 1 of chemotherapy, followed by dexamethasone alone on days 2 and 3, was well tolerated and produced a significantly higher rate of overall complete response than was seen in the control group without fosaprepitant (64% [95% confidence interval (CI): 16%–46%] vs. 47% [95% CI: 10%–36%]; p = .0015) [59]. These studies collectively suggest that a single dose of fosaprepitant enhances the antiemetic effects provided by conventional 5HT3 RA and corticosteroid therapy over conventional therapy alone and may provide a level of efficacy similar to that of the recommended 3-day aprepitant regimen.
Dosing Over Multiple Chemotherapy Cycles
To date, few studies have examined the efficacy of antiemetic drugs over multiple cycles of chemotherapy. Nevertheless, two studies reported the efficacy of aprepitant in protecting against CINV experienced over multiple cycles of cisplatin-based chemotherapy [41, 61].
The first study was a double-blind, placebo-controlled, parallel-group trial involving 202 patients randomly assigned to one of three antiemetic treatment regimens for up to a total of six chemotherapy cycles and showed that aprepitant-containing regimens provided superior control of CINV compared with treatment regimens without aprepitant [41]. Each group received standard therapy at each chemotherapy cycle, consisting of intravenous ondansetron 32 mg plus dexamethasone 20 mg, followed by dexamethasone 8 mg on days 2–5 [41]. Patients in the aprepitant treatment groups also received aprepitant 375 mg on day 1 and aprepitant 250 mg on days 2–5, or aprepitant 125 mg on day 1 and 80 mg on days 2 to 5, in addition to standard therapy [41]. The overall complete response rate of aprepitant 125 and 80 mg was significantly greater than that of the control group and was sustained over 6 treatment cycles, with 64% and 59% response rates between cycles 1 and 6, respectively [41]. In contrast, the response rate of the control group receiving standard therapy displayed a much more rapid rate of decline (49% in cycle 1, and 34% in cycle 6), with statistically significant differences between treatment groups at cycles 1 and 6 (p < .05, for both comparisons) [41]. Although no formal statistical comparisons were performed, rates of complete response were higher than for controls in the aprepitant 375 and 250 mg group (70% in cycle 1, and 65% in cycle 6), similar to those reported with aprepitant 125 and 80 mg [41].
The second study [61] analyzed extension data from the previously discussed phase III clinical trials published by Hesketh et al. [45] and Poli-Bigelli et al. [46] evaluating preventative efficacy of aprepitant against CINV over multiple rounds of chemotherapy. A total of 1,099 patients from these phase III studies continued to receive the same antiemetic agents they had been using for up to five additional cycles of chemotherapy [61]. With a combined exploratory endpoint of no emesis and no significant nausea (defined as nausea interfering with patients’ normal activities), aprepitant group rates over multiple cycles were consistently higher than those of the group receiving standard therapy (p ≤ .006 for all cycles) [61]. The rate of protection against CINV displayed minimal loss from cycle 1 to cycle 6, suggesting that antiemetic effects of aprepitant are maintained through multiple cycles of chemotherapy [61]. In addition, aprepitant was generally well tolerated with repeated dosing [61].
Adverse Events
A systematic review of 17 trials (n = 8,740) of NK1 RAs added to antiemetic regimens for prevention of CINV showed statistically significant but clinically trivial differences in fatigue and hiccups and lower constipation than controls [31]. The study also demonstrated a statistically significant increase in the risk of severe infection among patients who received NK1 RAs (odds ratio: 3.10; p < .001) [31]; however, the difference was derived almost exclusively from a single study that used high doses of dexamethasone [42].
Both standard 3-day dosing of aprepitant and single-dose fosaprepitant were demonstrated to be well tolerated after ondansetron and dexamethasone in patients receiving cisplatin [60]. The tolerability profiles of the two regimens were similar, except for a higher incidence of infusion-site AEs (2.2% vs. 0.4%) and significantly more thrombophlebitis (0.8% vs. 0.1%; p = .005) with fosaprepitant than with aprepitant. Furthermore, higher incidence of infusion-site AEs was observed in a retrospective review of 98 patients treated with fosaprepitant compared with 44 aprepitant recipients (34.7% vs. 2.3%) [62].
Special Populations
Dose modifications have not been recommended for patients with mildly to moderately impaired liver function or renal insufficiency [29]. No adjustment in aprepitant dose has been advised for patients of different sex, age, race, or body mass index [29]; however, it is important to note that data in pediatric populations are limited to date.
A study of aprepitant plasma pharmacokinetics in 44 Japanese cancer patients receiving cisplatin de novo, with oral aprepitant 125 mg administered before chemotherapy on day 1 and aprepitant 80 mg given on days 2 and 3, found that plasma aprepitant exposure was not correlated with age or sex [63]. In contrast, another pharmacokinetic study in a larger sample of 315 Japanese cancer patients reported that age had a mild effect on the oral clearance of aprepitant [64]. Comparison of aprepitant pharmacokinetics in healthy subjects versus those with impaired renal function revealed that renal insufficiency, renal failure, or hemodialysis did not affect the single-dose pharmacokinetics of aprepitant [65], and meta-analysis of data from four studies revealed that the antiemetic efficacy of aprepitant does not appear to be affected by patient age [66].
A recent phase III, placebo-controlled, double-blind study examined the use of a 3-day preventive therapy with an age- and weight-based regimen of aprepitant (in conjunction with ondansetron with or without dexamethasone) in 302 children and adolescents scheduled to receive HEC or MEC [67]. The use of aprepitant was associated with a greater proportion of patients achieving complete response across acute, delayed, and overall phases compared with ondansetron with or without dexamethasone alone (66.4% vs. 52.0%, 50.7% vs. 26.0%, and 40.1% vs. 20.0%, respectively; all p < .025). The AE profile was comparable between groups [67].
Aprepitant Use With High-Dose Chemotherapy and in Peripheral Stem Cell Transplantation
Patients undergoing stem cell transplantation (SCT) receive a vast array of conditioning drugs, including some highly emetogenic agents, necessitating the use of antiemetic therapy. Drugs used to condition patients for SCT can also potentially alter the pharmacokinetics of aprepitant [68]. Cyclophosphamide, a drug frequently used as a component of SCT conditioning, raises particular concern because it induces CYP3A activity, which could increase aprepitant metabolism [69]. A randomized, pharmacokinetic study of aprepitant versus placebo before chemotherapy or radiation for hematopoietic SCT conditioning showed that aprepitant was well absorbed in 14 patients receiving cyclophosphamide and did not alter the metabolism of cyclophosphamide [68]. In addition, aprepitant did not alter the pharmacokinetics of high-dose melphalan used as conditioning therapy before SCT in patients with multiple myeloma [70].
Aprepitant did not alter the pharmacokinetics of high-dose melphalan used as conditioning therapy before SCT in patients with multiple myeloma.
Highly emetogenic preparative regimens before autologous or allogenic SCT typically take up to 1 week to administer; therefore, the necessity to administer aprepitant for longer than the 3 days approved by the FDA might have safety implications. A randomized phase III study examined ondansetron and dexamethasone with or without aprepitant in 264 patients receiving such preparative regimens with the antiemetic regimen administered on each day of the preparative regimen plus over an additional 1–3 days [71]. Results showed significantly reduced emesis and nausea, without increased regimen-related toxicity, use of rescue medication, or effect on short-term survival [71]. Similarly, a study evaluating aprepitant in combination with palonosetron and dexamethasone showed the combination to be safe and effective for prevention of nausea and vomiting in patients receiving a high-dose BEAM regimen (carmustine, etoposide, cytarabine, melphalan) as conditioning therapy before undergoing hematopoietic SCT [72]. In addition, an aprepitant-based antiemetic regimen before high-dose cytarabine showed minimal effect on autologous peripheral blood stem cell mobilization [73].
Data on Drug-Drug Interactions
Aprepitant is metabolized extensively by liver enzymes, primarily CYP3A4; therefore, potent CYP3A4 inhibitors can increase aprepitant exposure, and potent CYP3A4 inducers can reduce aprepitant exposure [74]. Aprepitant is also, paradoxically, both an inducer and a moderate inhibitor of CYP3A4 [75]. Consequently, the potential for drug-drug interactions exists when aprepitant is coadministered with other drugs that are metabolized by CYP enzymes, including chemotherapeutic agents [76].
Nevertheless, results from several clinical efficacy trials and pharmacokinetic studies showed that most drug-drug interactions with aprepitant had little or no clinical consequence and that no differences in severe AEs were noted between treatment arms with or without aprepitant [48, 53, 76, 77]. Aprepitant had minimal effect on the area under the curve (AUC) of several chemotherapeutic agents tested, including cyclophosphamide, docetaxel, and vinorelbine [76]. Coadministration of aprepitant causes a significant increase in the AUC of some corticosteroids, including a 2.2-fold increase in dexamethasone and a 2.5-fold increase in oral methylprednisolone, necessitating up to 50% dose reduction of these drugs [76]. Nevertheless, aprepitant does not alter prednisolone pharmacokinetics in patients with non-Hodgkin’s lymphoma receiving R-CHOP, an AC-based chemotherapy regimen with a prednisolone component [78]. Aprepitant causes reduced AUC of oral contraceptives, and this has prompted the recommendation of a secondary barrier contraceptive for patients receiving aprepitant [76].
Current Guidelines
All clinical practice guidelines on antiemetic therapy currently recommend the addition of aprepitant to combination 5HT3 RA and dexamethasone for patients receiving HEC. Although all major clinical guidelines recommend aprepitant in combination with a 5HT3 RA and dexamethasone in patients treated with HEC or the combination of AC for prevention of acute emesis, not all guidelines recommend additional dexamethasone doses in the delayed phase for patients receiving AC. Clinical guidelines published by MASCC/ESMO, NCCN, and ASCO for prescription of aprepitant for control of CINV are summarized in Table 3 [16–18].
Table 3.
Summary of antiemetic guidelines for acute and delayed prevention
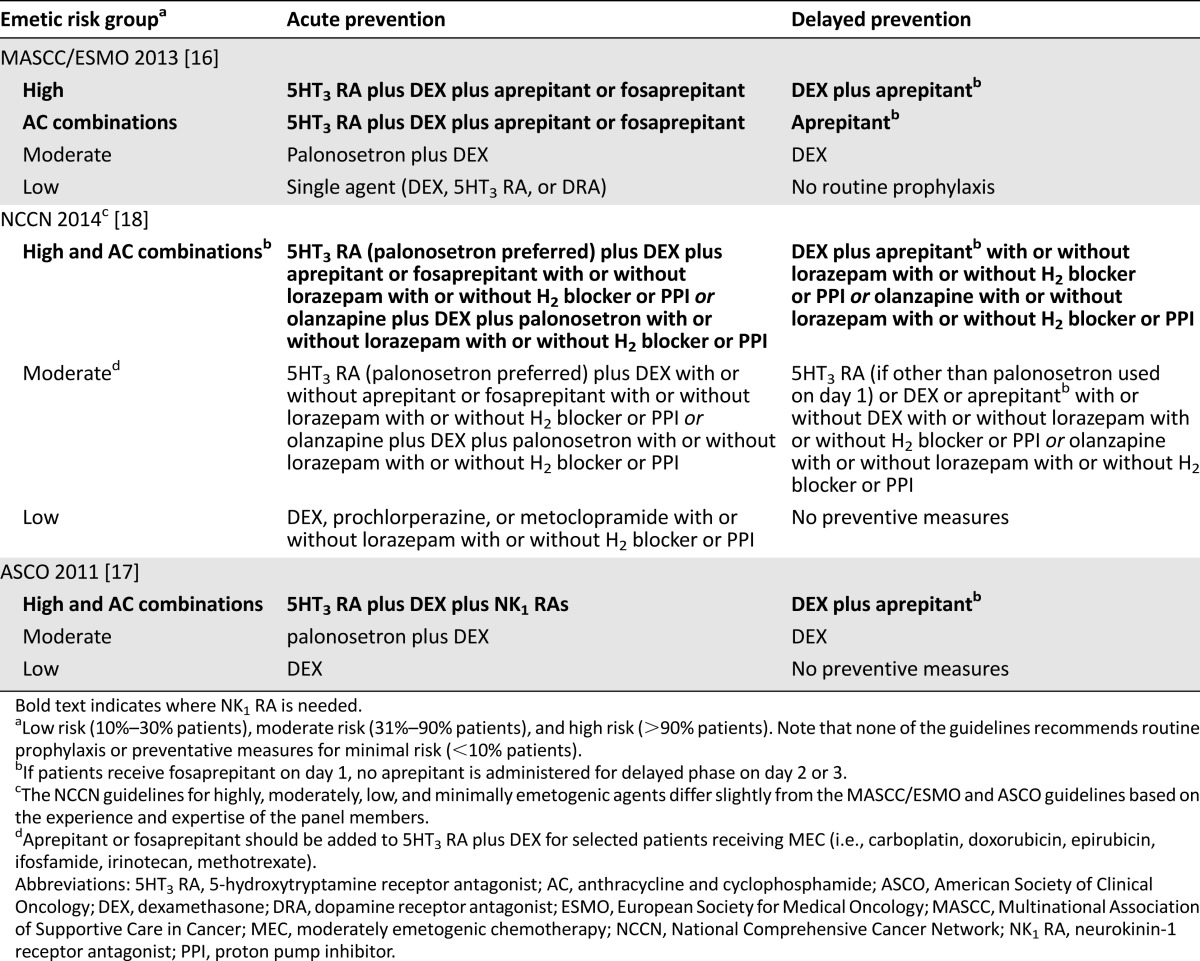
A phase III, multicenter, double-blind, placebo-controlled trial also confirmed good tolerability and superior CINV prevention with addition of aprepitant to standard antiemetic treatment regimens in Chinese patients receiving HEC [79]. In the People’s Republic of China, the most current clinical practice guidelines recommend aprepitant or fosaprepitant for patients receiving HEC and for high-risk patients receiving MEC [80]. In addition, patients treated with HEC should receive the combination of aprepitant, 5HT3 RA, and dexamethasone. Aprepitant or fosaprepitant is also recommended for patients receiving selected MEC, including carboplatin, cisplatin, doxorubicin, epirubicin, ifosfamide, irinotecan, and methotrexate, and for those receiving multiday cisplatin chemotherapy regimens. Many other countries have developed guidelines that closely follow the MASCC/ESMO publication and that are adapted for medication availability [81–83].
Implementation of antiemetic therapy consistent with clinical practice guidelines has been shown to produce better control of CINV [84]. A recent prospective observational study of more than 1,200 patients receiving initial treatment with HEC or MEC found that increased adherence to antiemetic clinical practice guidelines resulted in significantly reduced incidence of CINV [85]; however, adherence to antiemetic guidelines has been shown to be variable in clinical practice [84, 86]. Moreover, the incidence of nausea and emesis among patients receiving MEC has been found to be significantly underestimated by physicians and nurses [11]. These findings suggest that current management of CINV, given available knowledge and treatment options, is suboptimal in Europe, the U.S., and Asia [84, 86].
A recent prospective observational study of more than 1,200 patients receiving initial treatment with HEC or MEC found that increased adherence to antiemetic clinical practice guidelines resulted in significantly reduced incidence of CINV.
Future Perspectives
Aside from its current application in controlling CINV, aprepitant is currently being explored for potential use in treating pruritus in cancer patients, a common adverse effect of therapy with anti-epidermal growth factor antibody and tyrosine kinase inhibitors [27]. A prospective pilot study in 45 patients with pruritus resulting from biologic drug treatment showed that aprepitant significantly reduced symptoms associated with pruritus, including severe itch, compared with baseline in both refractory and treatment-naïve patients [27]. No treatment-related AEs were reported for aprepitant in this patient group [27]. Although the efficacy of aprepitant for alleviating pruritus has been supported by findings from a number of other studies, all studies were performed in small samples of patients [87, 88], necessitating further evaluation in larger sample sizes.
Several additional aprepitant and fosaprepitant trials are ongoing, including investigations into their use in CINV prevention for pediatric patients and in use of aprepitant for prevention of postoperative nausea and vomiting for both pediatric and adult populations, for antiviral activity in patients infected with HIV, and for its effects on heroin dependence (see http://www.ClinicalTrials.gov for additional details on these studies).
Conclusion
Antiemetic therapy has advanced over the past 10 years with the advent of NK1 RAs. Clinical studies of aprepitant and fosaprepitant for preventing CINV in patients treated with HEC or MEC over the past decade have consistently demonstrated that these agents enhance the effectiveness of the standard antiemetic combination of a corticosteroid and 5HT3 RA for controlling acute- and delayed-phase CINV. Aprepitant is effective in the presence of different chemotherapeutic agents [48] and can provide protection against CINV when given in multiple doses over several days or, potentially, when given as a single dose with palonosetron and dexamethasone before chemotherapy with the antiemetic effect persisting over several days after initial administration [54, 55]; however, the current 3-day packaging of the 125-mg capsule would entail substantial drug wastage. Aprepitant can also be used over several cycles of chemotherapy without appreciable loss of antiemetic protection [41, 61]. Extensive clinical trial data and long-term daily practice experience with aprepitant and fosaprepitant confirm their roles as standard antiemetic agents to be used according to guidelines.
Acknowledgments
Medical writing and editorial assistance was provided by Susan Quiñones and Dolores Matthews of ApotheCom in Yardley, Pennsylvania. This assistance was funded by Merck & Co. Inc., Kenilworth, New Jersey.
Author Contributions
Conception/Design: Matti Aapro, Alexandra Carides, Bernardo L. Rapoport, Hans-Joachim Schmoll, David Warr
Collection and/or assembly of data: Matti Aapro, Alexandra Carides, Hans-Joachim Schmoll, Li Zhang
Data analysis and interpretation: Matti Aapro, Alexandra Carides, Hans-Joachim Schmoll, Li Zhang
Manuscript writing: Matti Aapro, Alexandra Carides, Bernardo L. Rapoport, Hans-Joachim Schmoll, Li Zhang, David Warr
Final approval of manuscript: Matti Aapro, Alexandra Carides, Bernardo L. Rapoport, Hans-Joachim Schmoll, Li Zhang, David Warr
Disclosures
Matti Aapro: Helsinn, Merck, Sanofi, Roche, GlaxoSmithKline, Sandoz, Teva, Novartis (C/A, H), Helsinn, Sandoz (RF); Li Zhang: MSD (C/A); David Warr: Merck (RF, H). The other authors indicated no potential conflicts of interest.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Lindley CM, Hirsch JD, O’Neill CV, et al. Quality of life consequences of chemotherapy-induced emesis. Qual Life Res. 1992;1:331–340. doi: 10.1007/BF00434947. [DOI] [PubMed] [Google Scholar]
- 2.Grunberg SM, Boutin N, Ireland A, et al. Impact of nausea/vomiting on quality of life as a visual analogue scale-derived utility score. Support Care Cancer. 1996;4:435–439. doi: 10.1007/BF01880641. [DOI] [PubMed] [Google Scholar]
- 3.Osoba D, Zee B, Warr D, et al. Effect of postchemotherapy nausea and vomiting on health-related quality of life. Support Care Cancer. 1997;5:307–313. doi: 10.1007/s005200050078. [DOI] [PubMed] [Google Scholar]
- 4.Ballatori E, Roila F. Impact of nausea and vomiting on quality of life in cancer patients during chemotherapy. Health Qual Life Outcomes. 2003;1:46. doi: 10.1186/1477-7525-1-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laszlo J. Nausea and vomiting as major complications of cancer chemotherapy. Drugs. 1983;25(suppl 1):1–7. doi: 10.2165/00003495-198300251-00002. [DOI] [PubMed] [Google Scholar]
- 6.Di Maio M, Bria E, Banna GL, et al. Prevention of chemotherapy-induced nausea and vomiting and the role of neurokinin 1 inhibitors: From guidelines to clinical practice in solid tumors. Anticancer Drugs. 2013;24:99–111. doi: 10.1097/CAD.0b013e328359d7ba. [DOI] [PubMed] [Google Scholar]
- 7.Molassiotis A, Stamataki Z, Kontopantelis E. Development and preliminary validation of a risk prediction model for chemotherapy-related nausea and vomiting. Support Care Cancer. 2013;21:2759–2767. doi: 10.1007/s00520-013-1843-2. [DOI] [PubMed] [Google Scholar]
- 8.Sekine I, Segawa Y, Kubota K, et al. Risk factors of chemotherapy-induced nausea and vomiting: Index for personalized antiemetic prophylaxis. Cancer Sci. 2013;104:711–717. doi: 10.1111/cas.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleishman SB, Mahajan D, Rosenwald V, et al. Prevalence of delayed nausea and/or vomiting in patients treated with oxaliplatin-based regimens for colorectal cancer. J Oncol Pract. 2012;8:136–140. doi: 10.1200/JOP.2010.000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen L, de Moor CA, Eisenberg P, et al. Chemotherapy-induced nausea and vomiting: Incidence and impact on patient quality of life at community oncology settings. Support Care Cancer. 2007;15:497–503. doi: 10.1007/s00520-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 11.Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261–2268. doi: 10.1002/cncr.20230. [DOI] [PubMed] [Google Scholar]
- 12.Liau CT, Chu NM, Liu HE, et al. Incidence of chemotherapy-induced nausea and vomiting in Taiwan: Physicians’ and nurses’ estimation vs. patients’ reported outcomes. Support Care Cancer. 2005;13:277–286. doi: 10.1007/s00520-005-0788-5. [DOI] [PubMed] [Google Scholar]
- 13.Molassiotis A, Saunders MP, Valle J, et al. A prospective observational study of chemotherapy-related nausea and vomiting in routine practice in a UK cancer centre. Support Care Cancer. 2008;16:201–208. doi: 10.1007/s00520-007-0343-7. [DOI] [PubMed] [Google Scholar]
- 14.Bloechl-Daum B, Deuson RR, Mavros P, et al. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24:4472–4478. doi: 10.1200/JCO.2006.05.6382. [DOI] [PubMed] [Google Scholar]
- 15.Grunberg SM, Slusher B, Rugo HS. Emerging treatments in chemotherapy-induced nausea and vomiting. Clin Adv Hematol Oncol. 2013;11(suppl 1):1–18; quiz 2, 18. [PubMed] [Google Scholar]
- 16.MASCC/ESMO antiemetic guidelines. Available at http://www.mascc.org/antiemetic-guidelines. Updated 2013. Accessed December 19, 2014.
- 17.Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update [published correction appears in J Clin Oncol 2014;32:2117] J Clin Oncol. 2011;29:4189–4198. doi: 10.1200/JCO.2010.34.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NCCN clinical practice guidelines in oncology: Antiemesis. Version 1.2014. Available at http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed June 10, 2014.
- 19.Tattersall FD, Rycroft W, Francis B, et al. Tachykinin NK1 receptor antagonists act centrally to inhibit emesis induced by the chemotherapeutic agent cisplatin in ferrets. Neuropharmacology. 1996;35:1121–1129. doi: 10.1016/s0028-3908(96)00020-2. [DOI] [PubMed] [Google Scholar]
- 20.Gan TJ, Gu J, Singla N, et al. Rolapitant for the prevention of postoperative nausea and vomiting: A prospective, double-blinded, placebo-controlled randomized trial. Anesth Analg. 2011;112:804–812. doi: 10.1213/ANE.0b013e31820886c3. [DOI] [PubMed] [Google Scholar]
- 21.Apfel CC, Malhotra A, Leslie JB. The role of neurokinin-1 receptor antagonists for the management of postoperative nausea and vomiting. Curr Opin Anaesthesiol. 2008;21:427–432. doi: 10.1097/ACO.0b013e328301831c. [DOI] [PubMed] [Google Scholar]
- 22.Diemunsch P, Joshi GP, Brichant JF. Neurokinin-1 receptor antagonists in the prevention of postoperative nausea and vomiting. Br J Anaesth. 2009;103:7–13. doi: 10.1093/bja/aep125. [DOI] [PubMed] [Google Scholar]
- 23.Rost K, Fleischer F, Nieber K. Neurokinin 1 receptor antagonists—between hope and disappointment [in German] Med Monatsschr Pharm. 2006;29:200–205. [PubMed] [Google Scholar]
- 24.Alvaro G, Di Fabio R. Neurokinin 1 receptor antagonists—current prospects. Curr Opin Drug Discov Devel. 2007;10:613–621. [PubMed] [Google Scholar]
- 25.Keller M, Montgomery S, Ball W, et al. Lack of efficacy of the substance p (neurokinin1 receptor) antagonist aprepitant in the treatment of major depressive disorder. Biol Psychiatry. 2006;59:216–223. doi: 10.1016/j.biopsych.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Green SA, Alon A, Ianus J, et al. Efficacy and safety of a neurokinin-1 receptor antagonist in postmenopausal women with overactive bladder with urge urinary incontinence. J Urol. 2006;176:2535–2540; discussion 2540. doi: 10.1016/j.juro.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 27.Santini D, Vincenzi B, Guida FM, et al. Aprepitant for management of severe pruritus related to biological cancer treatments: A pilot study. Lancet Oncol. 2012;13:1020–1024. doi: 10.1016/S1470-2045(12)70373-X. [DOI] [PubMed] [Google Scholar]
- 28.Wickham R. Evolving treatment paradigms for chemotherapy-induced nausea and vomiting. Cancer Contr. 2012;19(suppl):3–9. doi: 10.1177/107327481201902s02. [DOI] [PubMed] [Google Scholar]
- 29.EMEND (aprepitant) [prescribing information]. Whitehouse Station, NJ: Merck Sharp & Dohme Corporation, 2013.
- 30.EMEND (fosaprepitant dimeglumine) [prescribing information]. Whitehouse Station, NJ: Merck Sharp & Dohme Corporation, 2013.
- 31.dos Santos LV, Souza FH, Brunetto AT, et al. Neurokinin-1 receptor antagonists for chemotherapy-induced nausea and vomiting: A systematic review. J Natl Cancer Inst. 2012;104:1280–1292. doi: 10.1093/jnci/djs335. [DOI] [PubMed] [Google Scholar]
- 32.Navari RM. Management of chemotherapy-induced nausea and vomiting: Focus on newer agents and new uses for older agents. Drugs. 2013;73:249–262. doi: 10.1007/s40265-013-0019-1. [DOI] [PubMed] [Google Scholar]
- 33.Hesketh PJ, Wright O, Rosati G, et al. Single-dose intravenous casopitant in combination with ondansetron and dexamethasone for the prevention of oxaliplatin-induced nausea and vomiting: A multicenter, randomized, double-blind, active-controlled, two arm, parallel group study. Support Care Cancer. 2012;20:1471–1478. doi: 10.1007/s00520-011-1235-4. [DOI] [PubMed] [Google Scholar]
- 34.Ruhlmann C, Herrstedt J. Casopitant: A novel NK(1)-receptor antagonist in the prevention of chemotherapy-induced nausea and vomiting. Ther Clin Risk Manag. 2009;5:375–384. doi: 10.2147/tcrm.s4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gralla RJ, Bosnjak SM, Hontsa A, et al. A phase III study evaluating the safety and efficacy of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting over repeated cycles of chemotherapy. Ann Oncol. 2014;25:1333–1339. doi: 10.1093/annonc/mdu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hesketh PJ, Rossi G, Rizzi G, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: A randomized dose-ranging pivotal study. Ann Oncol. 2014;25:1340–1346. doi: 10.1093/annonc/mdu110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aapro M, Rugo H, Rossi G, et al. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol. 2014;25:1328–1333. doi: 10.1093/annonc/mdu101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cocquyt V, Van Belle S, Reinhardt RR, et al. Comparison of L-758,298, a prodrug for the selective neurokinin-1 antagonist, L-754,030, with ondansetron for the prevention of cisplatin-induced emesis. Eur J Cancer. 2001;37:835–842. doi: 10.1016/s0959-8049(00)00416-0. [DOI] [PubMed] [Google Scholar]
- 39.Campos D, Pereira JR, Reinhardt RR, et al. Prevention of cisplatin-induced emesis by the oral neurokinin-1 antagonist, MK-869, in combination with granisetron and dexamethasone or with dexamethasone alone. J Clin Oncol. 2001;19:1759–1767. doi: 10.1200/JCO.2001.19.6.1759. [DOI] [PubMed] [Google Scholar]
- 40.Navari RM, Reinhardt RR, Gralla RJ, et al. Reduction of cisplatin-induced emesis by a selective neurokinin-1-receptor antagonist. L-754,030 Antiemetic Trials Group. N Engl J Med. 1999;340:190–195. doi: 10.1056/NEJM199901213400304. [DOI] [PubMed] [Google Scholar]
- 41.de Wit R, Herrstedt J, Rapoport B, et al. Addition of the oral NK1 antagonist aprepitant to standard antiemetics provides protection against nausea and vomiting during multiple cycles of cisplatin-based chemotherapy. J Clin Oncol. 2003;21:4105–4111. doi: 10.1200/JCO.2003.10.128. [DOI] [PubMed] [Google Scholar]
- 42.Chawla SP, Grunberg SM, Gralla RJ, et al. Establishing the dose of the oral NK1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting. Cancer. 2003;97:2290–2300. doi: 10.1002/cncr.11320. [DOI] [PubMed] [Google Scholar]
- 43.Grote T, Hajdenberg J, Cartmell A, et al. Combination therapy for chemotherapy-induced nausea and vomiting in patients receiving moderately emetogenic chemotherapy: Palonosetron, dexamethasone, and aprepitant. J Support Oncol. 2006;4:403–408. [PubMed] [Google Scholar]
- 44.Van Belle S, Lichinitser MR, Navari RM, et al. Prevention of cisplatin-induced acute and delayed emesis by the selective neurokinin-1 antagonists, L-758,298 and MK-869. Cancer. 2002;94:3032–3041. doi: 10.1002/cncr.10516. [DOI] [PubMed] [Google Scholar]
- 45.Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: A multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin—the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21:4112–4119. doi: 10.1200/JCO.2003.01.095. [DOI] [PubMed] [Google Scholar]
- 46.Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer. 2003;97:3090–3098. doi: 10.1002/cncr.11433. [DOI] [PubMed] [Google Scholar]
- 47.Schmoll HJ, Aapro MS, Poli-Bigelli S, et al. Comparison of an aprepitant regimen with a multiple-day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in high-dose cisplatin treatment. Ann Oncol. 2006;17:1000–1006. doi: 10.1093/annonc/mdl019. [DOI] [PubMed] [Google Scholar]
- 48.Rapoport BL, Jordan K, Boice JA, et al. Aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with a broad range of moderately emetogenic chemotherapies and tumor types: A randomized, double-blind study. Support Care Cancer. 2010;18:423–431. doi: 10.1007/s00520-009-0680-9. [DOI] [PubMed] [Google Scholar]
- 49.Boice J, Brown C, Taylor HJ. Aprepitant in the prevention of chemotherapy-induced nausea and vomiting associated with moderately or highly emetogenic chemotherapies in patients with gastrointestinal cancer. Ann Oncol. 2009;20(suppl 2):vii69. [Google Scholar]
- 50.Rapoport B, Boice JA, Brown C. Aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with moderately emetogenic (MEC) or highly emetogenic (HEC) chemotherapies in patients with lung cancer. Eur J Cancer. 2009;7(suppl 7):519–520. [Google Scholar]
- 51.Webb T, Hardwick J, Carides A. Aprepitant for the prevention of chemotherapy-induced nausea and vomiting (CINV) associated with moderately emetogenic (MEC) chemotherapy in patients with breast cancer. Cancer Res. 2009;69:1116. [Google Scholar]
- 52.Rapoport BL. Efficacy of a triple antiemetic regimen with aprepitant for the prevention of chemotherapy-induced nausea and vomiting: Effects of gender, age, and region. Curr Med Res Opin. 2014;30:1875–1881. doi: 10.1185/03007995.2014.925866. [DOI] [PubMed] [Google Scholar]
- 53.Warr DG, Hesketh PJ, Gralla RJ, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy [published correction appears in J Clin Oncol 2005;23:5851] J Clin Oncol. 2005;23:2822–2830. doi: 10.1200/JCO.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 54.Roila F, Ruggeri B, Ballatori E, et al. Aprepitant versus dexamethasone for preventing chemotherapy-induced delayed emesis in patients with breast cancer: A randomized double-blind study. J Clin Oncol. 2014;32:101–106. doi: 10.1200/JCO.2013.51.4547. [DOI] [PubMed] [Google Scholar]
- 55.Herrington JD, Jaskiewicz AD, Song J. Randomized, placebo-controlled, pilot study evaluating aprepitant single dose plus palonosetron and dexamethasone for the prevention of acute and delayed chemotherapy-induced nausea and vomiting. Cancer. 2008;112:2080–2087. doi: 10.1002/cncr.23364. [DOI] [PubMed] [Google Scholar]
- 56.Grunberg SM, Dugan M, Muss H, et al. Effectiveness of a single-day three-drug regimen of dexamethasone, palonosetron, and aprepitant for the prevention of acute and delayed nausea and vomiting caused by moderately emetogenic chemotherapy. Support Care Cancer. 2009;17:589–594. doi: 10.1007/s00520-008-0535-9. [DOI] [PubMed] [Google Scholar]
- 57.Lasseter KC, Gambale J, Jin B, et al. Tolerability of fosaprepitant and bioequivalency to aprepitant in healthy subjects. J Clin Pharmacol. 2007;47:834–840. doi: 10.1177/0091270007301800. [DOI] [PubMed] [Google Scholar]
- 58.Van Laere K, De Hoon J, Bormans G, et al. Equivalent dynamic human brain NK1-receptor occupancy following single-dose i.v. fosaprepitant vs. oral aprepitant as assessed by PET imaging. Clin Pharmacol Ther. 2012;92:243–250. doi: 10.1038/clpt.2012.62. [DOI] [PubMed] [Google Scholar]
- 59.Saito H, Yoshizawa H, Yoshimori K, et al. Efficacy and safety of single-dose fosaprepitant in the prevention of chemotherapy-induced nausea and vomiting in patients receiving high-dose cisplatin: A multicentre, randomised, double-blind, placebo-controlled phase 3 trial. Ann Oncol. 2013;24:1067–1073. doi: 10.1093/annonc/mds541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grunberg S, Chua D, Maru A, et al. Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with cisplatin therapy: Randomized, double-blind study protocol—EASE. J Clin Oncol. 2011;29:1495–1501. doi: 10.1200/JCO.2010.31.7859. [DOI] [PubMed] [Google Scholar]
- 61.de Wit R, Herrstedt J, Rapoport B, et al. The oral NK(1) antagonist, aprepitant, given with standard antiemetics provides protection against nausea and vomiting over multiple cycles of cisplatin-based chemotherapy: A combined analysis of two randomised, placebo-controlled phase III clinical trials. Eur J Cancer. 2004;40:403–410. [PubMed] [Google Scholar]
- 62.Leal AD, Kadakia KC, Looker S, et al. Fosaprepitant-induced phlebitis: A focus on patients receiving doxorubicin/cyclophosphamide therapy. Support Care Cancer. 2014;22:1313–1317. doi: 10.1007/s00520-013-2089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Motohashi S, Mino Y, Hori K, et al. Interindividual variations in aprepitant plasma pharmacokinetics in cancer patients receiving cisplatin-based chemotherapy for the first time. Biol Pharm Bull. 2013;36:676–681. doi: 10.1248/bpb.b12-01086. [DOI] [PubMed] [Google Scholar]
- 64.Nakade S, Ohno T, Kitagawa J, et al. Population pharmacokinetics of aprepitant and dexamethasone in the prevention of chemotherapy-induced nausea and vomiting. Cancer Chemother Pharmacol. 2008;63:75–83. doi: 10.1007/s00280-008-0713-y. [DOI] [PubMed] [Google Scholar]
- 65.Bergman AJ, Marbury T, Fosbinder T, et al. Effect of impaired renal function and haemodialysis on the pharmacokinetics of aprepitant. Clin Pharmacokinet. 2005;44:637–647. doi: 10.2165/00003088-200544060-00005. [DOI] [PubMed] [Google Scholar]
- 66.Chapell R, Aapro MS. Efficacy of aprepitant among patients aged 65 and over receiving moderately to highly emetogenic chemotherapy: A meta-analysis of unpublished data from previously published studies. J Geriatr Oncol. 2013;4:78–83. doi: 10.1016/j.jgo.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 67.Kang H, Loftus S, Taylor A, et al. Aprepitant for preventing chemotherapy induced nausea and vomiting. Support Care Cancer. 2014;22(suppl):S103. [Google Scholar]
- 68.Bubalo JS, Cherala G, McCune JS, et al. Aprepitant pharmacokinetics and assessing the impact of aprepitant on cyclophosphamide metabolism in cancer patients undergoing hematopoietic stem cell transplantation. J Clin Pharmacol. 2012;52:586–594. doi: 10.1177/0091270011398243. [DOI] [PubMed] [Google Scholar]
- 69.Lindley C, Hamilton G, McCune JS, et al. The effect of cyclophosphamide with and without dexamethasone on cytochrome P450 3A4 and 2B6 in human hepatocytes. Drug Metab Dispos. 2002;30:814–822. doi: 10.1124/dmd.30.7.814. [DOI] [PubMed] [Google Scholar]
- 70.Egerer G, Eisenlohr K, Gronkowski M, et al. The NK₁ receptor antagonist aprepitant does not alter the pharmacokinetics of high-dose melphalan chemotherapy in patients with multiple myeloma. Br J Clin Pharmacol. 2010;70:903–907. doi: 10.1111/j.1365-2125.2010.03792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stiff PJ, Fox-Geiman MP, Kiley K, et al. Prevention of nausea and vomiting associated with stem cell transplant: Results of a prospective, randomized trial of aprepitant used with highly emetogenic preparative regimens. Biol Blood Marrow Transplant. 2013;19:49–55, e1. doi: 10.1016/j.bbmt.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 72.Pielichowski W, Barzal J, Gawronski K, et al. A triple-drug combination to prevent nausea and vomiting following BEAM chemotherapy before autologous hematopoietic stem cell transplantation. Transplant Proc. 2011;43:3107–3110. doi: 10.1016/j.transproceed.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 73.Abidi MH, Tageja N, Ayash L, et al. Aprepitant for prevention of nausea and vomiting secondary to high-dose cyclophosphamide administered to patients undergoing autologous peripheral blood stem cells mobilization: A phase II trial. Support Care Cancer. 2012;20:2363–2369. doi: 10.1007/s00520-011-1341-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Drug interactions of aprepitant [slide presentation]. Available at http://www.fda.gov/ohrms/dockets/ac/03/slides/3928s1_03_fda-jarugula.ppt. Accessed June 13, 2014.
- 75.Scientific discussion: This module reflects the initial scientific discussion for the approval of Emend. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000527/WC500026534.pdf. Accessed June 13, 2014.
- 76.Aapro MS, Walko CM. Aprepitant: Drug-drug interactions in perspective. Ann Oncol. 2010;21:2316–2323. doi: 10.1093/annonc/mdq149. [DOI] [PubMed] [Google Scholar]
- 77.Warr DG, Grunberg SM, Gralla RJ, et al. The oral NK(1) antagonist aprepitant for the prevention of acute and delayed chemotherapy-induced nausea and vomiting: Pooled data from 2 randomised, double-blind, placebo controlled trials. Eur J Cancer. 2005;41:1278–1285. doi: 10.1016/j.ejca.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 78.Maie K, Okoshi Y, Takaiwa N, et al. Aprepitant does not alter prednisolone pharmacokinetics in patients treated with R-CHOP. Ann Oncol. 2014;25:298–299. doi: 10.1093/annonc/mdt477. [DOI] [PubMed] [Google Scholar]
- 79.Hu Z, Cheng Y, Zhang H, et al. Aprepitant triple therapy for the prevention of chemotherapy-induced nausea and vomiting following high-dose cisplatin in Chinese patients: A randomized, double-blind, placebo-controlled phase III trial. Support Care Cancer. 2014;22:979–987. doi: 10.1007/s00520-013-2043-9. [DOI] [PubMed] [Google Scholar]
- 80.China antiemetic guideline (2014 version) Chin Clin Oncol. 2014;19:261–273. [Google Scholar]
- 81.Poddubnaya I, Abramov M, Besova N, et al. Practical guidelines on prophylaxis and treatment of nausea and vomiting in patients receiving chemo- and radiation therapy. Practical guidelines for treatment of malignant tumors. Practical guidelines for supportive care in oncology. Moscow, Russia: Russian Society of Clinical Oncology; 2013. pp. 302–318. [Google Scholar]
- 82.Durand JF, Jovenin N. Prise en charge des nausees-vomissements chimio-induits NVCI. Available at http://www.afsos.org/IMG/pdf/130422_NVCI_MAJ_2012.pdf. Accessed June 13, 2014.
- 83.Ochoa-Carillo FJ, Caponero R, Cervantes-Sanchez MG, et al. [Latin-American guidelines for the management of emesis in oncology, haemato-oncology, and radiotherapy.] Rev Bras Ciudad Paliat. 2011;3:1–15. [Google Scholar]
- 84.Aapro M, Molassiotis A, Dicato M, et al. The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): The Pan European Emesis Registry (PEER) Ann Oncol. 2012;23:1986–1992. doi: 10.1093/annonc/mds021. [DOI] [PubMed] [Google Scholar]
- 85.Gilmore JW, Peacock NW, Gu A, et al. Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE Study. J Oncol Pract. 2014;10:68–74. doi: 10.1200/JOP.2012.000816. [DOI] [PubMed] [Google Scholar]
- 86.Kaiser R. Antiemetic guidelines: Are they being used? Lancet Oncol. 2005;6:622–625. doi: 10.1016/S1470-2045(05)70284-9. [DOI] [PubMed] [Google Scholar]
- 87.Torres T, Fernandes I, Selores M, et al. Aprepitant: Evidence of its effectiveness in patients with refractory pruritus continues. J Am Acad Dermatol. 2012;66:e14–e15. doi: 10.1016/j.jaad.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 88.Benecke H, Lotts T, Ständer S. Investigational drugs for pruritus. Expert Opin Investig Drugs. 2013;22:1167–1179. doi: 10.1517/13543784.2013.813932. [DOI] [PubMed] [Google Scholar]


