This network meta-analysis showed that denosumab, zoledronate, and pamidronate were generally effective in preventing skeletal-related events in cancer patients with bone metastasis. Denosumab and zoledronate were also associated with reductions in the risk of pathologic fractures and radiation compared with placebo. Denosumab was shown to be the most effective of the bone-targeted agents studied.
Keywords: Bisphosphonates, Denosumab, Skeletal-related events, Bone metastasis, Network meta-analysis
Abstract
Background.
Complications from skeletal-related events (SREs) constitute a challenge in the care of cancer patients with bone metastasis (BM).
Objectives.
This study evaluated the comparative effectiveness of pamidronate, ibandronate, zoledronate, and denosumab in reducing the morbidity of SREs in cancer patients with BM.
Methods.
Medline (1948 to January 2014), Embase (1980 to January 2014), the Cochrane Library (2014 issue 1), and Web of Science with Conference Proceedings (1970 to January 2014) were searched. Only randomized controlled trials assessing denosumab, bisphosphonates, or placebo in cancer patients with BM were included. The primary outcomes were SREs and SREs by type. The network meta-analysis (NMA) was performed with a random-effects Bayesian model.
Results.
The NMA included 14 trials with 10,192 patients. Denosumab was superior to placebo in reducing the risk of SREs (odds ratio [OR]: 0.49; 95% confidence interval [CI]: 0.31–0.75), followed by zoledronate (OR: 0.57; 95% CI: 0.41–0.77) and pamidronate (OR: 0.55; 95% CI: 0.41–0.72). Ibandronate compared with placebo could not reduce the risk of SREs. Denosumab was superior to placebo in reducing the risk of pathologic fractures (OR: 0.50; 95% CI: 0.32–0.79), followed by zoledronate (OR: 0.61; 95% CI: 0.43–0.86). Denosumab was superior to placebo in reducing the risk of radiation (OR: 0.51; 95% CI: 0.35–0.75), followed by pamidronate (OR: 0.67; 95% CI: 0.52–0.86) and zoledronate (OR: 0.70; 95% CI: 0.52–0.96).
Conclusion.
This NMA showed that denosumab, zoledronate, and pamidronate were generally effective in preventing SREs in cancer patients with BM. Denosumab and zoledronate were also associated with reductions in the risk of pathologic fractures and radiation compared with placebo. Denosumab was shown to be the most effective of the bone-targeted agents.
Implications for Practice:
Bone metastasis (BM) can lead to skeletal-related events (SREs), which can dramatically reduce patients’ quality of life and even shorten survival. We used network meta-analysis to evaluate the comparative effectiveness of bone-targeted agents (BTAs) in reducing the morbidity of SREs in cancer patients with BM. We found that denosumab, zoledronate, and pamidronate were generally effective (compared with placebo), and denosumab was shown to be the most effective BTA. Reduction in the incidence of pathologic fractures and radiation was the main cause of reduced risk of SREs. The findings of this study highlight the importance of the use of BTAs in cancer patients with BM.
Introduction
Bone is the most common site of metastasis in cancer, and cancer metastases to the bone are most prevalent among patients with advanced cancer of the breast (73%), prostate (68%), or lung (36%) [1]. Bone metastasis (BM) can lead to skeletal-related events (SREs), defined as pathologic fracture, spinal cord compression, requirement for radiation or surgery to bone, and hypercalcemia [2]. Data from the untreated arms of clinical trials indicate that SREs are most common in patients with BM secondary to breast cancer (2-year cumulative incidence of 68%), followed by prostate cancer (2-year cumulative incidence of 49%), and non-small cell lung cancer (NSCLC) and other solid tumors (OST; 21-month cumulative incidence of 48%) [3–5]. Observational studies yielded similar patterns, with a 1-year cumulative incidence of SREs after BM diagnosis of 46% in prostate cancer patients and 38% in female breast cancer patients [6, 7].
BM and subsequent SREs can be an important burden on a cancer patient’s quality of life (QOL) and overall health status [8]. SREs can dramatically reduce patients’ QOL and even shorten survival [9]. Treatment of BM includes orthopedic management, radiation, surgery, and systemic treatments (e.g., bone-targeting agents [BTAs], endocrine therapy, chemotherapy).
Currently licensed bisphosphonates include zoledronate (any advanced malignancy involving bone), pamidronate (breast cancer or multiple myeloma), clodronate (breast cancer or multiple myeloma), and ibandronate (breast cancer). Bisphosphonates are administered either intravenously (zoledronate, pamidronate, or ibandronate) or orally (clodronate or ibandronate) and have been associated with renal toxicity [10]. Denosumab (Xgeva; Amgen, Thousand Oaks, CA, http://www.amgen.com) is a RANK ligand inhibitor, licensed for the prevention of SREs in BM from solid tumors. It is administered by subcutaneous injection and does not require renal monitoring [11].
When comparing the effectiveness of two or more interventions, randomized clinical trials (RCTs) that compare the interventions directly (head-to-head trials) are often preferred for health technology assessment and reimbursement (HTA) decision making. Three RCTs have evaluated denosumab compared with zoledronate for the prevention of SREs [12–14]. No head-to-head trials have compared denosumab with other bisphosphonates or best supportive care. Such comparisons are important because of the wide variation in practice and HTA decision making. Some centers use only zoledronate, and some use a variety of bisphosphonates, whereas others do not use bisphosphonates at all (especially in cancer other than breast).
Modern statistical techniques, such as network meta-analysis (NMA), can simultaneously analyze direct comparisons of interventions within RCTs and indirect comparisons across trials based on a common comparator (e.g., placebo or some standard treatment) to overcome some of the challenges posed by the paucity of direct evidence [15]. The objective of our research was to evaluate the comparative effectiveness of BTAs including pamidronate, ibandronate, zoledronate, or denosumab in reducing the morbidity of SREs in cancer patients with BM.
Materials and Methods
Literature Search and Selection of Studies
Studies were identified by systematic searches of the following databases: Medline (1948 to January 2014), Embase (1980 to January 2014), the Cochrane Library (all sections of 2014 issue 1), and Web of Science with Conference Proceedings (1970 to January 2014). Additional meeting abstracts (2010 to 2014) were identified by searching the American Society of Clinical Oncology, the American Urological Association, and the San Antonio Breast Cancer Symposium. Reference lists of all included studies were scanned to identify additional potentially relevant studies. The titles and abstracts of all papers identified by the search strategy were screened, and full-text copies of all potentially relevant studies were obtained.
The following search strategy was used for Medline: step 1, exp Diphosphonates; step 2, RANK ligand; step 3, (denosumab or bisphosphonate* or ibandron* or pamidron* or zoledron*).tw.; step 4, or/1-3; step 5, exp Neoplasms; step 6, (solid tumor or solid tumor* or cancer or carcinoma).tw.; step 7, or/5-6; step 8, 4 and 7; step 9, exp Bone Neoplasms; step 10, (bone metast*).tw.; step 11, (skeletal or fracture*).tw.; step 12, or/9-11; step 13, 8 and 12; step 14, randomized controlled trial.pt.; step 15, 13 and 14; step 16, limit 15 to the English language. This search strategy was adapted, as appropriate, for the other databases.
Only RCTs evaluating denosumab, pamidronate, ibandronate, zoledronate, or placebo in reduction of SREs overall and by type were included. Screening was performed by two independent authors, and disagreements were resolved by discussion. After piloting a data-extraction form, data were extracted by one author and checked by a second. Data included study characteristics, inclusion and exclusion criteria, and results (SREs overall and by type).
Quality Score
The magnitude and heterogeneity of risk estimates may depend on the methodological quality associated with the underlying study and with the risk-estimate derivation. Similar to previous systematic review [16] of the association between BTAs and SREs, the authors used a quality score proposed by Higgins et al. [17] to assess the methodological quality of the studies and the consistency of the available evidence.
Main Statistical Analysis
The primary analyses were conducted with a Bayesian Markov Chain Monte Carlo method and fitted with the Bayesian software in WinBUGS version 1.4.3 (Medical Research Council Biostatistics Unit, Cambridge, U.K., http://www.mrc-bsu.cam.ac.uk) [18], the R2WinBUGS package [19] and GeMTC package in R software (R Foundation, Vienna, Austria, http://www.r-project.org) [20], and ADDIS 1.16.5 (drugis.org, Groningen, The Netherlands, http://drugis.org/software/addis1/index) [21]. For the analyses in WinBUGS, every sample consisted of 100,000 iterations with an initial burn-in period of 10,000 iterations [22]. The probability of the outcome was modeled with a binomial distribution, and each pair of treatments was compared by estimating the odds ratio (OR) of the outcome. We assumed that each of the log ORs had been sampled from a normal distribution and that treatment effects were wholly exchangeable within studies. Trials with zero cells in both arms or nodes in which there were no events were excluded from the evidence networks because they did not contribute information or allow interpretable information.
We gave vague prior information for all trial baselines, treatment effects, and between-trial variances. The autocorrelation plots showed that throughout the iterative process, the autocorrelation was satisfactorily reduced to a nominal amount, and the Brooks-Gelman-Rubin plots showed that the model had converged satisfactorily [22]. We assessed the fit of our model using the deviance information criterion, a measure of model fit that penalizes model complexity. This criterion advocates selecting the model with the lowest deviance information criterion value among a series of competing models for the same data because this model is believed to provide the best fit to the data [23].
All results for the NMA were reported as odds ratio with corresponding 95% credibility intervals (CrIs). Credibility intervals were the Bayesian equivalent of classic confidence intervals (CIs). To ensure consistency, we conducted direct pairwise comparison meta-analysis whenever possible and adjusted indirect comparisons according to the methods described by Bucher et al. [24]. For pairwise meta-analyses, we tested heterogeneity between trials with the I2 statistic, with I2 >50% indicating significant heterogeneity. A random-effects model (DerSimonian-Laird) was used if significant heterogeneity was detected. To address clinical heterogeneity and the underlying assumption of exchangeability, we performed a meta-regression analysis using Stata 10.1 (StataCorp LP, College Station, TX, http://www.stata.com) to see whether a difference in patient population affected the relative treatment effects [25].
Results
Literature Search
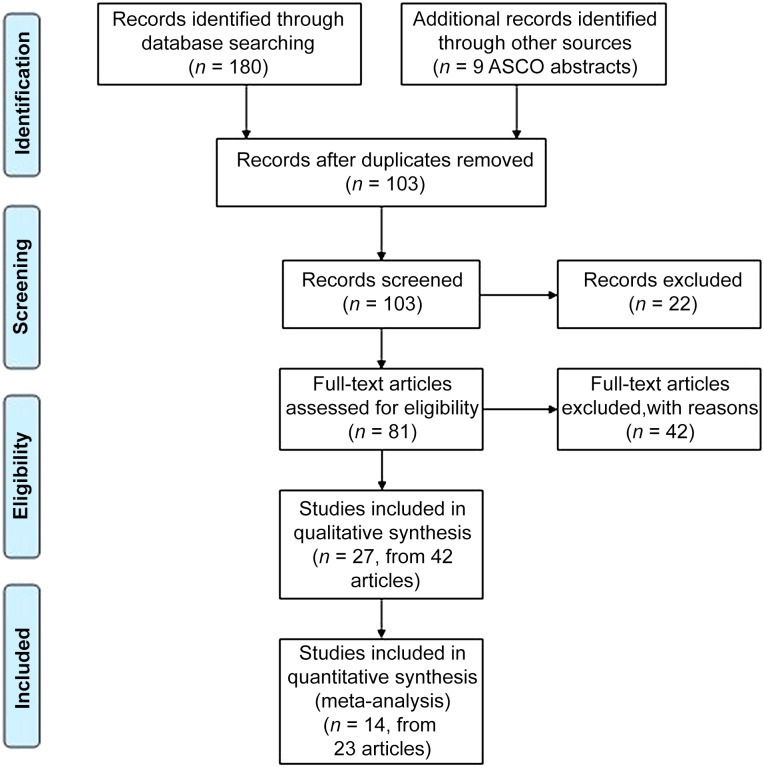
The studies were independently assessed for suitability for inclusion in the NMA. Results of the literature search are shown in Figure 1, and 14 studies met the inclusion criteria. The characteristics and results of the 14 studies included in the NMA are shown in Table 1.
Figure 1.
PRISMA flow diagram.
Abbreviation: ASCO, American Society of Clinical Oncology.
Table 1.
Characteristics of studies included in the Network Meta-Analysis
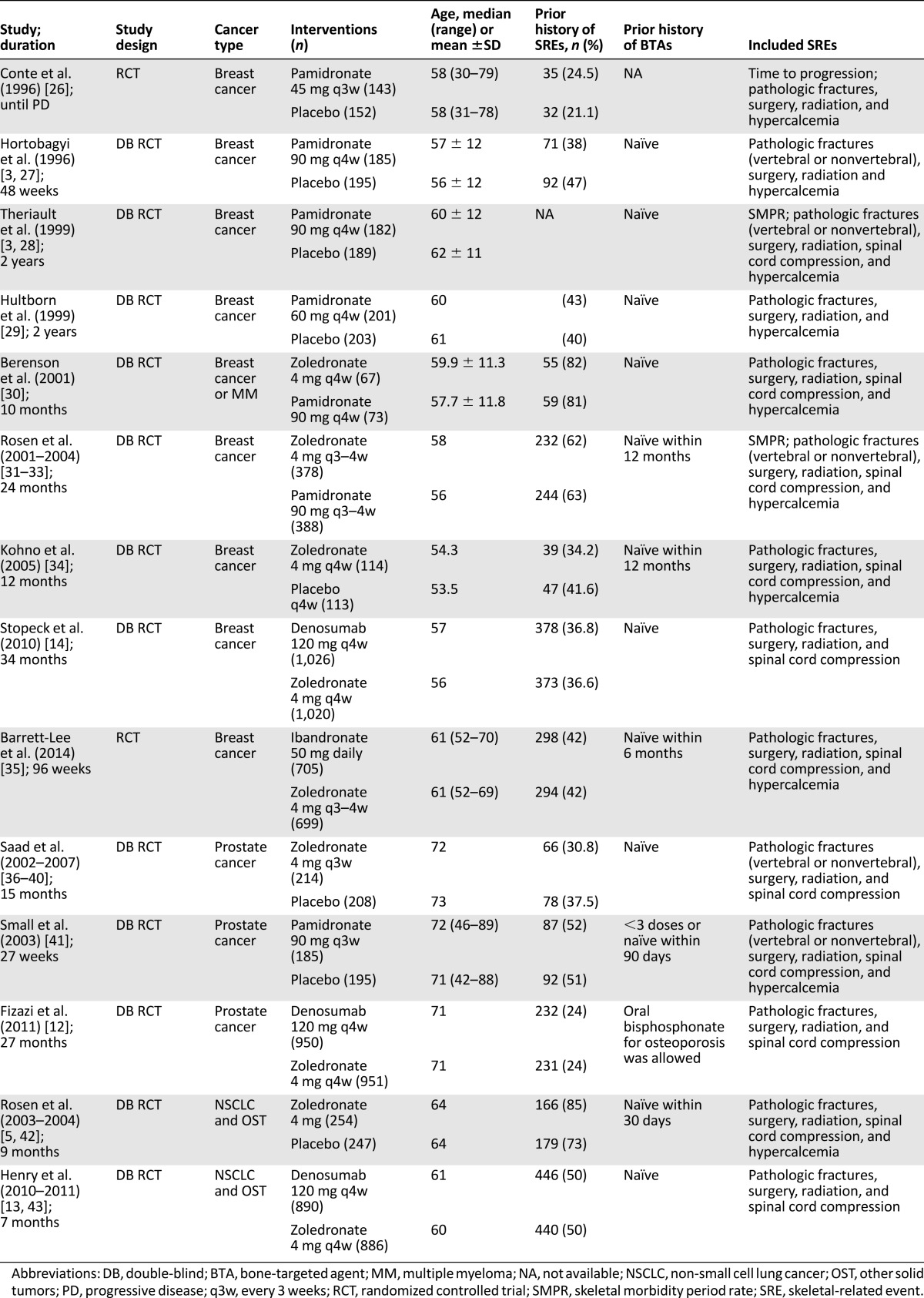
Study Characteristics
Nine studies included patients with breast cancer, three included patients with prostate cancer, and two included patients with NSCLC and OST. Three studies compared denosumab with zoledronate, three compared zoledronate with placebo, two compared zoledronate with pamidronate, one compared zoledronate with ibandronate, and four compared pamidronate with placebo.
Twelve studies were international, one study recruited only patients from Japan [34], and one study recruited patients from the U.K. [35]. Patients were youngest in the breast cancer studies and oldest in the prostate cancer studies. The proportion of patients with a previous SRE at baseline ranged from 24% [12] to 73% [5, 42].
Study Quality
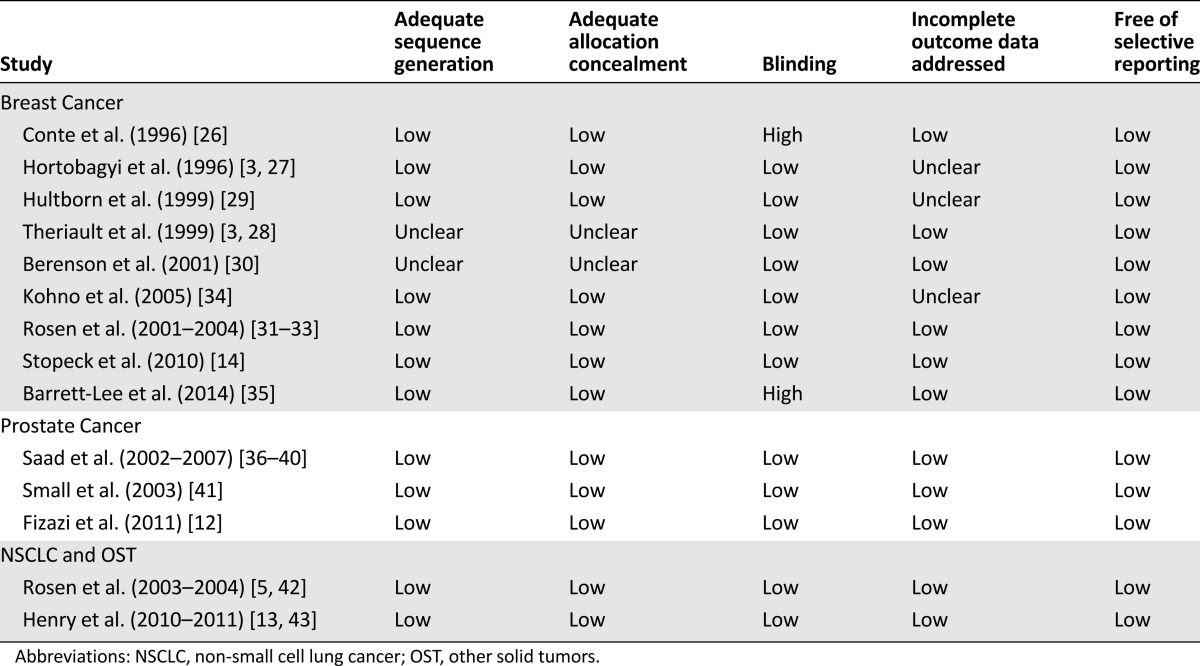
The quality of the studies included in the NMA was high (Table 2). There was a low risk of bias for the majority of categories. Most evidence was of moderate to good quality. Hortobagyi et al. [3, 27] and Berenson et al. [30] failed to describe sequence generation or allocation concealment. Hortobagyi et al. [3, 27], Hultborn et al. [29], and Kohno et al. [34] did not sufficiently address incomplete outcome data. In the study by Conte et al. [26], placebo infusions were not administered to control because of ethical objections in several countries, and the study by Barrett-Lee et al. [35] had an open-label design. Our searches were appropriate, but the possibility of publication bias cannot be excluded; however, it is unclear whether reporting biases would tend to favor any particular treatment.
Table 2.
Risk of bias of studies included in the network meta-analysis
Reduction in SREs in Cancer Patients With BM
Reduction in SREs Overall
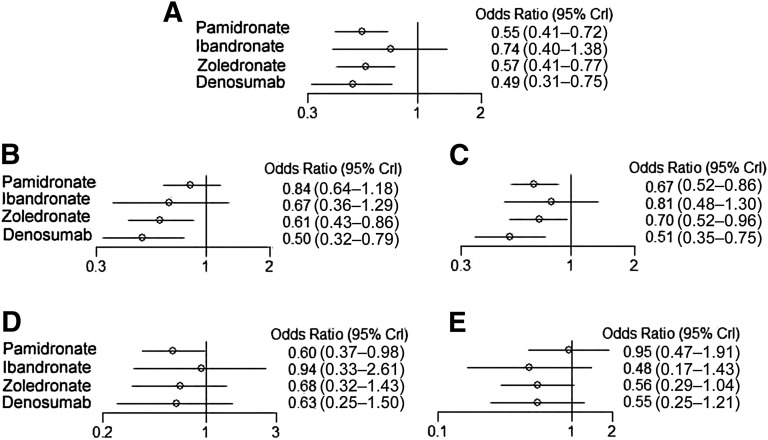
The NMA model converged, and there were no significant inconsistencies between the direct and indirect evidence within the NMA. Three BTAs were associated with significant reductions in SREs overall compared with the placebo in cancer patients with BM. Denosumab was superior to placebo in significantly reducing the risk of SREs (odds ratio [OR]: 0.49; 95% CI: 0.31–0.75), followed by zoledronate (OR: 0.57; 95% CI: 0.41–0.77) and pamidronate (OR: 0.55; 95% CI: 0.41–0.72). Ibandronate compared with placebo could not significantly reduce the risk of SREs overall (Fig. 2). The impact of BTAs in preventing SREs overall and by type (pathologic fractures, radiation, surgery, and spinal cord compression) compared with placebo are depicted in Figure 2.
Figure 2.
Odds ratios for skeletal-related events (A), pathologic fractures (B), bone radiation (C), bone surgery (D), and spinal cord compression (E) in Bayesian network meta-analysis versus placebo in cancer patients with bone metastases.
Abbreviation: CrI, credibility interval.
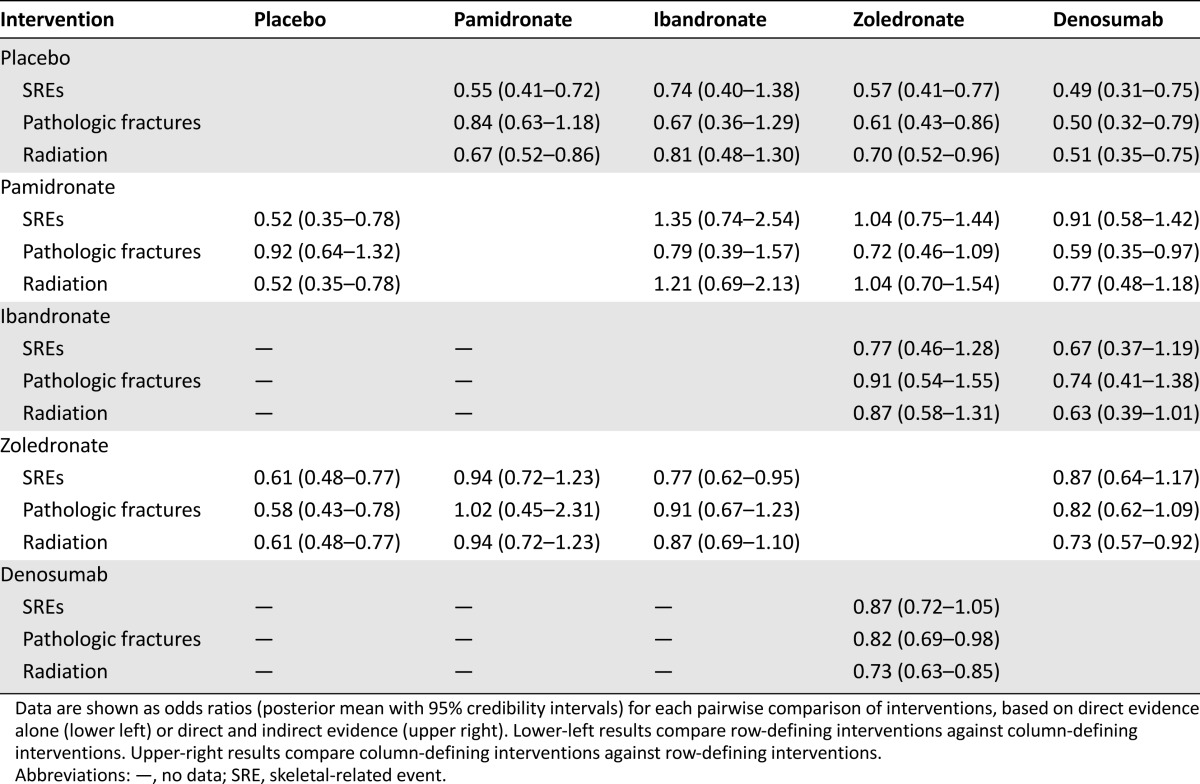
More extensive results for the relative efficacies of all BTAs are presented in Table 3. For each pairwise comparison of BTAs, the 95% CrIs for ORs were wide and included. The lower-left results in Table 3 presented ORs estimated from direct evidence alone, whereas the upper-right results presented ORs estimated from the NMA. We could not find that one BTA was superior to any other in reducing the risk of SREs.
Table 3.
Network meta-analysis results
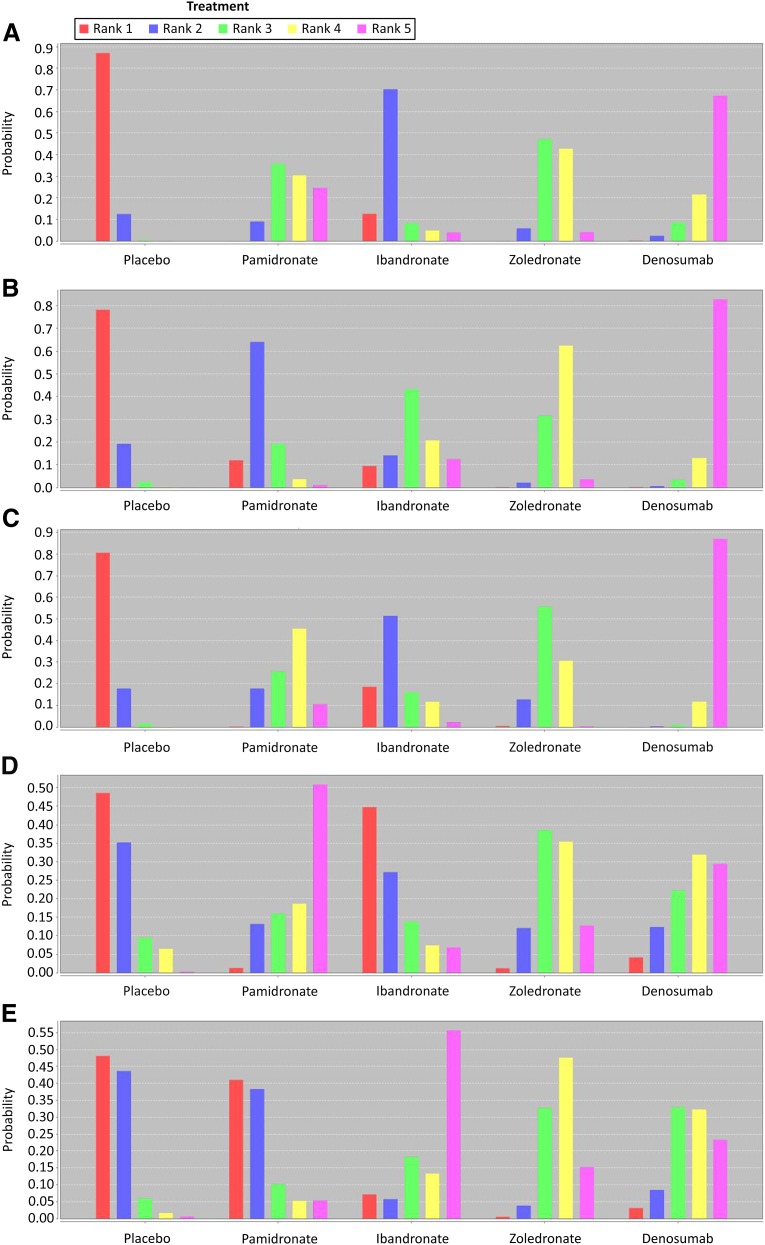
According to rank probability (Fig. 3), denosumab was the best alternative compared with the other BTAs because it had a much higher score on rank (score of 5), which indicated it had much lower incidence of SREs. In contrast, placebo was the worst, with rank of 1 being the highest and rank of 5 being the lowest for probability. The rank probabilities were summed to 1, both within a rank for all treatments and within a treatment for all ranks.
Figure 3.
The network meta-analysis rank probabilities for skeletal-related events (SREs) (A), pathologic fractures (B), bone radiation (C), bone surgery (D), and spinal cord compression (E) in cancer patients with bone metastases. The bar chart visualizes the (posterior) probability for each treatment to be best, second-best, and so forth, given the analysis model and the data. Rank of 1 is the worst, indicating the highest incidence of the condition, and rank of 5 is the best, indicating the lowest incidence of the condition.
Reduction in Pathologic Fractures
Denosumab and zoledronate were associated with significant reductions in risk of pathologic fractures compared with placebo. Denosumab was superior to placebo in significantly reducing the risk of pathologic fractures (OR: 0.50; 95% CI: 0.32–0.79), followed by zoledronate (OR: 0.61; 95% CI: 0.43–0.86). No significant reduction in the risk of pathologic fracture events was observed between pamidronate or ibandronate and placebo (Fig. 2; Table 3). Rank probability also showed that denosumab was the best alternative compared with the other BTAs, and zoledronate was the second best (Fig. 3).
Reduction in Radiation to Bone
Denosumab, zoledronate, and pamidronate were associated with significant reductions in risk of the need for radiation compared with placebo. Denosumab was superior to placebo in significantly reducing the risk of the need for radiation (OR: 0.51; 95% CI: 0.35–0.75), followed by pamidronate (OR: 0.67; 95% CI: 0.52–0.86) and zoledronate (OR: 0.70; 95% CI: 0.52–0.96). No significant reduction in the risk of the need for radiation was observed between ibandronate and placebo (Fig. 2; Table 3). Rank probability also showed denosumab was the best therapy compared with the other BTAs, and pamidronate was the second most effective therapy (Fig. 3).
Reduction in Bone Surgery
Only pamidronate was superior to placebo in significantly reducing the risk of bone surgery (OR: 0.60; 95% CI: 0.37–0.98), and thus the probability demonstrated that pamidronate was the best alternative. In contrast, denosumab, zoledronate, and ibandronate were not associated with significant reductions in the risk of surgery compared with placebo (Fig. 2, 3).
Reduction in Spinal Cord Compression
None of the four BTAs were associated with significant reductions in the risk of spinal cord compression versus placebo (Fig. 2).
Direct SRE Results in Breast Cancer Patients With BM
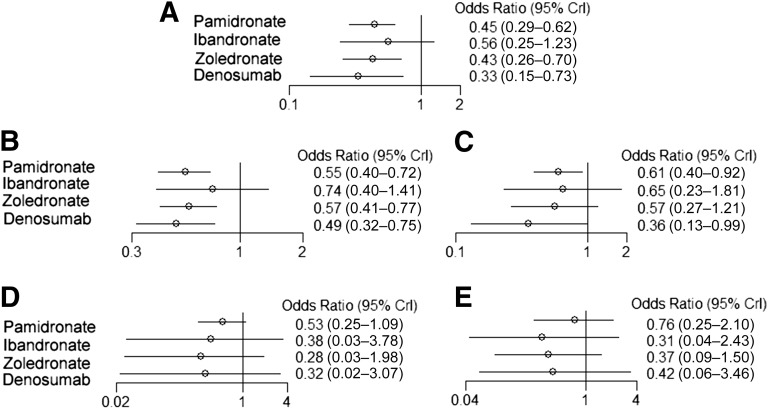
Similarly, denosumab was superior to placebo in significantly reducing the risk of SREs overall (OR: 0.33; 95% CI: 0.15–0.73), followed by zoledronate (OR: 0.43; 95% CI: 0.26–0.70) and pamidronate (OR: 0.45; 95% CI: 0.29–0.62). Ibandronate compared with placebo could not significantly reduce the risk of SREs (Fig. 4).
Figure 4.
Odds ratios for skeletal-related events (A), pathologic fractures (B), bone radiation (C), bone surgery (D), and spinal cord compression (E) in Bayesian network meta-analysis versus placebo in breast cancer patients with bone metastases.
Abbreviation: CrI, credibility interval.
Denosumab and pamidronate were associated with significant reduction of both pathologic fractures and the need for radiation compared with placebo in breast cancer patients with BM. The effect of zoledronate was limited to significantly reducing the risk of pathologic fractures in breast cancer patients with BM. No significant reduction in the risk of surgery or spinal cord compression was observed for BTAs compared with placebo.
Discussion
In this study, we performed an NMA to compare the efficacy of available BTAs for the prevention of SREs in cancer patients with BM. Denosumab, zoledronate, and pamidronate showed statistically significant efficacy compared with placebo. Denosumab demonstrated the highest probability of being the most efficacious treatment of all therapies analyzed. The evidence of the efficacy of these drugs in reducing the risks of SREs was related mainly to the reduction in the incidence of pathologic fractures and the need for radiation.
Strengths of the Study
Undertaking an NMA allows for estimation of effectiveness of therapies when no direct data comparison is available. This was the case for comparing placebo with pamidronate, ibandronate, zoledronate, and denosumab. NMAs provide a valid statistical alternative to direct head-to-head studies [18, 44]. An advantage of Bayesian NMAs, such as this study, over frequentist approaches is the ability to rank treatments according to the probability of being the best (i.e., most effective), which could be useful for clinical therapy decisions and HTA decision making [45–47].
A comprehensive and robust search strategy was used. Rigorous inclusion and exclusion criteria were used and included only high-quality evidence (RCTs). Not all studies identified in the Cochrane Review were included in our NMA because of our stricter inclusion criteria. We included, for example, only studies that evaluated the efficacy in reduction of SREs overall and by type; this excluded three studies that evaluated efficacy in reduction of skeletal morbidity period rate (SMPR) [48–50] and three denosumab phase II clinical trials [51–53]. We also excluded a study that treated patients after 12–15 months of zoledronate [54] because the focus of that study was patients with naïve BTAs treatment. Excluding studies with a different definition of what constitutes an SRE resulted in a smaller but more robust NMA. The quality of any NMA is only as good as the weakest link in the network. All studies included in this NMA were of moderate to good quality (Table 2), improving the validity of the NMA results. The included studies were similar with regard to average age of patients, sex, number of lesions at baseline, and other patient characteristics. Importantly, patient characteristics were not significantly correlated with treatment success. This reduced the potential for heterogeneity across trials and limited resulting bias in the NMA.
Stopeck et al. [14] and Henry et al. [13, 43] did not mention the number of SREs by type in their studies, whereas Fizazi et al. [12] listed the number of SREs by type. Consequently, SREs by type were analyzed only in prostate cancer in the meta-analysis by Ford et al. [55]. Lipton et al. [56] reported a combined analysis of these three phase III trials, and SREs by type were analyzed. We consulted Amgen and got the data about SREs by type in patients treated in the denosumab (Xgeva) group.
Limitations of the Study
Although RCTs provide the best available evidence for the relative treatment effect of a particular pairwise comparison, the identified RCTs were only placebo-controlled trials. To obtain insight into the relative efficacy of one BTA over another, we had to rely on indirect comparisons. NMA is a method by which multiple meta-analyses of different placebo-controlled (or pairwise) comparisons across a variety of different interventions are performed simultaneously. Consequently, indirect relative efficacy estimates were obtained. Although NMA allows indirect estimates to be calculated, they can be subject to potential biases and uncertainties. NMAs are not randomized comparisons but rather observational findings across studies and thus should be interpreted with caution.
A random-effects model was used for the NMA, which takes into account study heterogeneity and any differences in trial procedures and settings between the included studies that may have influenced results. Berenson et al. [30] treated patients with 0.4, 2.0, or 4.0 mg of zoledronate or 90 mg pamidronate, Rosen et al. [31–33] treated breast cancer patients with either 4 or 8 mg (reduced to 4 mg) of zoledronate or 90 mg pamidronate, Saad et al. [36–40] and Rosen et al. [5, 42] treated patients with either 4 or 8 mg (reduced to 4 mg) of zoledronate or placebo. In order to avoid study heterogeneity, we selected only patients treated with 4 mg of zoledronate, reducing the number of patients enrolled in the meta-analysis. Although >10,000 patients were included in the NMA, as is characteristic of many NMAs, the study was limited by the relatively small number of trials. Some published studies did not report full results, and thus some treatment effects were lost; for example, we obtained the results of only SREs and pathologic fractures from the studies by Rosen et al. [31–33].
Only one ibandronate trial was included because the outcome data reported in the literature were not compatible with those in the present meta-analysis. Despite many studies evaluating the efficacy of ibandronate in preventing SREs in patients with multiple myeloma [57] and prostate [58], colorectal [59], and breast cancer [48–50], those studies rarely reported outcome measures similar to the ones evaluated in this NMA. Clinical data from such studies were frequently reported as SMPR, defined as the number of 12-week periods with new SREs divided by the number of periods in the study. Tripathy et al. [60] pooled SMPR data from three studies and found a significant reduction in skeletal complications in patients receiving ibandronate compared with those receiving placebo (p = .004). They also reported that the risk of a new bone event was reduced by 40% with intravenous and 38% with oral forms of ibandronate in comparison with placebo.
Meaning of the Results
It is of key importance in both pairwise meta-analysis and NMA to not “break” randomization. It is incorrect to simply compare the absolute SRE risk observed with a BTA in one trial with the absolute risk observed with a comparator in another study (i.e., comparing without adjusting for differences in placebo risk). One reason is that part of the observed absolute effect can be attributed to the efficacy of the drug, whereas another part is the result of a placebo effect. One can compare only relative treatment effects, in this case, the relative effects of each BTA relative to placebo.
When looking at each BTA separately, there was a statistically significant difference in favor of denosumab, zoledronate, and pamidronate compared with placebo for the prevention of SREs in cancer patients with BM, whereas ibandronate did not show a difference compared with placebo. NMA showed that the evidence of the efficacy of these drugs in reducing the risks of SREs relates mainly to the reduction in the incidence of pathologic fractures and the need for radiation. A possible explanation for the lower SRE risk reduction observed with oral ibandronate could be poor gastrointestinal absorption of bisphosphonate. An alternative explanation is that only one ibandronate trial was included in the NMA. According to rank probability, denosumab was the best alternative compared with the other BTAs for preventing SREs and reducing the incidence of pathologic fractures and the need for radiation. Denosumab had the highest rank probability but also had a credibility interval larger than pamidronate; the reason for this may be that there were only three denosumab clinical trials and seven with pamidronate. In addition, in the trials, pamidronate was used mainly for breast cancer patients, with less heterogeneity among patient populations. There is currently a phase III clinical trial of denosumab in an Asian population. When we receive more data from this clinical trial, our results may be different. Based on direct evidence alone, pairwise comparison of BTAs showed that denosumab was superior to zoledronate in reducing the risk of pathologic fractures and radiation, in agreement with the results of Lipton et al. [56].
For the subgroup of breast cancer patients, denosumab and pamidronate also showed efficacy in reducing the risks of SREs and pathologic fractures; however, zoledronate could not reduce the risk of the need for radiation. The fact that we obtained the results of SREs and pathologic fractures from the studies by Rosen et al. [31–33] could be the factor responsible for the reduced effectiveness in terms of lowering the risk of need for radiation. We have contacted Novartis (Basel, Switzerland, http://www.novartis.com/index.shtml) to obtain the relevant data but have not yet received it. We hope to use these data, when received, for future studies.
Conclusion
Denosumab, zoledronate, and pamidronate were generally effective (compared with placebo) in preventing SREs in cancer patients with BM. Denosumab was shown to be the most effective of the bone-targeted agents. Reduction in the incidence of pathologic fractures and the need for radiation was the main cause of reduced risk of SREs. The findings of this study highlight the importance of the use of BTAs for cancer patients with BM.
Acknowledgment
This study was funded by Natural Science Foundation of China Grant No. 81201628.
Author Contributions
Conception/Design: Yang Yao, Hui Zhao
Provision of study material or patients: Zhiyu Wang, Dan Qiao, Yaohong Lu, Xiaoting Wen, Yang Yao, Hui Zhao
Collection and/or assembly of data: Zhiyu Wang, Dan Qiao, Yaohong Lu, Xiaoting Wen, Yang Yao, Hui Zhao
Data analysis and interpretation: Zhiyu Wang, Dan Qiao, Yaohong Lu, Dana Curtis, Xiaoting Wen, Yang Yao, Hui Zhao
Manuscript writing: Zhiyu Wang, Dan Qiao, Dana Curtis, Hui Zhao
Final approval of manuscript: Hui Zhao
Disclosures
The authors indicated no financial relationships.
References
- 1.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 2.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(suppl):1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Lipton A, Theriault RL, Hortobagyi GN, et al. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: Long term follow-up of two randomized, placebo-controlled trials. Cancer. 2000;88:1082–1090. doi: 10.1002/(sici)1097-0142(20000301)88:5<1082::aid-cncr20>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Saad F, McKiernan J, Eastham J. Rationale for zoledronic acid therapy in men with hormone-sensitive prostate cancer with or without bone metastasis. Urol Oncol. 2006;24:4–12. doi: 10.1016/j.urolonc.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 5.Rosen LS, Gordon D, Tchekmedyian NS, et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: A randomized, Phase III, double-blind, placebo-controlled trial. Cancer. 2004;100:2613–2621. doi: 10.1002/cncr.20308. [DOI] [PubMed] [Google Scholar]
- 6.Nørgaard M, Jensen AO, Jacobsen JB, et al. Skeletal related events, bone metastasis and survival of prostate cancer: A population based cohort study in Denmark (1999 to 2007) J Urol. 2010;184:162–167. doi: 10.1016/j.juro.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 7.Yong M, Jensen AO, Jacobsen JB, et al. Survival in breast cancer patients with bone metastases and skeletal-related events: A population-based cohort study in Denmark (1999-2007) Breast Cancer Res Treat. 2011;129:495–503. doi: 10.1007/s10549-011-1475-5. [DOI] [PubMed] [Google Scholar]
- 8.Weinfurt KPCL, Castel LD, Li Y, et al. Health-related quality of life among patients with breast cancer receiving zoledronic acid or pamidronate disodium for metastatic bone lesions. Med Care. 2004;42:164–175. doi: 10.1097/01.mlr.0000108746.69256.45. [DOI] [PubMed] [Google Scholar]
- 9.Costa L, Badia X, Chow E, et al. Impact of skeletal complications on patients’ quality of life, mobility, and functional independence. Support Care Cancer. 2008;16:879–889. doi: 10.1007/s00520-008-0418-0. [DOI] [PubMed] [Google Scholar]
- 10.Ross JR, Saunders Y, Edmonds PM, et al. A systematic review of the role of bisphosphonates in metastatic disease. Health Technol Assess. 2004;8:1–176. doi: 10.3310/hta8040. [DOI] [PubMed] [Google Scholar]
- 11.Xgeva [product information]. Annex 1: Summary of product characteristics. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002173/WC500110381.pdf. Accessed August 15, 2011.
- 12.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henry DH, Costa L, Goldwasser F, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 14.Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J Clin Oncol. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 15.Li T, Puhan MA, Vedula SS, et al. Network meta-analysis-highly attractive but more methodological research is needed. BMC Med. 2011;9:79. doi: 10.1186/1741-7015-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford JA, Jones R, Elders A, et al. Denosumab for treatment of bone metastases secondary to solid tumours: Systematic review and network meta-analysis. Eur J Cancer. 2013;49:416–430. doi: 10.1016/j.ejca.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Altman DG, Sterne JAC, eds. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Oxford, U.K.: Cochrane Collaboration, 2011. Available at http://www.cochrane-handbook.org. Accessed August 2011.
- 18.Jansen JP, Crawford B, Bergman G, et al. Bayesian meta-analysis of multiple treatment comparisons: An introduction to mixed treatment comparisons. Value Health. 2008;11:956–964. doi: 10.1111/j.1524-4733.2008.00347.x. [DOI] [PubMed] [Google Scholar]
- 19.Sturtz S, Ligges U, Gelman A. R2WinBUGS: A package for running WinBUGS from R. J Stat Softw. 2005;12:1–16. [Google Scholar]
- 20.R: A language and environment for statistical computing. Reference index version 2.12. Vienna, Austria: R Foundation.
- 21.van Valkenhoef G, Tervonen T, Zwinkels T, et al. ADDIS: A decision support system for evidence-based medicine. Decis Support Syst. 2013;55:459–475. [Google Scholar]
- 22.Toft N, Innocent GT, Gettinby G, et al. Assessing the convergence of Markov Chain Monte Carlo methods: An example from evaluation of diagnostic tests in absence of a gold standard. Prev Vet Med. 2007;79:244–256. doi: 10.1016/j.prevetmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Spiegelhalter DJ, Best NG, Carlin BP, et al. Bayesian measures of model complexity and fit. J R Statist Soc B. 2002;64:583–639. [Google Scholar]
- 24.Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–691. doi: 10.1016/s0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 25.Baker WL, White CM, Cappelleri JC, et al. Understanding heterogeneity in meta-analysis: The role of meta-regression. Int J Clin Pract. 2009;63:1426–1434. doi: 10.1111/j.1742-1241.2009.02168.x. [DOI] [PubMed] [Google Scholar]
- 26.Conte PF, Latreille J, Mauriac L, et al. Delay in progression of bone metastases in breast cancer patients treated with intravenous pamidronate: Results from a multinational randomized controlled trial. J Clin Oncol. 1996;14:2552–2559. doi: 10.1200/JCO.1996.14.9.2552. [DOI] [PubMed] [Google Scholar]
- 27.Hortobagyi GN, Theriault RL, Porter L, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med. 1996;335:1785–1791. doi: 10.1056/NEJM199612123352401. [DOI] [PubMed] [Google Scholar]
- 28.Theriault RL, Lipton A, Hortobagyi GN, et al. Pamidronate reduces skeletal morbidity in women with advanced breast cancer and lytic bone lesions: A randomized, placebo-controlled trial. Protocol 18 Aredia Breast Cancer Study Group. J Clin Oncol. 1999;17:846–854. doi: 10.1200/JCO.1999.17.3.846. [DOI] [PubMed] [Google Scholar]
- 29.Hultborn R, Gundersen S, Ryden S, et al. Efficacy of pamidronate in breast cancer with bone metastases: A randomized, double-blind placebo-controlled multicenter study. Anticancer Res. 1999;19:3383–3392. [PubMed] [Google Scholar]
- 30.Berenson JR, Rosen LS, Howell A, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer. 2001;91:1191–1200. doi: 10.1002/1097-0142(20010401)91:7<1191::aid-cncr1119>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 31.Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: A randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98:1735–1744. doi: 10.1002/cncr.11701. [DOI] [PubMed] [Google Scholar]
- 32.Rosen LS, Gordon D, Kaminski M, et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: A phase III, double-blind, comparative trial. Cancer J. 2001;7:377–387. [PubMed] [Google Scholar]
- 33.Rosen LS, Gordon DH, Dugan W, Jr, et al. Zoledronic acid is superior to pamidronate for the treatment of bone metastases in breast carcinoma patients with at least one osteolytic lesion. Cancer. 2004;100:36–43. doi: 10.1002/cncr.11892. [DOI] [PubMed] [Google Scholar]
- 34.Kohno N, Aogi K, Minami H, et al. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: A randomized, placebo-controlled trial. J Clin Oncol. 2005;23:3314–3321. doi: 10.1200/JCO.2005.05.116. [DOI] [PubMed] [Google Scholar]
- 35.Barrett-Lee P, Casbard A, Abraham J, et al. Oral ibandronic acid versus intravenous zoledronic acid in treatment of bone metastases from breast cancer: A randomised, open label, non-inferiority phase 3 trial. Lancet Oncol. 2014;15:114–122. doi: 10.1016/S1470-2045(13)70539-4. [DOI] [PubMed] [Google Scholar]
- 36.Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 37.Saad F, Gleason DM, Murray R, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 38.Saad F. Clinical benefit of zoledronic acid for the prevention of skeletal complications in advanced prostate cancer. Clin Prostate Cancer. 2005;4:31–37. doi: 10.3816/cgc.2005.n.009. [DOI] [PubMed] [Google Scholar]
- 39.Saad F, Lipton A, Cook R, et al. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;110:1860–1867. doi: 10.1002/cncr.22991. [DOI] [PubMed] [Google Scholar]
- 40.Saad F, Chen YM, Gleason DM, et al. Continuing benefit of zoledronic acid in preventing skeletal complications in patients with bone metastases. Clin Genitourin Cancer. 2007;5:390–396. doi: 10.3816/CGC.2007.n.022. [DOI] [PubMed] [Google Scholar]
- 41.Small EJ, Smith MR, Seaman JJ, et al. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol. 2003;21:4277–4284. doi: 10.1200/JCO.2003.05.147. [DOI] [PubMed] [Google Scholar]
- 42.Rosen LS, Gordon D, Tchekmedyian S, et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: A phase III, double-blind, randomized trial—the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol. 2003;21:3150–3157. doi: 10.1200/JCO.2003.04.105. [DOI] [PubMed] [Google Scholar]
- 43.Henry DH, von Moos R, Hungria V, et al. Delaying skeletal-related events in a randomized phase III study of denosumab versus zoledronic acid in patients with advanced cancer. J Clin Oncol. 2010;28(suppl):9133a. doi: 10.1007/s00520-013-2022-1. [DOI] [PubMed] [Google Scholar]
- 44.Ades AE, Sculpher M, Sutton A, et al. Bayesian methods for evidence synthesis in cost-effectiveness analysis. Pharmacoeconomics. 2006;24:1–19. doi: 10.2165/00019053-200624010-00001. [DOI] [PubMed] [Google Scholar]
- 45.Caldwell DM, Ades AE, Higgins JPT. Simultaneous comparison of multiple treatments: Combining direct and indirect evidence. BMJ. 2005;331:897–900. doi: 10.1136/bmj.331.7521.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 47.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J Clin Epidemiol. 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 48.Body JJ, Diel IJ, Lichinitzer M, et al. Oral ibandronate reduces the risk of skeletal complications in breast cancer patients with metastatic bone disease: Results from two randomised, placebo-controlled phase III studies. Br J Cancer. 2004;90:1133–1137. doi: 10.1038/sj.bjc.6601663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tripathy D, Lichinitzer M, Lazarev A, et al. Oral ibandronate for the treatment of metastatic bone disease in breast cancer: Efficacy and safety results from a randomized, double-blind, placebo-controlled trial. Ann Oncol. 2004;15:743–750. doi: 10.1093/annonc/mdh173. [DOI] [PubMed] [Google Scholar]
- 50.Body JJ, Diel IJ, Lichinitser MR, et al. Intravenous ibandronate reduces the incidence of skeletal complications in patients with breast cancer and bone metastases. Ann Oncol. 2003;14:1399–1405. doi: 10.1093/annonc/mdg367. [DOI] [PubMed] [Google Scholar]
- 51.Lipton A, Steger GG, Figueroa J, et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol. 2007;25:4431–4437. doi: 10.1200/JCO.2007.11.8604. [DOI] [PubMed] [Google Scholar]
- 52.Body JJ, Facon T, Coleman RE, et al. A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res. 2006;12:1221–1228. doi: 10.1158/1078-0432.CCR-05-1933. [DOI] [PubMed] [Google Scholar]
- 53.Fizazi K, Lipton A, Mariette X, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 54.Amadori D, Aglietta M, Alessi B, et al. Efficacy and safety of 12-weekly versus 4-weekly zoledronic acid for prolonged treatment of patients with bone metastases from breast cancer (ZOOM): A phase 3, open-label, randomised, non-inferiority trial. Lancet Oncol. 2013;14:663–670. doi: 10.1016/S1470-2045(13)70174-8. [DOI] [PubMed] [Google Scholar]
- 55.Ford J, Cummins E, Sharma P, et al. Systematic review of the clinical effectiveness and cost-effectiveness, and economic evaluation, of denosumab for the treatment of bone metastases from solid tumours. Health Technol Assess. 2013;17:1–386. doi: 10.3310/hta17290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lipton A, Fizazi K, Stopeck AT, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: A combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer. 2012;48:3082–3092. doi: 10.1016/j.ejca.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 57.Menssen HD, Sakalová A, Fontana A, et al. Effects of long-term intravenous ibandronate therapy on skeletal-related events, survival, and bone resorption markers in patients with advanced multiple myeloma. J Clin Oncol. 2002;20:2353–2359. doi: 10.1200/JCO.2002.02.032. [DOI] [PubMed] [Google Scholar]
- 58.Heidenreich A, Elert A, Hofmann R. Ibandronate in the treatment of prostate cancer associated painful osseous metastases. Prostate Cancer Prostatic Dis. 2002;5:231–235. doi: 10.1038/sj.pcan.4500574. [DOI] [PubMed] [Google Scholar]
- 59.Heras P, Karagiannis S, Kritikos K, et al. Ibandronate is effective in preventing skeletal events in patients with bone metastases from colorectal cancer. Eur J Cancer Care (Engl) 2007;16:539–542. doi: 10.1111/j.1365-2354.2007.00808.x. [DOI] [PubMed] [Google Scholar]
- 60.Tripathy D, Body JJ, Bergström B. Review of ibandronate in the treatment of metastatic bone disease: Experience from phase III trials. Clin Ther. 2004;26:1947–1959. doi: 10.1016/j.clinthera.2004.12.010. [DOI] [PubMed] [Google Scholar]