Significant controversy about the use of minimally invasive procedures for leiomyoma removal currently exists because of the potential risk of dissemination of unexpected sarcoma. The results of this study show that the risk of unexpected uterine sarcoma is projected to vary significantly by age. The proposed predictive model should be used by clinicians to counsel patients and to assist in providing guidelines for the surgical technique for leiomyoma.
Keywords: Leiomyosarcoma, Endometrial stromal sarcoma, Leiomyoma, Uterine myomectomy, Hysterectomy
Abstract
Background.
Estimates of unexpected uterine sarcoma following surgery for presumed benign leiomyoma that use age-stratification are lacking.
Patients and Methods.
A retrospective cohort of 2,075 patients that had undergone myomectomy was evaluated to determine the case incidence of unexpected uterine sarcoma. An aggregate risk estimate was generated using a meta-analysis of similar studies plus our data. Database-derived age distributions of the incidence rates of uterine sarcoma and uterine leiomyoma surgery were used to stratify risk by age.
Results.
Of 2,075 patients in our retrospective cohort, 6 were diagnosed with uterine sarcoma. Our meta-analysis revealed 8 studies from 1980 to 2014. Combined with our study, 18 cases of leiomyosarcoma are reported in 10,120 patients, for an aggregate risk of 1.78 per 1,000 (95% confidence interval [CI]: 1.1–2.8) or 1 in 562. Eight cases of other uterine sarcomas were reported in 6,889 patients, for an aggregate risk of 1.16 per 1,000 (95% CI: 0.5–4.9) or 1 in 861. The summation of these risks gives an overall risk of uterine sarcoma of 2.94 per 1,000 (95% CI: 1.8–4.1) or 1 in 340. After stratification by age, we predict the risk of uterine sarcoma to range from a peak of 10.1 cases per 1,000, or 1 in 98, for patients aged 75–79 years to <1 case per 500 for patients aged <30 years.
Conclusion.
The risk of unexpected uterine sarcoma varies significantly across age groups. Our age-stratified predictive model should be incorporated to more accurately counsel patients and to assist in providing guidelines for the surgical technique for leiomyoma.
Implications for Practice:
Significant controversy about the use of minimally invasive procedures for leiomyoma removal currently exists because of the potential risk of dissemination of unexpected sarcoma. To help quantify this risk, we presented an age-stratified incidence prediction of uterine sarcoma following surgery for presumed benign leiomyoma. Our results show that the risk of unexpected uterine sarcoma is projected to vary significantly by age, with a more than fivefold difference between the highest and lowest risk age groups. Our predictive model should be used by clinicians to more accurately counsel patients and to assist in providing guidelines for the surgical technique for leiomyoma.
Introduction
Uterine leiomyomas (fibroids or myomas) are the most common pelvic tumor in women. When symptomatic, surgical resection is often the management strategy. Minimally invasive techniques have been widely adopted for surgical management of leiomyoma because of decreased morbidity and length of hospital stay compared with the open technique [1]. To remove bulky tumors via a laparoscopic port, large tumors are often morcellated (i.e., cut into small pieces).
Uterine sarcomas are a group of rare neoplasms with an incidence of 3–7 per 100,000 women in the U.S. [2]. Because it is not generally feasible to distinguish a sarcoma from a leiomyoma by imaging and clinical characteristics alone, uterine sarcomas are often diagnosed only after a surgical procedure is performed for a mass that is presumed benign. When a uterine sarcoma is incidentally discovered after a surgical procedure, there is concern that morcellation could lead to unintended dissemination of malignant tissue throughout the peritoneum. Several retrospective series of patients with uterine leiomyosarcoma (uLMS) reported higher rates of dissemination and worse outcomes in patients who had undergone previous morcellation [3–6]. Given this concern, significant controversy and debate exists about the continued use of morcellation procedures for resection of presumed benign fibroids.
In April 2014, the U.S. Food and Drug Administration (FDA) issued a safety communication that “discourages the use of laparoscopic power morcellation” for the treatment of uterine fibroids and estimates an approximate risk of 1 in 350 cases for unsuspected uterine sarcoma [7]. In contrast, the Society of Gynecologic Oncology issued a position statement in December 2013 that emphasized the benefits of minimally invasive surgery for the treatment of fibroids and stated the risk of unsuspected uterine sarcoma to be “fewer than one out of 1,000” [8]. In a May 2014 task force report, the American College of Obstetricians and Gynecologists noted the “questionable applicability” of uterine sarcoma risk estimates because of the disease rarity and the lack of age stratification [9]. All expert groups and regulatory agencies agree that the benefits and risks of morcellation should be thoroughly discussed with patients before selecting a surgical approach for presumed benign fibroids.
Estimates of the risk of unsuspected uterine sarcoma come primarily from a collection of single-center retrospective analyses. The risk estimate in the FDA safety communication statement, for example, was based on nine such studies between 1980 and 2014 [10–18]. These studies ranged in size from 104 to 1,429 patients and estimated the risk of unsuspected uterine sarcoma to be between 0 and 4.9 cases per 1,000. In aggregate, the FDA reports that in these 9 studies, 26 cases of uterine sarcoma were reported for 9,160 patients, for a composite risk of 2.8 (95% confidence interval [CI]: 1.8–4.5) per 1,000 cases. Notably, despite combining all modern series on this topic, the confidence interval for risk remains wide. In addition, because there is a clear age-dependent pattern of incidence of uterine sarcoma, it is unlikely that the same risk estimate is applicable across all age groups.
Given these limitations, we aimed in this study to add precision to the risk assessment of uterine sarcoma in the setting of surgery for presumed benign leiomyoma and to add age stratification to our risk assessment. First, we retrospectively analyzed cases of myomectomy performed at our institution and have reported the largest series in the literature to date. Second, we combined our data with previous reports to calculate an aggregate risk. Finally, we used age-adjusted incidence rates of uterine sarcoma and uterine leiomyoma that require surgical management to generate an age-stratified risk model for unexpected uterine sarcoma at the time of surgery for presumed benign leiomyoma.
Methods
The Mount Sinai Data Warehouse (MSDW) is a database of clinical, operational, and financial data derived from patient care at the Mount Sinai Hospital and Mount Sinai Faculty Practice Associates. The MSDW contains data going back to 2003 and encompasses >3 million patients, approaching 1 billion facts. To select patients that had undergone myomectomy, the MSDW was queried for surgical procedure codes that corresponded with a myomectomy procedure. To identify potential cases of uterine sarcoma, we used two methods in parallel. First we searched the patients for a list of International Classification of Diseases, Ninth Revision (ICD-9) codes that could feasibly be used for uterine sarcoma (supplemental online Table 1). This list was intentionally broad so as to emphasize sensitivity at this stage. Second, we used an electronic medical record mining program [19] to search for the keyword sarcoma in the pathology reports of patients who had undergone myomectomy. The charts of the potential cases were manually reviewed by a physician familiar with sarcoma oncology to determine true cases. Approval was obtained from the institutional review board at the Mount Sinai School of Medicine for this chart review.
For our literature review, we replicated the methods used by the FDA for its recent safety communication [7]. In brief, we limited our analysis to English-language studies published from 1980 to July 2014. For the analysis, we included cohort and cross-sectional studies with the desired numerator (cases of uterine sarcoma and/or leiomyosarcoma) and denominator (surgery for presumed benign leiomyoma).
The Surveillance, Epidemiology, and End Results (SEER) incidence database of the National Cancer Institute, including cases between 1973 and 2011 (SEER 18, Nov 2013) was queried using the SEER*Stat tool (version 8.1.5) [20]. Uterine sarcoma cases were identified by the intersection of the ICD-0-3 and World Health Organization 2008 site code limited to the uterus (“Corpus and Uterus, NOS,” “Corpus Uteri,” or “Uterus, NOS”) and limited to ICD-0-3 malignant histology codes for leiomyosarcoma (8890-8897/3), endometrial stromal sarcoma (8930/3, 8931/3, 8935/3) or sarcoma not otherwise specified (NOS; 8800-8805/3). Age-stratified rates of uterine leiomyoma requiring surgery were extracted from a previously reported large cohort study [21].
All statistical calculations were performed using R software, version 3.0.2 (R Foundation, Vienna, Austria, http://www.r-project.org). CIs were calculated using the exact method for binomial distribution. For the sum of two binomial distributions with unequal sample sizes (i.e., sum of uterine leiomyosarcoma rate and other uterine sarcoma rate), we compared the exact CIs and found that they were very close to CIs derived from Gaussian approximation. Consequently, Gaussian approximation was used to calculate the CI of this summation of risk.
Results
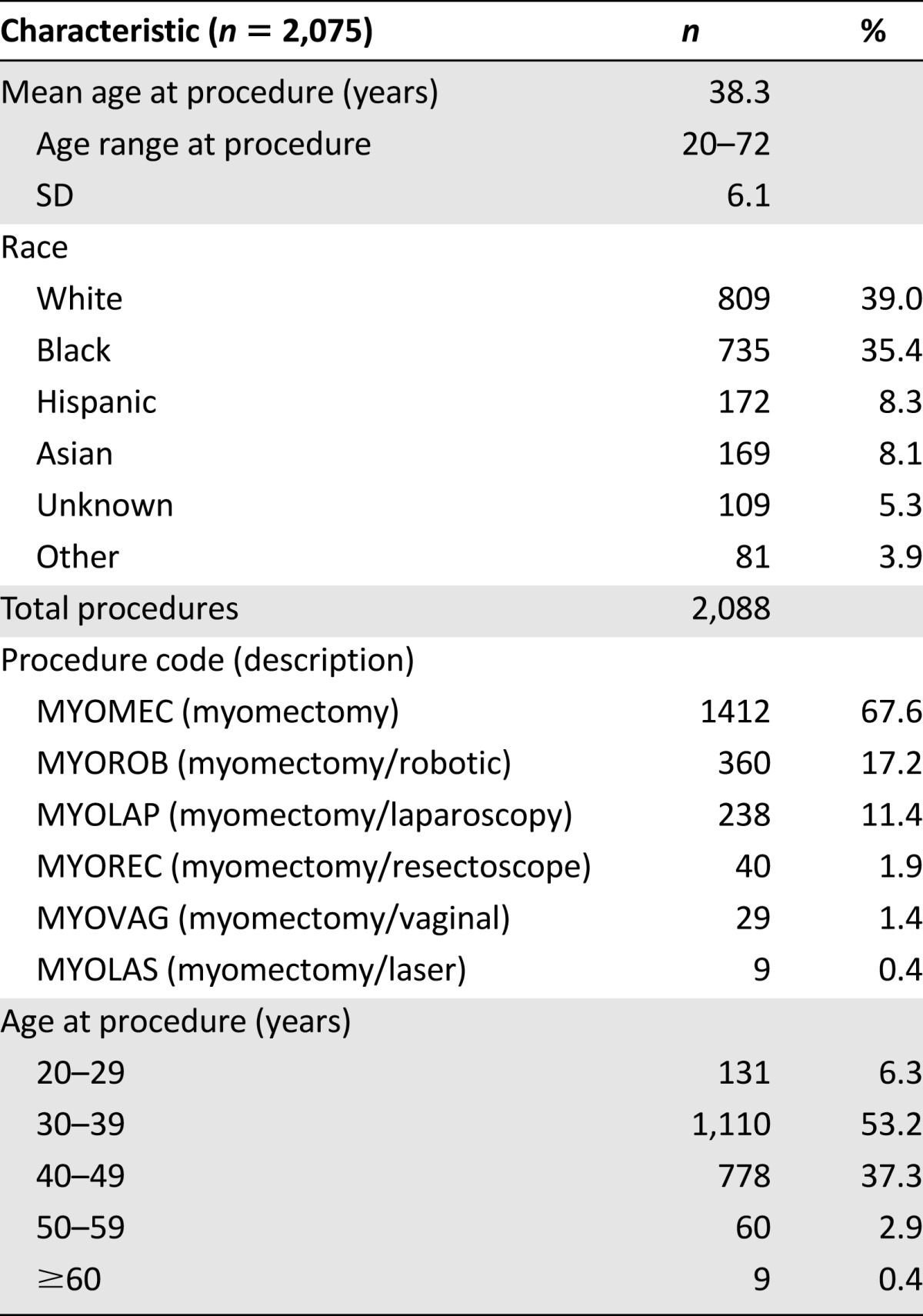
A total of 2,075 patients were identified as having undergone myomectomy between August 2005 and April 2014 at our institution. The mean age of patients having this procedure in this retrospective cohort was 38.3 years (SD: 6.1 years). Additional demographic information is detailed in Table 1. Our methods identified 30 potential cases of uterine sarcoma overlapping with myomectomy: 22 by ICD-9 code and 13 by keyword search by pathology, with an overlap of 5 found by both methods. After chart review of these patients, we identified a total of six true-positive cases of patients diagnosed with uterine sarcoma following myomectomy, two with leiomyosarcoma and four with endometrial stromal sarcoma (ESS). The most common reasons for false-positive identification included miscoding (n = 9), the keyword sarcoma appearing in an otherwise benign pathology report, for example, “rule out sarcoma” (n = 6), discovery of nonsarcoma uterine tumors (n = 5), and capture of nonuterine malignancies unrelated to myomectomy procedure (n = 3). Five of the true cases were identified by both of our methods. One case was identified only by the keyword search by pathology because this patient did not have an ICD-9 code of uterine neoplasm (or any other ICD-9 code suggestive of malignancy) in our medical record system.
Table 1.
Patient characteristics
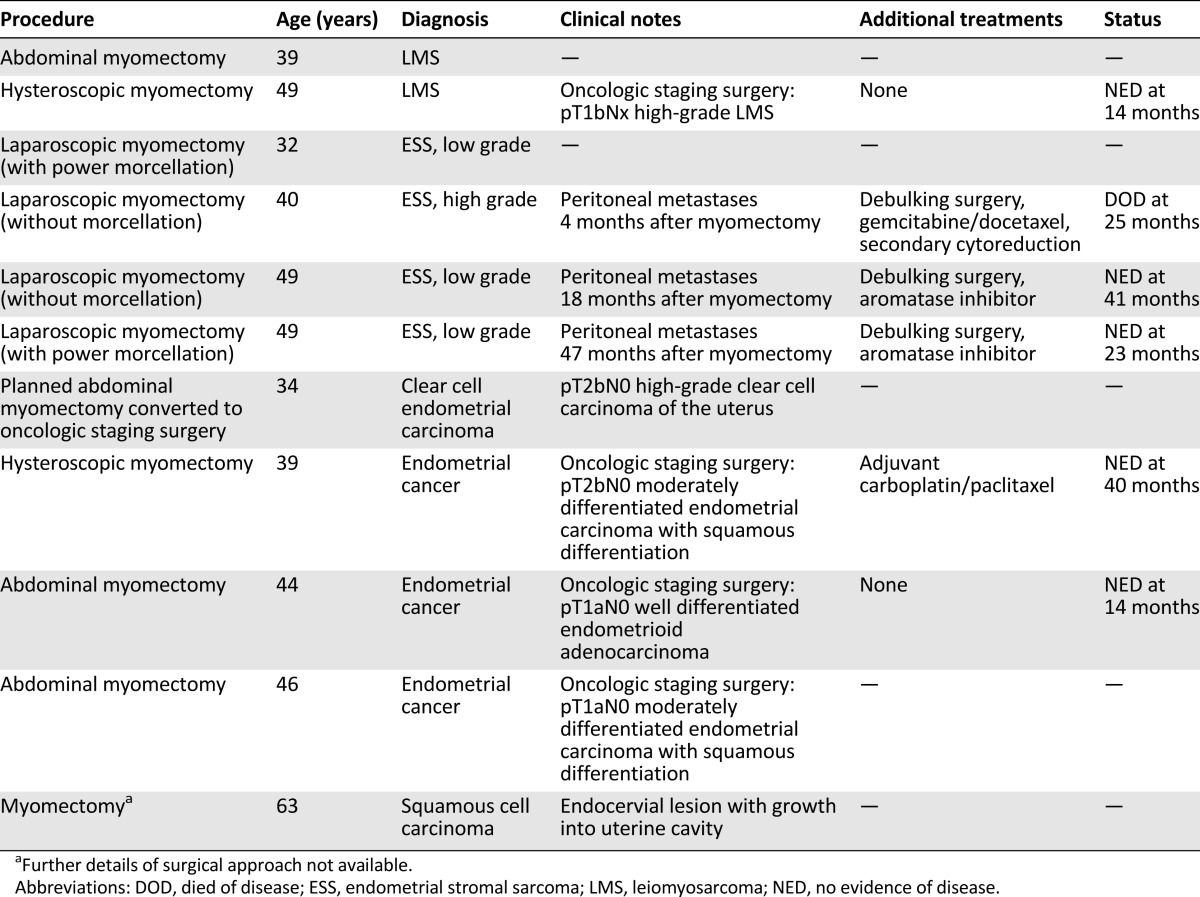
The two patients with uLMS were diagnosed by pathology of the myomectomy specimen immediately following the procedure. The first patient was aged 39 years at diagnosis. She underwent an open myomectomy for the indication of menorrhagia. Pathology revealed a 15 cm epithelioid leiomyosarcoma (mild to moderate nuclear atypia, 6 mitoses per 10 high-power fields, evidence of coagulative tumor cell necrosis). She received oncologic care elsewhere, and follow-up was not available for review. The second patient with uLMS was diagnosed at age 49 years. She underwent hysteroscopic myomectomy for the indication of menometrorrhagia. Pathology revealed fragments of high-grade smooth muscle neoplasm highly suspicious for leiomyosarcoma. She subsequently underwent oncologic staging surgery with pathology confirming high-grade LMS, stage pT1bN0. The patient did not receive adjuvant therapy and is alive without evidence of disease 14 months after her initial surgery.
Of the four patients diagnosed with ESS following myomectomy, only one was diagnosed in the immediate postoperative period from the initial procedure. The first patient underwent laparoscopic myomectomy with morcellation at age 32 years for the indication of pelvic pain and menorrhagia. Pathology revealed fragments of low-grade ESS. She received oncologic care elsewhere, and follow-up was not available for review. The remaining three patients, aged 40, 49, and 49 years, were all initially thought to have benign pathology on myomectomy but presented later with peritoneal dissemination of ESS. The first of these patients underwent laparoscopic myomectomy for the indication of menometrorrhagia. She was found to have metastatic high-grade ESS 4 months after myomectomy. She underwent debulking surgery and received postoperative chemotherapy. She recurred shortly after and underwent secondary cytoreduction but ultimately died of disease 25 months after her diagnosis. The next patient was found to have metastatic low-grade ESS 18 months after laparoscopic myomectomy for the indication of pelvic pain. She subsequently underwent debulking surgery and received hormonal therapy postoperatively. She is alive and without evidence of disease at 41 months after her cancer surgery. The remaining patient was diagnosed with metastatic low-grade ESS 47 months after undergoing laparoscopic myomectomy with morcellation for “symptomatic fibroid.” On her initial myomectomy pathology, she was noted as having a “benign spindle cell tumor” that, in retrospect, was re-reviewed as consistent with a low-grade ESS. She underwent debulking surgery followed by hormonal therapy and is alive and without evidence of disease at 23 months after her diagnosis.
In addition to the uterine sarcomas, we identified four patients who were incidentally diagnosed with endometrial carcinomas following myomectomy for presumed benign fibroid and one patient who was found to have an endocervical squamous cell carcinoma that mimicked a fibroid on imaging because of growth into the uterine cavity. Relevant clinicopathologic details for all gynecologic cancer patients in our series are summarized in Table 2.
Table 2.
Uterine cancer clinicopathologic details
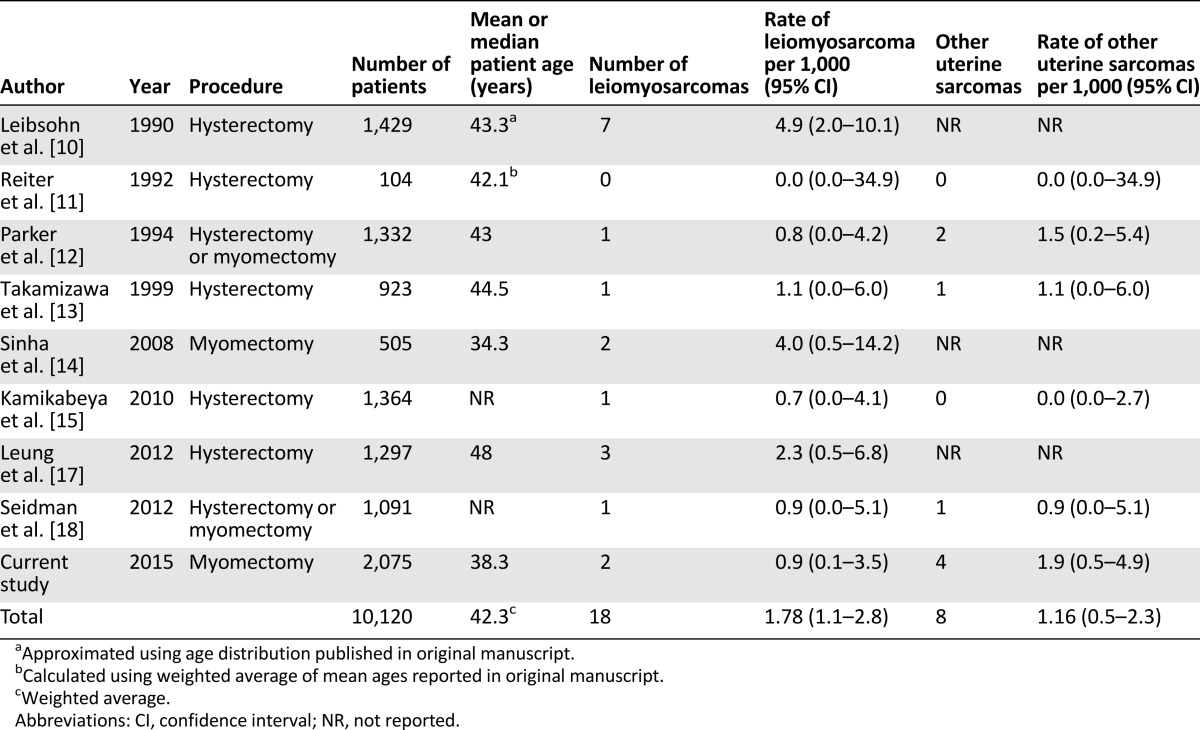
In our review of the scientific literature, we identified eight studies from 1980 to the present that we used to estimate the prevalence of uterine sarcoma following surgery for presumed benign leiomyoma [10–15, 17, 18]. All studies identified were retrospective cohorts or cross-sectional analyses. Our analysis was similar to the recent FDA report, with exceptions [7]. We excluded one study that was included in the FDA report as being surgery for “benign indications” rather than specifically for fibroids [16]. In three of the studies, it was unclear whether the authors assessed for nonleiomyosarcoma uterine sarcomas; therefore, we considered these studies only for the risk assessment of uLMS and not for non-uLMS uterine sarcoma risk [10, 14, 17]. Last, in one study [15], we excluded a case of carcinosarcoma that was counted as a uterine sarcoma by the FDA report; this histology is more appropriately grouped with carcinomas rather than a sarcoma. Including our series, 9 studies ranging in size from 104 to 2,075 patients are included in our analysis, for a total of 10,120 patients (Table 3). For clarity, we will refer to this group of 10,120 patients as the aggregate group for the remainder of our discussion. Overall, 18 cases of leiomyosarcoma were reported in 10,120 patients, for an aggregate risk of 1.78 per 1,000 (95% CI: 1.1–2.8) or 1 in 562. Eight cases of other uterine sarcomas, all ESS, were reported in 6,889 patients, for an aggregate risk of 1.16 per 1,000 (95% CI: 0.5–4.9) or 1 in 861. The summation of these risks gives a total risk estimate of uterine sarcoma of 2.94 per 1,000 (95% CI: 1.8–4.1) or 1 in 340.
Table 3.
Studies reporting incidence of unsuspected uterine sarcoma and leiomyosarcoma at the time of surgery for presumed benign leiomyoma, 1980–2014, plus current study
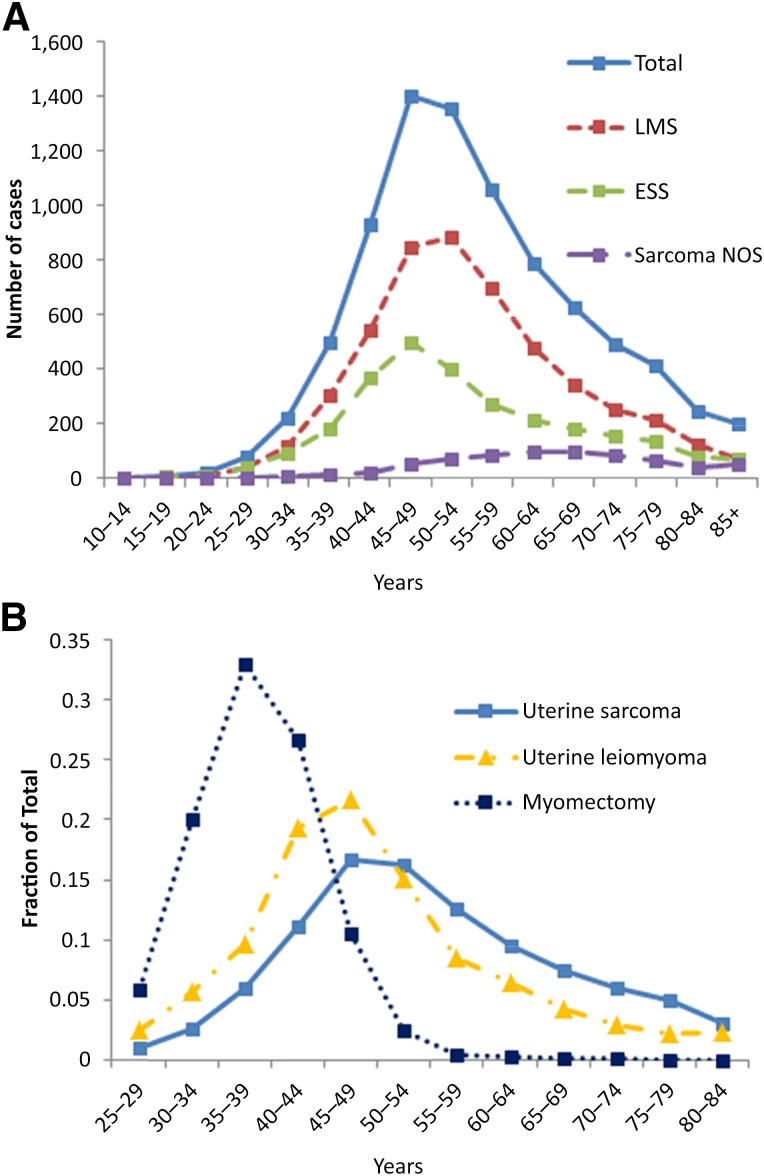
Our inquiry of the SEER 18 database revealed 8,365 total cases of uterine sarcoma [20]. Uterine leiomyosarcoma accounted for 59% of the cases, ESS accounted for 33%, and the remaining 8% were classified as sarcoma NOS. The peak incidences of all uterine sarcomas, uLMS, and ESS were in the perimenopausal age range of 45–54 years, with uLMS peaking in incidence at a slightly older age than ESS (Fig. 1A). Although lower in numbers overall, women with sarcoma NOS of the uterus were more commonly diagnosed in the postmenopausal age ranges.
Figure 1.
Age distribution of uterine sarcomas compared with uterine leiomyoma and myomectomy procedures. (A): Age distribution of uterine sarcoma subtypes in the SEER incidence database from 1973 to 2011 (SEER 18 [20]). (B): Comparison of age distributions of total uterine sarcoma incidence (SEER 18), incidence of leiomyoma requiring surgical management [21], and myomectomy procedures in our current series.
Abbreviations: ESS, endometrial stromal sarcoma; LMS, leiomyosarcoma; NOS, not otherwise specified.
We compared the age distribution of uterine sarcoma incidence with the age distribution of surgery for leiomyomas reported from a large prospective cohort study [21]. Although peaking in incidence at a similar age range, the overall distribution of uterine sarcomas trended older than uterine leiomyoma surgeries and, in particular, accounted for a higher percentage at postmenopausal ages (Fig. 1B). The ages of women at the time of myomectomy surgery in our retrospective cohort was also compared with the age distribution of women at the time of diagnosis of uterine sarcoma in the SEER database [20]. The age distribution of myomectomy procedures was shifted considerably younger compared with the incidence of uterine sarcomas (Fig. 1B).
To approximate the age-stratified risk of uterine sarcoma, we used the assumption that age-stratified risk following a myomectomy procedure should parallel the age-stratified ratio of incidence of uterine sarcoma to incidence of uterine leiomyoma requiring surgery. If, for example, the incidence of uterine sarcoma is doubled with a constant rate of leiomyoma requiring surgery, then the risk of having an incidentally discovered uterine sarcoma should be doubled; however, if the incidence rate of both uterine sarcoma and uterine leiomyoma requiring surgery are doubled, then the risk of incidental uterine sarcoma per procedure should be constant. We then used the SEER age-adjusted incidence of leiomyosarcoma [20], the published age-adjusted rate of leiomyomas requiring surgery [21], and the age distribution of procedures in our aggregate group to stratify the uterine sarcoma risk estimation.
where:
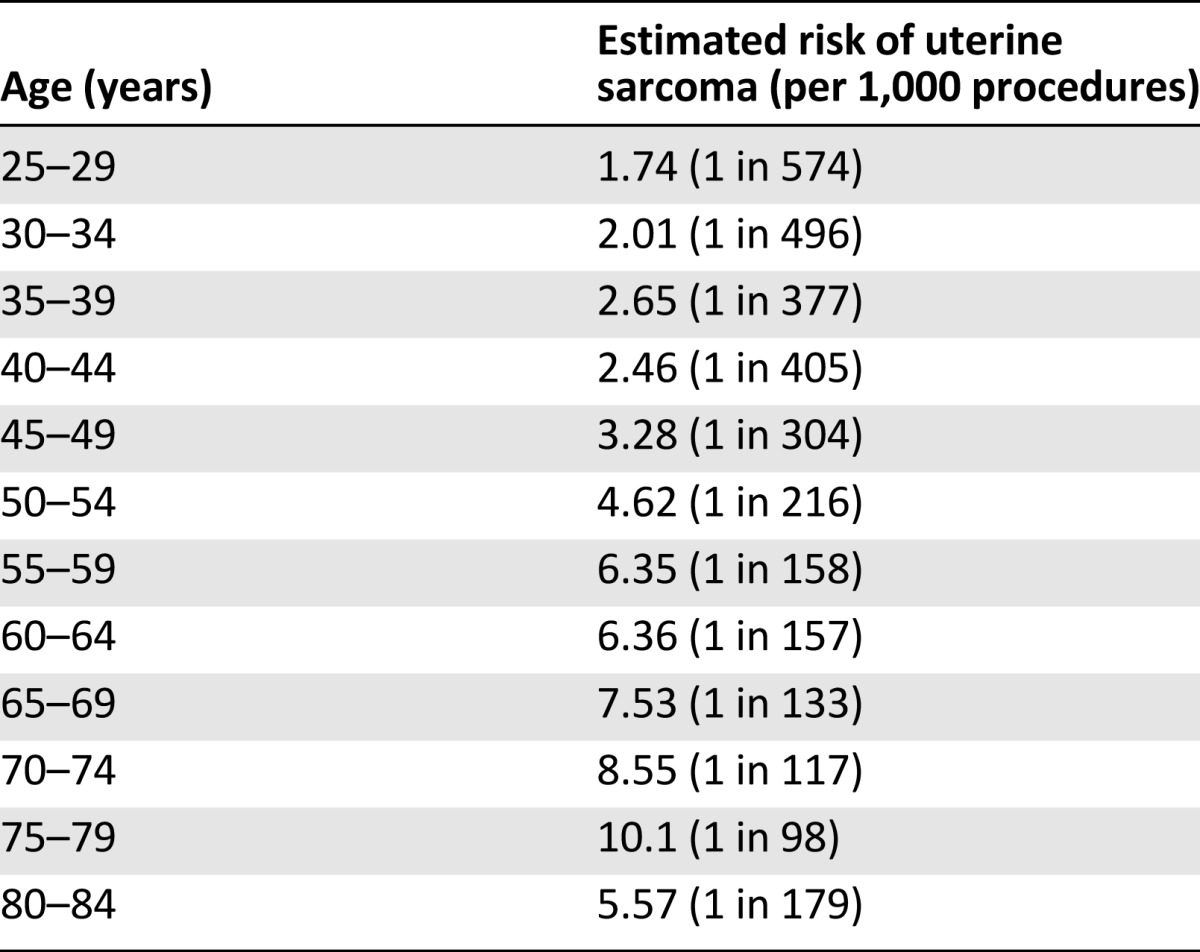
The mean age of myomectomy procedures performed at our institution was 4 years younger than the weighted mean age of the aggregate group used for overall risk calculation (Table 3). To estimate the age distribution of the aggregate group, we used the distribution of procedures in our retrospective cohort shifted upward by 4 years. Solving for x and calculating the age-based risk of uterine sarcoma yields the following results (Table 4). We predicted the highest risk to be 10.1 per 1,000, or 1 in 98, for women aged 75–79 years. All age groups >44 years have an estimated risk higher than the overall aggregate risk of 1 in 340. All age groups >54 years have an estimated risk >1 in 200. As expected, the risk decreases with decreasing age <45 years and is estimated to be <1 in 500 for patients aged <30 years. No risk is reported for age groups <25 or >84 years because these are the age limits of our data from at least one of the databases used.
Table 4.
Age-stratified risk of unsuspected uterine sarcoma at the time of surgery for presumed benign leiomyoma
Discussion
We report the largest institutional experience to date of the risk of uterine sarcoma following myomectomy for presumed benign leiomyoma. Our method of case identification by selecting patients by procedure and diagnosis codes and then manually confirming true cases by chart review highlights an important limitation of using only a coding database for this purpose. Of our 22 patients with matching ICD-9 diagnosis codes of interest, only 5 were true cases of uterine sarcoma, largely because of coding errors. In addition, we identified one case of uterine sarcoma by keyword search of the pathology report in which the patient lacked a malignant diagnosis code and otherwise would have been missed. In addition to the issue of miscoding, we noted that there was frequently a significant time delay between surgery and the diagnosis of uterine sarcoma, particularly in cases of endometrial stromal sarcoma.
In our retrospective cohort, we identified 6 cases of unexpected uterine sarcoma in 2,075 patients that had undergone a myomectomy procedure, for a rate of 2.89 per 1,000. Although our evaluation was limited to myomectomy procedures only, this incidence is similar to that seen in previously reported series of patients operated on for presumed benign leiomyoma either by hysterectomy or myomectomy, with published rates between 0 and 4.9 cases per 1,000 [10–15, 17, 18]. When combining the results from our series with previous reports, we used the data from >10,000 patients and estimated an aggregate risk of all uterine sarcomas of 2.94 per 1,000, or 1 in 340, and a risk specifically of leiomyosarcoma of 1.78 per 1,000, or 1 in 562. Of note, the fraction of uterine leiomyosarcoma to all uterine sarcomas in these aggregate predictions is 60.5%, almost exactly the same percentage of uterine sarcomas accounted for by uLMS in the SEER database.
Crucially, we show that age stratification significantly affects the risk prediction for uterine sarcoma. In the debate about the use of morcellation procedures for presumed fibroids, recent position statements by expert physician groups and a safety communication from the FDA quoted only overall risk predictions of unexpected uterine sarcoma in this context [9]. However, it is well known from epidemiological studies that uterine sarcoma risk peaks sharply in the perimenopausal age range [2]. Our analysis of the most recent SEER incidence database confirms this age distribution [20]. It is also known that benign uterine leiomyomas tend to regress after menopause and thus are much less likely to require surgery in this age range [21]. After applying our knowledge of the age distribution of sarcoma incidence and leiomyoma surgery incidence, we predict the risk of unexpected uterine sarcoma to range widely between age groups, from as high as ∼1 in 100 for patients aged 75–79 years to <1 in 500 for those aged <30 years. Importantly, there is a substantial difference in risk of uterine sarcoma even among the more narrow age ranges of women that are likely to undergo surgical management of fibroid, increasing from approximately 1 in 400 for women aged 35–44 years to 1 in 304 for women aged 45–49 years to 1 in 216 for women aged 50–54 years. Although both uterine sarcomas and uterine leiomyomas peak in incidence in the perimenopausal period, the incidence of surgery for benign leiomyomas peaks slightly earlier and has a more rapid decline in the postmenopausal ages, leading to this observed risk distribution.
Given that the risk of unexpected uterine sarcoma at the time of surgery increases with age, it is important to assess the age distribution of the population studied when analyzing and comparing studies on this topic. Studies that include women at younger ages would likely report lower risk of uterine sarcoma. Studies limited to myomectomy procedures, rather than hysterectomies or all surgical procedures, are likely to include younger populations. A limitation of our current analysis is that we did not have age data for the majority of the 10,120 patients in the aggregate group used for our overall risk calculation. To estimate the age distribution of procedures of this aggregate group, we used the age distribution of our retrospective myomectomy cohort shifted upward by 4 years to match the weighted mean age of the aggregate group. Had we not implemented this 4-year age shift and instead simply used the age distribution of our retrospective cohort to adjust for age, we would have calculated estimated risks to be approximately 15% higher across all age groups because of an overcorrection for the younger age tendency in our myomectomy patients.
Conclusion
Our study represents an advancement to better define the risk of unexpected uterine sarcoma in the setting of surgery for presumed benign leiomyoma. Our age-stratified predictive model may be incorporated to more accurately counsel patients in clinical practice and to assist in providing guidelines for the surgical technique for leiomyoma. Future studies on this topic should be undertaken to confirm our findings, to investigate the use of additional stratification variables, and to incrementally improve on our estimates.
See http://www.TheOncologist.com for supplemental material available online.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Supplementary Material
Acknowledgments
We thank the support staff of the Mount Sinai Data Warehouse for technical assistance and Dr. Jeremy Boal for bringing this problem to our attention. Drs. Li and Dudley are supported by funding from NIH/National Institute of Diabetes and Digestive and Kidney Diseases Grant 5R01DK098242-02. Drs. Brohl, Li, Hao, Dudley, and Kasarskis are supported by funding from the Icahn Institute. The study funders had no role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Author Contributions
Conception/Design: Andrew S. Brohl, Sarah G. Običan, Andrew Kasarskis, Robert G. Maki
Collection and/or assembly of data: Andrew S. Brohl, Li Li, Angela Cioffi
Data analysis and interpretation: Andrew S. Brohl, Li Li, Vaagn Andikyan, Ke Hao, Charles Ascher-Walsh
Manuscript writing: Andrew S. Brohl
Final approval of manuscript: Andrew S. Brohl, Li Li, Vaagn Andikyan, Sarah G. Običan, Angela Cioffi, Ke Hao, Joel T. Dudley, Charles Ascher-Walsh, Andrew Kasarskis, Robert G. Maki
Disclosures
Joel T. Dudley: Ayasdi, Inc. (C/A); Roche, AstraZeneca, GlaxoSmithKline (H); Janssen Pharmaceuticals (RF); NuMedii, Inc. (OI). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Bojahr B, Raatz D, Schonleber G, et al. Perioperative complication rate in 1706 patients after a standardized laparoscopic supracervical hysterectomy technique. J Minim Invasive Gynecol. 2006;13:183–189. doi: 10.1016/j.jmig.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Brooks SE, Zhan M, Cote T, et al. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989-1999. Gynecol Oncol. 2004;93:204–208. doi: 10.1016/j.ygyno.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 3.George S, Barysauskas C, Serrano C, et al. Retrospective cohort study evaluating the impact of intraperitoneal morcellation on outcomes of localized uterine leiomyosarcoma. Cancer. 2014;120:3154–3158. doi: 10.1002/cncr.28844. [DOI] [PubMed] [Google Scholar]
- 4.Park JY, Park SK, Kim DY, et al. The impact of tumor morcellation during surgery on the prognosis of patients with apparently early uterine leiomyosarcoma. Gynecol Oncol. 2011;122:255–259. doi: 10.1016/j.ygyno.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 5.Perri T, Korach J, Sadetzki S, et al. Uterine leiomyosarcoma: Does the primary surgical procedure matter? Int J Gynecol Cancer. 2009;19:257–260. doi: 10.1111/IGC.0b013e31819a1f8f. [DOI] [PubMed] [Google Scholar]
- 6.Morice P, Rodriguez A, Rey A, et al. Prognostic value of initial surgical procedure for patients with uterine sarcoma: Analysis of 123 patients. Eur J Gynaecol Oncol. 2003;24:237–240. [PubMed] [Google Scholar]
- 7.Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. Available at http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.Htm. Issued April 17, 2014. Accessed July 14, 2014.
- 8.SGO position statement: Morcellation. Available at http://www.sgo.org/newsroom/position-statements-2/morcellation. Issued December 2013. Accessed July 14, 2014.
- 9.Power morcellation and occult malignancy in gynecologic surgery: A special report. Available at http://www.acog.org/resources_and_publications/task_force_and_work_group_reports/power_morcellation_and_occult_malignancy_in_gynecologic_surgery. Issued May 2014. Accessed July 14, 2014.
- 10.Leibsohn S, d’Ablaing G, Mishell DR, Jr, et al. Leiomyosarcoma in a series of hysterectomies performed for presumed uterine leiomyomas. Am J Obstet Gynecol. 1990;162:968–974; discussion 974–976. doi: 10.1016/0002-9378(90)91298-q. [DOI] [PubMed] [Google Scholar]
- 11.Reiter RC, Wagner PL, Gambone JC. Routine hysterectomy for large asymptomatic uterine leiomyomata: A reappraisal. Obstet Gynecol. 1992;79:481–484. [PubMed] [Google Scholar]
- 12.Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83:414–418. [PubMed] [Google Scholar]
- 13.Takamizawa S, Minakami H, Usui R, et al. Risk of complications and uterine malignancies in women undergoing hysterectomy for presumed benign leiomyomas. Gynecol Obstet Invest. 1999;48:193–196. doi: 10.1159/000010172. [DOI] [PubMed] [Google Scholar]
- 14.Sinha R, Hegde A, Mahajan C, et al. Laparoscopic myomectomy: Do size, number, and location of the myomas form limiting factors for laparoscopic myomectomy? J Minim Invasive Gynecol. 2008;15:292–300. doi: 10.1016/j.jmig.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Kamikabeya TS, Etchebehere RM, Nomelini RS, et al. Gynecological malignant neoplasias diagnosed after hysterectomy performed for leiomyoma in a university hospital. Eur J Gynaecol Oncol. 2010;31:651–653. [PubMed] [Google Scholar]
- 16.Rowland M, Lesnock J, Edwards R, et al. Occult uterine cancer in patients undergoing laparoscopic hysterectomy with morcellation. Gynecol Oncol. 2012;127(suppl):S29. [Google Scholar]
- 17.Leung F, Terzibachian JJ. Re: “The impact of tumor morcellation during surgery on the prognosis of patients with apparently early uterine leiomyosarcoma”. Gynecol Oncol. 2012;124:172–173; author reply 173. doi: 10.1016/j.ygyno.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 18.Seidman MA, Oduyebo T, Muto MG, et al. Peritoneal dissemination complicating morcellation of uterine mesenchymal neoplasms. PLoS One. 2012;7:e50058. doi: 10.1371/journal.pone.0050058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LePendu P, Iyer SV, Bauer-Mehren A, et al. Pharmacovigilance using clinical notes. Clin Pharmacol Ther. 2013;93:547–555. doi: 10.1038/clpt.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.SEER*Stat Databases: November 2013 Submission. Available at http://seer.cancer.gov/data/seerstat/nov2013/. Accessed July 18, 2014.
- 21.Templeman C, Marshall SF, Clarke CA, et al. Risk factors for surgically removed fibroids in a large cohort of teachers. Fertil Steril. 2009;92:1436–1446. doi: 10.1016/j.fertnstert.2008.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.