The aim of this study was to determine the prevalence and role of BRCA1 and BRCA2 mutations in Lebanese women with breast cancer who were at high risk of harboring hereditary disease. The prevalence of deleterious BRCA mutations was lower than expected and does not support the hypothesis that BRCA mutations alone cause the observed high percentage of breast cancer in young women of Lebanese and Arab descent.
Keywords: Breast cancer, Young age, Family history, BRCA mutations, Haplotype, Lebanon, Arab countries
Abstract
Purpose.
Breast cancer is the most common malignancy among women in Lebanon and in Arab countries, with 50% of cases presenting before the age of 50 years.
Methods.
Between 2009 and 2012, 250 Lebanese women with breast cancer who were considered to be at high risk of carrying BRCA1 or BRCA2 mutations because of presentation at young age and/or positive family history (FH) of breast or ovarian cancer were recruited. Clinical data were analyzed statistically. Coding exons and intron-exon boundaries of BRCA1 and BRCA2 were sequenced from peripheral blood DNA. All patients were tested for BRCA1 rearrangements using multiplex ligation-dependent probe amplification (MLPA). BRCA2 MLPA was done in selected cases.
Results.
Overall, 14 of 250 patients (5.6%) carried a deleterious BRCA mutation (7 BRCA1, 7 BRCA2) and 31 (12.4%) carried a variant of uncertain significance. Eight of 74 patients (10.8%) aged ≤40 years with positive FH and only 1 of 74 patients (1.4%) aged ≤40 years without FH had a mutated BRCA. Four of 75 patients (5.3%) aged 41–50 years with FH had a deleterious mutation. Only 1 of 27 patients aged >50 years at diagnosis had a BRCA mutation. All seven patients with BRCA1 mutations had grade 3 infiltrating ductal carcinoma and triple-negative breast cancer. Nine BRCA1 and 17 BRCA2 common haplotypes were observed.
Conclusion.
Prevalence of deleterious BRCA mutations is lower than expected and does not support the hypothesis that BRCA mutations alone cause the observed high percentage of breast cancer in young women of Lebanese and Arab descent. Studies to search for other genetic mutations are recommended.
Implications for Practice:
This study provides new data to support discussion and referral of patients of Lebanese and Arab ancestry with high-genetic-risk breast cancer for BRCA counseling and testing. The probability of carrying a deleterious BRCA mutation in this population seems low at 5.6%. The absence of family history in patients aged ≤40 years reduces the possibility of BRCA mutations to only 1.4%. Young age combined with a positive family history raises the prevalence to 10.8% and increases the yield of testing. Further clarification of the 12.4% of cases with variants of uncertain significance and searches for alternative gene mutations are needed. This study adds missing information to the international BRCA population maps.
Introduction
Breast cancer is the most common cancer seen in women [1]. Many differences with respect to age, stage at presentation, and biological characteristics exist among various countries and ethnic groups. In the U.S., approximately 65% of all women newly diagnosed with breast cancer are aged >55 years, whereas in most low- and middle-income countries, almost half of women with newly diagnosed breast cancer are aged <50 years [2]. Although early stage breast cancer has become most common in industrialized nations, locally advanced and metastatic disease at presentation remain very common in the developing countries [2], with only recent reports indicating a shift toward early stages because of active widespread awareness and early detection campaigns [3].
Arab countries are home to >359 million people, according to the United Nations Development Program’s Arab Human Development Reports, and studies, reviews, and available cancer registries have shown that breast cancer is the most commonly diagnosed cancer among Arab women [2, 4, 5]. Although the age-standardized incidence rates (ASRs) are still below those of industrialized nations, they are rising. ASR for breast cancer in Lebanon, a country of 4 million residents, increased from 20 of 100,000 women per year in 1964 to 69 of 100,000 women in 2004 [6].
In several studies from Lebanon and neighboring countries, median age at onset of breast cancer was shown to range between 48 and 52 years [2, 4, 5]. Hospital-based registries and national collection of data by the Lebanese Ministry of Health showed that almost half of cases are diagnosed in women aged <50 years, with a significant number of cases diagnosed at <40 years and even <35 years [2, 5–7]. This presentation at young age has been reported to confer worse prognosis [8, 9].
Genetic susceptibility factors play important roles in predisposition to breast cancer. To date, mutations in BRCA1 or BRCA2 are the strongest known breast cancer predictors, and there is a wide variability in the specific types of mutations and their frequency in different geographic areas and ethnicities. Differences in study inclusion criteria and mutation-detection techniques can also partly explain differences in prevalence of BRCA1 and BRCA2 mutations between different populations [10].
Only a few small studies have looked at BRCA mutations in Arab countries. Data from Saudi Arabia, for example, showed a lack of association of BRCA1 and BRCA2 variants with100 breast cancer patients [11]. A study of 35 young patients from Sudan suggested an important role for BRCA 1 and BRCA2 genetic mutations [12]. Studies from North Africa looked at 36 selected patients found to have 4 BRCA1 and 2 BRCA2 frameshift mutations [13]. Moreover, 6%–36% BRCA1 mutations were described in a cohort of familial patients from Algeria [14] and 31% from Morocco [15]. Four BRCA1 exons and 1 BRCA2 exon were assessed in 60 selected and referred Egyptian women and showed a mutation prevalence rate of 25% [16]. As for Lebanon, a cohort of 72 women with early onset breast cancer and/or positive family history who were referred for testing showed deleterious BRCA1 and BRCA2 mutations in 12.5% of patients [17]. The variability of results from the above-mentioned small studies depends on patient-selection criteria and referral patterns.
The aim of our study was to determine the prevalence and roles of previously known and unknown BRCA1 and BRCA2 mutations in Lebanese women with breast cancer who were at a high risk of harboring hereditary disease because of presentation at young age and/or positive family history. This project is an important milestone in determining the prevalence of genetic predisposition to breast cancer in Lebanese women and other ethnic Arab and Middle Eastern populations.
Patients and Methods
Study
We tested for BRCA1 and BRCA2 genetic mutations in a cohort of breast cancer patients who were considered to be at high risk of having a hereditary breast cancer by virtue of their young age at presentation and/or positive family history of breast and ovarian cancer. The study won a competitive grant from the Ethnic Research Initiative, funded by GlaxoSmithKline. The study was approved by the American University of Beirut Medical Center (AUBMC) institutional review board (IRB). All patients signed informed consent.
Participants
Lebanese women diagnosed with breast cancer and considered to be at high risk for carrying BRCA1 or BRCA2 mutations were recruited at AUBMC between 2009 and 2012. Eligibility for enrollment was considered when patients fulfilled any of the following criteria: diagnosis at age ≤40 years, diagnosis at age ≤50 years with at least one relative diagnosed with breast cancer at age ≤50 years or diagnosed with ovarian cancer, diagnosis at any age with 2 or more relatives with breast cancer, diagnosis at any age with 2 or more relatives with ovarian cancer, or diagnosis at any age with a personal history of ovarian cancer.
We recruited 250 Lebanese women. For every participant, medical records were completed for disease stage, pathology, grade, and receptor status. Family history of breast and ovarian cancer was collected to construct multigenerational pedigrees.
BRCA1 and BRCA2 Genotyping
DNA was isolated from 0.6 mL peripheral blood using QIAamp DNA isolation kit (Qiagen, Venlo, The Netherlands, http://www.qiagen.com) and stored at −20°C. DNA was transferred to Centre Jean Perrin (CJP) in Clermont-Ferrand, France, for genotyping following AUBMC IRB approval and a material transfer agreement between the two institutions.
All coding exons and intron-exon boundaries of BRCA1 and BRCA2 were amplified using primers developed in the CJP laboratory and Fluidigm technology (primer sequences are available on request; Fluidigm, South San Francisco, CA, https://www.fluidigm.com). Bar codes for patient identification and universal sequencing primers were added by polymerase chain reaction before purification, quantification, and library preparation, according to the manufacturer’s instructions for GS FLX amplicon sequencing (Roche, Basel, Switzerland, http://www.roche.com). In addition to the Amplicon Variant Analyzer software provided by Roche, in-house software was used for analysis of sequencing data. Parameters included a minimum coverage depth of 40 reads required to validate an amplicon and a minimum of 20% variant reads plus variant reads present for each strand for a sequence variant to be called. Insertions-deletions in homopolymer stretches were analyzed with an in-house software to verify mono- versus bimodality of base number calls [29]. Complementary sequencing to verify mutations and/or unvalidated amplicons was performed using standard Sanger sequencing on a 3130xL genetic analyzer (Life Technologies; Thermo Fisher Scientific, Rockford, IL, https://www.lifetechnologies.com) and analyzed with SeqMan software (DNASTAR, Madison, WI, http://www.dnastar.com). Variants were annotated and submitted to in silico analysis with Alamut (Interactive Biosoftware, Rouen, France, http://www.interactive-biosoftware.com).
We screened for large deletions and duplications using reagents from MRC-Holland (kit P001 version C2 for BRCA1 and kit P090 version A4 for BRCA2; MRC-Holland, Amsterdam, The Netherlands, http://www.mlpa.com), according to the manufacturer’s instructions, and resolved on a 3130xL genetic analyzer (Life Technologies; Thermo Fisher Scientific). Coffalyser software (MRC-Holland) was used for normalization and quantitative analysis of peak data. All patients were tested for BRCA1 rearrangements using multiplex ligation-dependent probe amplification. BRCA2 was tested in 39 selected cases that were characterized by family history of ovarian cancer or male breast cancer. This selection was considered cost-effective based on the rarity of this type of mutation in the BRCA2 gene.
Analysis
Power Analysis
Deleterious mutations in BRCA1 and BRCA2 are rare in the general population but are much higher in hereditary breast and ovarian cancer families [10, 18]. It is estimated that mutations in these genes account for ∼20% of families with large clusters of breast cancers and 12.4% of young breast cancer patients (aged <35 years) [18]. Using a conservative estimate of 12% of patients with early onset and/or familial breast cancer carrying a BRCA mutation, a cohort of at least 250 unrelated subjects was calculated to measure the prevalence of BRCA1 and BRCA2 alleles in Lebanon with a 95% confidence interval.
Variant and Haplotype Analysis
Sequences were compared with the cDNA reference sequence of BRCA1 and BRCA2 (accession numbers NM_007294.2 and NM 000059.3, respectively), and nucleotide numbering of all variants is in reference to the coding sequence with the A of the start codon being 1. The significance of variants was verified using the Breast Cancer Information Core (BIC) [19] and the UMD-BRCA1 and UMD-BRCA2 mutations databases [20, 21]. Variants were classified into three categories: deleterious variants, variants of uncertain significance (VUS), and neutral variants. In silico prediction analysis was performed for VUS using several prediction algorithms (supplemental online Table 1). Deleterious mutations were confirmed on a second blood sample. Single nucleotide polymorphisms (SNPs) and common variants present in the exonic and near-exonic sequence were used to manually determine BRCA1 and BRCA2 haplotypes.
Data Analysis
Data were entered and analyzed using SPSS version 20.0.0 (IBM Corp, Armonk, NY, http://www-01.ibm.com/software/analytics/spss/). Patient and tumor characteristics were compared among patients who were positive for deleterious mutations, those with VUS, and those who were negative (i.e., have neutral or no variants). Nonparametric Mann-Whitney, Kruskal-Wallis, and Fisher's exact tests were used as appropriate. A p value of <.05 was considered statistically significant.
Results
Patients and Disease Characteristics
Of the total of 250 patients, with a mean age of 40.8 ± 9 years, 148 patients were aged ≤40 years, and of those, only 74 had a positive family history. All patients aged >40 years were required to have a positive family history. Family history was obtained from medical records during the process of screening for eligibility. Once the patients were considered eligible for the study, they were thoroughly and reliably interviewed for family history by our assigned study research physicians. We have breast cancer histological confirmation on all patients enrolled in the study. Family members who were reported to have a history of breast or ovarian cancer were treated at various hospitals in Lebanon, and some were treated overseas; this made it very difficult to check histopathology. This was true for the 17 patients who had a positive family history of ovarian cancer and for whom we had details about history of surgery and treatment for ovarian cancer, but histological confirmation was available on only the 3 who happened to have been treated at the American University of Beirut Medical Center.
Stages at diagnosis for the whole group were as follows: stage I, 20.4%; stage II, 46.9%; stage III, 24.9%; stage IV, 7.8%. The majority of patients (92.3%) had infiltrating ductal carcinoma, and 64.5% had positive hormone receptors, whereas 19.2% were HER2 overexpressive.
Mutations
For the BRCA1 gene, seven patients had deleterious mutations and six had VUS (Table 1). For the BRCA2 gene, seven patients had deleterious mutations and 19 had VUS (Table 2). The prevalence of deleterious BRCA mutations in high-risk Lebanese women with breast cancer was 5.6% (2.8% for BRCA1 and 2.8% for BRCA2), and the prevalence of VUS was 13.2% (3.2% for BRCA1 and 10% for BRCA2). No large deletions or duplications in BRCA1 or BRCA2 were observed. VUS are described in supplemental online Table 1.
Table 1.
Sequence mutations identified in the BRCA1 gene
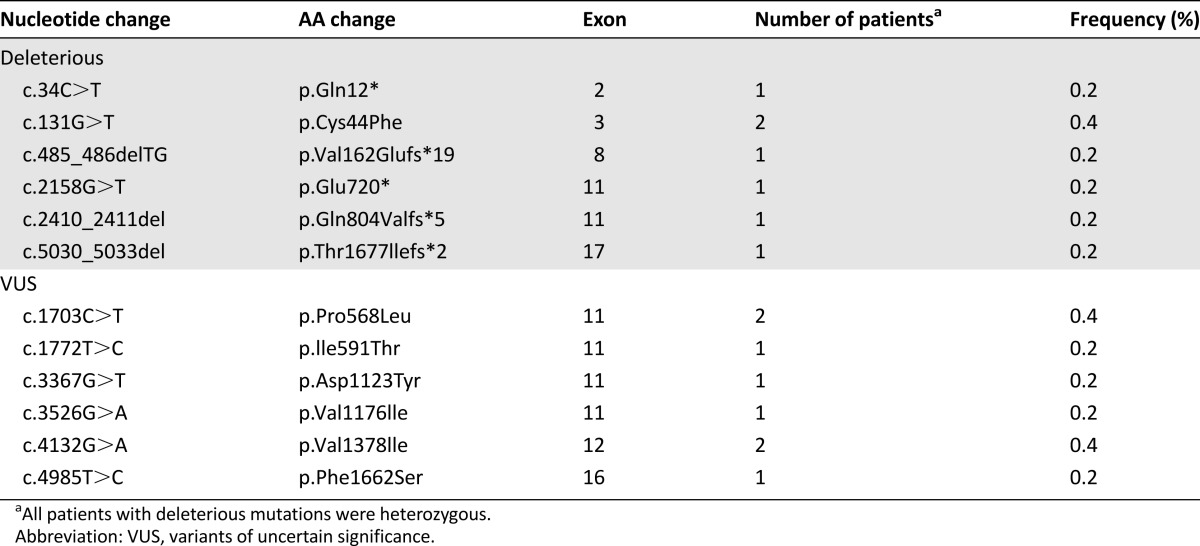
Table 2.
Sequence mutations identified in the BRCA2 gene

All patients with deleterious BRCA1 mutations had triple-negative grade 3 ductal breast carcinoma (Table 3).
Table 3.
Characteristics of patients with BRCA1 deleterious variants and variants of uncertain significance
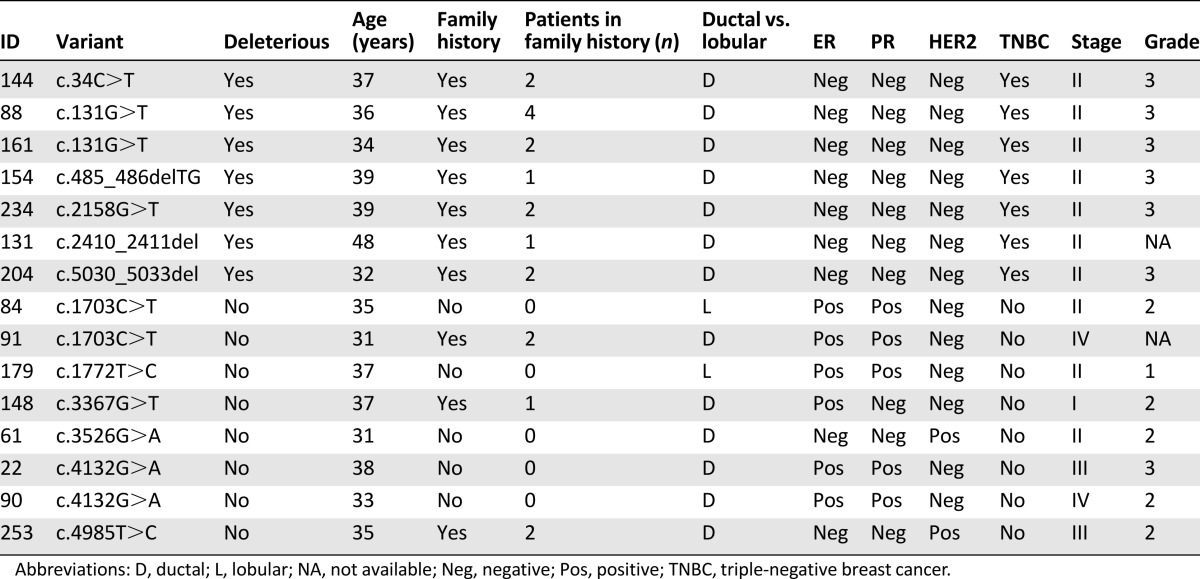
Patients with deleterious BRCA1 mutations were characterized by triple-negative grade 3 disease and positive family history (Table 3), whereas none of the BRCA2-positive patients had triple-negative breast cancer (Table 4).
Table 4.
Characteristics of patients with BRCA2 deleterious variants and variants of uncertain significance
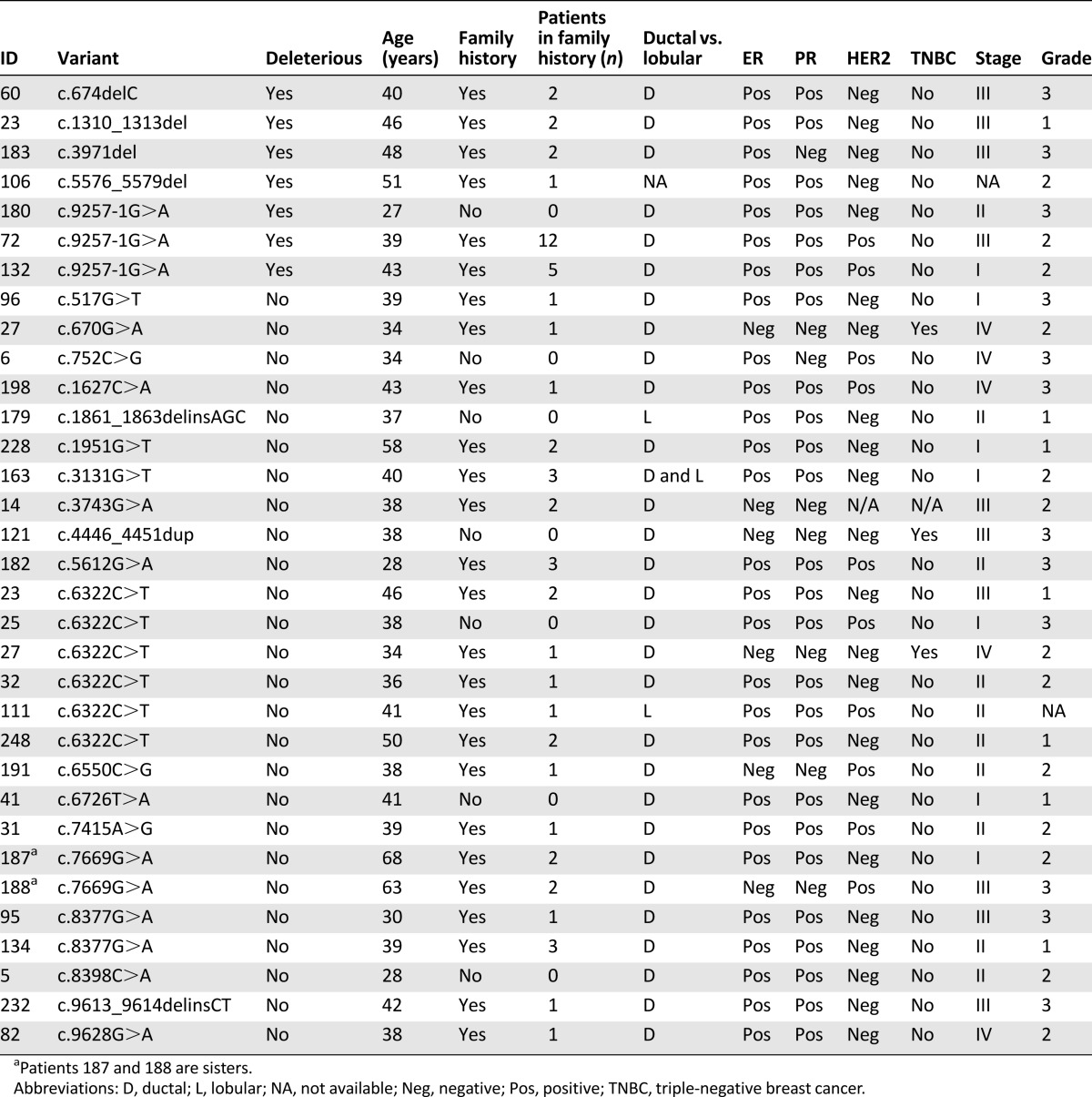
One BRCA2 VUS (c.6322C>T) was recurrent (n = 6) and co-occurred with a deleterious mutation (patient 23) (Table 4), suggesting this variant is benign. Patients with BRCA1 VUS were more likely nonmutated except for those who were slightly younger at diagnosis (the number of such cases was small) (Table 5).
Table 5.
Comparison of patient characteristics among negative, deleterious mutation, and VUS groups
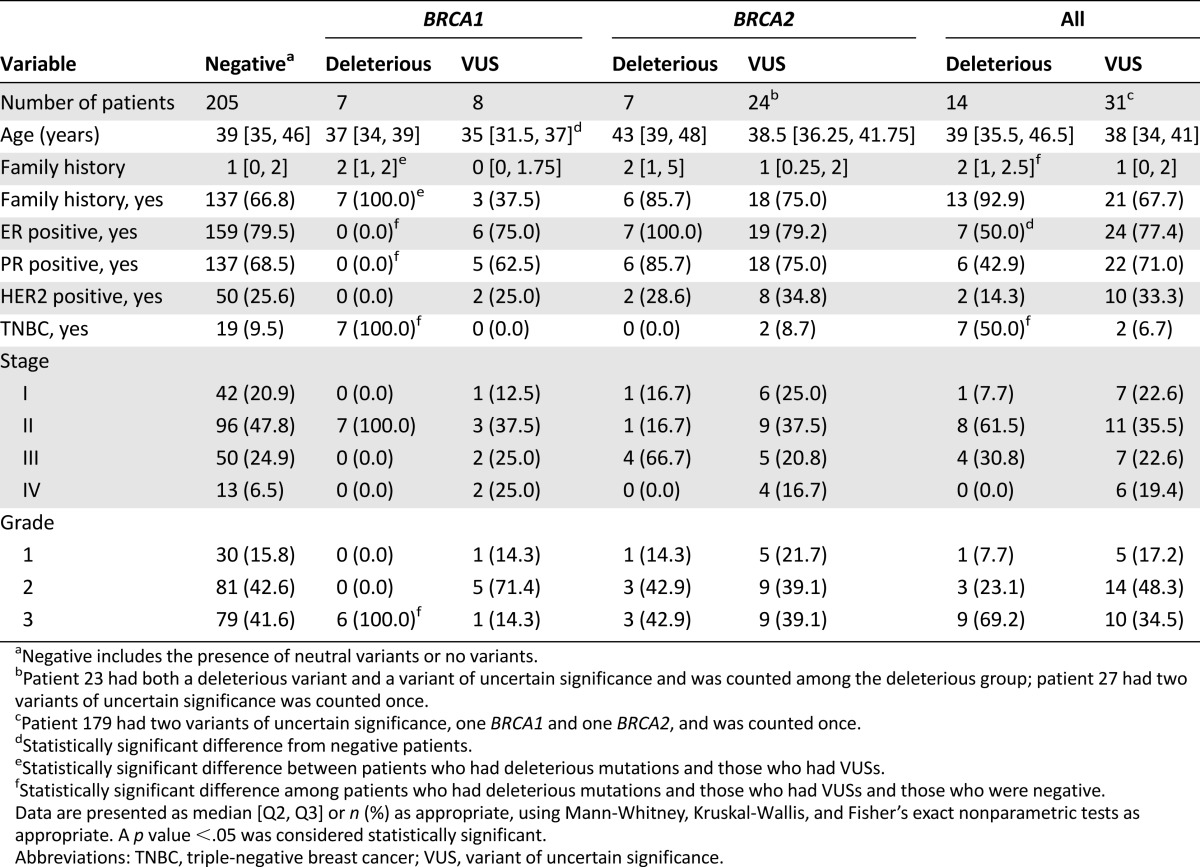
Of the 148 patients who were aged ≤40 years at diagnosis, 9 (6%) harbored deleterious mutations (6 BRCA1 and 3 BRCA2). When stratified by family history, 8 of 74 patients (10.8%) who were aged ≤40 years with positive family history had deleterious BRCA mutations (6 BRCA1 and 2 BRCA2). In contrast, only 1 of all 74 patients aged ≤40 years with negative family history (1.4%) had a deleterious BRCA2 mutation. Of the 75 patients aged 41–50 years with positive family history of ovarian and/or breast cancer, 4 (5.3%) had a deleterious mutation (1 BRCA1 and 3 BRCA2). Only 1 of 27 patients aged >50 years at diagnosis with positive family history had a BRCA2 deleterious mutation.
Eight patients reported family history of pancreatic cancer, and none were among those with BRCA2 mutations. Of the 21 patients with family history of prostate cancer, one was a BRCA1 carrier (c.34C>T) and one was a BRCA2 carrier (c.9257-1G>A).
Haplotypes
Eight BRCA1 haplotypes were identified at a frequency of ≥1% In addition to the most frequent haplotype (50% of alleles) that presented the default nucleotide at all positions (H1) (supplemental online Table 2), we observed 17 haplotypes with a frequency of >1%, with the most common at 27.5% (supplemental online Table 3). Fourteen major types accounted for 99% of all haplotypes; the remaining 1% consisted of 6 different rare combinations of alleles.
Discussion
This report is the first large study of BRCA mutations in high-risk hereditary breast and ovarian cancer patients in Lebanon and Arab countries. We observed 6 different deleterious mutations in BRCA1 and 5 in BRCA2 for a total of 14 mutation carriers using direct sequencing. The types of mutations observed were similar to those observed elsewhere, including stop codons, frame shifts, and splice-site mutations. No gene rearrangement and founder mutations were detected. Nine deleterious mutations were not reported previously, whereas five had been described previously by others: c.131G>T [22], c.485_486delTG [23], and c.5030_5033del [24] in BRCA1 and c.1310_1313del [25] and c.5576_5579del [24] in BRCA2. None of the 14 deleterious mutations were previously reported in Arab participants, including the BRCA2c.9257-1G>A variant that was detected in three families [10–17, 26–30]. Several of the detected VUS were novel because they were absent from the BIC database.
It has been estimated that the frequency of BRCA1 and BRCA2 mutations is around 1 in 800 in the general white population and reaches 1 in 40–50 in the Ashkenazi Jewish population [31]. Women carrying these mutations typically present at a young age (<40 years), especially carriers of BRCA1 mutations, and have a lifetime risk of developing breast and/or ovarian cancer in the range of 45%–85% for BRCA1 carriers and 40%–70% for BRCA2 carriers.
At 5.6%, the prevalence of deleterious mutations in our cohort of patients with high-genetic-risk breast cancer is lower than expected when compared with studies from industrialized nations. Using the same or similar case selection criteria, routine screening of cases at the CJP suggests 10%–25% of families carry a BRCA mutation if family history or young age at diagnosis is present. This finding suggests that we need to look for alternative gene mutations and other risk factors that may contribute to the development of breast cancer in these high-risk patients [32]. In addition, long-term follow-up of patients’ families is needed to estimate prognosis and penetrance. This aspect is important because penetrance and occurrence of breast cancer may be modified by other genetic mutations, reproductive factors, environmental factors, exercise, or diet. Recent increases in incidence rates in many countries, for example, have reflected changes showing that BRCA mutation carriers born after 1940 have higher breast cancer incidence than those born before 1940 (67% vs. 24%) [33].
The association of BRCA1 mutations with triple-negative grade 3 breast cancer was confirmed in our cohort, with all such mutation carriers having this particularly aggressive type of cancer. Only one patient had a positive family history of male breast cancer, but she did not carry a BRCA2 mutation.
Although this study did not include an extensive haplotype analysis, few different BRCA1 haplotypes were present in this cohort of Lebanese patients. Comparison with other published haplotypes of Moroccan populations showed that this haplotype accounted for 63% of haplotypes in Tunisia [13] and 59% of those in Algeria [14]; however, these studies did not include markers outside of a certain core. If we exclude those same markers from our estimate, grouping the L4 and L5 haplotypes with the canonical H1, 58% of Lebanese haplotypes correspond to the H1 reference. Both North African population studies observed the H7 and H10 haplotypes at 2%–5%, and these were present in the Lebanese sample at 1% and 3%, respectively [13, 14]. In addition to H1, H7, and H10, BRCA1 in our Lebanese sample can be described by five haplotypes. One of these, L1, represented 26% of haplotypes. Fifteen additional Tunisian and 12 additional Algerian BRCA1 haplotypes were absent from the Lebanese sample, despite the much larger population studied.
Very few publications are available for comparison of BRCA2 haplotype analysis using common SNPs in the coding region. A large study of Jewish populations looked at SNPs within and surrounding the BRCA2 locus, but only two markers were common with our data [34]. BRCA2 markers were not published for the Tunisian and Algerian populations described for BRCA1 above. As with BRCA1, using such a limited set of SNPs may oversimplify the haplotype structure present.
Conclusion
This report is the first large modern study of a cohort of Lebanese Arab women with breast cancer considered to be at a high risk for harboring hereditary disease. We showed a prevalence rate of 5.6% for having deleterious BRCA1 or BRCA2 mutations among this group of women. Prevalence is 10.8% in patients aged ≤40 years with positive family history (6 BRCA1 and 2 BRCA2 mutations) but is only 1.4% in patients aged ≤40 years with negative family history. Our study showed that BRCA mutations were lower than expected when compared with other populations and hence does not support the hypothesis that BRCA mutations alone are the cause of the high percentage of young women with breast cancer in Lebanon and in Arab countries. These results should help guide clinicians and policy makers with regard to genetic counseling and testing in Lebanon and Arab countries and in the Americas, Europe, and Australia, where large numbers of immigrants and citizens of Lebanese and Arab descent reside. Additional studies are needed to look for other mutations such as PALB2, CHEK2, and TP53.
See http://www.TheOncologist.com for supplemental material available online.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Supplementary Material
Acknowledgments
This study was funded by an Ethnic Research Initiative (ERI) competitive grant sponsored by GlaxoSmithKline. Presented in part at the 37th Annual San Antonio Breast Cancer Symposium, San Antonio, TX, December 9–13, 2014.
Footnotes
For Further Reading: Cynthia Villarreal-Garza, Christian Aguila, Maria C. Magallanes-Hoyos et al. Breast Cancer in Young Women in Latin America: An Unmet, Growing Burden. The Oncologist 2013;18:1298–1306.
Implications for Practice: This review illustrates that breast cancer (BC) among Latin American women is a growing burden throughout the region. The increased proportion of BC cases in young women is important because their diagnoses and tumor behavior are usually more aggressive than in their older counterparts. The findings of this study reveal that there is scarce information regarding this matter in Latin American countries, especially concerning the particular effects and complications that this group of women face during and after treatment. Also, there are no specific clinical or educational programs that focus on this population. A call to action from health policy planners, medical providers, researchers, BC patients, families, and the community in general is deserved for better care of this emergent challenge.
Author Contributions
Conception/Design: Nagi S. El Saghir, Nathalie K. Zgheib, Deborah K. Armstrong
Provision of study material or patients: Nagi S. El Saghir, Yannick Bidet, Georges M. Nemer, Ziad Salem, Ali Shamseddine, Arafat Tfayli, Jaber Abbas, Faek Jamali, Muhieddine Seoud, Nancy Uhrhammer
Collection and/or assembly of data: Nagi S. El Saghir, Nathalie K. Zgheib, Hussein A. Assi, Katia E. Khoury, Yannick Bidet, Sara M. Jaber, Stephanie Decousus, Pierre Romero, Georges M. Nemer, Nancy Uhrhammer
Data analysis and interpretation: Nagi S. El Saghir, Nathalie K. Zgheib, Hussein A. Assi, Katia E. Khoury, Yannick Bidet, Sara M. Jaber, Raghid N. Charara, Rania A. Farhat, Firas Y. Kreidieh, Stephanie Decousus, Pierre Romero, Deborah K. Armstrong, Yves-Jean Bignon, Nancy Uhrhammer
Manuscript writing: Nagi S. El Saghir, Nathalie K. Zgheib, Hussein A. Assi, Katia E. Khoury, Yannick Bidet, Raghid N. Charara, Rania A. Farhat, Firas Y. Kreidieh, Deborah K. Armstrong, Nancy Uhrhammer
Final approval of manuscript: Nagi S. El Saghir, Nathalie K. Zgheib, Hussein A. Assi, Katia E. Khoury, Yannick Bidet, Sara M. Jaber, Raghid N. Charara, Rania A. Farhat, Firas Y. Kreidieh, Stephanie Decousus, Pierre Romero, Georges M. Nemer, Ziad Salem, Ali Shamseddine, Arafat Tfayli, Jaber Abbas, Faek Jamali, Muhieddine Seoud, Deborah K. Armstrong, Yves-Jean Bignon, Nancy Uhrhammer
Disclosures
The authors indicated no financial relationships.
References
- 1.GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. Available at http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed February 24, 2015.
- 2.El Saghir NS, Khalil MK, Eid T, et al. Trends in epidemiology and management of breast cancer in developing Arab countries: A literature and registry analysis. Int J Surg. 2007;5:225–233. doi: 10.1016/j.ijsu.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 3.El Saghir NS, Assi HA, Jaber SM, et al. Outcome of breast cancer patients treated outside of clinical trials. J Cancer. 2014;5:491–498. doi: 10.7150/jca.9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chouchane L, Boussen H, Sastry KS. Breast cancer in Arab populations: Molecular characteristics and disease management implications. Lancet Oncol. 2013;14:e417–e424. doi: 10.1016/S1470-2045(13)70165-7. [DOI] [PubMed] [Google Scholar]
- 5.Shamseddine A, Sibai AM, Gehchan N, et al. Cancer incidence in postwar Lebanon: Findings from the first national population-based registry, 1998. Ann Epidemiol. 2004;14:663–668. doi: 10.1016/j.annepidem.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Lebanese national cancer registry. Available at http://www.moph.gov.lb/Prevention/Surveillance/Pages/Cancer.aspx. Accessed February 24, 2015.
- 7.El Saghir NS, Adib S, Mufarrij A, et al. Cancer in Lebanon: Analysis of 10,220 cases from the American University of Beirut Medical Center. J Med Liban. 1998;46:4–11. [PubMed] [Google Scholar]
- 8.Assi HA, Khoury KE, Dbouk H, et al. Epidemiology and prognosis of breast cancer in young women. J Thorac Dis. 2013;5(suppl 1):S2–S8. doi: 10.3978/j.issn.2072-1439.2013.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Saghir NS, Seoud M, Khalil MK, et al. Effects of young age at presentation on survival in breast cancer. BMC Cancer. 2006;6:194. doi: 10.1186/1471-2407-6-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karami F, Mehdipour P. A comprehensive focus on global spectrum of BRCA1 and BRCA2 mutations in breast cancer. Biomed Res Int. 2013;2013:928562. doi: 10.1155/2013/928562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasan TN, Shafi G, Syed NA, et al. Lack of association of BRCA1 and BRCA2 variants with breast cancer in an ethnic population of Saudi Arabia, an emerging high-risk area. Asian Pac J Cancer Prev. 2013;14:5671–5674. doi: 10.7314/apjcp.2013.14.10.5671. [DOI] [PubMed] [Google Scholar]
- 12.Awadelkarim KD, Aceto G, Veschi S, et al. BRCA1 and BRCA2 status in a central Sudanese series of breast cancer patients: Interactions with genetic, ethnic and reproductive factors. Breast Cancer Res Treat. 2007;102:189–199. doi: 10.1007/s10549-006-9303-z. [DOI] [PubMed] [Google Scholar]
- 13.Troudi W, Uhrhammer N, Sibille C, et al. Contribution of the BRCA1 and BRCA2 mutations to breast cancer in Tunisia. J Hum Genet. 2007;52:915–920. doi: 10.1007/s10038-007-0195-5. [DOI] [PubMed] [Google Scholar]
- 14.Uhrhammer N, Abdelouahab A, Lafarge L, et al. BRCA1 mutations in Algerian breast cancer patients: High frequency in young, sporadic cases. Int J Med Sci. 2008;5:197–202. doi: 10.7150/ijms.5.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laraqui A, Uhrhammer N, Lahlou-Amine I, et al. Mutation screening of the BRCA1 gene in early onset and familial breast/ovarian cancer in Moroccan population. Int J Med Sci. 2013;10:60–67. doi: 10.7150/ijms.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibrahim SS, Hafez EE, Hashishe MM. Presymptomatic breast cancer in Egypt: Role of BRCA1 and BRCA2 tumor suppressor genes mutations detection. J Exp Clin Cancer Res. 2010;29:82. doi: 10.1186/1756-9966-29-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jalkh N, Nassar-Slaba J, Chouery E, et al. Prevalance of BRCA1 and BRCA2 mutations in familial breast cancer patients in Lebanon. Hered Cancer Clin Pract. 2012;10:7. doi: 10.1186/1897-4287-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anglian Breast Cancer Study Group Prevalence and penetrance off BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Br J Cancer. 2000;83:1301–1308. doi: 10.1054/bjoc.2000.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breast Cancer Information Core: An open access on-line breast cancer mutation data base. Available at http://research.nhgri.nih.gov/bic/. Accessed February 24, 2015.
- 20.The UMD-BRCA1 mutations database. Available at http://www.umd.be/BRCA1/. Accessed February 24, 2015.
- 21.The UMD-BRCA2 mutations database. Available at http://www.umd.be/BRCA2/. Accessed February 24, 2015.
- 22.Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: A report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654–2663. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borg A, Haile RW, Malone KE, et al. Characterization of BRCA1 and BRCA2 deleterious mutations and variants of unknown clinical significance in unilateral and bilateral breast cancer: The WECARE study. Hum Mutat. 2010;31:E1200–E1240. doi: 10.1002/humu.21202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castéra L, Krieger S, Rousselin A, et al. Next-generation sequencing for the diagnosis of hereditary breast and ovarian cancer using genomic capture targeting multiple candidate genes. Eur J Hum Genet. 2014;22:1305–1313. doi: 10.1038/ejhg.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vos JR, Teixeira N, van der Kolk DM, et al. Variation in mutation spectrum partly explains regional differences in the breast cancer risk of female BRCA mutation carriers in the Netherlands. Cancer Epidemiol Biomarkers Prev. 2014;23:2482–2491. doi: 10.1158/1055-9965.EPI-13-1279. [DOI] [PubMed] [Google Scholar]
- 26.El-Harith el-HA, Abdel-Hadi MS, Steinmann D, et al. BRCA1 and BRCA2 mutations in breast cancer patients from Saudi Arabia. Saudi Med J. 2002;23:700–704. [PubMed] [Google Scholar]
- 27.Al-Moundhri MS, Al-Ansari A, Al-Mawali K, et al. BRCA1 gene molecular alterations in Omani breast cancer patients. Gulf J Oncolog. 2013;1:45–51. [PubMed] [Google Scholar]
- 28.Mahfoudh W, Bouaouina N, Ahmed SB, et al. Hereditary breast cancer in Middle Eastern and North African (MENA) populations: Identification of novel, recurrent and founder BRCA1 mutations in the Tunisian population. Mol Biol Rep. 2012;39:1037–1046. doi: 10.1007/s11033-011-0829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cherbal F, Bakour R, Adane S, et al. BRCA1 and BRCA2 germline mutations screening in Algerian breast/ovarian cancer families. Dis Markers. 2010;28:377–384. doi: 10.3233/DMA-2010-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadouri L, Bercovich D, Elimelech A, et al. A novel BRCA-1 mutation in Arab kindred from east Jerusalem with breast and ovarian cancer. BMC Cancer. 2007;7:14. doi: 10.1186/1471-2407-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rennert G, Dishon S, Rennert HS, et al. Differences in the characteristics of families with BRCA1 and BRCA2 mutations in Israel. Eur J Cancer Prev. 2005;14:357–361. doi: 10.1097/00008469-200508000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Antoniou AC, Casadei S, Heikkinen T, et al. Breast-cancer risk in families with mutations in PALB2. N Engl J Med. 2014;371:497–506. doi: 10.1056/NEJMoa1400382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 34.Im KM, Kirchhoff T, Wang X, et al. Haplotype structure in Ashkenazi Jewish BRCA1 and BRCA2 mutation carriers. Hum Genet. 2011;130:685–699. doi: 10.1007/s00439-011-1003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


