Abstract
Background.
Disulfiram, an alcohol aversion agent, has been in use for >50 years. Numerous authors have reported an anticancer effect of this drug in vitro and in mouse models. More recently, several reports have claimed that disulfiram also possesses anti-stem cell activity. We set out to obtain initial data regarding the safety of combining this drug with chemotherapy and the possible effectiveness of disulfiram in a combination regimen in non-small cell lung cancer (NSCLC).
Methods.
This phase II, multicenter, randomized, double-blinded study assessed the safety and efficacy of adding of disulfiram to cisplatin and vinorelbine for six cycles. Newly diagnosed NSCLC patients were recruited. Patients with either stage IV or what was considered at the time “wet IIIb” (since 2009, these patients have been considered stage IV) were recruited. The patients were treated with only chemotherapy, and none were treated with either surgery or chemoradiation. Disulfiram was administered at a dose of 40 mg three times daily.
Results.
Forty patients were treated for more than two cycles, half with and half without disulfiram, which was well tolerated. An increase in survival was noted for the experimental group (10 vs. 7.1 months). Interestingly, there were only two long-term survivors, both in the disulfiram group.
Conclusion.
The addition of disulfiram to a combination regimen of cisplatin and vinorelbine was well tolerated and appeared to prolong survival in patients with newly diagnosed non-small cell lung cancer. The results from this small study seem encouraging enough for assessment in larger trials. Disulfiram is an inexpensive and safe drug; if its addition to chemotherapy could be shown to prolong survival, an effective regimen could be established and used widely, even in resource-poor countries.
Author Summary
Discussion
Given preclinical data suggesting antitumor activity and its established safety, there is interest in the use of disulfiram as an anticancer drug [1, 2]. To our knowledge, this randomized trial is the first using disulfiram as an additional anticancer treatment in lung cancer. Although its precise mechanisms of action have yet to be established, preclinical studies have suggested that disulfiram possesses antiangiogenic activity and can inhibit the activity of ATP-binding cassette transporters [3]. Additional activity of disulfiram has been suggested recently: inhibition of cancer stem cells. The latter is thought to be a consequence of disulfiram’s inhibitory effect on aldehyde dehydrogenase, an enzyme that is highly expressed in what many believe are cancer stem cells [4]. Kim et al. recently demonstrated that the use of disulfiram can induce apoptosis in pancreatic cancer stem cells expressing high levels of aldehyde dehydrogenase [5]. This finding suggests that disulfiram’s anti-cancer stem cell activity may be of great importance, especially in tumors that have an initial response to the therapy.
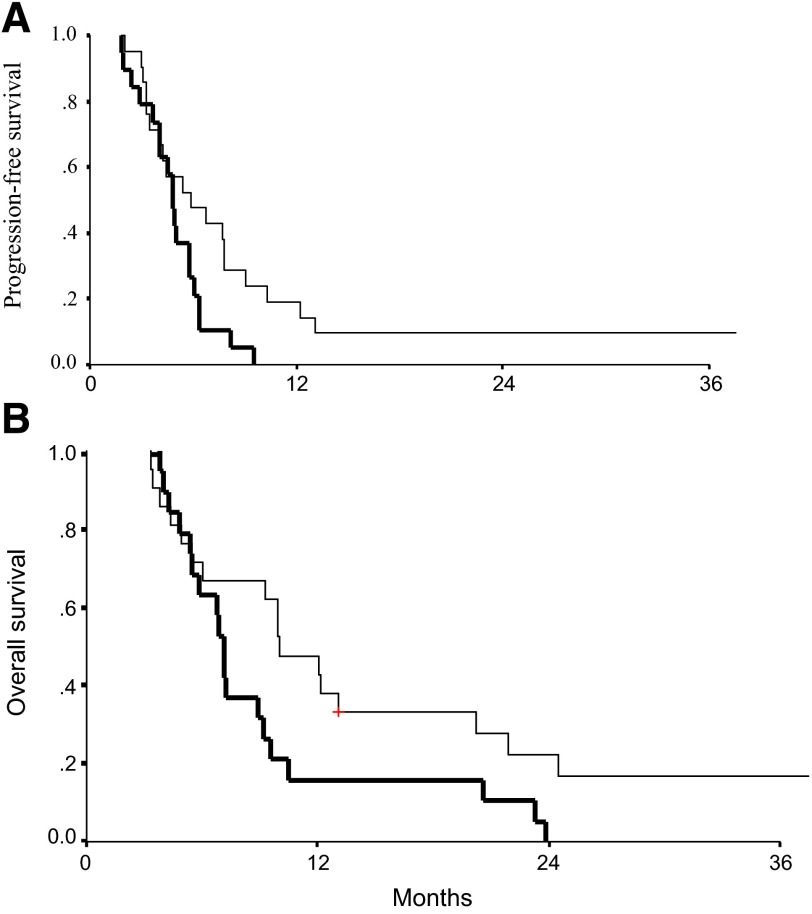
In this paper, we described a phase II trial of a regimen using cisplatin plus vinorelbine with or without disulfiram in lung cancer. Disulfiram was continued after cessation of chemotherapy after six cycles. Analysis was performed on the 40 patients who continued treatment beyond the first two cycles. The higher response rate (46% vs. 37%) in the disulfiram group was not statistically different. Quality of life was similar between the groups; however, it is noteworthy that both progression-free and overall survival curves separate, and because of the long-term response of a few patients in the active group, there is a statistically significant progression-free and overall survival advantage for the active group (Fig. 1).
Figure 1.
Progression-free survival (5.9 vs. 4.9 months; p = .043) (A) and overall survival (10.0 vs. 7.1 months; p = .041) (B) of patients in this phase II trial. Controls were treated with six cycles of cisplatin and vinorelbine (plus placebo tablets), and experimental groups were treated with the same treatment plus the addition of disulfiram, which was continued for stable or responding patients. Improvement in both progression-free and overall survival seems to have happened only after a few months.
The unusual result is the long-term survival of two patients in the active group, an event that is extremely rare in patients with stage IV lung cancer treated by chemotherapy alone. It is important to note that the difference in survival continued after cessation of chemotherapy and maintenance with disulfiram alone.
The drug is inexpensive, and its tolerability and safety have been demonstrated over years of clinical experience with a large number of patients. Our results support a larger phase III trial combining this drug with chemotherapy.
Supplementary Material
Footnotes
Access the full results at: Nechushtan-14-424.theoncologist.com
ClinicalTrials.gov Identifier: NCT00312819
Sponsor(s): Ora Bio Ltd.
Principal Investigators: Hovav Nechushtan, Yousef Hamamreh, Salim Nidal, Nili Peylan-Ramu
IRB Approved: Yes
Author disclosures and references available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.