Abstract
Needle track seeding following image guided needle biopsy is a known but uncommon complication in the workup of hepatocellular carcinoma. We present the case of a 55 year-old male who was found to have a recurrent hepatocellular carcinoma in the rectus sheath five years following a CT guided biopsy with the biopsy needle passing through the anterior abdominal wall muscles.
Keywords: Liver, hepatic, mass, percutaneous, biopsy, coaxial, needle, tract, seeding, hepatocellular, carcinoma, metastatic disease, transplant
CASE REPORT
A 50 year-old male with a history of alcohol abuse, hepatitis C, and cirrhosis underwent a screening CT which demonstrated a 2.5 × 2.6 × 2.5 cm enhancing hepatic mass. The mass was biopsied from an anterior approach, with a 20 gauge, 15 cm Temno Evolution biopsy needle (CareFusion) with 19 gauge introducer needle passing through the superior right rectus abdominis muscle (Figure 1). The biopsy results demonstrated hepatocellular carcinoma (HCC) (Figure 2A).
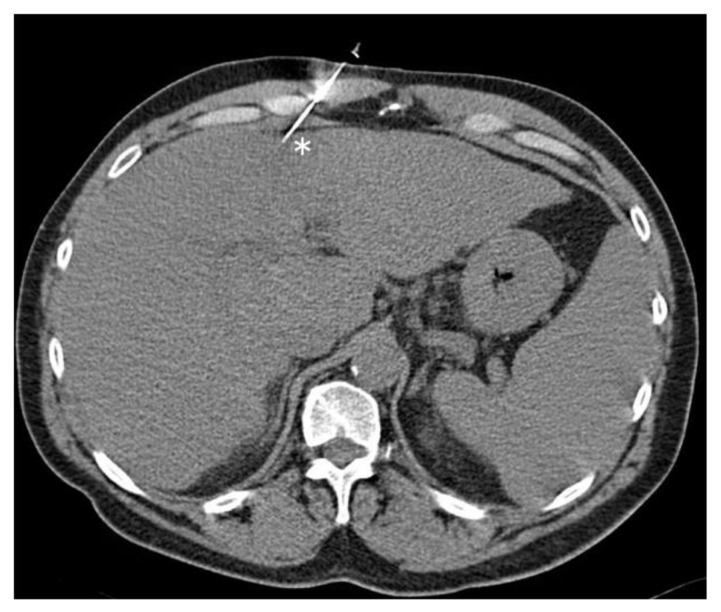
Figure 1.
Fifty year old male with hepatic lesion undergoing anterior approach CT guided liver biopsy. FINDINGS: The biopsy needle (asterisk) passes through the right rectus sheath. TECHNIQUE: Axial unenhanced CT, 120 kVp, 165 mAs, 3mm slice thickness, obtained on Siemens Volume Zoom.
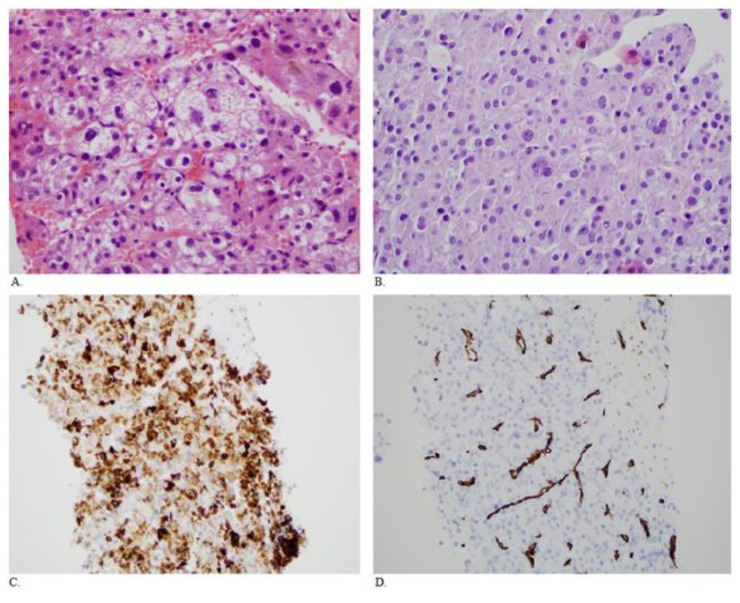
Figure 2.
Fifty year old male with HCC. A. Sections of the original liver needle core biopsies show a moderately differentiated hepatocellular carcinoma with a trabecular growth pattern. There are focally bizarre nuclei. (H&E, 400× original magnification) B. Sections of the rectus mass core biopsy show similar morphology to the original biopsy taken over 5 years previously (H&E, 400× original maginification). C. Immunohistochemistry shows the tumor cells are positive for the hepatocyte marker Hep Par 1 (clone OCH1E5) (200× original magnification). D. There is also CD34 immunopositivity in the endothelial cells, typical of hepatocellular carcinoma (200× original magnification). The morphology and the stains support the diagnosis of a metastatic hepatocellular carcinoma.
One year following the biopsy, the patient underwent orthotopic liver transplantation. Due to HCC with vascular invasion noted on the explanted liver, the patient underwent chemotherapy for 4 months after transplantation. Subsequently, he developed recurrent hepatitis C as well as a right lung mass demonstrated to be squamous cell carcinoma which was resected and the patient received adjuvant chemotherapy for 6 weeks.
A follow up CT scan of the abdomen approximately five years after the initial liver biopsy demonstrated a rounded soft tissue mass measuring 4.3 × 3.8 × 6.1 cm in diameter centered within the right rectus sheath, involving the right rectus abdominis muscle, and abutting the costochondral cartilage of the right anterior chest wall. The mass demonstrated moderate heterogeneous enhancement on venous phase CT images and enhancement similar to the liver on delayed phase CT images (Figure 3). On MRI, the mass was slightly hyperintense to muscle on T2-weghted images, isointense to muscle on T1-weighted images, and demonstrated moderate enhancement (Figure 4). The mass was biopsied, with the pathology results consistent with metastatic HCC (Figure 2B–D). Subsequently, the mass was excised (Figure 5).
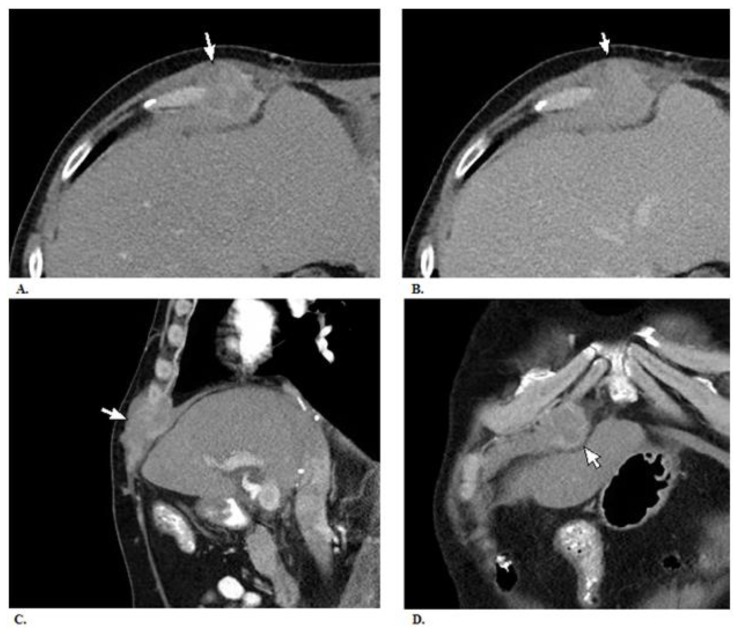
Figure 3.
Fifty five year old male with biopsy needle tract seeding from HCC. A. There is a mass measuring 4.3 × 3.8 × 6.1 cm within the right rectus sheath (arrow). The mass demonstrates heterogeneous enhancement on venous phase images. B. Its enhancement is less intense and similar to that of the liver on delayed phase images. C. & D. Sagittal and coronal reconstructions of venous phase images showing the mass. It appears to invade the adjacent costochondral cartilage. Mild thickening of the anterior right hemidiaphragm suggests possible involvement. No obvious invasion of the underlying liver is seen, when accoutning for volume averaging artifact. There is no evidence of spread into the subcutaneous fat. TECHNIQUE: Contrast enhanced CT (120 cc iohexol) CT images in venous phase (100 kVp, 295 mAs, 5mm slice thickness, scan obtained 60 seconds following injection of contrast) and delayed phase (100 kVp, 261 mAs, 5mm slice thickness, scan obtained 3 minutes following injection of contrast) obtained on Siemens SOMATOM Definition AS.
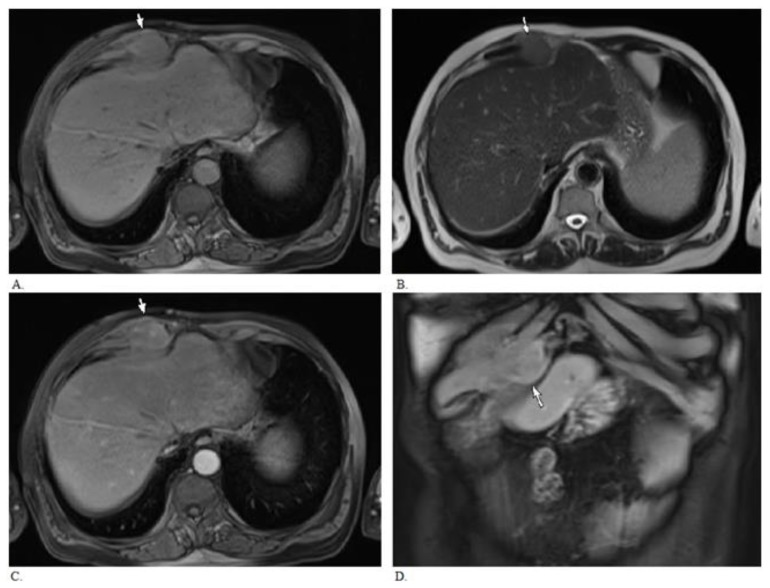
Figure 4.
MRI of 55 year old male with needle tract seeding from HCC 5 years following percutaneous biopsy. MRI demonstrates right rectus sheath enhancing mass (arrow) abutting and possibly invading the adjascent costochondral cartilage. There is no obvious invasion of the adjacent liver. A. Axial T1 weighted Volumetric Interpolated Breath Hold Examination image (VIBE, TR 7.22, TE 3.38, 4mm slice thickness, 1.5 Tesla). B. Axial T2 weighted Half-Fourier Acquisition Single-Shot Turbo Spin-Echo image (HASTE, TR 1000, TE 80, 6mm slice thickness, 1.5 Tesla). C. & D. Axial and coronal post gadolinium T1 weighted images (VIBE, arterial phase after administration of 19 cc gadodiamide intravenously, TR 7.22, TE 3.38, 4 mm slice thickness).
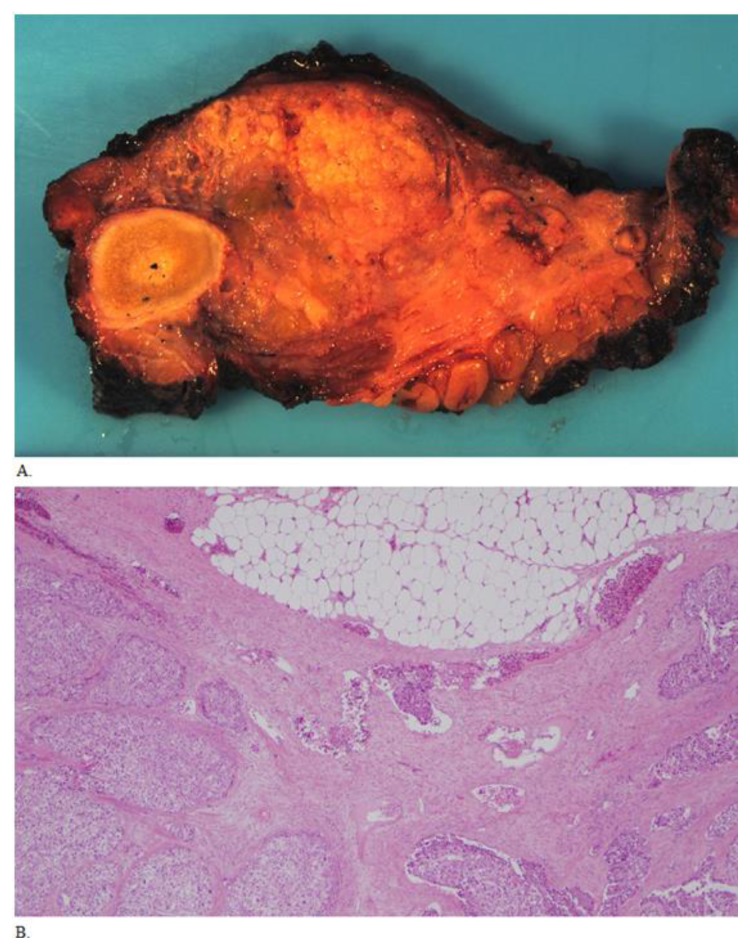
Figure 5.
Fifty five year old male with biopsy needle tract seeding by HCC, status post resection of the metastatic mass. A. Grossly, the abdominal wall mass resection specimen had tan-pink lobular cut surfaces with no bony invasion. B. Histologic sections show similar morphology compared to the two previous biopsies. Extensive lymphovascular invasion is present, and is seen in the center of the field (40× original magnification).
DISCUSSION
Clinical & imaging findings
Needle tract seeding invariably presents with one or multiple rounded enhancing nodules along the needle biopsy tract. Nodules can be located within the peritoneum, along the abdominal muscles which were penetrated by the needle, or in the subcutaneous and cutaneous tissues[1]. Seeding metastases demonstrate similar enhancement characteristics, appearance, and doubling time as intrahepatic HCC[2], which typically demonstrates strong arterial phase enhancement with “washout” on venous and delayed phases. Appearance is generally nonspecific, with heterogeneous echogenicity, variable T1 signal intensity, usually high T2 signal intensity, and hypermetabolic activity on PET scan (Table 1). Intrahepatic HCC is typically positive with gallium scintigraphy, and would probably be positive with needle tract seeding, although this study was not performed in the case reported here. Radiographs are most likely to appear normal although a soft tissue mass or rib erosion could potentially be seen.
Table 1.
Differential diagnosis for needle tract seeding by HCC and imaging characteristics of each entity.
| Needle tract seeding by HCC | Soft tissue sarcoma | Metastatic squamous cell carcinoma | |
|---|---|---|---|
| X-ray | Nonspecific soft tissue mass and bone erosion (if present) may be visible although most likely normal appearing radiographs | ||
| Ultrasound | Mixed echogenicity mass | ||
| CT | Soft tissue density mass which may contain hypodensity/necrosis and calcification | ||
| MRI | Variable T1 intensity, usually T2 hyperintense in relation to liver | Variable appearance, high (fat) and low (calcification) T1 and T2 signal. Hemorrhage may be present. | Variable appearance, may be T1 iso/hypointense and T2 moderately hyperintense |
| Enhancement pattern | Enhances strongly on arterial phase with progressive “washout” on venous and delayed phases | Enhances heterogeneously | Enhances heterogeneously; early enhancement |
| Scintigraphy | Intrahepatic HCC is typically positive with gallium scan | May concentrate thallium | Nonspecific findings |
| PET | Typically hypermetabolic, however, may be similar to liver FDG uptake | ||
Differential Diagnoses
In addition to needle tract seeding by HCC, differential diagnosis includes soft tissue sarcoma and metastatic disease from the patient’s previous squamous cell carcinoma. The imaging findings are similar for these lesions. Soft tissue sarcoma may concentrate thallium.
Treatment & prognosis
The patient was treated with local excision of the mass. No gross residual tumor was noted at surgery. However, upon microscopic examination, tumor free margins were less than 1mm and lymphovascular invasion was noted to be present. For this reason, the patient was started on sorafenib which was later discontinued due to an adverse drug reaction (scalp burning). Subsequently, the patient was started on sunitinib. There was no evidence of new neoplastic disease 6 months after mass resection. Radiaton therapy was not administered after resection.
In prior case reports, recurrence has been readily treated by excision, embolization, or radiation[3]. However, neoplastic spread due to seeding has also been deemed inoperable in some reported cases[4]. Prognosis for patients with needle tract seeding has not been studied extensively. One study found that none of six patients who underwent en-bloc wide excision developed local recurrence at the site of seeding, while the two patients who underwent mass excision both developed local recurrence [5]. To our knowledge, gender or age has not been associated with likelihood of biopsy tract seeding (Table 2).
Table 2.
Summary table for needle tract seeding by HCC.
| Etiology | Adhesion of tumor cells to biopsy needle and relocation of tumor cells via post-biopsy bleeding |
| Incidence | Exact incidence unknown. Estimates vary from 0 to 11%. Probably approximately 1.7% or less. |
| Gender ratio | No known gender predilection. |
| Age predilection | No known age predilection. |
| Risk factors | Possible risk factors for needle tract seeding include larger needle diameter, more needle passes, patient immunosuppression, and tumor aggressiveness |
| Treatment | Excision, embolization, chemotherapy, and/or radiation therapy. |
| Prognosis | Not extensively studied. Wide en-bloc excision improves prognosis. |
| Findings on imaging | Enhancing nodule along biopsy tract. Similar characteristics as intrahepatic HCC. |
Etiology & demographics
Approximately thirty thousand new cases of liver cancer are estimated to have occured in the United States in 2013, with more than 80% of these cases being HCC[6]. The primary burden of this disease is seen in developing countries, however, incidence of HCC in the United States has tripled during the past 2 decades. This has been primarily attributed to increased prevalence of Hepatitis C virus (HCV) infection, but also to alcoholic hepatitis and non-alcoholic fatty liver disease[7]. The introduction of contrast enhanced cross sectional imaging has decreased the need for percutaneous needle biopsy (PNB). However, the high sensitivity and specificity of PNB is still useful when diagnosis is uncertain[8].
Complications of PNB are uncommon, but can include malignant seeding, hematoma formation, sepsis, infection, pneumothorax and death. The incidence of seeding has been the subject of a number of studies, with reported incidence varying widely from 0 to 11%[9,10,1]. A literature review performed by Stigliano et al concluded that the overall mean risk of seeding is 1.73%, based on results from 2242 patients. The exact incidence of needle tract seeding is difficult to accurately determine as there have only been case reports and small retrospective studies but no prospective studies.
Proposed mechanisms for needle track seeding include adhesion of tumor cells to biopsy needle and relocation of tumor cells via post-biopsy bleeding. Possible risk factors for needle tract seeding include larger needle diameter, more needle passes, patient immunosuppression, and tumor aggressiveness[4]. However, smaller needle diameter could lead to more needle passes in order to obtain enough material for histologic analysis[11]. Risk of seeding appears to decrease when biopsy is combined with percutaneous ethanol ablation or radiofrequency ablation[9].
One study of 128 patients with HCC who had undergone image guided biopsy found no evidence of needle tract seeding when using a coaxial cutting needle system[3]. However, our report demonstrates such a case. One potential source for seeding is the distal portion of the needle which could protrude past the needle introducer, if left within the introducer needle as the introducer needle is being withdrawn. The needle introducer tip itself could also hypothetically seed the tract, if it was inserted into the border of the mass or if malignant cells are transferred to the tip.
It is important to perform PNB only when necessary in order to minimize risks associated with the procedure [4]. However, a study examining the Model for End-Stage Liver Disease allocation policy determined that 31% of patients undergoing liver transplantation for stage 1 HCC lesions had no pathological evidence of HCC in the explanted liver and were misdiagnosed before transplantation [12]. This creates a quandary as to what is the best course of action. Tumor recurrence due to the low risk of needle tract seeding can pose a potentially deadly complication, however, better pretransplant assessment would lead to less unnecessary transplantation operations and better allocation of available organs. It is important to be aware of the possibility of needle tract seeding following HCC biopsy in order to provide optimal follow up surveillance and treatment.
TEACHING POINT
Needle tract seeding with neoplastic disease is a rare but serious complication that may occur following hepatocellular carcinoma biopsy, even with co-axial cutting needle biopsy systems. The benefits of diagnostic certainty need to be weighed against the risk of complications with percutaneous needle biopsy.
ABBREVIATIONS
- CT
computed tomography
- HASTE
Half-Fourier Acquisition Single-Shot Turbo Spin-Echo
- HCC
hepatocellular carcinoma
- MRI
magnetic resonance imaging
- PNB
percutaneous needle biopsy
- VIBE
Volumetric Interpolated Breath Hold Examination
REFERENCES
- 1.Chang S, Kim SH, Lim HK, et al. Needle Tract Implantation after Percutaneous Interventional Procedures in Hepatocellular Carcinomas: Lessons Learned from a 10-year Experience. Korean J Hepatol. 2008;9:268–274. doi: 10.3348/kjr.2008.9.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang S, Kim SH, Lim HK, Lee WJ, Choi D, Lim JH. Needle tract implantation after sonographically guided percutaneous biopsy of hepatocellular carcinoma: evaluation of doubling time, frequency, and features on CT. AJR Am J Roentgenol. 2005;185:400–405. doi: 10.2214/ajr.185.2.01850400. [DOI] [PubMed] [Google Scholar]
- 3.Maturen KE, Nghiem HV, Marrero JA, et al. Lack of Tumor Seeding of Hepatocellular Carcinoma After Percutaneous Needle Biopsy Using Coaxial Cutting Needle Technique. AJR. 2006;187:1184–1187. doi: 10.2214/AJR.05.1347. [DOI] [PubMed] [Google Scholar]
- 4.Stigliano R, Burroughs AK. Should we biopsy each liver mass suspicious for HCC before liver transplantation? - No, please don’t. J Hepatol. 2005;43(4):563–568. doi: 10.1016/j.jhep.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Ahn DW, Shim JH, Yoon JH, et al. Treatment and clinical outcome of needle-track seeding from hepatocellular carcinoma. Korean J Hepatol. 2011;17:106–112. doi: 10.3350/kjhep.2011.17.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Cancer Society. Cancer Facts and Figures 2013. Atlanta, Ga: American Cancer Society; 2013. [Last accessed August 25, 2013]. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf. [Google Scholar]
- 7.El-Serag HB. Hepatocellular Carcinoma. N Engl J Med. 2011;365(12):1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 8.Caturelli E, Ghittoni G, Roselli P, De Palo M, Anti M. Fine Needle Biopsy of Focal Liver Lesions: The Hepatologist’s Point of View. Liver Transpl. 2004;10(2 Suppl 1):S26–29. doi: 10.1002/lt.20037. [DOI] [PubMed] [Google Scholar]
- 9.Stigliano R, Marelli L, Yu D, Davies N, Patch D, Burroughs AK. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev. 2007;33(5):437–447. doi: 10.1016/j.ctrv.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Silva MA, Hegab B, Hyde C, Guo B, Buckels JAC, Mirza DF. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut. 2008;57:1592–1596. doi: 10.1136/gut.2008.149062. [DOI] [PubMed] [Google Scholar]
- 11.Schotman SN, De Man RA, Stoker J, Zondervan PE, Ijzermans JN. Subcutaneous seeding of hepatocellular carcinoma after percutaneous needle biopsy. Gut. 1999;45:626–627. doi: 10.1136/gut.45.4.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiesner RH, Freeman RB, Mulligan DC. Liver transplantation for hepatocellular cancer: the impact of the MELD allocation policy. Gastroenterology. 2004;127(suppl 1):S261–S267. doi: 10.1053/j.gastro.2004.09.040. [DOI] [PubMed] [Google Scholar]