Abstract
Soft tissue aneurysmal bone cyst is a rare entity, with about 20 cases reported in literature, only 3 of which are in patients over 40 years of age. We present a case of a 41 year old Latin American female who presented for evaluation of atraumatic chest pain with radiation to the left shoulder. Her initial workup was negative, including radiographic imaging of the chest and left shoulder. 4 months later, she presented to her orthopedic surgeon with a palpable mass and mild left shoulder pain. Radiographs acquired at that time demonstrated a 7.0 × 5.5 × 6.7 cm mass with rim calcification in the region of the upper triceps muscle. Subsequent CT imaging showed central areas of hypodensity and thin septations, a few of which were calcified. MR evaluation showed hemorrhagic cystic spaces with multiple fluid-fluid levels and enhancing septations. Surgical biopsy was performed and pathology was preliminarily interpreted as cystic myositis ossificans, however on final review the diagnosis of soft tissue aneurysmal bone cyst was made. The lesion was then surgically excised and no evidence of recurrence was seen on a 3 year post-op radiograph. Following description of our case, we conduct a literature review of the imaging characteristics, diagnosis, and treatment of soft tissue aneurysmal bone cyst.
Keywords: Soft tissue aneurysmal bone cyst, extraskeletal aneurysmal bone cyst, aneurysmal bone cyst, STABC, ABC, soft tissue mass, CT imaging MR imaging
CASE REPORT
We present the case of a 41 year old female with no significant past medical history who originally presented to the emergency department with slowly progressive left upper arm pain over several weeks with radiation to the chest without a known history of trauma. The patient went to the emergency department for evaluation since she was concerned of a cardiac origin and did not have a primary care physician. Her vital signs and laboratory values (including cardiac enzymes) were normal. Initial radiographs of the chest and left shoulder were obtained and were essentially unremarkable (figures 1 and 2a–2b). The patient was discharged to home and told to follow up with orthopedic surgery as needed for her arm pain.
Figure 1.
41 year old female with a soft tissue aneurysmal bone cyst.
Findings: PA radiograph of the chest obtained at the patient’s initial presentation to the Emergency Department showed no acute cardiopulmonary process.
Technique: Radiograph acquired on a GE® CR digital radiography unit. X-ray tube was set at 125kVp.
Figure 2.
41 year old female with a soft tissue aneurysmal bone cyst.
Findings: AP (figure 2a) and axial (figure 2b) radiographs of the left shoulder obtained at the patient’s initial presentation to the Emergency Department showed no acute pathology. No mass was seen at that time.
Technique: Radiographs were acquired on a GE® CR digital radiography unit with the x-ray tube set at 70 kVp.
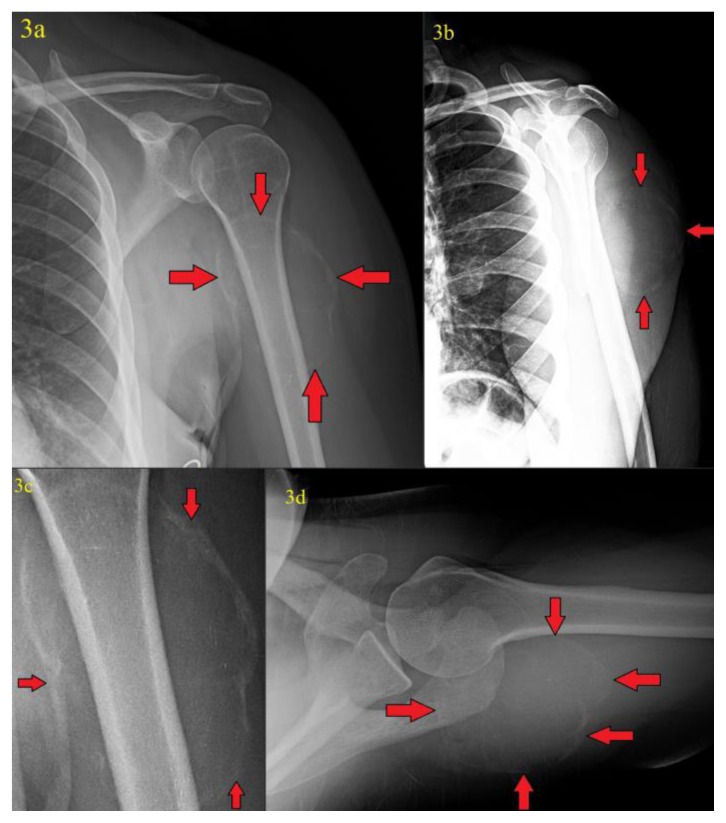
The patient opted to see an orthopedic surgeon 4 months later because she experienced occasional mild/dull pain in her left upper arm and was able to palpate a growing mass. A new series of left shoulder radiographs was performed which showed a 7.0 (transverse) × 5.5 (anteroposterior) × 6.7 (craniocaudad) cm mass with rim calcification in the region of the posterior deltoid/upper triceps muscles (figures 3a–3d). Due to the fairly rapid growth of the lesion, a computed tomography (CT) scan of the chest was ordered to better evaluate the lesion and check for any other sites of involvement.
Figure 3.
41 year old female with a soft tissue aneurysmal bone cyst.
Findings: Internal rotation (figure 3a), scapular Y-view (figure 3b), magnification (figure 3c), and axial (figure 3d) radiographs of the left shoulder obtained 4 months after the patient’s initial presentation showed interim development of a 7.0 (transverse) cm × 5.5 (anteroposterior) cm × 6.7 cm (craniocaudad) cm peripherally calcified mass in the region of the posterior deltoid/upper triceps muscles (red arrows).
Technique: Radiographs were acquired on a GE® CR digital radiography unit with x-ray tube set at 70 kVp. Figure 3c is magnification view from figure 3a.
On CT, the mass again showed peripheral calcification. Central areas of hypodensity and a few calcified thin septations were also seen (figures 4a–4c). Magnetic resonance imaging (MRI) subsequently performed at an outside institution demonstrated a mass localized to the proximal triceps muscle and showed blood filled spaces of varying sizes with numerous fluid-fluid levels, T1/T2 hypointense septa, and a T1/T2 hypointense calcified rim with some septal enhancement (figures 5a–5f). There was no osseous involvement. A differential diagnosis of sarcoma and myositis ossificans was raised.
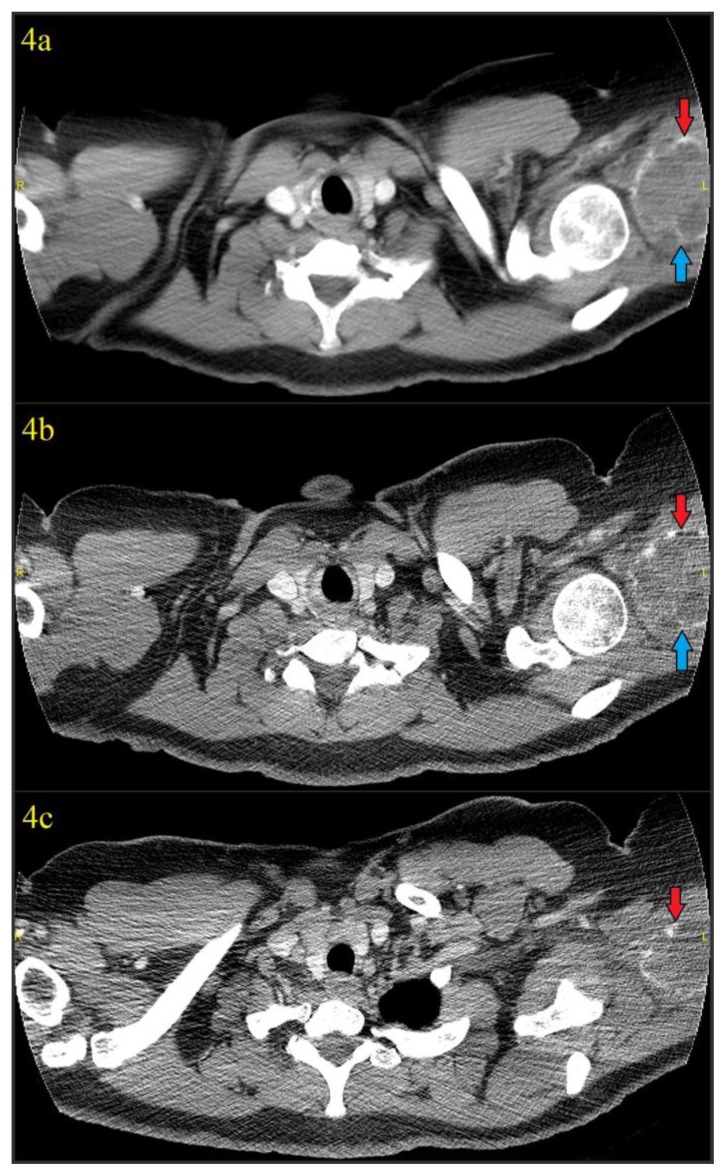
Figure 4.
41 year old female with a soft tissue aneurysmal bone cyst.
Findings: Figures 4a–4c: Axial images from non-contrast chest CT (thus the incomplete inclusion of the lesion) obtained 4 days after discovery of the calcified mass in the soft tissues of the left shoulder using soft tissue window which again showed the 7.0 (transverse) cm × 5.5 (anteroposterior) cm × 6.7 cm (craniocaudad) cm mass with rim calcification (red arrows) in the region of the posterior deltoid/upper triceps muscles. A thin calcified septation was also seen (blue arrows). Hypodense areas could be seen in the lesion, suggesting the underlying blood filled cystic spaces characteristic of an aneurysmal bone cyst.
Technique: CT was performed on a GE® 64-slice CT scanner. All images are from a non-contrast chest CT using soft tissue windows (width = 350, center = 40 for figure 4a, width = 400, center = 40 for figures 4b and 4c). Figure 4a is a 5mm slice using standard soft tissue algorithm, while figures 4b and 4c are high resolution 1.25mm slices from the edge-enhanced lung algorithm. 120 kVp was used with variable mAs, which ranged from 456 mAs to 457 mAs for the provided axial images.
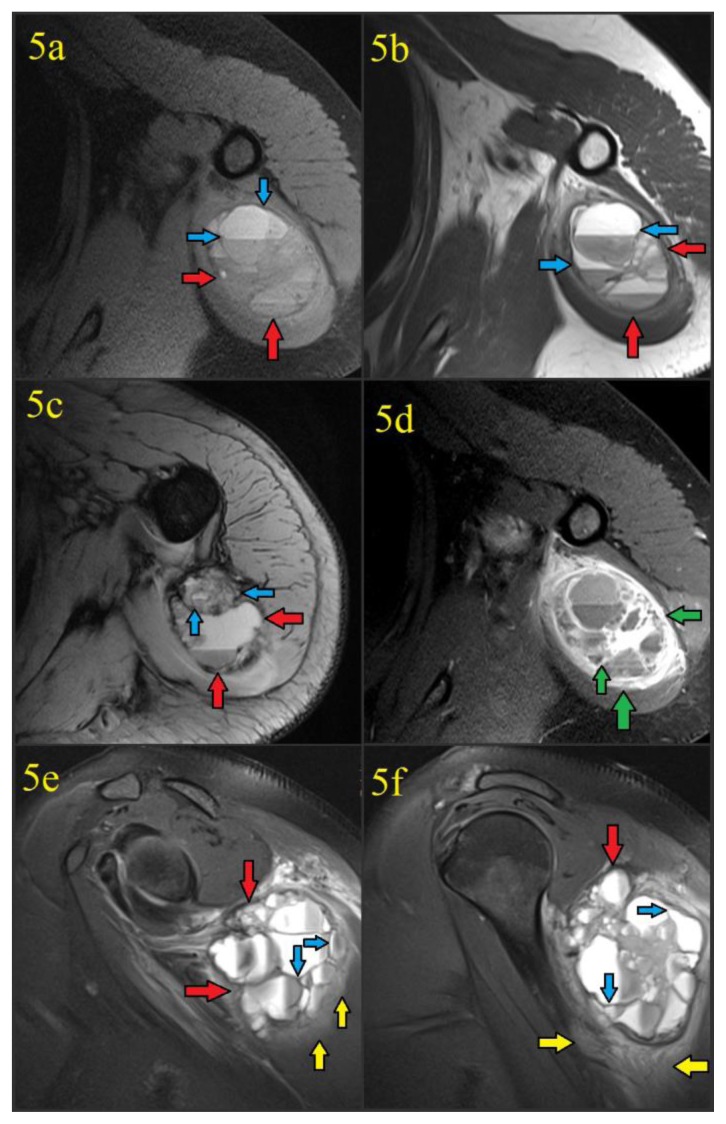
Figure 5.
41 year old female with a soft tissue aneurysmal bone cyst. The MR examination was performed 5 days after discovery of a calcified mass in the soft tissues of the left shoulder. Intravenous gadolinium contrast was administered prior to the acquisition of figure 5d.
Findings: Figure 5a: Noncontrast T1-weighted fat suppressed axial image acquired at the level of the upper triceps demonstrated a 6.8 (transverse) cm × 5.9 (anteroposterior) cm × 7.0 cm (craniocaudad) cm well-circumscribed mass (red arrows) with hypointense capsule/septations (blue arrows) and multiple fluid-fluid levels. No invasion of bone or muscular fat planes is seen.
Figure 5b: Proton density axial image acquired at the level of the upper triceps again showed the mass (red arrows) with hypointense capsule/septations (blue arrows) and multiple fluid-fluid levels, without bone invasion.
Figure 5c: T2-weighted axial image acquired at a slightly lower level of the triceps again showed the known mass (red arrows) with hypointense capsule/septations (blue arrows) and multiple fluid-fluid levels from hematocrit layering in the blood-filled cystic spaces.
Figure 5d: Post-contrast T1-weighted fat suppressed axial image acquired at the level of the upper triceps following the administration of intravenous gadolinium contrast showed avid enhancement of the soft tissue aneurysmal bone cyst fibrous capsule and septations (green arrows) in a “honeycomb” appearance. There was no enhancement of the blood filled cystic spaces themselves. Fluid-fluid levels were again seen.
Figures 5e and 5f: Proton density fat suppressed sagittal images provided craniocaudad demonstration of the 6.8 (transverse) cm × 5.9 (anteroposterior) cm × 7.0 cm (craniocaudad) cm well-circumscribed soft tissue ABC (red arrows) with hypointense capsule/septations (blue arrows) and multiple fluid-fluid levels. Edema was seen in the adjacent triceps and deltoid musculature (yellow arrows).
Technique: MR scanner and contrast details are unknown since the examination was performed at an outside institution. Parameters were as follows: figure 5a: TR=720.0, TE=9.1, FA=170.0, figure 5b TR=3500.0, TE=33.0, FA=160.0, figure 5c: TR=577.0, TE=15.0, FA=30.0, figure 5d: TR=720.0, TE=9.1, FA=170.0, figures 5e and 5f: TR=3100.0, TE=42.0, FA=150.0.
A surgical biopsy was then performed. The patient was placed prone on the operating table and a longitudinal incision was made over the midpoint of the palpable mass. The incision was carried through the subcutaneous tissue down to the triceps muscle, where the muscle fibers were then separated to reveal the mass. The mass capsule was noted to have a grayish/fleshy appearance on visual inspection. Following incision of the mass, the interior was noted to be friable with several fluid pockets which drained blood and dark serous material. Despite difficulty encountered from the calcified nature of the mass, several biopsies were obtained. Samples of the drained fluid were also sent for cytology. The mass capsule, subcutaneous tissues, and skin were then closed with sutures.
On gross pathologic inspection, the submitted specimen was noted to be tan/pinkish in color and rubbery in consistency. Histologic examination showed fragments of lamellar bone, fibroblasts, giant cells, and osteoid (figure 6). Mitotic figures were low, and a lack of atypical nuclei and sarcomatous features were noted. An initial diagnosis of cystic myositis ossificans was made, however, on final review a soft tissue aneurysmal bone cyst was diagnosed.
Figure 6.
41 year old female with a soft tissue aneurysmal bone cyst.
Findings: Figures 6a and 6b (both 10× magnification) provided an overview of the lesion, with a calcified rim of lamellar bone (red arrows), fibrous capsule/septations (blue arrows), collapsed and hemorrhagic internal cystic spaces (green arrows), occasional giant cells (yellow arrows) and osteoid. Figure 6c provided a more magnified (40× magnification) demonstration of the mature lamellar bone that made up the STABC rim. Figure 6d provided a higher magnification (40× magnification) of the lesion interior, improving visualization of giant cells, fibroblasts, osteoid, and hemorrhagic cystic spaces. Figures 6c and 6d also documented a lack of atypical nuclei, active mitoses, or other worrisome/sarcomatous features.
Technique:
Photomicrographs were obtained from surgical biopsy specimens and prepared using hematoxylin and eosin (H&E) staining.
The patient consented to subsequent surgical removal of the mass which was performed at an outside institution. The operative technique summary and pathology report from the outside institution were not available. The diagnosis of soft tissue aneurysmal bone cyst was made.
The patient recovered well from the surgery and presented three years after the excision to our emergency department for shortness of breath associated with an upper respiratory infection. A portable AP chest radiograph obtained at that time showed surgical clips in the region of the prior soft tissue aneurysmal bone cyst with no recurrent palpable or calcified mass (figure 7). She denied any residual pain, palpable abnormality, or any other complaints related to her resected lesion.
Figure 7.
44 year old female with a history of prior soft tissue aneurysmal bone cyst.
Findings: Figure 7: Portable AP radiograph of the chest obtained 3 years after excision of a soft tissue aneurysmal bone cyst showed surgical clips in the region of the left axilla (purple arrow), with no evidence of calcified mass recurrence.
Technique: The radiograph was acquired on a GE® CR digital radiography unit with x-ray tube set at 125 kVp.
DISCUSSION
Aneurysmal bone cysts (ABCs) were first described by Jaffe and Lichtenstein in 1942 [1]. For decades, the lesion was thought of as a benign lesion of bone with an aggressive, lytic appearance typically seen in long bone metaphyses or spinal posterior elements in patients under the age of 30 [1–5]. However, in 1972, Salm and Sissons described “giant cell tumors of soft tissues,” with pathologic features remarkably similar to aneurysmal bone cysts, indicating that, unbeknownst to the authors, these cases may have been the first cases of soft tissue aneurysmal bone cysts (STABCs) to be described in the literature [6–7]. Aneurysmal bone cysts were originally thought to be reactive lesions caused by venous hypertension leading to vascular dilatation [7–10]. However, several studies have provided evidence that aneurysmal bone cysts are actually neoplastic in nature [7, 11–17]. Several groups confirmed the research initially conducted by Paoutsakopoulos and associates in 1999 which found recurrent t(16;17)(q22;p13) translocations in primary aneurysmal bone cysts and more recently several studies have demonstrated ubiquitin specific protease (USP) 6 rearrangements on chromosome 17p13 in both osseous and soft tissue aneurysmal bone cysts, which has been found to have effects on cell adhesion and actin remodeling [7,11–18].
Soft tissue aneurysmal bone cysts do not have a definite gender or racial predilection. Although osseous ABCs are typically seen in patients under 30 years of age, they can rarely be seen in older patients [2,19]. STABCs appear to have a similar age distribution, with our case representing only the fourth reported case in a patient over the age of 40 years according to our searches of the medical literature [4]. Typical osseous ABC locations include the spine (16%), metaphyses of long bones (13% in the femur, 24% in the lower leg, 21% in the upper extremity, 3% in the foot), and occasionally in flat bones (4% in the skull/mandible, 12% in the pelvis/sacrum, 5% in the ribs/clavicles) [20]. Of the 20 known cases of STABC in the literature, 75% (15 of 20) are seen in the proximal extremities (with 9 of 20 in a proximal upper extremity and 6 of 20 in a proximal lower extremity), with the remainder in the groin, abdominal wall, pelvis, and common carotid artery [4].
Histologically, aneurysmal bone cysts (both osseous and soft tissue) are comprised of blood filled cysts with fibrous septa, which also contain giant cells, reactive woven bone, and fibroblasts [3–5]. The hemorrhagic cystic spaces with peripheral woven bone is what causes aneurysmal bone cysts (both soft tissue and osseous) to have their well-defined, expansile, lytic appearance with peripheral calcification on radiographs and CT.
On MRI, STABCs again have an essentially identical appearance to traditional osseous ABCs. Like our case, blood filled cystic spaces produce characteristic fluid-fluid levels which can usually be appreciated on all sequences (but are best seen on more fluid sensitive sequences), and typically do not enhance following intravenous gadolinium administration. Septations usually have low T1 and T2 signal as a result of their fibrous (and sometimes calcified) nature and should enhance on post-contrast images, producing a “honeycomb” type appearance if there is an extensive enough network of septations. The STABC capsule/margin should be well defined and low in signal from its calcified, sclerotic rim [4,21]. According to our searches, there has not been data on the ability of a soft tissue aneurysmal bone cyst to restrict diffusion.
Although not performed for our patient, it is intuitive that a mature STABC with a calcified periphery should produce the characteristic “doughnut” sign of a traditional osseous ABC on bone scan scintigraphy as a result of osteoblastic activity in the calcified rim and central photopenia from central cystic spaces [22].
Additional entities in the differential diagnosis for STABC include myositis ossificans, nodular fasciitis with osteoclast type giant cells, ossifying fibromyxoid tumor of soft parts, calcified hematoma, extraskeletal telangiectatic osteosarcoma, and giant cell tumor of soft tissue. A combination of clinical history, imaging characteristics, and pathologic examination can allow differentiation of each.
The fibrous septa of STABCs may feature occasional osteoclast type giant cells with macrophages and lymphocytes, which can potentially cause histopathologic confusion of the entity for nodular fasciitis with osteoclast type giant cells [23]. However, the characteristic peripheral calcification of STABC is not seen in nodular fasciitis, and thus the entities should be readily differentiated by radiologic-pathologic correlation. On CT, nodular fasciitis lesions will appear as a nonspecific soft tissue mass, and MRI appearances are variable depending on the cellularity of the lesion. Predominately fibrous lesions will be hypointense to skeletal muscle on T1 and T2 weighted sequences, and more cellular lesions will be isointense to hyperintense to muscle on T1 weighted images and hyperintense to muscle on T2 weighted images [24,25].
Like STABC, an ossifying fibromyxoid tumor may also feature peripheral woven/lamellar bone, but in a more lobular and aggressive pattern on radiographs/CT as opposed to the characteristically smoother margins of STABC [23,26–27]. The features of ossifying fibromyxoid tumors on MRI vary according to the cellularity of the tumor, but often feature generalized T2 hyperintensity and heterogeneous T1 signal [27]. Predominately fibrous lesions will appear hypointense on T1/T2 weighted images. Ossifying fibromyxoid tumors featuring blood filled cystic spaces and fluid-fluid levels on MRI have not been reported.
Differentiation of STABC from giant cell tumor of soft tissue is particularly difficult. Both can occur at any age, although giant cell tumors usually occur in those over 20 years of age while STABCs usually (but as our case shows, not always) occur in patients under 20 years. Additionally, giant cell tumors are often associated with aneurysmal bone cysts, since ABCs usually feature a large number of giant cells [4]. Radiologically, giant cell tumors of soft tissue do not tend to have a calcified rim like STABC. Giant cell tumors of soft tissue can rarely feature cystic change, necrosis, and/or hemorrhage with formation of fluid-fluid levels, although not usually to the extent of a STABC [4,28–29]. On histopathology, STABC usually contains more reactive osteoid and woven bone but fewer giant cells than a giant cell tumor of soft tissue or giant cell tumor of the tendon sheath [4].
STABC and mature myositis ossificans appear very similar on radiography and CT, as both entities feature a thin rim of ossification and a lucent/hypodense center [4,30]. However, clinical history will often (but not always) yield an antecedent history of trauma for myositis ossificans and its imaging features will vary with the age of the lesion more than STABC. Shortly after trauma (days to 4 weeks), myositis ossificans will appear as a soft tissue mass with developing central amorphous osteoid on radiographs and CT, while formation of sharper, more mature peripheral cortical bone will start at about 4–6 weeks and mature by about 5–6 months [21]. MR imaging of myositis ossificans will also vary with the maturity of the lesion and will have isointense T1 signal and hyperintense T2 signal in early and intermediate stages, with bone-like increased T1 signal and decreased T2 signal in the late stage [21]. Early stage myositis ossificans will usually feature avid central enhancement due to osteoid formation and periosteal reaction. As the lesion matures in its later stages, enhancement will no longer be present. The characteristic fluid-fluid levels and septated “honeycomb” enhancement pattern of STABC should not be seen [4,21,30].
Differentiation of STABC from extraskeletal telangiectatic osteosarcoma is exceptionally important, as their management is very different. Extraskeletal telangiectatic osteosarcoma has a wide age range, but usually occurs in patients over 30 years of age [4,31]. On radiography and CT, extraskeletal telangiectatic osteosarcoma will typically feature a wider zone of transition and more aggressive/invasive behavior as a result of its malignancy. On MRI, extraskeletal telangiectatic osteosarcoma usually features fluid-fluid levels, but will tend to have more nodularity and soft tissue components, as opposed to the “honeycomb” septated appearance of STABC. Microscopically, extraskeletal telangiectatic osteosarcoma will be much more aggressive and feature anaplastic tumor cells and atypical mitoses, which should not be present in STABC [4]. When ultimately diagnosed, the recommended treatment of STABC is surgical excision as opposed to the more radical surgical treatment with chemotherapy for extraskeletal osteosarcoma with telangiectatic features [4–5].
The purpose in presenting this case is to introduce the reader to a rare diagnosis which he/she may have been previously unfamiliar with and to prompt the radiologist to consider soft tissue aneurysmal bone cyst when encountering a peripherally calcified soft tissue mass instead of automatically defaulting to myositis ossificans. Additionally, according to our searches, our case is only the fourth reported case of STABC in a patient over the age of 40 years, which highlights that the entity is not limited to patients less than 30 years of age. Moreover, our case is unique in that it demonstrates the ability of the lesion to rapidly form, mature, and calcify in as little as 4 weeks, and that this kind of behavior is not necessarily indicative of the more ominous extraskeletal telangiectatic osteosarcoma.
TEACHING POINT
There is a differential diagnosis for peripherally calcified soft tissue masses, and thus the diagnosis of myositis ossificans should not be automatic. Soft tissue aneurysmal bone cysts can also occur in any age group and typically appear as peripherally calcified soft tissue masses with lucent/hypodense centers on radiographs/CT, and hemorrhagic spaces with fluid-fluid levels and enhancing “honeycomb” septations on MRI.
Table 1.
Differential table for soft tissue aneurysmal bone cyst
| Diagnosis | Radiography | CT | MRI |
|---|---|---|---|
| Soft tissue aneurysmal bone cyst | Soft tissue mass with calcified periphery and more lucent center | Soft tissue mass with peripheral calcification and hypodense center which may have discernable septations (and can calcify) | Blood filled cystic spaces produce characteristic fluid-fluid levels which are best seen on more fluid sensitive sequences. The capsule and septations usually have low T1 and T2 signal as a result of their fibrous (and sometimes calcified) nature and should enhance on post-contrast images, producing a “honeycomb” appearance |
| Extraskeletal telangiectatic osteosarcoma | Usually feature a wider zone of transition and more aggressive/invasive behavior as a result of its malignancy | Usually feature a wider zone of transition and more aggressive/invasive behavior as a result of its malignancy | Will feature fluid-fluid levels, but will tend to have more nodularity and soft tissue components as opposed to the “honeycomb” septated appearance of STABC |
| Giant cell tumor of soft tissue | Do not tend to have a calcified rim like STABC | Do not tend to have a calcified rim like STABC | Can rarely feature cystic change, necrosis, and/or hemorrhage with formation of fluid-fluid levels, although not usually to the extent of STABC |
| Nodular fasciitis with osteoclast type giant cells | No calcified rim like STABC | No calcified rim like STABC | Cystic changes characteristic of STABC not usually seen. Appearance is variable depending on lesion cellularity. Predominately fibrous lesions usually hypointense on all sequences. More cellular lesions are usually hyperintense to muscle on T2 and isointense to muscle on T1 weighted sequences. |
| Ossifying fibromyxoid tumor | Can have peripheral mature bone, but usually appears more lobular/aggressive than the smoother margins of STABC | Can have peripheral mature bone, but usually appears more lobular/aggressive than the smoother margins of STABC | Blood filled cystic spaces and fluid- fluid levels on MRI have not been reported. MRI appearances vary according to tumor cellularity, but often are T2 hyperintense and heterogeneous on T1 weighted images. More fibrous lesions will be hypointense on T1 and T2. |
| Myositis ossificans | Appearance varies with myositis ossificans age; mature myositis ossificans has a very similar appearance to STABC as both feature a thin rim of ossification and a more lucent center, whereas young myositis ossificans (days to 4 weeks) will appear as a soft tissue mass with developing central amorphous osteoid | Appearance varies with myositis ossificans age; mature myositis ossificans has a very similar appearance to STABC as both feature a thin rim of ossification and a more lucent center, whereas young myositis ossificans (days to 4 weeks) will appear as a soft tissue mass with developing central amorphous osteoid | Appearance varies with myositis ossificans age; myositis ossificans will have isointense T1 signal and hyperintense T2 signal in early and intermediate stages, with bone-like increased T1 signal and decreased T2 signal in the late stage. Unlike STABC, central contrast enhancement will be avid due to the solid nature of myositis ossificans and fluid-fluid levels or a septated “honeycomb” enhancement pattern are not typical |
Table 2.
Summary table for soft tissue aneurysmal bone cyst
| Etiology | Originally thought to be reactive lesions caused by venous hypertension leading to vascular dilatation, however several studies have provided evidence that aneurysmal bone cysts are actually neoplastic in nature |
| Incidence | Rare, only 20 reported cases according to our searches |
| Gender ratio | No gender predilection |
| Age predilection | Can occur at almost any age, case reports exist in patients from 7 – 73 years of age |
| Risk factors | No established risk factors currently in the literature |
| Treatment | Surgical excision |
| Prognosis | Excellent |
| Findings on imaging | Radiography- Soft tissue mass with peripheral calcification CT- Soft tissue mass with peripheral calcification and a hypodense center which may have discernable septations (and can calcify) MRI- Blood filled cystic spaces produce characteristic fluid-fluid levels which are best seen on more fluid sensitive sequences. The capsule and septations usually have low T1 and T2 signal as a result of their fibrous (and sometimes calcified) nature and should enhance on post-contrast images, producing a “honeycomb” appearance. Scintigraphy- Intuitively, STABC should be similar to osseous ABC and produce a “doughnut” sign as a result of osteoblastic activity in the calcified rim with central photopenia from the central blood-filled cystic spaces. Ultrasound- Evaluation of the interior of the mass will likely be limited by shadowing from peripheral calcification. However, if the interior is visualized then intuitively, there would be hypoechoic cystic spaces with echogenic fibrous septa. |
ACKNOWLEDGEMENTS
The authors would like to acknowledge Dr. Nipun Gupta, who devised the idea to present this case.
ABBREVIATIONS
- ABC
aneurysmal bone cyst
- CR
computed radiography
- CT
computed tomography
- DWI
diffusion weight imaging
- FA
flip angle
- H&E
hematoxylin and eosin
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- STABC
soft tissue aneurysmal bone cyst
- TE
echo time
- TR
repetition time
- US
ultrasound
- USP
ubiquitin specific protease
REFERENCES
- 1.Jaffe HL, Lichtenstein L. Solitary unicameral bone cyst: with emphasis on the roentgen picture, the pathologic appearance and the pathogenesis. Arch Surg. 1942;44:1004–1025. doi: 10.1001/archsurg.1942.01210240043003. [DOI] [Google Scholar]
- 2.Helms C. Benign Cystic Bone Lesions. In: Brant WE, Helms CA, editors. Fundamentals of Diagnostic Radiology. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 1063–1085. [Google Scholar]
- 3.Rodriguez-Peralto JL, Lopez-Barea F, Sanchez-Herrera S, Atienza M. Primary aneurysmal cyst of soft tissues (extraosseous aneurysmal cyst) Am J Surg Pathol. 1994 Jun;18(6):632–636. doi: 10.1097/00000478-199406000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Hao Y, Wang L, Yan M, Jin F. Soft tissue aneurysmal bone cyst in a 10-year-old girl. Oncol Lett. 2012 Mar;3(3):545–548. doi: 10.3892/ol.2011.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nielsen GP, Fletcher CD, Smith MA, Rybak L, Rosenberg AE. Soft tissue aneurysmal bone cyst: a clinicopathologic study of five cases. Am J Surg Pathol. 2002 Jan;26:64–69. doi: 10.1097/00000478-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Salm R, Sissons HA. Giant-cell tumours of soft tissues. J Pathol. 1972 May;107(1):27–39. doi: 10.1002/path.1711070106. [DOI] [PubMed] [Google Scholar]
- 7.Pietschmann MF, Oliveira AM, Chou MM, Ihrler S, Niederhagen M, Baur-Melnyk A, Dürr HR. Aneurysmal bone cysts of soft tissue represent true neoplasms: a report of two cases. J Bone Joint Surg Am. 2011 May 4;93(9):e45. doi: 10.2106/JBJS.J.00534. [DOI] [PubMed] [Google Scholar]
- 8.Clough JR, Price CH. Aneurysmal bone cyst: pathogenesis and long term results of treatment. Clin Orthop Relat Res. 1973 Nov-Dec;(97):52–63. doi: 10.1097/00003086-197311000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Martinez V, Sissons HA. Aneurysmal bone cyst. A review of 123 cases including primary lesions and those secondary to other bone pathology. Cancer. 1988 Jun 1;61(11):2291–2304. doi: 10.1002/1097-0142(19880601)61:11<2291::aid-cncr2820611125>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Kransdorf MJ, Sweet DE. Aneurysmal bone cyst: concept, controversy, clinical presentation, and imaging. AJR Am J Roentgenol. 1995 Mar;164(3):573–580. doi: 10.2214/ajr.164.3.7863874. [DOI] [PubMed] [Google Scholar]
- 11.Baruffi MR, Neto JB, Barbieri CH, Casartelli C. Aneurysmal bone cyst with chromosomal changes involving 7q and 16p. Cancer Genet Cytogenet. 2001 Sep;129(2):177–180. doi: 10.1016/s0165-4608(01)00453-8. [DOI] [PubMed] [Google Scholar]
- 12.Althof PA, Ohmori K, Zhou M, Bailey JM, Bridge RS, Nelson M, Neff JR, Bridge JA. Cytogenetic and molecular cytogenetic findings in 43 aneurysmal bone cysts: aberrations of 17p mapped to 17p13.2 by fluorescence in situ hybridization. Mod Pathol. 2004 May;17(5):518–525. doi: 10.1038/modpathol.3800090. [DOI] [PubMed] [Google Scholar]
- 13.Panoutsakopoulos G, Pandis N, Kyriazoglou I, Gustafson P, Mertens F, Mandahl N. Recurrent t(16;17)(q22;p13) in aneurysmal bone cysts. Genes Chromosomes Cancer. 1999 Nov;26:265–266. doi: 10.1002/(sici)1098-2264(199911)26:3<265::aid-gcc12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira AM, Hsi BL, Weremowicz S, et al. USP6 (Tre2) fusion oncogenes in aneurysmal bone cyst. Cancer Res. 2004 Mar 15;64:1920–1923. doi: 10.1158/0008-5472.can-03-2827. [DOI] [PubMed] [Google Scholar]
- 15.Sciot R, Dorfman H, Brys P, et al. Cytogenetic-morphologic correlations in aneurysmal bone cyst, giant cell tumor of bone and combined lesions. A report from the CHAMP study group. Mod Pathol. 2000 Nov;13:1206–1210. doi: 10.1038/modpathol.3880224. [DOI] [PubMed] [Google Scholar]
- 16.Ye Y, Pringle LM, Lau AW, et al. TRE17/USP6 oncogene translocated in aneurysmal bone cyst induces matrix metalloproteinase production via activation of NF-kappaB. Oncogene. 2010 Jun 24;29:3619–3629. doi: 10.1038/onc.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dal Cin P, Kozakewich HP, Goumnerova L, Mankin HJ, Rosenberg AE, Fletcher JA. Variant translocations involving 16q22 and 17p13 in solid variant and extraosseous forms of aneurysmal bone cyst. Genes Chromosomes Cancer. 2000 Jun;28:233–234. [PubMed] [Google Scholar]
- 18.Masuda-Robens JM, Kutney SN, Qi H, Chou MM. The TRE17 oncogene encodes a component of a novel effector pathway for Rho GTPases Cdc42 and Rac1 and stimulates actin remodeling. Mol Cell Biol. 2003 Mar;23(6):2151–2161. doi: 10.1128/MCB.23.6.2151-2161.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leithner A, Windhager R, Lang S, Haas OA, Kainberger F, Kotz R. Aneurysmal bone cyst. A population based epidemiologic study and literature review. Clin Orthop Relat Res. 1999 Jun;(363):176–179. [PubMed] [Google Scholar]
- 20.Rosai J. Rosai J Rosai and Ackerman’s Surgical Pathology. 10th ed. Vol. 2. Edinburgh: Mosby Elsevier; 2011. Bone and joints; pp. 2013–2105. [Google Scholar]
- 21.Manaster BJ. Aneurysmal Bone Cyst. Statdx Premier. [Accessed February 13, 2014]. Available at: https://my.statdx.com/STATdxMain.jsp?rc=false#dxContent;aneurysmal_bone_cyst_dx.
- 22.Hudson TM. Scintigraphy of aneurysmal bone cysts. AJR Am J Roentgenol. 1984 Apr;142(4):761–765. doi: 10.2214/ajr.142.4.761. [DOI] [PubMed] [Google Scholar]
- 23.Karkuzhali P, Bhattacharyya M, Sumitha P. Multiple soft tissue aneurysmal cysts: An occurrence after resection of primary aneurysmal bone cyst of fibula. Indian J Orthop. 2007 Jul-Sep;41(3):246–249. doi: 10.4103/0019-5413.33693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim ST, Kim HJ, Park SW, Baek JH, Byun HS, Kim YM. Nodular fasciitis in the head and neck: CT and MR imaging Findings. AJNR Am J Neuroradiol. 2005 Nov-Dec;26(10):2617–2623. [PMC free article] [PubMed] [Google Scholar]
- 25.Leung LY, Shu SJ, Chan ACL, Chan MK, Chan CHS. Nodular fasciitis: MRI appearance and literature review. Skeletal Radiol. 2002 Jan;31(1):9–13. doi: 10.1007/s002560100411. [DOI] [PubMed] [Google Scholar]
- 26.Ogose A, Otsuka H, Morita T, Kobayashi H, Hirata Y. Ossifying fibromyxoid tumor resembling parosteal osteosarcoma. Skeletal Radiol. 1998 Oct;27(10):578–580. doi: 10.1007/s002560050441. [DOI] [PubMed] [Google Scholar]
- 27.Schaffler G, Raith J, Ranner G, Weybora W, Jeserschek R. Radiographic appearance of fibromyxoid tumor of soft parts. Skeletal Radiol. 1997 Oct;26(10):615–618. doi: 10.1007/s002560050296. [DOI] [PubMed] [Google Scholar]
- 28.Meana Moris AR, Garcia Gonzalez P, Fuente Martin E, Gonzalez Suarez C, Moro Barrero L. Primary giant cell tumor of soft tissue: fluid-fluid levels at MRI. Eur Radiol. 2010 Jun;20(6):1539–1543. doi: 10.1007/s00330-009-1528-x. [DOI] [PubMed] [Google Scholar]
- 29.Bu An S, Choi JA, Chung JH, Oh JH, Kang HS. Giant Cell Tumor of Soft Tissue: a Case with Atypicaly US and MRI Findings. Korean J Radiol. 2008 Sep-Oct;9(5):462–465. doi: 10.3348/kjr.2008.9.5.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ajilogba KA, Kaur H, Duncan R, McFarlane JH, Watt AJ. Extraossesous aneurysmal bone cyst in a 12-year-old girl. Pediatr Radiol. 2005;35:1240–1242. doi: 10.1007/s00247-005-1560-1. [DOI] [PubMed] [Google Scholar]
- 31.Lee KH, Joo JK, Kim DY, Lee JS, Choi C, Lee JH. Mesenteric extraskeletal osteosarcoma with telangiectatic features: a case report. BMC Cancer. 2007;7:82. doi: 10.1186/1471-2407-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]