Abstract
Objective
Consuming smaller, more frequent meals is often advocated as a means of controlling body weight, but studies demonstrating a mechanistic effect of this practice on factors associated with body weight regulation are lacking. The purpose of this study was to compare the effect of consuming three (3M) vs. six meals (6M) per day on 24-h fat oxidation and subjective ratings of hunger.
Design and Methods
Lean (body mass index <25 kg/m2) subjects (7M, 8F) were studied in a whole-room calorimeter on two occasions in a randomized cross-over design. Subjects were provided isoenergetic, energy balanced diets with a 1- to 2-week washout between conditions. Hunger, fullness, and “desire to eat” ratings were assessed throughout the day using visual analog scales and quantified as area under the curve (AUC).
Results
There were no differences (P < 0.05) in 24-h energy expenditure (8.7 ± 0.3 vs. 8.6 ± 0.3 mj d−1), 24-h respiratory quotient (0.85 ± 0.01 vs. 0.85 ± 0.01), or 24-h fat oxidation (82 ± 6 vs. 80 ± 7 g day−1) between 3M and 6M, respectively. There was no difference in fullness 24-h AUC, but hunger AUC (41850 ± 2255 vs. 36612 ± 2556 mm.24 h, P = 0.03) and “desire to eat” AUC (47061 ± 1791 vs. 41170 ± 2574 mm.24 h, P = 0.03) were greater during 6M than 3M.
Conclusion
We conclude that increasing meal frequency from three to six per day has no significant effect on 24-h fat oxidation, but may increase hunger and the desire to eat.
Introduction
Popular advice for weight control advocates the consumption of small, frequent meals. However, the science supporting this as an effective weight control strategy is lacking, and studies examining the effects of eating frequency on body weight have shown mixed results. Although some cross-sectional studies have reported an inverse relationship of habitual meal frequency with body weight, body mass index (BMI), or percentage body fat (1–5), others have found no associations (6–11). Prospective studies have also shown no association between eating frequency and weight change (12–14). Furthermore, Palmer et al. (15) reviewed intervention studies of the effects of meal frequency on weight-loss or weight-maintenance and concluded that meal frequency had no effect on weight loss or weight loss maintenance.
One hypothesized mechanism by which frequent meal consumption leads to lower body weight is by increasing energy expenditure (EE). The effects of meal frequency on EE have been investigated under isocaloric conditions, using room calorimetry (16–22) or doubly-labeled water (21). In these studies, EE did not differ between large (one to two meals/d), normal (three meals/d), or small frequent (more than five meals/d) patterns. Another possible mechanism is that increasing meal frequency increases fat oxidation. However, only two studies reported effects of meal frequency on 24-h fat oxidation (17,19), with discordant results. It has also been hypothesized that consumption of small frequent meals maintains plasma glucose concentrations relatively constant throughout the day, possibly leading to better appetite control (23,24). Insulin may also play a role in control of appetite regulation through the central nervous system (25), with increases in insulin being associated with satiety. Thus, consuming more frequent meals might be expected to lead to higher concentrations of insulin and increased satiety. However, the effect of varying meal frequency on glucose and insulin responses over the course of a day has not been well-studied.
Surprisingly, no studies have compared the effects of a traditional meal consumption pattern (three meals per day) versus smaller more frequent meals on fat oxidation and perceived hunger. Thus, the aim of this study was to compare the acute effects of consuming three meals a day vs. six meals a day on 24-h fat oxidation and perceived hunger.
Methods
Institutional approval
This randomized, cross-over study was conducted at the University of Colorado (UCD) Anschutz Medical Campus from April to December, 2010. All subjects were recruited and studied during this period. Procedures followed were in accordance with the ethical standards of the responsible institutional or regional committee on human experimentation or in accordance with the Helsinki Declaration of 1975 as revised in 1983. The study was approved by the Colorado Multiple Institutional Review Board and the Scientific Advisory Board of the Clinical Translation Research Center at the UCD.
Subjects
Healthy adult men and women were recruited for this study. Respondents were queried about their age, current and past body weight, physical activity patterns, and health history during an initial telephone screening. Inclusion criteria were age (18–60 years), BMI (19–25 kg/m2), and self-reported weight stability (less than 5% change in body weight over the previous 6 months). Exclusion criteria were self-reported acute or chronic disease (e.g., hypo- or hyperthyroidism, diabetes, and heart disease), current use of over-the-counter or prescribed medications known to affect appetite, food intake or intermediary metabolism (e.g., appetite suppressants, antidepressants), and tobacco use. Additional exclusion criteria for women were pregnancy, lactation, amenorrhea (absence of three or more consecutive menstrual cycles), and self-reported abnormal menstrual cycle length (<26 days or >32 days). Volunteers who passed the initial screening provided informed written consent and were then invited to participate in a health history and physical examination to confirm a good state of health. BMI was confirmed by measuring height and weight while wearing only socks, undergarments, and a hospital gown. After acceptance into the study, resting metabolic rate (RMR) and body composition were measured, as described previously (26). The study sampled consisted of eight females (four Caucasian and four Asian) and seven males (three Caucasian, three Asian, and one Hispanic). The characteristics of the 15 subjects are presented in Table 1.
TABLE 1.
Subject descriptive characteristics
| Age (y) | Wt (kg) | BMI (kg/m2) | % fat | |
|---|---|---|---|---|
| Male (N=7) | 27 (5) | 70.0 (3.3) | 21.5 (1.9) | 15.4 (3.2) |
| Female (N=8) | 30 (5) | 57.0 (9.3) | 20.7 (2.1) | 26.6 (4.7) |
Mean (SD).
Study design
This was a randomized cross-over trial comparing the acute effects of varying meal frequency. Subjects were studied under two conditions, three meals (3M) and six meals (6M) a day, with a 1- to 2-week washout between study conditions. The order of the meal conditions was counterbalanced. Each condition lasted 4 days. Subjects consumed a controlled outpatient diet on days 1–3 (to stabilize energy and macronutrient intake) and were studied in the room calorimeter on day 4. Subjects were instructed to consume three meals per day during the outpatient phase; meal frequency was varied only during the calorimeter study day (day 4). Subjects were instructed to maintain their normal physical activity patterns during the outpatient phase, but physical activity during the calorimeter study day was tightly controlled (described below).
Study diets
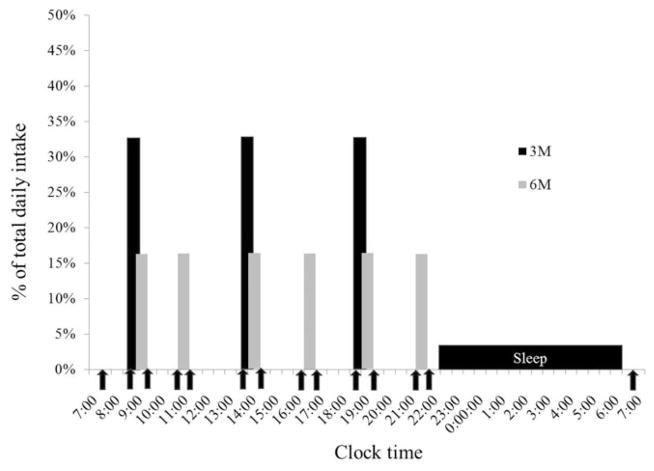
Subjects were provided all meals during the 4-day trials. Study diets consumed during 3M and 6M were matched for energy and macronutrient content (30% of energy from fat, 55% carbohydrate, and 15% protein). The energy content of the diets was estimated to meet free-living energy requirements (RMR × 1.5) and maintain body weight stability. All diets were designed by a trained nutritionist using ProNutra software (Viocare Nic, Princeton, NJ). During the outpatient period (days 1–3), meals were packaged and taken with the subject. All food was required to be consumed, and no other food was permitted. Two optional food modules (200 kcal each) were provided in the event that the subject experienced hunger. To verify compliance to the diets, subjects were required to return the empty food containers to the study personnel. The energy and macronutrient content of the calorimeter diet (day 4) was identical to that of the outpatient diet. Meal times were scheduled at equidistant time points (Figure 1); that is, during 3M, meals were served at 5-h intervals (0900, 1400, and 1900), and during 6M, meals were provided at 2.5-h intervals (0900, 1130, 1400, 1630, 1900, and 2130). Three of the meals during 6M were provided at the same time as the meals during 3M. The distribution of energy was 33.3% at each meal during 3M and 16.7% at each meal during 6M.
FIGURE 1.
Calorimeter protocol. Vertical shaded bars indicate the time and energy value (as a percentage of total daily intake) of meals during the three meals per day (3M, black bars) and six meals per day (6M, gray bars) conditions. Clock time is indicated on the horizontal axis. Solid arrows indicate times when hunger scales were administered and blood was sampled.
Calorimeter protocol
Subjects entered the calorimeter at 0800 h and exited at 0700 h the following day. Two bouts of bench-stepping exercise (20 min at 72 steps/min) were performed at 0830 and 1730 h to mimic free-living physical activity. Subjects were free to move about the calorimeter during other times of the day, but this time was primarily spent in sedentary behavior (reading, writing, computer use, and watching TV). Subjects were instructed to remain awake and not to nap or perform any exercise other than that prescribed by the protocol, and to go to bed at the same time during each calorimeter stay. Subjects recorded their time to bed, and all subjects reported going to bed before 2300 h. Physical activity during 3M and 6M was measured using an accelerometer-based physical activity monitor (Actigraph GT1M, Actigraph LLC, Pensacola, FL). Subjects wore the activity monitor during the entire calorimeter stay, except for during sleep, which was confirmed during data downloading. Physical activity and step counts were accumulated in 1-min intervals and totaled over the measurement period and expressed as counts/day and steps/day. Nonexercise activity and step counts were also calculated by subtracting the counts that accumulated during the prescribed bench-stepping bouts.
To assess the effects of meal frequency on hunger and satiety, subjects completed visual analog scale (VAS) questions (hunger, fullness, and desire to eat) during each calorimeter stay (27). Subjects were asked to complete the VAS questions before the first meal (fasting), before and after each meal, and on waking the second morning (fasting). The scales were recorded using a computer program. Scales were administered at the same clock time during each calorimeter stay, that is, at the 6M premeal and post meal times (Figure 1). Hunger was rated on a line preceded by the question, “How hungry are you right now?” and anchored on the left by “not at all hungry” and by “extremely hungry” on the right. Other questions addressed fullness (“How full do you feel right now?) and desire to eat (“How much food could you eat right now?) with the anchors “not at all …” and “extremely …” (fullness) and “nothing” and “a great deal” (desire). After each VAS was completed, a blood samples (5 ml) was obtained and analyzed for serum concentrations of glucose, insulin, and free fatty acids. To obtain blood samples, an indwelling venous catheter were inserted prior to entering the calorimeter, and blood samples were obtained by asking subjects to extend their arm through a leak-proof port in the side of the calorimeter. Whole blood (2.5 ml) was added to 40 ml of preservative (3.6 mg EDTA plus 2.4 mg glutathione in distilled water). The sample was allowed to clot and the serum was separated after spinning. Serum was stored at −80°C until analyzed. All samples were assayed for glucose, insulin, glycerol, and fatty acid (FFA). Glucose concentrations were determined using the hexokinase method (Roche Indianapolis, IN). Insulin concentrations were measured using standard, double antibody radioimmunoassay (Diagnostic Systems Laboratory, Webster, CT). FFA concentrations (Wako Chemical, Richmond, VA) were determined using direct enzymatic/ calorimetric assays (COBRA Mira Plus Chemistry analyzer).
Data reduction
Calorimeter data were extrapolated to 24 h values, based on average minute values. 24-h EE and total substrate oxidation were determined from oxygen consumption (VO2), carbon dioxide production (VCO2), and urinary nitrogen excretion, as previously described (28). Integrated VAS and blood responses during the waking hours (0730–2200) were determined by calculating the integrated area under the curve (AUC) using the trapezoid rule.
Statistical analysis
Statistical analyses were carried out using Graphpad Prism (Version 5.03, La Jolla, CA). Prior to all analyses, data were examined graphically for unusual or extreme values, and appropriate remedies applied (e.g., double check unusual values, log transform to reduce skew). Primary outcomes (24-h fat oxidation, 24-h AUC for hunger, fullness, and desire to eat) and secondary (24-h EE) outcomes were analyzed using paired t-tests. Correlations were determined using the Pearson product moment formula. Twenty-four blood and VAS profiles were analyzed using a two-factor (condition, time) repeated measures ANOVA to determine the specific timepoints where differences occurred between 3M and 6M; to account for multiple comparisons, the Bonferroni adjustment was applied. All statistical tests were two-tailed with significance set at P < 0.05. Data are presented as mean (SE), unless otherwise noted.
Sample size was estimated based on the primary outcome of fat oxidation using a two-sided level 0.05 paired t-test (a simplified version of the cross-over model) and a beta level of 0.20. Calculations were done using SAS 9.2 software (SAS Institute Inc., Cary, NC). The necessary standard deviations were obtained from the previous study, where 24-h fat oxidation was measured during energy-balanced sedentary and exercise conditions (26). The pooled standard deviation for difference in fat oxidation between conditions was 27.5 g/day. Effect size was estimated from the same study, where the difference in fat oxidation between an exercise and nonexercise day was ~25 g/day. Twelve subjects would be required to detect a similar difference in 24-h fat oxidation in the current study. In the current study, 15 subjects were studied, which provided more than 80% power to detect a difference in 24-h fat balance of ~22 g/day between the two meal conditions. If increasing meal frequency from three to six meals per day results in a similar increase in fat oxidation and this were maintained over a period of 1 month, this would result in a cumulative increase in fat oxidation of ~660 g (0.66 kg). We would consider this to be a clinically meaningful effect.
Results
Prestudy diets
Subjects returned all empty food containers, as instructed. There were no differences in consumption of the optional food modules between conditions. Subjects consumed on average 0.90 ± 0.20 modules per day during 3M and 1.00 ± 0.20 modules per day during 6M (P = 0.80).
24-h EE and fat oxidation
Twenty-four hour EE (8.7 ± 0.3 vs. 8.6 ± 0.3 mj d−1P = 0.08), energy balance (0.07 ± 0.01 vs. 0.02 ± 0.01 mj d−1, P = 0.07), respiratory quotient (RQ) (0.85 ± 0.01 vs. 0.85 ± 0.01, P = 0.90), and fat oxidation (82 ± 6 vs. 80 ± 7 g d−1 P = 0.72) did not differ between 3M and 6M, respectively. These measures were also highly and significantly correlated between conditions (all r ≥ 0.82, P < 0.0001). There were no differences in total activity counts (139,343 ± 8,099 vs. 133,399 ± 7,741, P = 0.35) or total steps (2,640 ± 111 vs. 2,671 ± 96, P = 0.78) during 3M and 6M, respectively. There were also no differences in nonexercise activity counts (57,116 ± 7,041 vs. 53,548 ± 5,313, P = 0.47) or steps (1,048 ± 69 vs. 1,058 ± 58, P = 0.85). These data demonstrate that physical activity levels were similar between the two study conditions.
Blood markers
Differences in glucose, insulin, and FFA profiles were analyzed using repeated measures ANOVA. There were no differences in prebreakfast measurements of glucose (Figure 2), Insulin (Figure 3), or FFA (Figure 4). Consumption of six meals per day resulted in more frequent peaks in glucose concentrations, but there was no difference in the glucose AUC between 3M and 6M (P = 0.08). Although glucose concentrations fell between meals, concentrations did not fall below 4.6 mmol during either 3M or 6M. Glucose concentrations prior to exiting the calorimeter on the second day returned to baseline and were not different between 3M and (4.7 ± 0.1 mmol) and 6M (4.9 ± 0.1 mmol). Similar to glucose, consumption of six meals per day resulted in more frequent peaks in insulin concentrations (Figure 3); however, the insulin AUC response was lower during 6M (P = 0.03). Insulin concentrations prior to exiting the calorimeter on the second day returned to baseline and were not different between 3M and (90.7 ± 6.6 pmol/L) and 6M (89.8 ± 4.8 pmol/L). During 3M, FFA concentrations fell following the consumption of each meal and rose prior to the subsequent meal. However, during 6M, FFA concentrations fell following consumption of the first meal, and remained below fasting levels throughout the remainder of the day. As a result, the FFA AUC was lower during 6M (P = 0.01). FFA concentrations prior to exiting the calorimeter on the second day returned to baseline and were not different between 3M and (0.3 ± 0.02 mmol) and 6M (0.3 ± 0.02 mmol).
FIGURE 2.
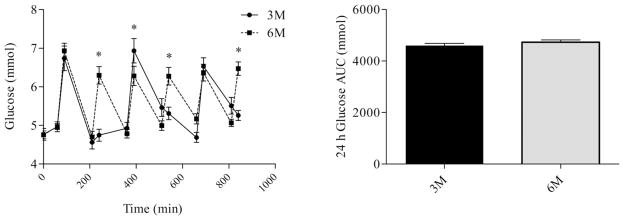
Glucose profiles (left) and glucose AUC (right) during the waking hours. The first sample was obtained at 0730 (prebreakfast) and the last sample was obtained at 2200 (corresponding to 30 min after the last meal in the six meal condition). * indicates significant difference between conditions, as determined by ANOVA. 3M = three meals per day, 6M = six meals per day.
FIGURE 3.
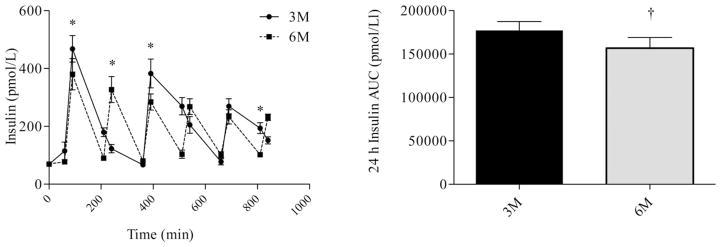
Insulin profiles (left) and insulin AUC (right) during the waking hours. The first sample was obtained at 0730 (prebreakfast) and the last sample was obtained at 2200 (corresponding to 30 min after the last meal in the six meal condition). * indicates significant difference between conditions, as determined by ANOVA. † indicates significant difference, as determined by paired t-test. 3M = three meals per day, 6M = six meals per day.
FIGURE 4.
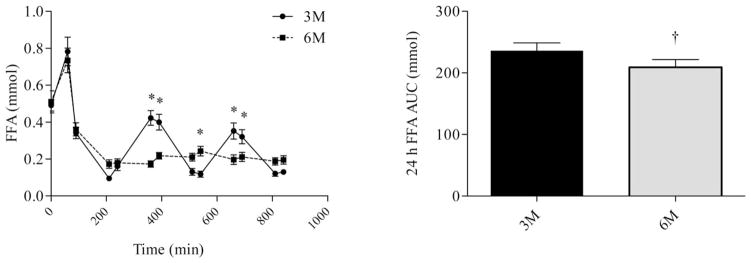
Fatty acid (FFA) profiles (left) and FFA AUC (right) during the waking hours. The first sample was obtained at 0730 (prebreakfast) and the last sample was obtained at 2200 (corresponding to 30 min after the last meal in the six meal condition). * indicates significant difference between conditions, as determined by ANOVA. † indicates significant difference, as determined by paired t-test. 3M = three meals per day, 6M = six meals per day.
Perceived hunger, fullness, and desire to eat
Differences in hunger, fullness, and fullness profiles (Figures 5–7) were analyzed using repeated measures ANOVA. There were no differences in morning measures of appetite or fullness. Fullness AUC (Figure 6) did not differ between 3M (37,220 ± 2,492 mm−24 h) and 6M (34,261 ± 2,447 mm−24 h, P = 0.22). Consumption of six meals per day, however, tended to blunt the post meal fall in perceived hunger and desire to eat and rise in fullness. As a result, perceived hunger AUC (41,850 ± 2,255 vs. 36,612 ± 2,556 mm.24 h, P = 0.03) and desire to eat AUC (47,061 ± 1,791 vs. 41,170 ± 2,574 mm.24 h, P = 0.03) were greater during 6M compared to 3M.
FIGURE 5.
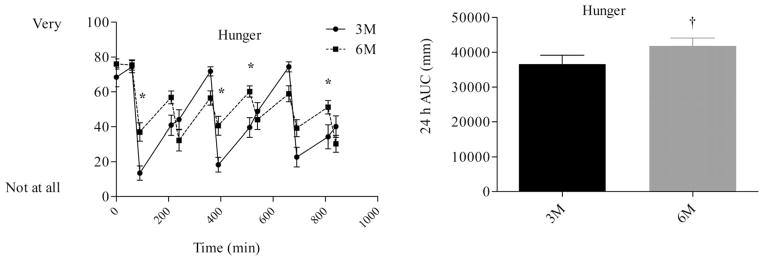
Hunger profiles (left) and hunger AUC (right) during the waking hours. The first measurement was obtained at 0730 (prebreakfast) and the last measurement was obtained at 2200 (corresponding to 30 min after the last meal in the 6 meal condition). * indicates significant difference between conditions, as determined by ANOVA. † indicates significant difference, as determined by paired t-test. 3M = three meals per day, 6M = six meals per day.
FIGURE 7.
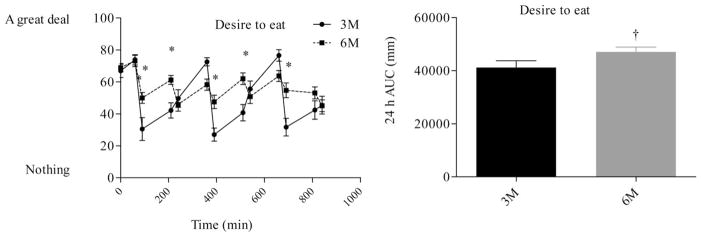
Desire to eat profiles (left) and desire to eat AUC (right) during the waking hours. The first measurement was obtained at 0730 (prebreakfast) and the last measurement was obtained at 2200 (corresponding to 30 min after the last meal in the six meal condition). * indicates significant difference between conditions, as determined by ANOVA. † indicates significant difference, as determined by paired t-test. 3M = three meals per day, 6M = six meals per day.
FIGURE 6.
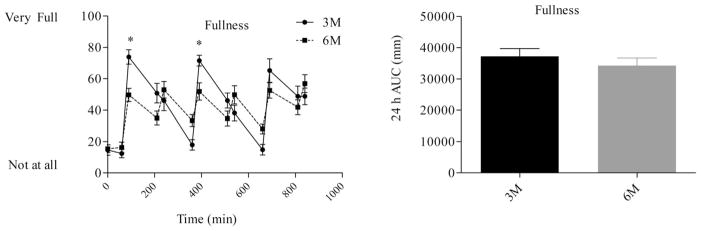
Satiety profiles (left) and satiety AUC (right) during the waking hours. The first measurement was obtained at 0730 (prebreakfast) and the last measurement was obtained at 2200 (corresponding to 30 min after the last meal in the six meal condition). * indicates significant difference between conditions, as determined by ANOVA. † indicates significant difference, as determined by paired t-test. 3M = three meals per day, 6M = 6 meals per day.
Discussion
The present study examined the effect of consuming smaller more, frequent meals (6M) on 24-h fat oxidation and perceived hunger compared with a traditional meal consumption pattern (3M). We observed no differences in 24-h EE, 24-h RQ, or 24-h fat oxidation. There was a strong, positive correlation in 24-h EE (r = 0.97), and energy balance (r = 0.87) between conditions, suggesting that subjects were studied in a similar energetic state, minimizing the effects of differences in energy balance on fat oxidation. These results suggest that there is no effect of meal frequency on EE or fat oxidation under isoenergetic states, which is in agreement with results from previous results (17,19). However, hunger AUC and the “desire to eat” AUC were significantly greater during 6M compared to 3M. With the more frequent meal pattern, subjects did not experience the same decreases in hunger or increases in fullness between meals (Figures 5–7). Energy intake was controlled in this study so we could study the specific effects of meal pattern. However, we can speculate that if subjects are hungrier and less satiated when consuming more frequent meals during isoenergetic conditions, they would be likely to consume more total calories under ad libitum conditions. This hypothesis should be tested in a separate, ad libitum feeding study. Thus, we conclude that consuming smaller, more frequent meals has no advantages in terms of its effects on metabolism and appetite and may, in fact, even have adverse effects on hunger and fullness.
Only two previous studies (17,19) examined the effect of meal frequent patterns on 24-h fat oxidation. Consistent with the results of the current study, Verboeket-van de Venne and Westerterp (19) reported no difference in 24-h RQ or fat oxidation with consumption of two or seven meals per day. Conversely, Smeets et al. (17) reported a lower 24-h RQ (and higher fat oxidation) with consumption of three (0.86 ± 0.04) compared to two meals/d; 0.88 ± 0.03). They speculated that consuming fewer, but larger meals, increases glycogen storage and the availability of oxidizable carbohydrate, leading to a reduction in fat oxidation. We are unable to reconcile factors that may have contributed to the differences amongst these studies, which is further complicated by the different meal patterns in each study. However, it is possible that small differences in energy balance may be a contributing factor. In the study of Smeets et al. (17), energy balance was positive (0.90 MJ) and slightly (~0.2 MJ), albeit nonsignificantly, lower during the 3M condition, which may have contributed to the higher fat oxidation during this condition. In comparison, energy balance in the current study was nearly zero and identical during both conditions. Thus, we conclude that under isoenergetic conditions, meal frequency has no measurable effect on 24-h fat oxidation. This conclusion is consistent with our previous studies of the effect of exercise on 24-h fat oxidation that clearly demonstrated that energy balance is the primary determinant of 24-h fat oxidation (26,29,30).
Our observation that consumption of smaller more frequent meals is associated with higher appetite ratings is consistent with recent findings in obese and overweight men during weight loss (31), but in contrast to previous findings that increased meal frequency is associated with enhanced appetite control (32,33). However, the latter studies were short in duration, and compared only the effects of splitting a single meal into several small meals. One of the mechanisms via which increased meal frequency is hypothesized to enhance appetite control is by preventing large decreases in plasma glucose between meals. In our sample of lean individuals, glucose AUC between tended to be higher during 6M than 3M (Figure 2, P = 0.08). It is worth noting that because glucose had not returned to baseline concentrations by the time of the last sample, this difference may have become significant had a sample been obtained at a later time point. However, glucose profiles in the current study (Figure 2) demonstrated that plasma glucose reached a similar nadir between meals regardless of meal frequency. The nadir in glucose (~4.8 mmol) was well above the threshold for the clinical hypoglycemia (3.9 mmol). These results are not surprising, given that plasma glucose is tightly controlled and well-maintained; for example, during prolonged fasting in healthy individuals, plasma glucose is maintained in excess of 4 mmol for up 40 h (34). Previous research has demonstrated that the rate of decline in plasma glucose, rather than the absolute plasma glucose concentration, is associated with meal initiation (35). These studies used continuous glucose monitoring to precisely determine the rate of decline in plasma glucose. Although this was not done in the current study, our data (Figure 2) do not suggest that the rate of decline in plasma glucose was altered by meal frequency in the postprandial period. Another mechanism by which smaller, more frequent meals has been suggested to reduce hunger is by sustaining higher levels of between meal insulin concentrations, which would be expected to lead to reduced hunger (25). However, in the current study, insulin AUC was significantly lower during 6M than 3M. Furthermore, insulin tended to fall to similar concentrations after the consumption of each meal. Thus, the current results suggest that it is unlikely that increasing meal frequency from 3M to 6M alters the rate of decline in glucose or insulin concentrations between meals in a manner that would influence appetite and food intake patterns. Future work in this field should include continuous or more frequent measurements of glucose and insulin to better study these effects.
Although increasing meal frequency caused more frequent peaks in glucose and insulin with only minor effects on AUC responses, the effects of FFA was markedly different. Increasing meal frequency from 3M to 6M caused a marked suppression in FFA concentrations between meals, resulting in a significantly lower (~11%) FFA AUC (Figure 4). It is likely that the marked suppression of FFA during 6M was due to insulin-mediated suppression of lipolysis. In lean, healthy individuals, half-maximal suppression of lipolysis occurs at insulin concentrations of ~100 pmol/L (36). In the current study, consumption of more frequent meals caused insulin concentrations to be sustained above this concentration throughout much of the day (Figure 3). Interestingly, despite the reduction in plasma FFA, there was no difference in 24-h fat oxidation between conditions. Previous studies have shown that increasing plasma FFA can lead to increases in fat oxidation (37). Results of the current study suggest that the differences in plasma FFA between the 3M and 6M conditions was not sufficient to induce changes in fat oxidation. Furthermore, as FFA is the major source of fat oxidized, these results are counter to the hypothesis that increasing meal frequency causes increases in fat oxidation.
Few well-controlled, long-term studies have examined the effects of meal frequency on body weight during a period of weight loss. However, results from a recent study indicate that increasing meal frequency during behavioral weight loss programs does not enhance weight loss. Bachman and Raynor (38) randomized obese men and women participating in a 6-month behavioral weight loss intervention to either a grazing group or a group that consumed their meals in a traditional three meal per day pattern. The grazing group was instructed to consume at least 100 kcals every 2–3 h. At 6 months, the grazing group reported consuming 5.8 ± 1.1 meals per day. Perceived hunger following the primary meals (breakfast, lunch, and dinner) decreased only in the grazing group. Despite this difference in perceived hunger, weight loss at 6 months did not differ between the two groups. In another recent study, Leidy et al. (31) studied overweight and obese men during a behavioral weight loss program. During week 7 of the 12-week intervention, subjects were instructed to consume their diets as either 3M or 6M in a randomized, crossover fashion; each meal pattern was to be followed for 3 days. Consistent with results of the current study, the 6M pattern led to reduced fullness throughout the day. Importantly, only 60% of the participants were able to adhere to the 6M condition in that study, which suggests that this may be a difficult meal pattern to sustain. It has also been suggested that because higher meal frequencies increase the exposure to foods throughout the day, this would lead to intake being regulated more by environment than physiological cues (39), which may counter the attempts to reduce overall energy intake. Taken together with the results of the current study, these observations suggest that it is unlikely that higher meal frequencies will lead to greater weight loss in individuals attempting to lose weight via caloric restriction.
There were several limitations in the present study. Our study examined only the acute effects of meal frequency on 24-h fat oxidation and perceived hunger. Additionally, we only studied lean individuals, and thus, our conclusions cannot be extended to obese individuals. For example, although we observed no differences in plasma glucose AUC in our sample of lean individuals, Leidy et al. (31) reported that plasma glucose concentrations were lower during 6M than 3M in their sample of overweight and obese individuals. Although we studied women, we did not attempt to study all of them in the same phase of the menstrual cycle. However, given that we observed no differences in the response between men and women, and the observed high correlation between repeated measurements, we find it unlikely that doing so would lead to different conclusions. We also did not measure differences in appetite-related hormones, which may have provided more objective information about changes in physiological markers of appetite. Finally, we performed our studies under isocaloric conditions to specifically study the effects of meal frequency, but given the higher ratings of hunger and lower fullness during 6M, we would expect intake to be increased during 6M under ad libitum conditions, an effect which needs to be studied directly.
In summary, although consuming smaller, more frequent meals is often advocated as a means of controlling body weight, the present study suggests that this practice has no obvious advantages in terms of its effects on metabolism and appetite, and may, in fact, even have adverse effects on hunger and satiety.
Acknowledgments
Funding agencies: Dr. Ohkawara was supported by Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists. Research support was provided by the University of Colorado Clinical and Translational Science Award (1UL1 RR025780) and Nutrition and Obesity Research Center (P30 DK048520).
We thank the volunteers, as well as the Nursing, Clinical Lab, and Bionutrition Staffs of the University of Colorado CTRC.
Footnotes
Disclosure: The authors had no financial or personal conflicts of interests.
References
- 1.Drummond SE, Crombie NE, Cursiter MC, Kirk TR. Evidence that eating frequency is inversely related to body weight status in male, but not female, non-obese adults reporting valid dietary intakes. Int J Obes Relat Metab Disord. 1998;22:105–112. doi: 10.1038/sj.ijo.0800552. [DOI] [PubMed] [Google Scholar]
- 2.Lioret S, Touvier M, Lafay L, Volatier JL, Maire B. Are eating occasions and their energy content related to child overweight and socioeconomic status? Obesity. 2008;16:2518–2523. doi: 10.1038/oby.2008.404. [DOI] [PubMed] [Google Scholar]
- 3.Ma Y, Bertone ER, Stanek EJ, III, et al. Association between eating patterns and obesity in a free-living US adult population. Am J Epidemiol. 2003;158:85–92. doi: 10.1093/aje/kwg117. [DOI] [PubMed] [Google Scholar]
- 4.Ruidavets JB, Bongard V, Bataille V, Gourdy P, Ferrieres J. Eating frequency and body fatness in middle-aged men. Int J Obes Relat Metab Disord. 2002;26:1476–1483. doi: 10.1038/sj.ijo.0802143. [DOI] [PubMed] [Google Scholar]
- 5.Toschke AM, Kuchenhoff H, Koletzko B, von Kries R. Meal frequency and childhood obesity. Obes Res. 2005;13:1932–1938. doi: 10.1038/oby.2005.238. [DOI] [PubMed] [Google Scholar]
- 6.Andersson I, Rossner S. Meal patterns in obese and normal weight men: the “Gustaf” study. Eur J Clin Nutr. 1996;50:639–646. [PubMed] [Google Scholar]
- 7.Duval K, Strychar I, Cyr MJ, Prud’homme D, Rabasa-Lhoret R, Doucet E. Physical activity is a confounding factor of the relation between eating frequency and body composition. Am J Clin Nutr. 2008;88:1200–1205. doi: 10.3945/ajcn.2008.26220. [DOI] [PubMed] [Google Scholar]
- 8.Hartline-Grafton HL, Rose D, Johnson CC, Rice JC, Webber LS. The influence of weekday eating patterns on energy intake and BMI among female elementary school personnel. Obesity. 2010;18:736–742. doi: 10.1038/oby.2009.249. [DOI] [PubMed] [Google Scholar]
- 9.Howarth NC, Huang TT, Roberts SB, Lin BH, McCrory MA. Eating patterns and dietary composition in relation to BMI in younger and older adults. Int J Obes (Lond) 2007;31:675–684. doi: 10.1038/sj.ijo.0803456. [DOI] [PubMed] [Google Scholar]
- 10.Mills JP, Perry CD, Reicks M. Eating frequency is associated with energy intake but not obesity in midlife women. Obesity. 2011;19:552–559. doi: 10.1038/oby.2010.265. [DOI] [PubMed] [Google Scholar]
- 11.Summerbell CD, Moody RC, Shanks J, Stock MJ, Geissler C. Relationship between feeding pattern and body mass index in 220 free-living people in four age groups. Eur J Clin Nutr. 1996;50:513–519. [PubMed] [Google Scholar]
- 12.Cameron JD, Cyr MJ, Doucet E. Increased meal frequency does not promote greater weight loss in subjects who were prescribed an 8-week equi-energetic energy-restricted diet. Br J Nutr. 2010;103:1098–1101. doi: 10.1017/S0007114509992984. [DOI] [PubMed] [Google Scholar]
- 13.Garrow JS, Durrant M, Blaza S, Wilkins D, Royston P, Sunkin S. The effect of meal frequency and protein concentration on the composition of the weight lost by obese subjects. Br J Nutr. 1981;45:5–15. doi: 10.1079/bjn19810072. [DOI] [PubMed] [Google Scholar]
- 14.Iwao S, Mori K, Sato Y. Effects of meal frequency on body composition during weight control in boxers. Scand J Med Sci Sports. 1996;6:265–272. doi: 10.1111/j.1600-0838.1996.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 15.Palmer MA, Capra S, Baines SK. Association between eating frequency, weight, and health. Nutr Rev. 2009;67:379–390. doi: 10.1111/j.1753-4887.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- 16.Dallosso HM, Murgatroyd PR, James WP. Feeding frequency and energy balance in adult males. Hum Nutr Clin Nutr. 1982;36C:25–39. [PubMed] [Google Scholar]
- 17.Smeets AJ, Westerterp-Plantenga MS. Acute effects on metabolism and appetite profile of one meal difference in the lower range of meal frequency. Br J Nutr. 2008;99:1316–1321. doi: 10.1017/S0007114507877646. [DOI] [PubMed] [Google Scholar]
- 18.Taylor MA, Garrow JS. Compared with nibbling, neither gorging nor a morning fast affect short-term energy balance in obese patients in a chamber calorimeter. Int J Obes Relat Metab Disord. 2001;25:519–528. doi: 10.1038/sj.ijo.0801572. [DOI] [PubMed] [Google Scholar]
- 19.Verboeket-van de Venne WP, Westerterp KR. Influence of the feeding frequency on nutrient utilization in man: consequences for energy metabolism. Eur J Clin Nutr. 1991;45:161–169. [PubMed] [Google Scholar]
- 20.Verboeket-van de Venne WP, Westerterp KR. Frequency of feeding, weight reduction and energy metabolism. Int J Obes Relat Metab Disord. 1993;17:31–36. [PubMed] [Google Scholar]
- 21.Verboeket-van de Venne WP, Westerterp KR, Kester AD. Effect of the pattern of food intake on human energy metabolism. Br J Nutr. 1993;70:103–115. doi: 10.1079/bjn19930108. [DOI] [PubMed] [Google Scholar]
- 22.Wolfram G, Kirchgessner M, Muller Hl, Hollomey S. Thermogenesis in humans after varying meal time frequency. Ann Nutr Metab. 1987;31:88–97. doi: 10.1159/000177255. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins DJ, Wolever V, Vuksan F, et al. Nibbling versus gorging: metabolic advantages of increased meal frequency. N Engl J Med. 1989;321:929–934. doi: 10.1056/NEJM198910053211403. [DOI] [PubMed] [Google Scholar]
- 24.Jones PJ, Leitch CA, Pederson RA. Meal-frequency effects on plasma hormone concentrations and cholesterol synthesis in humans. Am J Clin Nutr. 1993;57:868–874. doi: 10.1093/ajcn/57.6.868. [DOI] [PubMed] [Google Scholar]
- 25.Pliquett RU, Fuhrer D, Falk S, Zysset S, von Cramon DY, Stumvoll M. The effects of insulin on the central nervous system—focus on appetite regulation. Horm Metab Res. 2006;38:442–446. doi: 10.1055/s-2006-947840. [DOI] [PubMed] [Google Scholar]
- 26.Melanson EL, Gozansky WS, Barry DW, Maclean PS, Grunwald GK, Hill JO. When energy balance is maintained, exercise does not induce negative fat balance in lean sedentary, obese sedentary, or lean endurance-trained individuals. J Appl Physiol. 2009;107:1847–1856. doi: 10.1152/japplphysiol.00958.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rolls BJ. Carbohydrates, fats, and satiety. Am J Clin Nutr. 1995;61:960S–967S. doi: 10.1093/ajcn/61.4.960S. [DOI] [PubMed] [Google Scholar]
- 28.Melanson EL, Ingebrigtsen JP, Bergouignan A, Ohkawara K, Kohrt WM, Lighton JR. A new approach for flow-through respirometry measurements in humans. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1571–R1579. doi: 10.1152/ajpregu.00055.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melanson EL, MacLean PS, Hill JO. Exercise improves fat metabolism in muscle but does not increase 24-h fat oxidation. Exerc Sport Sci Rev. 2009;37:93–101. doi: 10.1097/JES.0b013e31819c2f0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melanson EL, Sharp TA, Seagle HM, et al. Effect of exercise intensity on 24-h energy expenditure and nutrient oxidation. J Appl Physiol. 2002;92:1045–1052. doi: 10.1152/japplphysiol.00706.2001. [DOI] [PubMed] [Google Scholar]
- 31.Leidy HJ, Tang M, Armstrong CL, Martin CB, Campbell WW. The effects of consuming frequent, higher protein meals on appetite and satiety during weight loss in overweight/obese men. Obesity. 2011;19:818–824. doi: 10.1038/oby.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Speechly DP, Buffenstein R. Greater appetite control associated with an increased frequency of eating in lean males. Appetite. 1999;33:285–297. doi: 10.1006/appe.1999.0265. [DOI] [PubMed] [Google Scholar]
- 33.Speechly DP, Rogers GG, Buffenstein R. Acute appetite reduction associated with an increased frequency of eating in obese males. Int J Obes Relat Metab Disord. 1999;23:1151–1159. doi: 10.1038/sj.ijo.0801046. [DOI] [PubMed] [Google Scholar]
- 34.Stannard SR, Thompson MW, Fairbairn K, Huard B, Sachinwalla T, Thompson CH. Fasting for 72 h increases intramyocellular lipid content in nondiabetic, physically fit men. Am J Physiol Endocrinol Metab. 2002;283:E1185–E1191. doi: 10.1152/ajpendo.00108.2002. [DOI] [PubMed] [Google Scholar]
- 35.Melanson KJ, Westerterp-Plantenga MS, Saris WH, Smith FJ, Campfield LA. Blood glucose patterns and appetite in time-blinded humans: carbohydrate versus fat. Am J Physiol. 1999;277:R337–R345. doi: 10.1152/ajpregu.1999.277.2.R337. [DOI] [PubMed] [Google Scholar]
- 36.Campbell PJ, Carlson MG, Nurjhan N. Fat metabolism in human obesity. Am J Physiol. 1994;266:E600–E605. doi: 10.1152/ajpendo.1994.266.4.E600. [DOI] [PubMed] [Google Scholar]
- 37.Hawley JA. Effect of increased fat availability on metabolism and exercise capacity. Med Sci Sports Exerc. 2002;34:1485–1491. doi: 10.1097/00005768-200209000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Bachman JL, Raynor HA. Effects of manipulating eating frequency during a behavioral weight loss intervention: a pilot randomized controlled trial. Obesity. 2012;20:985–992. doi: 10.1038/oby.2011.360. [DOI] [PubMed] [Google Scholar]
- 39.McCrory MA, Howarth NC, Roberts SB, Huang TT. Eating frequency and energy regulation in free-living adults consuming self-selected diets. J Nutr. 2011;141:148–153. doi: 10.3945/jn.109.114991. [DOI] [PubMed] [Google Scholar]