Abstract
Objective
Restenosis following endovascular treatment of the femoro-popliteal (fem-pop) segment is associated with the inflammatory response produced in the artery wall at the time of the procedure. While local drug delivery to the superficial femoral and popliteal arteries promises improved patency, data are currently limited. We hypothesized that improved percutaneous delivery of an anti-inflammatory compound into the adventitia of the fem-pop at the time of endovascular treatment would be safe, feasible and decrease the inflammatory response.
Methods
This was a prospective, investigator-initiated, phase I, first-in-man study testing the safety and feasibility of percutaneous adventitial delivery of dexamethasone. Following successful intervention, an adventitial micro-infusion catheter was advanced over a 0.014″ wire to the treated segment. Its micro-needle (0.9 mm long × 140 μm diameter) was deployed into the adventitia to deliver dexamethasone (DEX, 4 mg/ml) mixed with contrast agent (80:20 ratio), providing fluoroscopic visualization. The primary safety outcome measure was freedom from vessel dissection, thrombosis or extravasation while the primary efficacy outcome was duplex -determined binary restenosis defined as a peak systolic velocity ratio >2.5.
Results
Twenty patients with Rutherford clinical category 2–5 enrolled in this study. The mean age was 66 and 55% had diabetes mellitus. Treated lesion length was 8.9 ± 5.3 cm and 50% were chronic total occlusions. Eighty percent of treated lesions were in the distal superficial femoral or popliteal arteries. All lesions were treated by balloon angioplasty with provisional stenting (N=6) for suboptimal result. Three patients were treated with atherectomy as well. A mean of 1.6 ± 1.1 mg (0.5 ± 0.3 mL) of dexamethasone sodium phosphate was injected per centimeter of lesion length. In total, a mean of 12.1 ± 6.1 mg of DEX was injected per patient. The mean number of injections required per lesion was 3.0 ± 1.3 cm, minimum 1 and maximum 6 injections. There was 100% technical success of drug delivery and no procedural or drug-related adverse events. The mean Rutherford score decreased from 3.1 ± .7 (median 3.0) pre-operatively to .5 ± .7 at 6 months (median 0.0), P<.00001. Over this same time interval the index leg ABI increased from .68 ± .15 to .89 ± .19, P=.0003. The pre-operative C-reactive protein in this study was 6.9 ± 8.5 indicating severe baseline inflammation which increased to 14.0 ± 23.1 mg/l (103% increase) at 24 hours following the procedure. However, this increase did not reach statistical significance p=.14. Two patients met the primary efficacy endpoint of loss of primary patency by re-occluding their treated segment of the index lesion during the follow up period.
Conclusion
Adventitial drug delivery via a micro-infusion catheter is a safe and feasible alternative to intimal-based methods for adjunctive treatment in the femoro-popliteal segment. The 6-month preliminary results suggest perivascular dexamethasone treatment may improve outcomes following angioplasty to the femoral and popliteal arteries, and support further clinical investigation of this approach .
Introduction
Balloon angioplasty superimposes an acute barotraumatic injury on a chronically inflamed artery, resulting in the recruitment of neutrophils and macrophages into the site of injury. (4, 6, 7) These cells, in concert with intrinsic vascular cells, participate in injury-induced stress responses which ultimately determine the fate of the revascularization procedure. (8, 9) Vascular injury programs, mediated in part by the coordinated activity of the inflammatory transcription factors, nuclear factor κB (NFκB) and activated protein-1 (AP-1), lead to the expression of a broad spectrum of inflammatory proteins resulting in cellular proliferation, fibrous protein production, and remodeling of the vascular wall. (10) The magnitude of the inflammatory response has been linked to subsequent restenosis in the femoropopliteal artery suggesting that therapies which mitigate the initial inflammatory cascade might improve patency. (11)
Glucocorticoids are powerful anti-inflammatory, immune-suppressive and anti-proliferative compounds used to treat a variety of immune-mediated diseases. (12) Dexamethasone is a long-acting synthetic glucocorticoid approved by the FDA to treat inflammatory conditions and is 25 times more potent than hydrocortisone (cortisol) and has no mineralocorticoid cross over activity. (12) While systemic delivery of steroids,(19, 20) following percutaneous coronary intervention have had mixed results in part related to systemic side effects, dexamethasone-coated stents have been more successful demonstrating a reduction in binary restenosis and clinical events. (21–23) However, wide spread adoption has been limited given the older generation stent platforms and the propensity for crystallization on the stent surface.
Endovascular adventitial delivery, through a microinfusion syringe is an attractive alternative for local drug delivery because the drug can be injected directly into the artery at the site of injury without the need for a permanent implanted stent or partitioning from a balloon surface. Adventitial delivery also establishes an outside-in concentration gradient with the majority of the drug distributed in the adventitia and media but relative sparing of endothelial cells. Specifically targeting the adventitia is also of interest as this outer vessel layer actively participates in inflammatory cell recruitment, arterial remodeling, and contribution of cells to neo-intima formation. (2–5) Animal studies have demonstrated that adventitial microinfusion is one of the most efficient means of local drug delivery by maximizing tissue concentration and minimizing plasma levels and non-target organ distribution of drug. (24) Consequently, pilot clinical trials have utilized this approach in the coronary arteries to deliver bone marrow derived stem cells. (25) However, this approach has not been utilized in peripheral arteries. We hypothesized that percutaneous delivery of dexamethasone with a micro-infusion catheter to the adventitia of symptomatic patients with peripheral artery disease would be safe and feasible.
Methods
Study design and patient population
This was a first-in-human study to test the safety and feasibility of dexamethasone administration through a microinfusion catheter (Bullfrog, Mercator MedSystems, Inc, San Leandro, Ca.) into the superficial femoral and popliteal artery (URL:http://www.clinicaltrials.gov. Unique identifier: NCT 01507558). The study design was a prospective, single-center, investigator-initiated study which enrolled consecutive patients who met eligibility requirements from the San Francisco Veteran Affairs Medical Center. This study was approved by the Committee for Human Research and the University of California Clinical and Translational Science Institute. Safety data and outcomes were monitored by a Data Safety and Monitoring Committee which convened on a quarterly basis or as needed.
The primary inclusion criteria were patients suffering from moderate to severe disabling claudication, ischemic rest pain, or minor tissue loss secondary to atherosclerotic lower extremity occlusive disease with TASC II A-D lesions of the SFA or popliteal arteries. The minimal reference vessel lumen diameter was required to be 3–6 mm and the patient was required to have at least one infra-popliteal runoff vessel. Exclusion criteria included serum creatinine ≥ 2.5 mg/dl, prior revascularization of the target limb, known allergy to contrast agents or dexamethasone, estimated life-expectancy less than one year, or other concurrent illness in which the investigators thought would limit the patient’s ability to follow the schedule of assessments.
Micro-infusion catheter
The Bullfrog Micro-Infusion Catheter (Mercator MedSystems, San Leandro, CA) is a rapid-exchange, wire-guided catheter with a balloon-sheathed 0.9 mm long, 35-gauge (140 μm diameter) needle that delivers infusions to adventitial and perivascular tissues, Figure 1. It is FDA 510(k)-cleared for use in coronary and peripheral arteries. It is advanced through a 6-Fr sheath over a 0.014″ wire and can treat vessels from 3–6mm in diameter (Figure 2). Three radio-opaque markers on the catheter allow for proper orientation of the needle. Using standard angioplasty inflation equipment, the balloon is inflated exposing the needle. When the balloon contacts the arterial wall opposite the needle tip, contact pressure forces the needle through the vessel wall and into the adventitia and perivascular tissues. The contact pressure of the balloon against the artery wall is limited to 2 atmospheres by a pressure release valve, which prevents damage to the artery wall. A mixture of infusate and contrast (4:1) is then delivered under fluoroscopic guidance into the adventitia. A test injection of 0.1 ml is made to confirm proper adventitial placement of the microinfusion needle tip. If resistance is met, or the test injection enters the blood stream, the balloon is deflated and the injection is attempted in another location by moving the catheter a few millimeters proximally or distally or rotating the catheter a few degrees. Once adventitia placement is confirmed, the remainder of the infusate is delivered at a rate of 1 ml/min. When the infusion is complete, the balloon is deflated, sheathing the needle, and allowing the catheter to be withdrawn (Figure 2). Injections were administered approximately every 3 cm along the length of the treated arterial segment. Because the drug:contrast admixture can be visualized on both sides of the arterial wall, only one fluoroscopic view is necessary to confirm circumferential arterial coverage in the majority of cases.
Figure 1.
The Bullfrog micro-infusion catheter, A. Diffusion grading scale, B. Figure 1A supplied courtesy of Mercator Medsystems, San Leandro, Cal.
Figure 2.
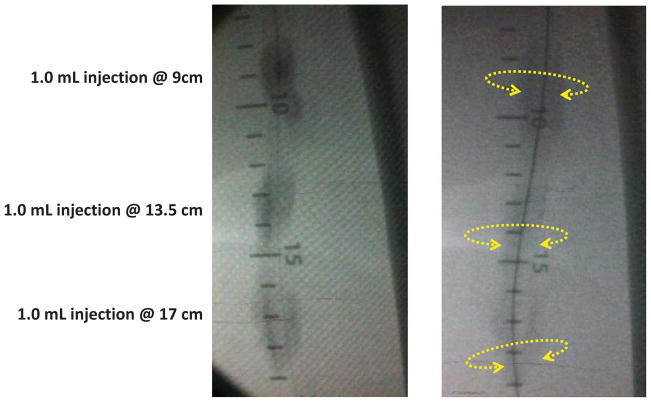
Endovascular treatment with adjunctive dexamethasone. This is a 49 year old man with severe disabling claudication and a 16 cm superficial femoral artery occlusion. Following securing access across the lesion with a glide wire, the lesion was treated with balloon angioplasty. Following successful angioplasty, four 1.0 ml injections were performed along the length of the lesion (only 3 shown). In the left panel there is a discreet contrast blush seen at each injection site. Note that the contrast appears circumferentially at each injection site. Three minutes later, the drug-contrast admixture can be seen to have diffused longitudinally to fully cover the treated segment. The patient is now 2 years from his index procedure and remains and complains of only mild claudication with heavy exertion. He has an ankle-brachial index of 1.09 and a peak systolic velocity ratio of less than 2.5.
Procedure
Patients not taking aspirin or clopidogrel before study enrollment received 325 mg of aspirin 12 hours prior the procedure. Post-procedure, patients were prescribed 81 mg/day of aspirin to be taken indefinitely and 75 mg/day of clopidogrel daily for 12 weeks. Vascular access was accomplished by either the contralateral or ipsilateral (anterograde) approach. Patients received a bolus of 5,000 IU of heparin after insertion of the sheath in the common femoral artery and their activated clotting time was kept above 250 seconds with additional heparin as needed. In the case of chronic total occlusions, all lesions were crossed sub-intimally with a glide wire and glide catheter (Terumo, Somerset, NJ). After securing access across the lesion with a guidewire, the target lesion was treated according to physician preference. All patients were treated with balloon angioplasty. If a flow-limiting dissection or residual stenosis was determined to require a stent the protocol specified for treatment with dexamethasone prior to stent placement. In all cases, the microinfusion catheter was advanced to the treatment site following angioplasty to deliver dexamethasone into the arterial adventitia.
Following the procedure, all patients were admitted for a 23-hour observation period for access site, adverse event, and revascularization monitoring. Prior to discharge, ABIs and arterial duplex ultrasound studies were performed in the vascular laboratory. Blood was drawn at baseline and at 24 hours following the procedure to assess the inflammatory response.
Dexamethasone dosing and rationale
The dosage utilized in this protocol was an off-the-shelf concentration of dexamethasone sodium phosphate for injection USP, 4 mg/ml, which is approved for reducing soft tissue inflammation. Specifically dexamethasone is indicated for soft tissue injection of 0.4 to 6 mg to treat acute exacerbations in a variety of inflammatory conditions. Based on these similar uses of the drug to treat localized inflammation, it was postulated that a similar dose (2 to 6 mg) should be used to treat each 3 cm of lesion (0.7 to 2 mg/cm), allowing for multiple infusions in the case of long lesions. The 3 cm benchmark was chosen based on typical longitudinal peri-vascular diffusion patterns in preclinical ex vivo cadaveric femoral artery studies (unpublished). The dexamethasone sodium phosphate for injection USP, which contains 4.0 mg dexamethasone phosphate per milliliter, was mixed 80%:20% with an iso-osmolar iodinated contrast medium (iodixanol 320 mg I/ml, GE HealthCare, Cork, Ireland) resulting in a final concentration of 3.2 mg dexamethasone phoshate and 60–74 mg of iodine in each milliliter of solution. The final dosing target was therefore determined to be approximately 0.5 ml of the diluted drug per centimeter of lesion or 1.6 mg/cm. The co-infusion of contrast medium with the drug allowed the x-ray fluoroscopic visualization required to positively assess infusion success, Figure 2 and 3. All infusions were graded based on the circumferential and longitudinal distribution of the drug/contrast infusate and the coverage of the target lesion, Figure 1. For example, an infusion that was completely circumferential and extended 3 cm in either direction from the point of injection would be considered a diffusion grade A. If infusions were only partially circumferential or partially longitudinal then a grade of B was given and so on according to the figure.
Figure 3.
Examples of typical dexamethasone-contrast diffusion patterns in patients treated in this study.
Patient Assessment and Endpoint definitions
Medical history was obtained before the procedure, including concomitant medication use, Rutherford clinical category, resting ankle brachial index, and laboratory results for baseline C-reactive protein, serum creatinine, and lipids. Adverse event evaluation was performed at the end of the index procedure and at each follow up visit. Patients were then reassessed with vascular history and physical examination, ABIs, and duplex arterial ultrasound examinations at 1, 3, and 6 months. The primary safety endpoint was freedom from death, vessel perforation, dissection, thrombosis, or pseudoaneurysm formation within 30 days following the procedure. The primary feasibility endpoint was procedural success for adventitial infusion of dexamethasone and contrast at the target lesion as determined by the relationship of the fluoroscopic blush to the treatment segment. While not powered for an efficacy signal, the primary efficacy endpoint was primary patency rate defined as freedom from the combined endpoints of target lesion revascularization (TLR), occlusion, or > 50% restenosis in the treated lesion. Duplex ultrasonography was performed to assess restenosis and >50% restenosis was defined by a peak systolic velocity ratio (PSVR) >2.5. Rates of TLR, death, and amputation endpoints were also analyzed. Secondary endpoints were change in Rutherford classification and ankle-brachial index (ABI) from baseline to 6 months.
Inflammation as detected by plasma CRP has been linked to restenosis following peripheral intervention. As one of our intended goals was to reduce inflammation following vascular intervention, serum CRP was measured at baseline and 24 hours following the procedure.
Statistical analysis
This study was not powered for clinical outcomes. Normally distributed continuous variables were expressed as mean and standard deviation and evaluated with the Student’s t test or one way analysis of variance where appropriate. Proportions were evaluated by the chi-squared test. Rutherford classification and categorical variables were assessed by the Kruskal-Wallis test. Safety parameters were collected and assessed qualitatively or summarized quantitatively by descriptive statistics. Statistical significance was set at the 2-tailed .05 level.
Results
Patient Profile and Lesion Characteristics
Demographic and lesion characteristics are presented in Table 1. In brief, twenty male patients were enrolled in this study with 35% African American and 50% caucasian. The mean age of this cohort was 66.5 ± 9.8 years and 55% had diabetes mellitus. Eighty percent of the patients had claudication, the majority had a pre-operative Rutherford score of 3 (65%) and the mean pre-operative ankle brachial index was .68 ± .15. Lesion characteristics treated in this study are presented in table 2. The mean lesion length was 8.9 ± 5.3 cm (2.3 – 25.2 cm) and 50% of treated lesions were chronic total occlusions. Eighty percent of lesions were located in the distal SFA and/or popliteal artery. The mean reference vessel diameter was 4.8 ± .1 mm. Six patients (30%) required the placement of a self-expanding stent due to residual stenosis or flow limiting dissection following balloon angioplasty. The lesion characteristics of the patients who received stents including percentage occlusions or lesion length was not different than those who were not stented.
Table 1.
Baseline Patient Demographics and Clinical Characteristics
| Age (mean ± SD) | 66 ± 10 |
|
| |
| Gender (male,%) | 20 (100%) |
|
| |
| Race | |
| Caucasian (N, %) | 10 (50%) |
| African American (N, %) | 7 (35%) |
| Hispanic (N, %) | 2 (10%) |
| Asian (N,%) | 1 (5%) |
|
| |
| Diabetes mellitus (N, %) | 11 (55%) |
|
| |
| Coronary artery disease (N,%) | 11 (55%) |
|
| |
| Hypertension (N, %) | 19 (95%) |
|
| |
| Hyperlipidemia (N, %) | 20 (100%) |
|
| |
| Body mass index (mean ± SD, kg/m2) | 27.4 ± 4.5 |
|
| |
| Creatinine (mean ± SD, mg/dl) | 1.0 ± .34 |
|
| |
| C-reactive protein (mean ± SD, mg/l) | 6.9 ± 8.5 |
|
| |
| Total cholesterol (mean ± SD, mg/dl) | 149.1 ± 37.5 |
|
| |
| Rutherford classification | 3 = Moderate claudication 13 = Severe claudication 3 = Ischemic rest pain 1 = Minor tissue loss |
|
| |
| Index limb ankle brachial index (mean ± SD) | .68 ± .15 |
Table 2.
Baseline Lesion Characteristics
| SFA location (N, %) | Proximal SFA (2, 10%) Mid SFA (2, 10%) Distal SFA (8, 40%) Popliteal (8, 40%) |
| Mean lesion length cm (mean ± SD)a | 8.9 ± 5.3 |
| Reference vessel diameter (mean ± SD, mm) | 4.8 ± .1 |
| Diameter stenosis (%) | 78.5 |
| Occlusion (%) | 10 (50%) |
| Mean % occlusion (mean ± SD) | 88 ± 12 |
| TASC II classification | A = 5 B = 11 C = 2 D = 2 |
| Revascularization method | PTA in 20 patients (100%) + atherectomy in 3 patients (15%) + provisional stent in 6 patient (30%) |
SD, standard deviation; SFA superficial femoral artery
Normal-to-normal lesion length as assessed by principal investigator
Safety of Adventitial Delivery of Dexamethasone
There were no device-related adverse events in this study. There were no amputations or deaths at 30-days or during follow up. There were no dissections, pseudoaneurysm formation, or 30 day thrombosis. There was one case of hyperglycemia following dexamethasone treatment in a long-standing diabetic patient who did not receive his hypoglycemic medications.
Technical Considerations and Feasibility of Femoropopliteal Adventitial Delivery
In all cases dexamethasone was able to be delivered to the adventitia of the target lesion. The mean number of injections required per lesion was 3.0 ± 1.3 cm, minimum 1 and maximum 6 injections. Each injection was graded on an ordinal descriptive scale as depicted in Figure 1. In 19 out of 20 subjects there was complete circumferential coverage of the lesion with the infusate as assessed immediately after the infusion (grade = A). In 1 patient there was only partial coverage noted by contrast distribution (grade = B). The mean volume injected was 3.8 ± 1.9 ml which contained a mean of 12.1 ± 6.1 mg of dexamethasone sodium phosphate and .80 ± .4 ml of contrast. This equated to a mean of 1.6 ± 1.1 mg of dexamethasone sodium phosphate per centimeter of lesion length. The minimal dose was 3.2 mg and the maximal dose a patient received was 24 mg of dexamethasone sodium phosphate. Accordingly there was a positive linear correlation between the amount of dexamethasone received and length of lesion treated, R2=.27, P=.019.
Inflammatory Response
The post-intervention immune response following femoropopliteal intervention has been shown to be independently associated with subsequent restenosis. The pre-operative CRP for subjects in this study was 6.9 ± 8.5 indicating severe baseline inflammation which increased to 14.0 ± 23.1 mg/l (103% increase) at 24 hours following the procedure indicating that there was an inflammatory response following peripheral intervention. However, this increase did not reach statistical significance p=.14.
Effectiveness
Two patients in this study reached the primary endpoint of loss of primary patency by duplex ultrasound-determined binary restenosis by 6 months. The first, a 77-year old man who had an 11.9 cm CTO involving the distal SFA extending into the popliteal artery treated with balloon angioplasty and a 7 by 100 mm Everflex stent (Covidien, Plymouth MN, USA) was found to have re-occluded his lesion at 172 days following the procedure. The second patient is a 63-year old man that had a 10 cm popliteal artery occlusion which was treated by angioplasty and was found to have re-occluded 182 days following his procedure. The mean pre-operative Rutherford score decreased from 3.1 ± .71 (median 3.0) pre-operatively to .5 ± .70 (median 0) at 6 months, P<.00001. Over this same time interval the pre-operative index leg ABI increased from .68 ± .15 (range .22 to .89) to .89 ± .19 (range .49 to 1.2), P=.0003, Figure 4.
Figure 4.
The mean ankle-brachial index (ABI) is significantly improved from baseline across all time points post procedure.
Discussion
This first-in-human study establishes the safety and feasibility of adventitial delivery of dexamethasone into the femoropopliteal artery to augment patency after therapeutic interventions. An additional novel feature of this methodology is that the drug-contrast admixture can be seen “staining” the area of interest thereby providing visual confirmation of drug delivery. Because the Bullfrog catheter is a microinfusion device, the dose applied can be titrated to deliver a desired concentration of drug, and scaled to the length of artery treated rather than relying on passive elution from a fixed-length stent or balloon surface. Therefore this represents a significant departure from intimal-based drug delivery methods and is the first application of this technology to the peripheral vasculature.
Additional novelty is found in the ability to percutaneously deliver the anti-inflammatory drug dexamethasone to the adventitia, hence establishing a reverse concentration gradient with maximal drug concentration located in the adventitia and outer media with relatively sparing of the endothelial cell layer. This outside-in approach of drug delivery specifically targets the adventitia which has been shown to be an active participant in vascular remodeling after angioplasty. (4, 6) Once thought to be a passive layer within the arterial wall, the tunica adventitia is now known to be extremely metabolically active and capable of regulating vascular homeostasis. (3) Following vascular injury, resident adventitial fibroblasts undergo transformation into the myofibroblasts which have been shown to contribute to neo-intimal growth and vessel contraction. In porcine models of balloon angioplasty, injections with 5-bromo-2-deoxyuridine (BrDU) have identified the majority of the first wave of proliferating cells to be located in the adventitia as early as 2 days following injury. (27, 28) Further Shi et al, demonstrated that adventitial myofibroblast both contribute to synthesis of extracellular matrix including collagen and migrate into the developing neointima following balloon injury. (29, 30) Finally utilizing in vivo reporter gene transfer studies into adventitial cells, Siow et al demonstrated adventitial myofibroblast migration into the media and neointima following balloon injury. (5) Collectively, these studies provide important biological rationale supporting the targeting the adventitia to reduce the rate of restenosis.
The hypothesis that restenosis principally occurs in those whose inflammation associated with vascular healing is excessive or is not appropriately turned off is intriguing but has not been clinically confirmed. However, it has been known for over 25 years that inflammation is involved in nearly every step in restenosis and that adventitia harbors a rich array of inflammatory cells including macrophages, dendritic cells, lymphocytes, neutrophils and mast cells. (31) Within hours following vascular wall injury, additional inflammatory cells home to the site of injury and liberate cytokines, growth factors and reactive oxygen species (ROS) that contribute to the proliferation of smooth muscle cells (SMC) and adventitial cells. (32) There is increasing evidence that the magnitude of inflammation at baseline as well as the spike in inflammation following vascular intervention is correlated with the subsequent restenosis. (26) In this regard Schillinger et al, demonstrated that plasma CRP increased in patients without restenosis by 113% whereas CRP increased by 155% at 24 hours in patients who subsequently developed restenosis of their treated lesions. (26) Accordingly therapies specifically targeting inflammation may inhibit or dampen the proliferative response.
Glucocorticoids are ideal candidates to repress angioplasty-induced immune response programs by inhibiting inflammatory transcription factors, NFκB and AP-1, and their downstream mediators. (10, 13) Preclinical studies have demonstrated that dexamethasone down-regulates monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor α (TNF-α) and interleukin 1β in nano- and micromolar concentrations in vascular smooth muscle cells. (14, 15) In the specific context of neointimal hyperplasia and vascular restenosis, dexamethasone has been shown to inhibit the proliferation and migration of smooth muscle and inflammatory cells as well as adventitial myofibroblasts through effects on thymidine kinase, matrix metalloproteinases, and retinoblastoma protein. (16–18) Clincally DEX has been shown to reduce soluble inflammatory proteins following vascular intervention. For example, patients with unstable angina treated with dexamethasone-eluting stents had lower plasma concentrations of CRP, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 post-intervention compared to patients treated with a bare metal stent. (33, 34) Dexamethasone induces the anti-inflammatory proteins annexin 1 and mitogen activated kinase phosphatase 1 while repressing the transcription of pro-inflammatory molecules such as cyclooxygenase 2. (35) Dexamethasone also markedly inhibits the production of ROS by mononuclear cells and polymorphonuclear leukocytes in vivo. Dexamethasone increases the immune-modulatory cytokine interleukin 10 which is known to inhibit the pro-inflammatory TH1 cells. (36) Finally by delivering an anti inflammatory drug shown to improve endothelial cell migration, the need for dual anti-platelet therapy required after a limus or taxol eluting stent or balloon may not be necessary.
Limitations
This was a pilot safety and feasibility study with short follow up of a heterogeneous group of symptomatic patients with PAD. The lesions were distributed in the SFA and popliteal artery and treated with a variety of interventions including angioplasty and atherectomy. Provisional stenting was applied when necessary. The intent of the study was to determine the safety and feasibility of adventitial steroid delivery in the periphery. Nevertheless this was an extremely morbid cohort reflected by a mean CRP > 6 mg/l with challenging lesion characteristics. The treated lesion was over 8 cm and 50% of lesions were chronic total occlusions. Further, 80% of treated lesions included the distal SFA and popliteal arteries which have traditionally been considered no stent zones. No core facility was employed to either adjudicate duplex ultrasound or angiographic results in this study. While the 6 month patency results were considered to be excellent, no conclusions about efficacy can be drawn from a pilot trial design.
Conclusions
In conclusion, this pilot study establishes that adventitial delivery of dexamethasone using a micro-infusion catheter following femoropopliteal intervention is safe and feasible. This method of local drug delivery represents an alternative to intimal-based delivery platforms and may have unique advantages. Further study is required to determine the efficacy of this approach to improve vessel patency.
Acknowledgments
This work is supported by the National Institutes of Health K23 HL-92163 and by the National Center for Advancing Translational Sciences, National Institutes of Health, through the University of California San Francisco Clinical and Translational Science Institute grant number KL2 TR000143. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. This work was performed through Vascular Integrated Physiology and Experimental Therapeutics Laboratory.
The authors would like to gratefully thank Mercator MedSystems for donating The Bullfrog micro-infusion devices used in this project, and Kirk Seward, PhD, from Mercator MedSystems for guidance in the use of the product and scientific discussions.
Footnotes
Author conflict of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tzafriri AR, Vukmirovic N, Kolachalama VB, Astafieva I, Edelman ER. Lesion complexity determines arterial drug distribution after local drug delivery. J Control Release. 2010;142(3):332–8. doi: 10.1016/j.jconrel.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu Y, Xu Q. Adventitial biology: differentiation and function. Arterioscler Thromb Vasc Biol. 2011;31(7):1523–9. doi: 10.1161/ATVBAHA.110.221176. [DOI] [PubMed] [Google Scholar]
- 3.Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovasc Res. 2007;75(4):640–8. doi: 10.1016/j.cardiores.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okamoto E, Couse T, De Leon H, Vinten-Johansen J, Goodman RB, Scott NA, et al. Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation. 2001;104(18):2228–35. doi: 10.1161/hc4301.097195. [DOI] [PubMed] [Google Scholar]
- 5.Siow RC, Mallawaarachchi CM, Weissberg PL. Migration of adventitial myofibroblasts following vascular balloon injury: insights from in vivo gene transfer to rat carotid arteries. Cardiovasc Res. 2003;59(1):212–21. doi: 10.1016/s0008-6363(03)00292-x. [DOI] [PubMed] [Google Scholar]
- 6.Wilcox JN, Okamoto EI, Nakahara KI, Vinten-Johansen J. Perivascular responses after angioplasty which may contribute to postangioplasty restenosis: a role for circulating myofibroblast precursors? Ann N Y Acad Sci. 2001;947:68–90. doi: 10.1111/j.1749-6632.2001.tb03931.x. dicussion -2. [DOI] [PubMed] [Google Scholar]
- 7.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 8.Moreno PR, Bernardi VH, Lopez-Cuellar J, Newell JB, McMellon C, Gold HK, et al. Macrophage infiltration predicts restenosis after coronary intervention in patients with unstable angina. Circulation. 1996;94(12):3098–102. doi: 10.1161/01.cir.94.12.3098. [DOI] [PubMed] [Google Scholar]
- 9.Farb A, Weber DK, Kolodgie FD, Burke AP, Virmani R. Morphological predictors of restenosis after coronary stenting in humans. Circulation. 2002;105(25):2974–80. doi: 10.1161/01.cir.0000019071.72887.bd. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez MV, Jimenez B, Berciano MT, Gonzalez-Sancho JM, Caelles C, Lafarga M, et al. Glucocorticoids antagonize AP-1 by inhibiting the Activation/phosphorylation of JNK without affecting its subcellular distribution. J Cell Biol. 2000;150(5):1199–208. doi: 10.1083/jcb.150.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schillinger M, Exner M, Mlekusch W, Rumpold H, Ahmadi R, Sabeti S, et al. Vascular inflammation and percutaneous transluminal angioplasty of the femoropopliteal artery: association with restenosis. Radiology. 2002;225(1):21–6. doi: 10.1148/radiol.2251011809. [DOI] [PubMed] [Google Scholar]
- 12.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335(1):2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107(2):135–42. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruun JM, Lihn AS, Pedersen SB, Richelsen B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab. 2005;90(4):2282–9. doi: 10.1210/jc.2004-1696. [DOI] [PubMed] [Google Scholar]
- 15.Franchimont D, Martens H, Hagelstein MT, Louis E, Dewe W, Chrousos GP, et al. Tumor necrosis factor alpha decreases, and interleukin-10 increases, the sensitivity of human monocytes to dexamethasone: potential regulation of the glucocorticoid receptor. J Clin Endocrinol Metab. 1999;84(8):2834–9. doi: 10.1210/jcem.84.8.5931. [DOI] [PubMed] [Google Scholar]
- 16.Longenecker JP, Kilty LA, Johnson LK. Glucocorticoid influence on growth of vascular wall cells in culture. J Cell Physiol. 1982;113(2):197–202. doi: 10.1002/jcp.1041130203. [DOI] [PubMed] [Google Scholar]
- 17.Reil TD, Kashyap VS, Sarkar R, Freishlag J, Gelabert HA. Dexamethasone inhibits the phosphorylation of retinoblastoma protein in the suppression of human vascular smooth muscle cell proliferation. J Surg Res. 2000;92(1):108–13. doi: 10.1006/jsre.2000.5942. [DOI] [PubMed] [Google Scholar]
- 18.Longenecker JP, Kilty LA, Johnson LK. Glucocorticoid inhibition of vascular smooth muscle cell proliferation: influence of homologous extracellular matrix and serum mitogens. J Cell Biol. 1984;98(2):534–40. doi: 10.1083/jcb.98.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribichini F, Tomai F, De Luca G, Boccuzzi G, Presbitero P, Pesarini G, et al. Immunosuppressive therapy with oral prednisone to prevent restenosis after PCI. A multicenter randomized trial. Am J Med. 2011;124(5):434–43. doi: 10.1016/j.amjmed.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 20.Ribichini F, Tomai F, Pesarini G, Zivelonghi C, Rognoni A, De Luca G, et al. Long-term clinical follow-up of the multicentre, randomized study to test immunosuppressive therapy with oral prednisone for the prevention of restenosis after percutaneous coronary interventions: Cortisone plus BMS or DES veRsus BMS alone to EliminAte Restenosis (CEREA-DES) Eur Heart J. 2013;34(23):1740–8. doi: 10.1093/eurheartj/eht079. [DOI] [PubMed] [Google Scholar]
- 21.Konig A, Leibig M, Rieber J, Schiele TM, Theisen K, Siebert U, et al. Randomized comparison of dexamethasone-eluting stents with bare metal stent implantation in patients with acute coronary syndrome: serial angiographic and sonographic analysis. Am Heart J. 2007;153(6):979, e1–8. doi: 10.1016/j.ahj.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Huang Y, Hanet C, Vandormael M, Legrand V, Dens J, et al. Study of antirestenosis with the BiodivYsio dexamethasone-eluting stent (STRIDE): a first-inhuman multicenter pilot trial. Catheter Cardiovasc Interv. 2003;60(2):172–8. doi: 10.1002/ccd.10636. discussion 9. [DOI] [PubMed] [Google Scholar]
- 23.Park YM, Han SH, Lee K, Suh SY, Oh PC, Chung WJ, et al. Dexamethasone-eluting stents had sustained favorable ischemic driven target lesion revascularization rates over 5 years: a randomized controlled prospective study. Int J Cardiol. 2013;165(2):359–62. doi: 10.1016/j.ijcard.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 24.Karanian JW, Peregoy JA, Chiesa OA, Murray TL, Ahn C, Pritchard WF. Efficiency of drug delivery to the coronary arteries in swine is dependent on the route of administration: assessment of luminal, intimal, and adventitial coronary artery and venous delivery methods. J Vasc Interv Radiol. 2010;21(10):1555–64. doi: 10.1016/j.jvir.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 25.Penn MS, Ellis S, Gandhi S, Greenbaum A, Hodes Z, Mendelsohn FO, et al. Adventitial delivery of an allogeneic bone marrow-derived adherent stem cell in acute myocardial infarction: phase I clinical study. Circ Res. 2012;110(2):304–11. doi: 10.1161/CIRCRESAHA.111.253427. [DOI] [PubMed] [Google Scholar]
- 26.Schillinger M, Haumer M, Schlerka G, Mlekusch W, Exner M, Ahmadi R, et al. Restenosis after percutaneous transluminal angioplasty in the femoropopliteal segment: the role of inflammation. J Endovasc Ther. 2001;8(5):477–83. doi: 10.1177/152660280100800509. [DOI] [PubMed] [Google Scholar]
- 27.Scott NA, Cipolla GD, Ross CE, Dunn B, Martin FH, Simonet L, et al. Identification of a potential role for the adventitia in vascular lesion formation after balloon overstretch injury of porcine coronary arteries. Circulation. 1996;93(12):2178–87. doi: 10.1161/01.cir.93.12.2178. [DOI] [PubMed] [Google Scholar]
- 28.Wilcox JN, Waksman R, King SB, Scott NA. The role of the adventitia in the arterial response to angioplasty: the effect of intravascular radiation. Int J Radiat Oncol Biol Phys. 1996;36(4):789–96. doi: 10.1016/s0360-3016(96)00299-4. [DOI] [PubMed] [Google Scholar]
- 29.Shi Y, O’Brien JE, Jr, Ala-Kokko L, Chung W, Mannion JD, Zalewski A. Origin of extracellular matrix synthesis during coronary repair. Circulation. 1997;95(4):997–1006. doi: 10.1161/01.cir.95.4.997. [DOI] [PubMed] [Google Scholar]
- 30.Shi Y, O’Brien JE, Fard A, Mannion JD, Wang D, Zalewski A. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation. 1996;94(7):1655–64. doi: 10.1161/01.cir.94.7.1655. [DOI] [PubMed] [Google Scholar]
- 31.Macdonald RG, Panush RS, Pepine CJ. Rationale for use of glucocorticoids in modification of restenosis after percutaneous transluminal coronary angioplasty. Am J Cardiol. 1987;60(3):56B–60B. doi: 10.1016/0002-9149(87)90486-3. [DOI] [PubMed] [Google Scholar]
- 32.Rey FE, Pagano PJ. The reactive adventitia: fibroblast oxidase in vascular function. Arterioscler Thromb Vasc Biol. 2002;22(12):1962–71. doi: 10.1161/01.atv.0000043452.30772.18. [DOI] [PubMed] [Google Scholar]
- 33.Patti G, Chello M, Pasceri V, Colonna D, Carminati P, Covino E, et al. Dexamethasone-eluting stents and plasma concentrations of adhesion molecules in patients with unstable coronary syndromes: results of the historically controlled SESAME study. Clin Ther. 2005;27(9):1411–9. doi: 10.1016/j.clinthera.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Patti G, Pasceri V, Carminati P, D’Ambrosio A, Carcagni A, Di Sciascio G. Effect of dexamethasone-eluting stents on systemic inflammatory response in patients with unstable angina pectoris or recent myocardial infarction undergoing percutaneous coronary intervention. Am J Cardiol. 2005;95(4):502–5. doi: 10.1016/j.amjcard.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 35.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711–23. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 36.Dandona P, Mohanty P, Hamouda W, Aljada A, Kumbkarni Y, Garg R. Effect of dexamethasone on reactive oxygen species generation by leukocytes and plasma interleukin-10 concentrations: a pharmacodynamic study. Clin Pharmacol Ther. 1999;66(1):58–65. doi: 10.1016/S0009-9236(99)70054-8. [DOI] [PubMed] [Google Scholar]